Abstract
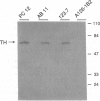
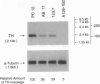
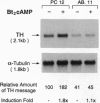
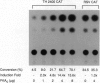
Tyrosine hydroxylase (TH) catalyzes the conversion of L-tyrosine to 3,4-dihydroxy-L-phenylalanine, the first and rate-limiting step in catecholamine biosynthesis. The cAMP-dependent protein kinase (PKA) phosphorylates and activates the TH enzyme and is thought to mediate transcriptional induction of the TH gene. To better understand the functional role of PKA in TH gene regulation, we studied TH gene expression at the transcriptional, translational, and post-translational levels in several PKA-deficient cell lines derived from rat PC12 pheochromocytoma cells. Strikingly, all PKA-deficient cell lines analyzed in this study showed substantial deficits in basal TH expression as measured by TH enzymatic activity, level of TH immunoreactivity, TH protein level, and steady-state mRNA level. Interestingly, the steady-state level of mRNA correlated well with levels of TH activity, immunoreactivity, and protein. In addition, PKA-deficient cell lines lacked transcriptional induction of the TH gene following treatment with dibutyryl cAMP. Cotransfection of PKA-deficient cells with an expression plasmid for the catalytic subunit of PKA fully reversed transcriptional defect, as indicated by robust transcriptional induction of a reporter construct containing 2400 bp of TH upstream sequence in all PC12 cells tested. These data indicate that the PKA system regulates both the basal and the cAMP-inducible expression of the TH gene primarily at the transcriptional level in PC12 cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cambi F., Fung B., Chikaraishi D. 5' flanking DNA sequences direct cell-specific expression of rat tyrosine hydroxylase. J Neurochem. 1989 Nov;53(5):1656–1659. doi: 10.1111/j.1471-4159.1989.tb08567.x. [DOI] [PubMed] [Google Scholar]
- Correll L. A., Woodford T. A., Corbin J. D., Mellon P. L., McKnight G. S. Functional characterization of cAMP-binding mutations in type I protein kinase. J Biol Chem. 1989 Oct 5;264(28):16672–16678. [PubMed] [Google Scholar]
- Costa E., Guidotti A., Hanbauer I. Do cyclic nucleotides promote the trans-synaptic induction of tyrosine hydroxylase? Life Sci. 1974 Apr 1;14(7):1169–1188. doi: 10.1016/0024-3205(74)90425-1. [DOI] [PubMed] [Google Scholar]
- Costa E., Kurosawa A., Guidotti A. Activation and nuclear translocation of protein kinase during transsynaptic induction of tyrosine 3-monooxygenase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1058–1062. doi: 10.1073/pnas.73.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Erny R., Wagner J. A. Adenosine-dependent activation of tyrosine hydroxylase is defective in adenosine kinase-deficient PC12 cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4974–4978. doi: 10.1073/pnas.81.15.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. P., Yoon S. O., Chikaraishi D. M. Sequences that direct rat tyrosine hydroxylase gene expression. J Neurochem. 1992 Jun;58(6):2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Glowacka D., Bader D. S., Hidaka H., Wagner J. A. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991 Sep 15;266(26):17454–17458. [PubMed] [Google Scholar]
- Ginty D. D., Glowacka D., DeFranco C., Wagner J. A. Nerve growth factor-induced neuronal differentiation after dominant repression of both type I and type II cAMP-dependent protein kinase activities. J Biol Chem. 1991 Aug 15;266(23):15325–15333. [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Harrington C. A., Lewis E. J., Krzemien D., Chikaraishi D. M. Identification and cell type specificity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2363–2384. doi: 10.1093/nar/15.5.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Lin Y. S., Pearlberg J., Green M. R., Goodman H. M. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988 Oct;8(10):4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Park D. H., Reis D. J. Direct phosphorylation of brain tyrosine hydroxylase by cyclic AMP-dependent protein kinase: mechanism of enzyme activation. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4744–4748. doi: 10.1073/pnas.75.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lewis E. J., Harrington C. A., Chikaraishi D. M. Transcriptional regulation of the tyrosine hydroxylase gene by glucocorticoid and cyclic AMP. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia K. R., Rasmussen K., Terwilliger R. Z., Haycock J. W., Nestler E. J., Duman R. S. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992 Feb;58(2):494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenroth V. H., 3rd, Hegstrand L. R., Roth R. H., Greengard P. Evidence for involvement of protein kinase in the activation by adenosine 3':5'-monophosphate of brain tyrosine 3-monooxygenase. J Biol Chem. 1975 Mar 10;250(5):1946–1948. [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Nestler E. J. Molecular mechanisms of drug addiction. J Neurosci. 1992 Jul;12(7):2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. H., Park H. S., Joh T. H., Anwar M., Ruggiero D. A. Strain differences between albino and pigmented rats in monoamine-synthesizing enzyme activities of brain, retina and adrenal gland. Brain Res. 1990 Feb 5;508(2):301–304. doi: 10.1016/0006-8993(90)90412-5. [DOI] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Tank A. W., Curella P., Ham L. Induction of mRNA for tyrosine hydroxylase by cyclic AMP and glucocorticoids in a rat pheochromocytoma cell line: evidence for the regulation of tyrosine hydroxylase synthesis by multiple mechanisms in cells exposed to elevated levels of both inducing agents. Mol Pharmacol. 1986 Nov;30(5):497–503. [PubMed] [Google Scholar]
- Taylor S. S. cAMP-dependent protein kinase. Model for an enzyme family. J Biol Chem. 1989 May 25;264(15):8443–8446. [PubMed] [Google Scholar]
- Van Buskirk R., Corcoran T., Wagner J. A. Clonal variants of PC12 pheochromocytoma cells with defects in cAMP-dependent protein kinases induce ornithine decarboxylase in response to nerve growth factor but not to adenosine agonists. Mol Cell Biol. 1985 Aug;5(8):1984–1992. doi: 10.1128/mcb.5.8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels-Reiker M., Haycock J. W., Howlett A. C., Strong R. Vasoactive intestinal polypeptide induces tyrosine hydroxylase in PC12 cells. J Biol Chem. 1991 May 25;266(15):9347–9350. [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]