Summary
Background
Sputum obtained either under instruction from a health-care worker or through induction can improve case detection of active tuberculosis. However, the best initial sputum sampling strategy for adults with suspected smear-negative or sputum-scarce tuberculosis in primary care is unclear. We compared these two methods of sample acquisition in such patients.
Methods
In this randomised controlled trial, we enrolled adults (age ≥18 years) with sputum-scarce or smear-negative suspected tuberculosis from three primary care clinics in Cape Town, South Africa. Patients were randomly assigned (1:1) to receive either health-care worker instruction or induction to obtain sputum samples. Neither patients nor investigators were masked to allocation. The primary outcome was the proportion of patients who had started treatment after 8 weeks in a modified intention-to-treat population. Secondary outcomes were proportions starting treatment within different time periods, proportion of patients producing sputum for diagnosis, adverse effects, sputum samples’ quality, and case detection by diagnostic method. This study is registered with ClinicalTrials.gov, number NCT01545661.
Findings
We enrolled 481 patients, of whom 213 were assigned to health-care worker instruction versus 268 assigned to induction. The proportion of patients who started treatment in the 8 weeks after enrolment did not differ significantly between groups (53/213 [25%] vs 73/268 [27%]; OR 0.88, 95% CI 0.57–1.36; p=0.56). A higher proportion of instructed versus induced patients initiated empiric treatment based on clinical and radiography findings (32/53 [60%] vs 28/73 [38%]; p=0.015). An adequate sputum sample ≥1 mL was acquired in a lower proportion of instructed versus induced patients (164/213 [77%] vs 238/268 [89%]; p<0.0001), and culture-based diagnostic yield was lower in instructed versus induced patients (24/213 [11%] vs 51/268 [19%]; p=0.020). However, same-day tuberculosis case detection was similar in both groups using either smear microscopy (13/213 [6%] vs 22/268 [8%]; p=0.38) or Xpert-MTB/RIF assay (13/89 [15%] vs 20/138 [14%]; p=0.98). No serious adverse events occurred in either group; side-effects related to sample acquisition were reported in 32 of 268 (12%) patients who had sputum induction and none who had instruction. Cost per procedure was lower for instructed than for induced patients (US$2.14 vs US$7.88).
Interpretation
Although induction provides an adequate sample and a bacteriological diagnosis more frequently than instruction by a health-care worker, it is more costly, does not result in a higher proportion of same-day diagnoses, and—because of widespread empiric treatment—may not result in more patients starting treatment. Thus, healthcare worker instruction might be the preferred strategy for initial collection of sputum samples in adults with suspected sputum-scarce or smear-negative tuberculosis in a high burden primary care setting.
Funding
South African National Research Foundation, European Commission, National Institutes of Health, European and Developing Countries Clinical Trials Partnership, Discovery Foundation.
Introduction
Tuberculosis kills more than 1 million people in Africa every year.1 Several hurdles hamper effective control of tuberculosis, but an inability to access new and accurate diagnostic instruments is a major unmet need that is crucial to achieving the Millennium Development Goals.2 Confirmation of tuberculosis requires not only an effective diagnostic test, but also acquisition of a biological sample of adequate volume and quality. Thus, obtaining such a sample is as important as having access to an accurate diagnostic device, especially in regions with high HIV prevalence, where most notified cases of tuberculosis are smear-negative or sputum-scarce (patient is unable to produce sputum),1 use of empiric tuberculosis treatment is common, and tuberculosis-related mortality is high.3 Moreover, people with a negative smear are more likely to be admitted to hospital and have delays in diagnosis than are people with a positive smear.3 WHO has recommended the Xpert MTB/RIF assay (Cepheid; CA, USA) for the frontline diagnosis of active tuberculosis in people with HIV.4 However, around one in five people with both HIV and tuberculosis will have a negative result,5–7 and this assay can only be used in patients who are able to provide a sputum sample. Thus, despite the advent of new diagnostic techniques, interventions to improve sample acquisition are urgently needed in primary care, where early diagnosis will have the greatest effect.8
Sputum can be safely acquired from sputum-scarce or smear-negative patients through induction using ultrasonic nebulisation with hypertonic saline.9–11 Low cost, outdoor sputum induction booths with adequate infection control could help to make induction more feasible in resource-limited, HIV-prevalent primary care settings, and several already operate in South African primary care clinics. An alternative effective sputum-sampling method is instruction by a health-care worker,12,13 in which a healthcare worker provides simple training to the patient in sputum expectoration. However, which of these strategies is best for people with sputum-scarce or smear-negative tuberculosis in primary care is unclear. Furthermore, the effects of either technique on patient-oriented outcomes—eg, treatment initiation and time to treatment—in settings where empiric treatment is common, are unknown. Assessment of diagnostic strategies using patient-centred outcomes as primary endpoints is recognised by WHO advisory groups as essential for endorsement and scale-up.14 Therefore, we did a randomised controlled trial to compare these two sampling strategies for adults with smear-negative or sputum-scarce tuberculosis in primary care.
Methods
Study design and participants
We did this open-label pragmatic randomised controlled trial in three primary care clinics in Cape Town, South Africa. The first patient was enrolled on Aug 7, 2009, and follow-up was completed on May 5, 2012. The study was approved by the University of Cape Town Human Research Ethics Committee.
Eligibility criteria were: age at least 18 years, ongoing symptoms suggestive of tuberculosis, and either an inability to self-expectorate a sputum sample or two negative sputum smear-microscopy samples (self-expectorated within the preceeding 4 weeks). We included both HIV-positive and HIV-negative patients. Patients were excluded if their initial spontaneous sputum samples were assessed with MTB/RIF assay rather than smear microscopy. Patients were compensated 50 rand for transport and absence from work when attending follow-up, non-routine, study clinic visits. Written informed consent was obtained from all patients and the standard of care was not altered by study participation.
Randomisation and masking
Patients were referred for study screening by the designated nursing staff at each primary care clinic, after which they were assessed by a study research nurse. We used a simple randomisation strategy without stratification or masking. 600 unmarked, opaque envelopes each containing an intervention group assignment (in a 1:1 ratio) were made by personnel not involved in patient enrolment. The unmarked envelopes were shuffled by hand and distributed to each clinic in batches of 100. At the clinic, enrolment was done by the study nurse before either a doctor’s assessment or a chest radiograph. After providing informed consent and completing a detailed clinical record form, patients selected an envelope to determine their allocation. Intervention group cards were then stored with patient clinical record forms and frequent unannounced checks were made by the researcher to confirm adherence to the randomisation protocol.
Procedures
Patients allocated to receive health-care worker instruction were individually instructed in their native language by the study nurse. Sputum induction was done by a trained study nurse using ultrasonically nebulised 5% hypertonic saline in an outdoor, open-air ventilated booth. Both instruction and induction occurred only once after enrolment. The appendix shows full step-by-step details of both procedures, as well as sample processing and laboratory methods.
All patients were asked to provide two spot sputum samples. Samples of at least 1 mL, irrespective of visual quality, were sent for processing at the National Health Laboratory (Cape Town, South Africa). Results for smear microscopy were available within 24 h. If a patient provided two samples they were randomly labelled sputum 1 and sputum 2. Sputum 1 was processed with N-acetyl-L-cysteine and sodium hydroxide, centrifuged, and resuspended in 1.5 mL phosphate buffer. The sample was subjected to auramine O staining and fluorescence microscopy; 0.5 mL of the sediment was inoculated into a Mycobacterial Growth Indicator Tube (Becton Dickinson Diagnostics; Franklin Lakes, NJ, USA) and incubated for no more than 8 weeks. Sputum 2 was unprocessed and frozen at −20°C within 6 h of acquisition. Xpert MTB/RIF testing was unavailable at enrolment. After the study was completed, available sputum 2 specimens were thawed and tested with the Xpert MTB/RIF assay15 If patients provided only a single sputum sample, this was processed as sputum 1.
As per standard clinic guidelines, patients had chest radiography after enrolment and sputum sampling, and were scheduled to return to the clinic for a doctor’s assessment. All patients—except those who were lost to follow-up or who had a positive sputum smear—were assessed by a doctor at least once, but usually twice; first, as soon as possible after enrolment (usually within 7 days), and second, at the 8 week follow-up visit (unless otherwise specified). Chest radiographs, treatment regimens, and culture results of all sputum smear-positive patients referred directly for treatment were reviewed by the study doctor. If extrapulmonary tuberculosis was suspected, additional non-sputum samples were taken at the doctor’s discretion. The timing and initiation of treatment was decided by the attending doctor, and the basis for starting treatment (smear microscopy, clinical or chest radiography [empiric], or culture) was recorded. Throughout the study, a specialist physician or pulmonologist reviewed the medical files of all patients who were treated, any patient of concern to the attending doctor, and a random selection of remaining, untreated patients. We calculated the cost of each sputum sampling strategy by an ingredients approach (total expenditure is presented as a sum of the components).16 Costs are expressed in 2012 $US at an exchange rate of $1=ZAR8.20 based on the UN rate of exchange in September, 2012 (appendix).
The primary outcome was the proportion of patients who started treatment for tuberculosis during the 8 week study period. Secondary outcomes were: time-specific proportions of patients starting treatment within 3, 5, 7, 10, 14, 21, and 56 days from enrolment, the proportion of patients producing sputum for diagnostic testing, adverse effects related to sampling procedures, the quality of sputum samples as measured by the Bartlett score,17 and tuberculosis case detection by diagnostic method (smear microscopy, MTB/RIF assay, or culture).
Statistical analysis
Published9 and unpublished data suggested that 15–20% of the study population would have a positive culture. Thus, we chose a target sample size of 500 patients, which would provide at least 80% power to detect a 10% difference in the proportion of patients starting treatment (overall and at prespecified points), assuming roughly 15% treatment initiation in the instructed group and 25% in the induction group, with 5% type 1 error. We used STATA IC (version 10) for all statistical anlayses. We did a modified intention-to-treat analysis with the χ2 and Wilcoxon rank-sum tests to compare groups, with no corrections for mulitple testing made for secondary outcomes. We calculated point estimates and odds ratios (ORs) with 95% CIs together with p values, all of which were two-sided.
The study is registered with ClinicalTrials.gov, number NCT01545661.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile. We screened 517 patients and included 481 in the analysis (213 assigned to healthcare worker instruction, 268 assigned to sputum induction). Table 1 shows baseline characteristics. 262 patients were male and 171 were HIV positive. In patients who were HIV positive at enrolment, median CD4 cell count was 242 cells per mL (IQR 146–358) and 37 of 171 (22%) were receiving antiretroviral therapy. Baseline charac teristics did not differ substantially between groups. Cough duration and phlegm production were the only differences between patients producing one and two sputum samples (appendix).
Figure 1. Trial profile.
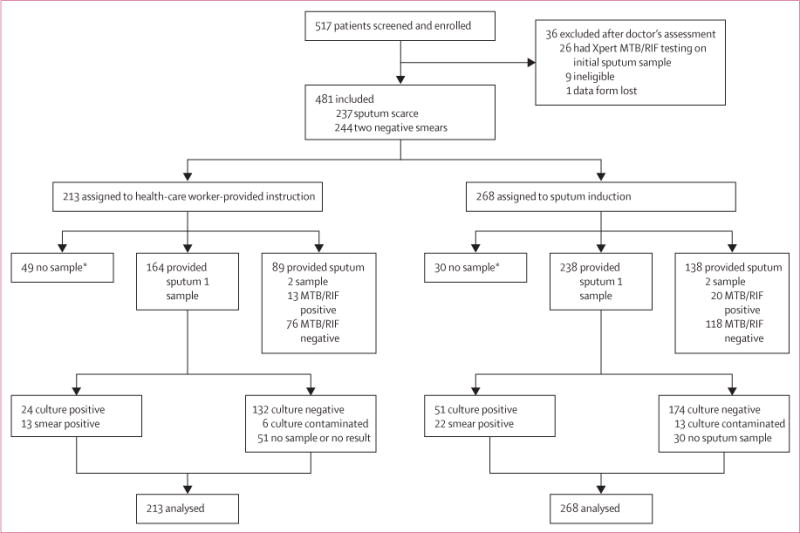
*Unable to provide a sputum sample for diagnostic testing.
Table 1.
Baseline characteristics
| Health-care worker instruction (n=213) | Induction (n=268) | |
|---|---|---|
| Age (years) | 40 (31–49) | 38 (29–49) |
|
| ||
| Men | 122 (57%) | 140 (52%) |
|
| ||
| HIV-positive | 75 (35%) | 96 (36%) |
| CD4 cell count (cells per mL) | 239 (136–345) | 247 (149–379) |
| Taking antiretrovirals | 15 (20%) | 22 (23%) |
|
| ||
| History of tuberculosis | 82 (38%) | 98 (37%) |
|
| ||
| Diagnostic categorisation | ||
| Two negative sputum smears | 117 (55%) | 127 (47%) |
| Unable to produce sputum | 96 (45%) | 141 (53%) |
|
| ||
| Cough >2 weeks | 189 (89%) | 241 (90%) |
|
| ||
| Productive cough | 141 (66%) | 170 (63%) |
|
| ||
| Night sweats | 152 (71%) | 192 (72%) |
|
| ||
| Weight loss | 145 (68%) | 190 (71%) |
|
| ||
| Appetite loss | 114 (54%) | 139 (52%) |
|
| ||
| Bodyweight (kg) | 62 (54–72) | 63 (55–72) |
| Chest radiography compatible with tuberculosis | 85 (40%) | 94 (35%) |
Data are median (IQR) or n (%).
53 of 213 (25%) patients who had health-care worker instruction versus 73 of 268 (27%) who had induction started treatment by week 8 (OR 0.88, 95% CI 0.57–1.36; p=0.56; table 2, figure 2A). At 3, 5, 7, 14, 21, and 56 days after enrolment the proportion of patients in each group who had started treatment did not differ significantly (figure 2B). At 10 days—the median time of the doctor’s first visit—40 (75%) of 53 instructed patients compared with 43 (59%) of 73 induced patients had started treatment (p=0.053). The median time to start of treatment was shorter for instructed versus induced patients (4 days, IQR 2–9 vs 7 days, IQR 2–27; p=0.029). However, neither the proportion of patients who started treatment by a specific time nor median times to treatment differed significantly between groups if the analysis included only patients in whom a sputum sample was acquired for diagnostic testing (appendix).
Table 2.
Outcomes stratified by method of diagnosis
| Health-care worker instruction | Induction | OR (95% CI) | p value | |
|---|---|---|---|---|
| Total patients starting treatment | 53/213 (25%) | 73/268 (27%) | 0.88 (0.57–1.36) | 0.56 |
|
| ||||
| Diagnosis and treatment initiation based on smear microscopy | ||||
| Patients treated | 13/53 (25%) | 22/73 (30%) | 0.75 (0.31–1.80) | 0.49 |
| Median time to treatment (days) | 2 (2–6) | 3 (2–6) | 0.03 (0.00–27.15) | 0.68 |
|
| ||||
| Diagnosis based on clinical and radiologcal presentation with empiric treatmlent initiation | ||||
| Patients treated | 32/53 (60%) | 28/73 (38%) | 2.45 (1.12–5.40) | 0.015 |
| Patients with HIV* | 18/32 (56%) | 17/28 (61%) | 0.83 (0.26–2.57) | 0.73 |
| Patients without HIV | 14/32 (44%) | 11/28 (39%) | 1.20 (0.38–3.83) | 0.73 |
| Median time to treatment (days) | 4 (1–9) | 7 (3–10) | 0.01 (0.00–4.01) | 0.15 |
|
| ||||
| Diagnosis and treatment initiation based on culture | ||||
| Patients treated based on sputum 1 culture result | 6/53 (11%) | 18/73 (25%) | 0.39 (0.12–1.14) | 0.060 |
| Patients treated based on other (sputum 2 or non-sputum) culture results | 1/53 (2%) | 2/73 (3%) | 0.68 (0.01–13.47) | 0.76 |
| Median time to treatment (days) | 34 (29–48) | 42 (20–56) | 0.26 (0.00–5.48) | 0.90 |
| Culture-positive patients not given any tuberculosis treatment during study | 3/213 (1%) | 4/268 (2%) | 0.94 (0.14–5.61) | 0.93 |
One patient in the health-care worker instruction group and three in the sputum induction group were missing data for reason for treatment initiation.
Initiation of tuberculosis treatment was based on the 2007 WHO smear-negative diagnostic and treatment algorithm for HIV-positive ambulatory patients.
Figure 2. Proportions of patients receiving tuberculosis diagnosis and treatment and time to treatment initiation.
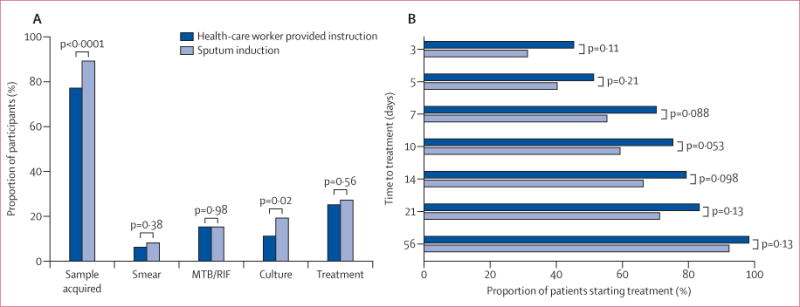
(A) shows proportions of samples acquired, diagnosis by smear microscopy, Xpert MTB/RIF assay, and culture, and proportions of patients given treatment.
(B) shows time to start of treatment Groups were compared with χ2.
Irrespective of intervention group, patients unable to produce a sputum sample, and thus not waiting for a diagnostic test result, received a doctor’s assessment and empiric treatment quicker than did those who could produce a sample (median 3 days, IQR 1–7 vs 6 days, 2–8 days; p=0.004). Furthermore, if the analysis was restricted to sputum-scarce or HIV-positive patients, or repeated with a random sample that had balanced patient numbers from each study group (n=200; appendix) time-specific proportions of patients starting treatment did not differ between groups. We also did a secondary time-to-event analysis, comparing the time to start of treatment between groups (appendix). Overall (p=0.4), and when the analysis was restricted to patients who definitely had tuberculosis (p=0.7), the groups did not differ significantly.
In our analysis by reason for starting treatment, a similar proportion of patients started treatment in the instructed group versus the induced group, whether treatment initiation was based on positive sputum smear microscopy or positive culture (table 2). By contrast, more instructed patients compared with induced patients received treatment empirically based on clinical and radiological findings (table 2). 60% of empirically treated patients were HIV positive and treatment was started in accordance with the 2007 WHO guidelines.18
A smaller proportion of instructed patients compared with induced patients successfully produced a sputum sample of at least 1 mL for diagnostic testing (164/213 [77%] vs 238/268 [89%]; p<0.0001; figure 2). However, the proportion of samples that were of good quality—as assessed by the Bartlett score—was much the same between groups (table 3).
Table 3.
Diagnostic outcomes
| Instruction by health-care worker (n=213) | Sputum induction (n=268) | p value | |
|---|---|---|---|
|
Sample volume and quality
| |||
| Patients providing at least one sputum sample of ≥1 mL for laboratory testing* | 164 (77%) | 238 (89%) | <0.0001 |
| Sputum samples considered to be adequate quality | 117/136 (86%) | 181/216 (84%) | 0.57 |
| Side-effects from sampling procedure | 0 (0%) | 32 (12%)† | <0.0001 |
|
Diagnostic yield and accuracy
| |||
| Smear microscopy yield | 13 (6%) | 22 (8%) | 0.38 |
| Tuberculosis culture yield | 24 (11%) | 51 (19%) | 0.020 |
| Median time to positivity for tuberculosis culture (IQR; days) | 14 (11–18) | 13 (9–18) | 0.54 |
| MTB/RIF assay diagnostic yield (sputum sample 2) | 13/89 (15%) | 20/138 (14%) | 0.98 |
| MTB/RIF assay sensitivity‡ | |||
| All culture positive sensitivity (n/N; %; 95% CI) | 9/12 (75%; 51–99) | 16/25 (64%; 45–83) | 0.50 |
| Sputum culture positive sensitivity (n/N; %; 95% CI) | 4/7 (57%; 20–94) | 8/16 (50%; 26–75) | 0.75 |
19 sputum specimens undergoing liquid culture were contaminated: six in the health-care worker-provided instruction group and 13 in the sputum induction group.
Includes nausea or vomiting, headache, dizziness, and shortness of breath.
Calculated using liquid tuberculosis culture from a paired sample as the reference standard.
27 induced patients (10%) and 31 (15%) instructed patients who provided a sputum specimen did not return for an initial doctor’s assessment or to collect their diagnostic test results (p=0.1). After 2 months, 12 of the initial 27 induced patients (4/12 were culture positive) and 13 of the 31 instructed patients (3/13 were culture positive) were still lost to follow-up (p=0.8). Diagnostic yield from smear microscopy was similar in instructed and induced patients (table 3), as was diagnostic yield from MTB/RIF assay (table 3). In view of the overall similarities between patients producing one and two sputum samples (appendix), we calculated an estimated MTB/RIF diagnostic yield for sputum 1 (ie, adjusting for the success of sample acquisition), which provided much the same diagnostic yield in instructed patients (24/213 [11%]) versus induced patients (43/268 [16%]; p=0.1). By contrast, culture-based diagnostic yield was lower in instructed patients compared with induced patients (table 3), although culture-based diagnostic yield did not differ significantly if analysis was restricted to patients providing a sputum sample for diagnostic testing (23/164 [14%] vs 51/238 [21%]; p=0.060). Among culture-positive patients, median time to culture positivity was similar in instructed and induced patients (table 3).
Side-effects related to sample acquisition were reported in 32 of 268 (12%) patients who had sputum induction and none who had health-care worker instruction (table 3). The most common side-effects were: shortness of breath (n=11), dizziness (n=9), headache (n=8), and nausea or vomiting (n=7). Sputum induction was stopped if patients had side-effects and all side-effects resolved without the need for review by a doctor.
Health-care worker-provided instruction cost $2.14 per sampling procedure versus $7.88 for sputum induction. The higher cost of sputum induction is a result of the additional consumables used and staff time needed for nebulisation (appendix).
Discussion
To our knowledge, this study is the first pragmatic randomised controlled trial to compare health-care worker instruction with induction for sputum sampling in adults with suspected tuberculosis who are smear-negative or sputum-scarce in a primary care practice (panel). Our findings have important clinical and public health policy implications. In regions where HIV is common, smear-negative and sputum-scarce tuberculosis presents a diagnostic challenge. Our study supports the use of health-care worker instruction as the initial sputum sampling strategy. Nurses should instruct patients how to take a sample before patients are empirically treated or given sputum induction. Although sputum sampling by induction provided an adequate specimen volume and microbiological diagnosis in a higher proportion of patients than did health-care worker instruction, it was more costly and did not result in more patients starting treatment. Notably, sputum induction did not result in a higher proportion of case detection using same-day diagnostic methods (smear microscopy and Xpert MTB/RIF), probably because of the paucibacillary nature of induced sputum. This result, combined with the high rates of empiric treatment initiation based on clinical and radiological findings, meant that the benefits of sputum induction failed to affect either the proportion of patients starting treatment or the time to start of treatment. Thus, health-care worker instruction had an equivalent effect on treatment initiation compared with induction, at a substantially lower cost and with fewer adverse events.
Panel: Research in context.
Systematic review
We searched PubMed for studies about either sputum induction or health-care worker instruction published in English up to March 13, 2013. We combined search terms that could indicate sputum induction or health-care worker instruction (“sputum induction, induced sputum, sputum expect*, sputum sampl*, sputum/*microbiology”) with “TB” We identified two systematic reviews of sputum induction9,11 and a large study19 of adult patients with suspected smear-negative and sputum-scarce tuberculosis. We identified four studies12,13,20,21 involving health-care worker supervision or instruction during sputum sampling.
Interpretation
To our knowledge, our study is the only randomised controlled trial to assess the role of sputum induction in the diagnosis of tuberculosis, and is the first study to directly compare two sputum sampling strategies with treatment uptake as the primary outcome. Previous studies of sputum induction in adults with suspected smear-negative and sputum-scarce tuberculosis from settings with high HIV and tuberculosis prevalence are heterogeneous, with varying estimates of culture-based diagnostic yield (8–66%) and smear microscopy sensitivity (32–60%).19,22–25 Two studies of health-care-provided instruction show increased diagnosis of tuberculosis by smear for adults with suspected pulmonary tuberculosis,12,13 but two studies using sequential combinations of instruction and induction provided conflicting results.20,22 Our study confirms that sputum induction— although more costly—offers better sputum sampling and increased culture-based diagnosis compared with simple instruction. However, use of sputum induction did not result in more patients being treated. Health-care worker-provided instruction should be the preferred initial sputum sampling strategy for adults with suspected smear-negative and sputum-scarce tuberculosis, especially where sputum induction facilities are unavailable or where culture-based diagnosis is unlikely to alter treatment decision making. Advocacy to improve the training of health-care workers in sputum sampling instruction and the incorporation of simple instruction into diagnostic algorithms of primary care clinics is warranted.
In previous studies, health-care worker instruction12,13 and nurse-specific educational outreach improved rates of tuberculosis case detection with smear microscopy in people with persistent cough in primary care.26,27 In HIV-positive Malawian patients thought to have tuberculosis, health-care worker instruction offered better diagnostic yield for smear microscopy and culture than did alternative acquisition methods.22 However, before this study, no comparative randomised controlled studies of sampling strategies were available and despite its simplicity, health-care worker instruction is neither routinely used nor is it a formalised step in smearnegative tuberculosis diagnostic procedures. Our study findings suggest that national tuberculosis programmes should include health-care worker instruction as the first strategy for smear-negative or sputum-scarce patients and thus, they should urgently provide widespread training to health-care workers and nurses about this sputum sampling strategy.
Other studies9,11,20 have shown sputum induction to be an excellent and safe sampling method for culture-based diagnosis. Although our study does not change this conclusion, we have found that sputum induction does not necessarily affect treatment initiation because of the long delays associated with culture-based diagnosis. Furthermore, because Xpert MTB/RIF assay performs suboptimally when using induced sputum specimens, the use of Xpert MTB/RIF assay as a replacement for smear microscopy would probably not have affected the primary outcome. Thus, sputum induction has limitations for adults with smear-negative and sputum-scarce tuberculosis, particularly in settings where other investigations—eg, chest radiography and high empiric treatment use—are routinely done.
Although not assessed in this study, a step-wise approach might be best for diagnosis of smear-negative or sputum-scarce patients, with routine use of sputum induction reserved for when instruction has been unsuccessful or when a culture-based diagnosis is essential—eg, in a suspected case of multidrug-resistant tuberculosis.28,29 More studies are needed to assess such an approach. In addition, sputum induction is still an important sampling strategy for children and asymptomatic HIV-positive patients who are being screened for tuberculosis before starting antiretroviral therapy.21,30
Our study has some limitations. An open-label design can be prone to bias but this was chosen for its simplicity because of the location and infrastructure of the clinics and the nature of the intervention. However, we did regular unannounced checks—to ensure that the protocol was adhered to—and patient characteristics did not differ between groups, suggesting no bias. The different number of patients randomly assigned to each group—although statistically plausible given the simple randomisation method—might have introduced selection bias. However, baseline characteristics were similar and the main conclusions were the same when we analysed a random sample of 200 patients with balanced groups. The exclusion of 36 patients from the primary analysis is another important limitation, with most excluded because of programmatic implementation of Xpert MTB/RIF instead of smear microscopy for testing pre-enrolment sputum specimens at some study sites in the final few months of enrolment. Sensitivity analyses showed no differences between excluded and included patients, and power calculations suggest that the analysis had a greater than 80% power to detect a 12% difference between study groups for the primary outcome taking exclusions into account.
No validated sputum quality scoring system exists for induced sputum samples. Thus, Bartlett scoring is not ideal and conclusions about differences in sample quality between groups should be interpreted with caution. Empiric treatment was more common among instructed patients and whether this constituted appropriate treatment or over-treatment is difficult to ascertain. The exact specificity of empiric treatment is unknown, and estimates from studies of WHO algorithms for smear-negative tuberculosis in high tuberculosis and HIV settings range from 44% to 95%.31–34 In our study, 60% of empirically treated patients were HIV-positive and qualified for treatment in accordance with the WHO smear-negative tuberculosis algorithm.22 Xpert MTB/RIF assay was used on stored sputum samples when available and not for treatment decisions. Because many patients did not have a second sputum specimen these findings should be interpreted cautiously. Our findings are applicable to settings in which HIV is common and further studies are needed to assess their usefulness elsewhere.
Our data support the use of nurse-driven health-care worker instruction as the initial sputum sampling method for adults with suspected smear-negative or sputum-scarce tuberculosis in a high-burden primary care setting. Sputum induction is an important sampling strategy when the need for a microbiologically confirmed diagnosis of tuberculosis is essential. More effort should be made to formalise and incorporate sputum instruction and supervision in the education of primary clinic healthcare workers in regions where HIV and tuberculosis are common.
Supplementary Material
Acknowledgments
This work was supported by the South African National Research Foundation, a TBSusgent grant from the European Commission, the National Institutes of Health, the European and Developing Countries Clinical Trials Partnership, and the Discovery Foundation. We thank the research nursing staff who were involved in recruitment of patients and collection of sputum samples. In addition, the assistance and support of the health-care workers at the primary care clinics of Langa, Gugulethu, and Chapel Street was greatly appreciated.
Footnotes
Contributors
JGP and KD designed the study. JGP, JT, and MP collected data. JP, GT, and AP analysed the data. JGP wrote the first draft of the report and all authors gave input to the final version.
Conflicts of interest
We declare that we have no conflicts of interest.
See Online for an interview with Jonathan Peter
References
- 1.WHO. WHO Report 2011: global tuberculosis control. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Lonnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–29. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 3.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–49. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 5.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theron G, Peter J, Dheda K. Xpert MTB/RIF test for tuberculosis. Lancet. 2011;378:481. doi: 10.1016/S0140-6736(11)61242-7. [DOI] [PubMed] [Google Scholar]
- 7.Theron G, Pooran A, Peter J, et al. Do adjunct TB tests, when combined with Xpert MTB/RIF, improve accuracy and the cost of diagnosis in a resource-poor setting? Eur Respir J. 2012;40:161–68. doi: 10.1183/09031936.00145511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328:856–61. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 9.Hepple P, Ford N, McNerney R. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. Int J Tuberc Lung Dis. 2012;16:579–88. doi: 10.5588/ijtld.11.0617. [DOI] [PubMed] [Google Scholar]
- 10.Schoch OD, Rieder P, Tueller C, et al. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am J Respir Crit Care Med. 2007;175:80–86. doi: 10.1164/rccm.200608-1092OC. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo Y, Wiysonge CS, Geldenhuys H, et al. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2012;31:1619–30. doi: 10.1007/s10096-011-1485-6. [DOI] [PubMed] [Google Scholar]
- 12.Alisjahbana B, van Crevel R, Danusantoso H, et al. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis. 2005;9:814–17. [PubMed] [Google Scholar]
- 13.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet. 2007;369:1955–60. doi: 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 14.Cobelens F, van den Hof S, Pai M, Squire BS, Ramsay A, Kimmerling ME. Which new diagnostics for tuberculosis, and when? J Infect Dis. 2012;205(suppl 2):S191–98. doi: 10.1093/infdis/jis188. [DOI] [PubMed] [Google Scholar]
- 15.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 17.Bartlett RC. Medical microbiology: quality, cost and clinical relevance. New York: John Wiley and Sons; 1974. [Google Scholar]
- 18.WHO. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Geneva: World Health Organization; 2006. [Google Scholar]
- 19.Peter JG, Theron G, Singh N, Singh A, Dheda K. Sputum induction to aid the diagnosis of smear-negative or sputum-scarce TB in adults from a HIV-endemic setting. Eur Respir J. 2013 doi: 10.1183/09031936.00198012. published online March 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang KC, Leung CC, Yew WW, Tam CM. Supervised and induced sputum among patients with smear-negative pulmonary tuberculosis. Eur Respir J. 2008;31:1085–90. doi: 10.1183/09031936.00122907. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell DJ, Dacombe R, Graham SM, et al. Simple measures are as effective as invasive techniques in the diagnosis of pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2009;13:99–104. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10:31–38. [PubMed] [Google Scholar]
- 24.Morse M, Kessler J, Albrecht S, et al. Induced sputum improves the diagnosis of pulmonary tuberculosis in hospitalized patients in Gaborone, Botswana. Int J Tuberc Lung Dis. 2008;12:1279–85. [PubMed] [Google Scholar]
- 25.Parry CM, Kamoto O, Harries AD, et al. The use of sputum induction for establishing a diagnosis in patients with suspected pulmonary tuberculosis in Malawi. Tuber Lung Dis. 1995;76:72–76. doi: 10.1016/0962-8479(95)90583-9. [DOI] [PubMed] [Google Scholar]
- 26.Fairall L, Bachmann MO, Zwarenstein M, et al. Cost-effectiveness of educational outreach to primary care nurses to increase tuberculosis case detection and improve respiratory care: economic evaluation alongside a randomised trial. Trop Med Int Health. 2010;15:277–86. doi: 10.1111/j.1365-3156.2009.02455.x. [DOI] [PubMed] [Google Scholar]
- 27.Fairall LR, Zwarenstein M, Bateman ED, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. BMJ. 2005;331:750–54. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dheda K, Warren RM, Zumla A, Grobusch MP. Extensively drug-resistant tuberculosis: epidemiology and management challenges. Infect Dis Clin North Am. 2010;24:705–25. doi: 10.1016/j.idc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 30.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev. 2011;12:16–21. doi: 10.1016/j.prrv.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alamo ST, Kunutsor S, Walley J, et al. Performance of the new WHO diagnostic algorithm for smear-negative pulmonary tuberculosis in HIV prevalent settings: a multisite study in Uganda. Trop Med Int Health. 2012;17:884–95. doi: 10.1111/j.1365-3156.2012.03003.x. [DOI] [PubMed] [Google Scholar]
- 32.Huerga H, Varaine F, Okwaro E, et al. Performance of the 2007 WHO algorithm to diagnose smear-negative pulmonary tuberculosis in a HIV prevalent setting. PLoS One. 2012;7:e51336. doi: 10.1371/journal.pone.0051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soto A, Solari L, Gotuzzo E, Acinelli R, Vargas D, Van der Stuyft P. Performance of an algorithm based on WHO recommendations for the diagnosis of smear-negative pulmonary tuberculosis in patients without HIV infection. Trop Med Int Health. 2011;16:424–30. doi: 10.1111/j.1365-3156.2010.02715.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilson D, Mbhele L, Badri M, et al. Evaluation of the World Health Organization algorithm for the diagnosis of HIV-associated sputum smear-negative tuberculosis. Int J Tuberc Lung Dis. 2011;15:919–24. doi: 10.5588/ijtld.10.0440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


