Abstract
The Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) conduct post-licensure vaccine safety monitoring using the Vaccine Adverse Event Reporting System (VAERS), a spontaneous (or passive) reporting system. This means that after a vaccine is approved, CDC and FDA continue to monitor safety while it is distributed in the marketplace for use by collecting and analyzing spontaneous reports of adverse events that occur in persons following vaccination. Various methods and statistical techniques are used to analyze VAERS data, which CDC and FDA use to guide further safety evaluations and inform decisions around vaccine recommendations and regulatory action. VAERS data must be interpreted with caution due to the inherent limitations of passive surveillance. VAERS is primarily a safety signal detection and hypothesis generating system. Generally, VAERS data cannot be used to determine if a vaccine caused an adverse event. VAERS data interpreted alone or out of context can lead to erroneous conclusions about cause and effect as well as the risk of adverse events occurring following vaccination. CDC makes VAERS data available to the public and readily accessible online.
We describe fundamental vaccine safety concepts, provide an overview of VAERS for healthcare professionals who provide vaccinations and might want to report or better understand a vaccine adverse event, and explain how CDC and FDA analyze VAERS data. We also describe strengths and limitations, and address common misconceptions about VAERS. Information in this review will be helpful for healthcare professionals counseling patients, parents, and others on vaccine safety and benefit-risk balance of vaccination.
Keywords: Vaccination, vaccine adverse event, adverse event following immunization, adverse reaction, adverse effect, spontaneous reporting, passive surveillance, vaccine safety, Vaccine Adverse Event Reporting System (VAERS)
Introduction
The Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA) conduct post-licensure safety monitoring of U.S. licensed vaccines. This means that after a vaccine is approved, CDC and FDA continue to monitor safety while it is distributed in the marketplace for use. CDC and FDA co-administer the Vaccine Adverse Event Reporting System (VAERS), a spontaneous (or passive) reporting system [1]. Spontaneous surveillance means that no active effort is made to search for, identify and collect information, but rather information is passively received from those who choose to voluntarily report their experience. Therefore, VAERS relies on the intuition and experience of healthcare professionals in particular, but likewise for patients, parents and caregivers, to recognize and report unusual or unexpected events following vaccination or suspected vaccine safety problems. CDC and FDA also independently administer large-linked electronic health record-based surveillance systems [2,3]. Various methods and statistical techniques are used to analyze VAERS data, which CDC and FDA use to guide further safety evaluations and inform decisions around vaccine recommendations and regulatory action. Furthermore, VAERS transmits its vaccine adverse event reports to the Uppsala Monitoring Center, the World Health Organization collaborating center for international drug and vaccine safety monitoring [4,5], in order to contribute to the global pharmacovigilance effort along with other countries that employ passive vaccine safety monitoring systems. VAERS data must be interpreted with caution due to the inherent limitations of passive surveillance. VAERS is primarily a safety signal detection and hypothesis generating system. VAERS data interpreted alone or out of context can lead to erroneous conclusions about cause and effect or the risk of adverse events after vaccination.
We describe fundamental vaccine safety concepts, provide an overview of VAERS for healthcare professionals who provide vaccinations and might want to report or better understand a vaccine adverse event, and explain how CDC and FDA analyze VAERS data. We also describe strengths and limitations, and address common misconceptions about VAERS. Information in this review will be helpful for healthcare professionals counseling patients, parents, and others on vaccine safety and benefit-risk balance of vaccination.
What is a vaccine adverse event or adverse event following immunization?
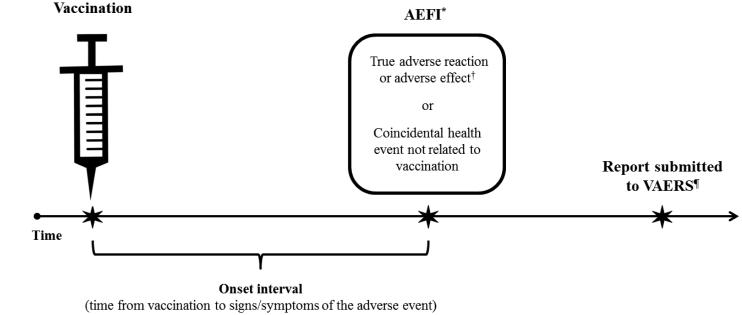
A “vaccine adverse event,” also referred to as an “adverse event following immunization” (AEFI), is an adverse health event or health problem that occurs following (Figure 1) or during administration of a vaccine. Adverse events are temporally associated events, which might be caused by a vaccine or might be coincidental and not related to vaccination [6]. The Council for International Organizations of Medical Sciences (CIOMS) defines an AEFI as “… any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease” [7]. CIOMS also defines AEFI related to product quality defects, vaccination errors and anxiety-related reactions, in addition to those related to inherent properties of a vaccine. In contrast to the term “event”, a vaccine adverse “reaction” and vaccination adverse “effect,” like “adverse drug reaction” used in pharmacovigilance for drug safety monitoring [8], are synonymous terms that indicate a reasonable body of scientific evidence exists to suggest an adverse health event was caused by vaccination [6,9]. Examples of common vaccine adverse reactions are pain and redness at the injection site.
Figure 1. Adverse event following immunization (AEFI) and the VAERS reporting timeline.
*“Adverse event following immunization” (AEFI) indicates only that the event happened after vaccination (i.e., a temporal association).
†“Vaccine adverse reaction” and “vaccination adverse effect” are also AEFIs, but imply that the vaccine caused the event (i.e., a causal association).
¶There are no deadlines or time limits for the submission of a VAERS report, but reports should be submitted promptly after an adverse event occurs to facilitate surveillance and review. The National Vaccine Injury Compensation Program (VICP) is administered by the Health Resources and Services Administration (HRSA). The VICP is separate from the VAERS program and reporting an adverse event to VAERS does not constitute filing a claim for compensation to the VICP (see www.hrsa.gov/vaccinecompensation/index.html).
Why do the CDC and the FDA monitor vaccine safety?
The FDA requires extensive testing to evaluate safety and efficacy of a vaccine before granting licensure. The final phase of pre-licensure clinical trials might involve hundreds to thousands of volunteer study subjects [10]. Pre-licensure clinical trials are effective at identifying and characterizing the most common adverse events associated with a particular vaccine; examples include injection site reactions and post-vaccination fever. However, clinical trials might not be large enough to detect rare adverse events, which may be seen only after tens or hundreds of thousands of people are vaccinated. The limited patient follow-up period for clinical trials also constrains the ability to identify possible adverse events with delayed onset. Clinical trials generally conduct active follow-up on participants for up to a full year after vaccination, and often extended follow-up for periods beyond one a year. This level of follow-up is sufficient to assess most acute and delayed onset adverse events of interest for vaccine safety, but is not sufficient to assess conditions with onset multiple years following exposure. Additionally, clinical trials for initial licensure usually include only healthy individuals, so data on special populations, like those with chronic illnesses or pregnant women, are limited. Therefore, after a vaccine is licensed and distributed for widespread use it is necessary to conduct monitoring to further evaluate safety [11].
Apart from scientific and methodological issues, policy considerations also influence CDC and FDA determinations on vaccine safety monitoring. Vaccines are generally given to healthy individuals to prevent disease, whereas drugs are primarily given for treatment of illness. Sick patients, or parents of sick children, might be more willing to accept safety risks of drugs used to treat illnesses compared to vaccines used to prevent possible future illnesses. Furthermore, many state and local governments require vaccination for school attendance and healthcare facilities are increasingly requiring vaccination as a condition of employment [12,13]. These mandates place additional emphasis on vaccine safety and adverse event monitoring.
What is the Vaccine Adverse Event Reporting System (VAERS)?
VAERS is a national early warning system to detect possible safety problems in U.S. licensed vaccines. It is a spontaneous, voluntary reporting system for adverse events [1,14,15], and therefore no effort is made to search for individuals who experience adverse events and actively collect data, but rather VAERS passively receives information on adverse events from those who choose to report. VAERS is most useful as a hypothesis generating system with the primary goal to detect safety signals [9] that might be related to vaccination. The main objectives of VAERS are to: 1.) detect new, unusual, or rare adverse events, 2.) monitor reporting trends that might reflect true increases in known adverse events, 3.) identify potential risk factors for particular types of adverse events, 4.) assess the safety of newly licensed vaccines and new recommendations for existing vaccines, 5.) detect and address possible reporting clusters (e.g., suspected localized [temporally or geographically] or product-/batch-/lot-specific adverse event reporting), 6.) detect persistent safe-use problems and administration errors, and 7.) provide a national safety monitoring system that extends to the entire general population for response to public health emergencies, such as a large-scale pandemic influenza vaccination program [16].
VAERS was established in 1990 [17,18] to fulfill a requirement of the National Childhood Vaccine Injury Act of 1986 [19]. By law, vaccine manufacturers are required to report adverse events that come to their attention, and healthcare professionals are required to report adverse events that are considered a contraindication to further doses of vaccine and those specified in the VAERS Table of Reportable Events Following Vaccination [20-23]. The National Childhood Vaccine Injury Act of 1986 also authorized establishment of the National Vaccine Injury Compensation Program [24]. Adverse events on the VAERS Table of Reportable Events Following Vaccination mirror the “illness, disability, injury or condition covered” conditions in the National Vaccine Injury Compensation Program’s Vaccine Injury Table [25] used to help adjudicate petitioner claims of vaccine related injury.
Anyone can report an adverse event to VAERS, including healthcare professionals, vaccine manufacturers, patients, parents and caregivers, and others. Reports are submitted voluntarily either directly from individual reporters, who may be reporting for themselves or others, or secondarily from vaccine manufacturers, that also receive spontaneous reports and in turn submit them to VAERS. Reporting is encouraged for any clinically important or unexpected adverse event, even if the reporter is not sure if a vaccine caused the event [20]. VAERS accepts all reports without rendering judgment on clinical importance or whether vaccine(s) might have caused the adverse event.
How does VAERS work?
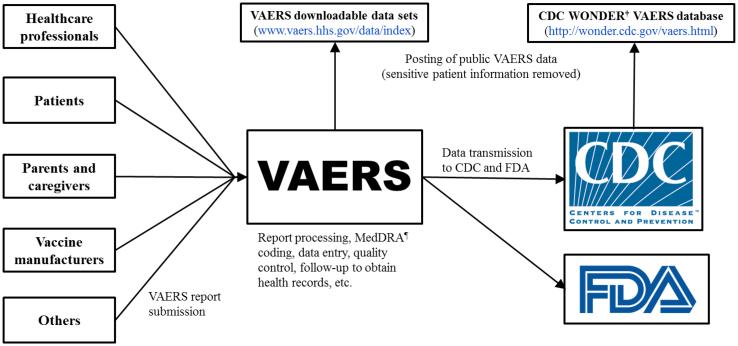
VAERS currently receives reports on a standard form via mail or fax, or through a secure online submission process (www.vaers.hhs.gov/esub/index). The VAERS form includes data fields for patient demographic information and medical history, information on the reporter and the facility where vaccine(s) were given, description of the adverse event and health outcomes, date of vaccination, vaccine(s) administered, onset of adverse event symptoms, recovery status, and other relevant information. VAERS reports are received at a central facility that is managed by a private contractor under the direction of CDC and FDA (Figure 2). Here, staff specialized in coding case report information review reports and assign medical terms for adverse events using the Medical Dictionary for Regulatory Activities (MedDRA) [26], a widely used and accepted standardized medical terminology for adverse events. MedDRA terms are not confirmed medical diagnoses, but rather serve as the classification scheme to systematically encode information reported to VAERS. VAERS uses certified MedDRA coders and software programs to facilitate consistency in the capture and coding of signs and symptoms in reports. Reports are categorized as either serious or non-serious according to an FDA regulatory definition. Serious reports include at least one of the following: death following vaccination, life-threatening health event, hospitalization following vaccination or prolonged hospitalization if a vaccine was administered while the patient was already hospitalized, or lasting disability [21].
Figure 2. Vaccine Adverse Event Reporting System (VAERS) report submission* and data flow.
*During the time period 2011-2014, healthcare professionals submitted 38% of U.S. reports, patients and parents submitted 14%, vaccine manufacturers submitted 30%, and others (e.g., friends/acquaintances of the patient, 3rd party reporters who became aware of adverse events from the media, lawyers, etc.) submitted 12% (CDC unpublished data). There is variability in reporter type across different types and brands of vaccines.
†Wide-ranging Online Data for Epidemiologic Research
¶Medical Dictionary for Regulatory Activities
For VAERS reports submitted by the public, the primary reporter receives an acknowledgement letter or email and a request to provide additional information if there is missing or incomplete essential information on the report. For reports classified as serious, the VAERS contractor requests associated health records, including hospital discharge summaries, medical and laboratory results, and death certificates and autopsy reports for deaths. Additional MedDRA terms might be added based on information obtained through follow-up. Also, for serious reports where the patient has not recovered from the adverse event by the time the report was filed or recovery status was unknown, a follow-up letter is sent to the reporter at one year requesting information on recovery status if that information is still not known. Vaccine manufacturers are responsible for attempting to obtain follow-up information on serious and unexpected adverse event reports that they submit to VAERS [21].
Information in each report, along with assigned MedDRA terms, is entered into an electronic database and sent to CDC and FDA for analysis. Data are continuously updated as new reports come in and follow-up information for existing reports is received. CDC and FDA receive a cumulative dataset every business day that contains all VAERS reports including recently entered reports and refreshed (or updated) reports. In addition, copies of original reports, any health records, and other associated documents are electronically maintained in an image database that CDC and FDA staff use to clinically review individual case reports. If errors or inconsistencies in reported information are detected during the course of follow-up or during routine analysis, corrections are made to the VAERS database. VAERS data from the primary reports, with sensitive patient information removed, are publicly available on the VAERS website (www.vaers.hhs.gov/data/index) and through CDC’s Wide-ranging Online Data for Epidemiologic Research (WONDER) tool (http://wonder.cdc.gov/vaers.html) (Figure 2). Due to patient privacy protections, additional information obtained during follow-up on individual VAERS reports is not included in the publicly available data.
During 2011-2014, VAERS averaged around 30,000 U.S. reports annually, with 7% classified as serious. Healthcare professionals submitted 38% of reports, vaccine manufacturers 30% and patients and parents 14%. Reporter type and percent of serious reports vary across vaccines, age of vaccine recipient and how long the vaccine has been in use. During this same time period VAERS averaged around 6,000 foreign source reports annually. Vaccine manufacturers, which accounted for >99% of foreign source reporting, are required by law to submit foreign source adverse event reports that are both serious and unexpected [21], but not other types of foreign source reports. Given the vaccine manufacturer reporting requirements and the minimal amount of direct public reporting, it is not surprising that a relatively high percentage (48%) of foreign source reports are classified as serious. This likely represents selective reporting based on regulatory requirements rather than any substantial differences in safety profiles of foreign vaccines.
How do CDC and FDA analyze VAERS data?
CDC and FDA use several methods to analyze VAERS data to detect vaccine safety signals. CDC focuses on public health priority vaccines, like influenza vaccine which is given in large quantities during a compressed time period, and newly licensed and recommended vaccines during their initial uptake period. The data needs of the Advisory Committee on Immunization Practices [27] often drive CDC’s monitoring priorities. FDA monitors all U.S. licensed vaccines and regularly submits mandated post-licensure safety reports to its advisory committees. When necessary, CDC, FDA and state and local health departments collaborate on investigations of unusual or unexpected reports or concerning patterns of reporting (e.g., clusters). The joint monitoring efforts of CDC and FDA ensure that U.S. licensed vaccines are continuously monitored, with emphasis on high use vaccines, new vaccines, and when new recommendations are implemented for existing vaccines. Some key methods include:
Descriptive analysis, historical comparisons and reporting trends over time
The basic analyses of VAERS data are intended to detect concerning patterns or unusual and unexpected changes in adverse event reporting that might indicate a safety problem in a specific vaccine or vaccine type. CDC and FDA physicians, epidemiologists and statisticians assess numbers of reports, types of reports based on serious and non-serious status, the most common adverse events, current versus historical data, and reporting trends over time, such as comparisons of influenza vaccine reports across multiple consecutive influenza seasons. Analysis also includes evaluation of reporting rates of adverse events in the context of vaccine doses distributed for use in the U.S. marketplace. Vaccine doses distributed provides a proxy measure of persons vaccinated. Reporting rates enable comparison with background rates of adverse events from the literature or other sources, but they must be interpreted cautiously since vaccine doses distributed are not all actually administered. Even if they do not exceed known background rates, reporting rates for specific adverse events that approach the background rates might indicate a safety problem due to the known underreporting of adverse events to VAERS.
Disproportionality analysis
Disproportionality analysis involves statistical techniques like empirical Bayesian data mining and the proportional reporting ratio to assess for disproportional reporting of specific vaccine-adverse event combinations [28-30]. VAERS is not able to provide incidence of adverse events. As a passive, numerator-only surveillance system, VAERS lacks information on total number of individuals vaccinated and total number who experience an adverse event, as well as incidence of adverse events in unvaccinated individuals. However, the proportion of reports involving a specific adverse event and a specific vaccine can be compared to the proportion of reports involving the same adverse event and other vaccines. An example would be comparing the proportion of live attenuated influenza vaccine (LAIV)-nasal congestion reports (a known causal association [31]) to the proportion of inactivated influenza vaccine-nasal congestion reports. Here we might expect to see a higher proportion of LAIV reports with nasal congestion than for inactivated influenza vaccine, for which there is no known causal association. In this case, disproportional reporting observed in post-licensure surveillance would not be considered a safety signal because nasal congestion is already a known, well characterized adverse reaction that was observed in clinical trials. A mathematical representation of the proportional reporting ratio illustrates the concept:
| Adverse event of interest |
All other adverse events |
|
| Vaccine of interest |
ViAEi | ViAEx |
| Comparator vaccine(s) |
VxAEi | VxAEx |
In this equation, the proportion of reports involving the vaccine of interest and the adverse event of interest in relation to all adverse event reports involving the vaccine of interest is divided by the proportion of reports involving comparator vaccine(s) with the adverse event of interest in relation to all adverse event reports for comparator vaccine(s). The mathematical criteria used for a statistical signal is a proportional reporting ratio ≥2, chi-square ≥4 and number of reports in a cell ≥3 [30].
Disproportionality analysis complements clinical reviews and other analyses to identify adverse events that may be more frequently associated with a particular vaccine. A result that exceeds a pre-specified statistical alerting threshold might warrant further evaluation, such as clinical review of reports, but does not definitively demonstrate a true increased incidence of an adverse event, a causal association, or a safety problem. If, after an initial evaluation, CDC and FDA determine that a safety signal requires further assessment, epidemiologic studies can be conducted using other, more robust data sources to assess for causality [2,3]. An illustrative example of signal detection in VAERS using disproportionality analysis for febrile seizures in young children following inactivated influenza vaccine, with follow-on assessment using clinical review of VAERS reports and an epidemiologic study in another data source is described in the final section of this paper.
Clinical review of reports
CDC and FDA physicians review serious reports, selected reports based on results of descriptive analysis and disproportionality analysis, and reports for selected conditions of interest. Clinical reviews are conducted to characterize the completeness and quality of reports, verify diagnoses if possible, characterize clinical and laboratory features, assess other potential risk factors (e.g., co-administration of vaccines, underlying health conditions), and evaluate the interval between vaccination and the adverse event. Reviewers use clinical judgment to detect concerning patterns or unusual and unexpected adverse events. CDC physicians generally conduct clinical reviews of selected types of vaccines and conditions of interest for particular vaccines (e.g., serious and pregnancy-related reports for influenza vaccines). FDA physicians structure clinical reviews of serious reports around individual vaccine brands with a regulatory focus. CDC and FDA regularly share information on clinical review findings. For selected adverse events of interest that are the focus of enhanced surveillance (e.g., anaphylaxis following inactivated influenza vaccine in egg allergic patients), Brighton Collaboration case definitions [32] are used when available. The Brighton Collaboration is a global research network with a mission to “…enhance the science of vaccine research by providing standardized, validated, and objective methods for monitoring safety profiles and benefit to risk ratios of vaccines.” (https://brightoncollaboration.org/public/who-we-are.html). The Brighton Collaboration generates standardized adverse event case definitions in order to enhance data consistency and comparability across systems and studies.
What are the strengths of VAERS?
VAERS is national in scope and is able to receive information from the entire U.S. population. Because of the large and diverse population available to report, VAERS is able to rapidly detect possible safety problems and rare adverse events [1,14,15]. VAERS reports often include detailed information on vaccines given, characteristics of the individual vaccinated, and the adverse event itself. Furthermore, follow-up to obtain health records, when necessary, is possible. Due to direct reporting capability and the speed at which reports and follow-up information can be processed and analyzed, VAERS can often provide the earliest information on potential vaccine safety problems. VAERS is less impacted by data lags and delayed access to health records than claims-based monitoring systems, although these types of systems often compliment VAERS by allowing for more sophisticated follow-on signal assessment due to availability of numerator and denominator data. Lastly, VAERS data are made available online to the public, which affords an important level of transparency. This service allows the public to see the amount and nature of spontaneous adverse event reporting data that CDC and FDA collect and analyze to guide further safety evaluations and inform decisions around vaccine recommendations and regulatory action.
What are the limitations of VAERS?
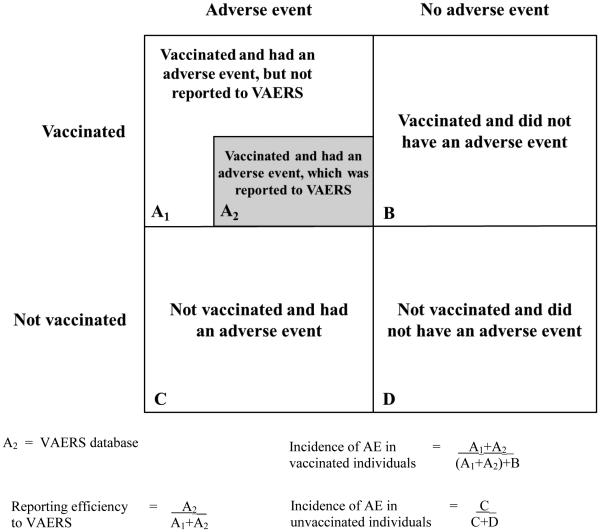
Like all spontaneous public health reporting systems, VAERS has limitations [1,14]. VAERS is subject to reporting bias, including underreporting of adverse events – especially common, mild ones [33,34] – and stimulated reporting, which is elevated reporting that might occur in response to intense media attention and increased public awareness, such as during the 2009 H1N1 pandemic influenza vaccination program [35]. Quality and completeness of VAERS reports are variable and many reports lack valid medical diagnoses. The amount of VAERS reporting (30,000 U.S. reports annually) makes it impractical to conduct detailed follow-up on all reports to obtain missing and incomplete information and correct inconsistencies and errors. Because VAERS data do not include an unvaccinated comparison group, it is not possible to calculate and compare rates of adverse events in vaccinated versus unvaccinated individuals and determine if vaccination is associated with an increased risk of an adverse event (Figure 3). Reporting efficiency, which is the proportion of adverse events that actually get reported to VAERS, is unknown, but is believed to be higher for clinically serious conditions. In a 1995 study, reporting sensitivities ranged from 68% for vaccine-associated polio following oral poliovirus vaccine to <1% for rash following MMR vaccine [33]. Although underreporting is a limitation, VAERS is capable of detecting possible safety problems through disproportionality analyses and the other methods described above.
Figure 3.
2×2 contingency table illustrating a hypothetical single vaccine and adverse event (AE) combination scenario
Except in unambiguous biologically plausible cases (like pain and redness at the injection site), it generally cannot be determined if a vaccine caused an adverse event using VAERS data [11,18]. On rare occasions, a detailed VAERS report with documentation of conclusive clinical or laboratory evidence might be sufficient to establish causality. For example, there have been case reports where vaccine strain rotavirus has been isolated from a stool specimen in a vaccinated infant experiencing severe gastroenteritis who was later diagnosed with severe combined immunodeficiency [36]. There have also been case reports documenting anaphylaxis occurring within an appropriate onset interval following vaccination with no other obvious environmental exposure triggers [37].
Misconceptions about VAERS
Perhaps the two most common misconceptions about VAERS are that temporally associated reports represent true adverse reactions caused by vaccination, and that VAERS reports equate to rates of adverse events or indicate risk of adverse events associated with vaccination. The VAERS website has specific guidance on interpreting case report information, which includes the statement: “When evaluating data from VAERS, it is important to note that for any reported event, no cause-and-effect relationship has been established … VAERS collects data on any adverse event following vaccination, be it coincidental or truly caused by a vaccine” [38]. Despite this cautionary guidance, VAERS reports have been misinterpreted and erroneously communicated as definitive evidence of causally associated adverse events. For example, during the U.S. multi-state measles outbreak of 2015 [39], unsubstantiated claims of over 100 deaths caused by MMR vaccine in the United States during the previous decade began circulating on the Internet [40,41]. The claim was based on VAERS reports in the public data. The authors of the Internet article further stated that no measles related deaths had been reported in the United States during the same time period, implying that MMR vaccine was doing more harm than good. In fact, many of the death reports after MMR vaccination involved children with serious preexisting medical conditions or were likely unrelated to vaccination (e.g., accidents). The complete VAERS reports and accompanying health records, autopsy reports and death certificates were reviewed in depth by CDC and FDA physicians and no concerning patterns emerged that would suggest a causal relationship with MMR vaccination and death [42].
The relatively rapid increase in numbers of reports to VAERS following the introduction and initial uptake of a new vaccine, an expected occurrence [43], has been misinterpreted as actual increases in incidence of adverse events and vaccine related risk. This has been the case with VAERS reports following quadrivalent human papillomavirus (HPV4) vaccination [44], which as expected, increased as uptake of HPV4 vaccine increased following licensure in 2006. However, post-licensure epidemiologic studies have consistently demonstrated the safety of HPV4 vaccine [45-51], confirming the limitations of passive surveillance systems like VAERS.
Closing thoughts
VAERS has been used to monitor adverse events since 1990 and continues to ably serve as the nation’s frontline post-licensure vaccine safety monitoring system. VAERS has successfully detected safety signals that required further evaluation [36,52-59] and has also provided reassurance on the safety of vaccines [60-63]. One of the earliest successes in signal detection and assessment in VAERS involved the first rotavirus vaccine, RotaShield®. Within nine months of its licensure in the United States in August 1998, reports to VAERS raised suspicion of a possible safety problem with intussusception, a type of bowel obstruction, in infants [52]. Further evaluation of the signal, which combined estimated RotaShield® doses administered with known background rates of infant intussusception, indicated that the observed number of intussusception reports to VAERS within one week of receipt of RotaShield® was approaching what would be expected by chance alone. Given the known underreporting of adverse events to VAERS, these findings were concerning enough for CDC to suspend its recommendation for RotaShield® vaccination and initiate further investigation [64]; shortly thereafter the vaccine was withdrawn from the market by the manufacturer [65]. More recently, VAERS detected disproportional reporting for febrile seizures in young children following an inactivated influenza vaccine during the 2010-2011 influenza season [58,59]. Clinical review of the VAERS reports indicated the cases were typical of uncomplicated febrile seizures and all children fully recovered. A related finding was later detected using sequential monitoring methods in a separate CDC surveillance system that uses large-linked electronic health record databases, and the increased risk was assessed and quantified in an epidemiologic study [66]. The information was quickly communicated to the public along with reassurances on the benefit-risk balance of vaccinating children against influenza [67].
CDC and FDA are currently updating the VAERS reporting form and enhancing electronic methods for reporting to improve the public health and regulatory value of VAERS data. These data adjustments and system enhancements are necessary responses to changes in the U.S. immunization program that have made some VAERS data fields obsolete and have imposed other needs such as information on adverse events following maternal vaccination. Additionally, CDC and FDA are implementing processes to improve and facilitate online reporting and to transition vaccine manufacturers to reporting using standardized messages through electronic data interchange [68-71]. A major impetus for improving electronic reporting and increasing automation in VAERS was the 2009 influenza pandemic experience where 10,000 influenza A (H1N1) monovalent (pandemic) vaccine reports were submitted to VAERS during the 2009-2010 influenza season [72]. Other future initiatives might include incorporating adverse event reporting reminders [73] and VAERS reporting capability directly into the software of electronic health records systems [74].
While near real-time sequential monitoring using large-linked electronic health record databases has become increasingly prominent in post-licensure vaccine safety surveillance [75], VAERS will continue to remain a foundation of the U.S. vaccine safety monitoring infrastructure. Understanding the purpose, strengths, and limitations of VAERS is essential when interpreting VAERS data and when responding to concerns from patients, parents, and others about adverse event reports to VAERS and vaccine safety in general. Healthcare professionals reporting to VAERS is arguably the most broad-based, cost-effective, and timely way to obtain real world feedback on vaccine safety. Often healthcare professionals, relying on experience and intuition, are the first to suspect a medical product problem and bring it to the attention of public health and regulatory officials [76,77]. CDC and FDA encourage reporting of clinically important or unexpected adverse events to VAERS following any U.S. licensed vaccines.
Acknowledgments
The authors thank Paige Lewis from the Immunization Safety Office at the Centers for Disease Control and Prevention for her contributions to this paper.
Funding source: No external sources of funding
Abbreviations
- VAERS
Vaccine Adverse Event Reporting System
- AEFI
adverse event following immunization
- CDC
Centers for Disease Control and Prevention
- FDA
U.S. Food and Drug Administration
- MedDRA
Medical Dictionary for Regulatory Activities
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration
Conflicts of interest: None of the authors have any conflicts of interest
References
- [1].Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, Chen RT. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23(4):287–94. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- [2].Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, Naleway A, Klein NP, Baxter R, Belongia E, Glanz J, Hambidge SJ, Jacobsen SJ, Jackson L, Nordin J, Weintraub E. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(Suppl 1):S45–53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- [3].Nguyen M, Ball R, Midthun K, Lieu TA. The Food and Drug Administration's Post-Licensure Rapid Immunization Safety Monitoring program: strengthening the federal vaccine safety enterprise. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):291–7. doi: 10.1002/pds.2323. [DOI] [PubMed] [Google Scholar]
- [4].The Uppsala Monitoring Centre Safer Medicines, Safer Use of Medicines, Safer Patients: What UMC is doing to help it happen. Available at: http://www.who-umc.org/graphics/27916.pdf. Accessed June 5, 2015.
- [5].Uppsala Monitoring Centre (UMC) Report from the WHO Collaborating Centre for International Drug Monitoring: Activities July 2013-June 2014. Available at: http://www.who-umc.org/graphics/28368.pdf. Accessed June 5, 2015.
- [6].Centers for Disease Control and Prevention Understanding the Vaccine Adverse Event Reporting System (VAERS) 2013 Feb; Available at: http://www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-vaers-color-office.pdf. Accessed June 5, 2015.
- [7].Council for International Organizations of Medical Sciences (CIOMS) Definition and Application of Terms for Vaccine Pharmacovigilance: Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance. WHO Press, World Health Organization; Geneva, Switzerland: 2012. [Google Scholar]
- [8].Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Guideline for Industry. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. 1995 Mar; Available at: http://www.fda.gov/downloads/Drugs/Guidances/ucm073087.pdf. Accessed June 5, 2015.
- [9].Council for International Organizations of Medical Sciences (CIOMS) Practical aspects of signal detection in pharmacovigilance: Report of CIOMS working group VIII. Geneva, Switzerland: 2010. [Google Scholar]
- [10].Marshall V, Baylor NW. Food and Drug Administration regulation and evaluation of vaccines. Pediatrics. 2011;127(Suppl 1):S23–30. doi: 10.1542/peds.2010-1722E. [DOI] [PubMed] [Google Scholar]
- [11].Chen RT, Davis RL, Rhodes PH. Special methodological issues in pharmacoepidemiology studies of vaccine safety. In: Strom BL, editor. Pharmacoepidemiology. 4th John Wiley & Sons; Sussex: 2005. [Google Scholar]
- [12].Centers for Disease Control and Prevention State School Immunization Requirements and Vaccine Exemption Laws. 2015 Feb-Mar; Available at: http://www.cdc.gov/phlp/docs/school-vaccinations.pdf. Accessed June 5, 2015.
- [13].Centers for Disease Control and Prevention State Immunization Laws for Healthcare Workers and Patients. 2014 Nov; Searchable database available at: http://www2a.cdc.gov/vaccines/statevaccsApp/default.asp. Accessed June 5, 2015.
- [14].Iskander JK, Miller ER, Chen RT. The role of the Vaccine Adverse Event Reporting system (VAERS) in monitoring vaccine safety. Pediatr Ann. 2004;33(9):599–606. doi: 10.3928/0090-4481-20040901-11. [DOI] [PubMed] [Google Scholar]
- [15].Singleton JA, Lloyd JC, Mootrey GT, Salive ME, Chen RT. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. VAERS Working Group. Vaccine. 1999;17(22):2908–17. doi: 10.1016/s0264-410x(99)00132-2. [DOI] [PubMed] [Google Scholar]
- [16].Centers for Disease Control and Prevention . Manual for the surveillance of vaccine-preventable diseases. Centers for Disease Control and Prevention; Atlanta, GA: Oct, 2011. Surveillance for Adverse Events Following Immunization Using the Vaccine Adverse Event Reporting System (VAERS) [Google Scholar]
- [17].Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System–United States. MMWR Morb Mortal Wkly Rep. 1990;39(41):730–3. [PubMed] [Google Scholar]
- [18].Chen RT, Rastogi SC, Mullen JR, Hayes SW, Cochi SL, Donlon JA, Wassilak SG. The Vaccine Adverse Event Reporting System (VAERS) Vaccine. 1994;12(6):542–50. doi: 10.1016/0264-410x(94)90315-8. [DOI] [PubMed] [Google Scholar]
- [19]. 42 U.S. Code §§ 300aa-1 to 300aa-34. National Childhood Vaccine Injury Act (1986)
- [20].What can be reported to VAERS? Available at: https://vaers.hhs.gov/about/faqs#what. Accessed June 5, 2015.
- [21].U. S. Code of Federal Regulations, 21 CFR 600.80 Postmarketing reporting of adverse experiences. 2014 Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=600.80. Accessed June 5, 2015.
- [22].Recording and Reporting of Information. 1999 42 U.S. Code § 300aa-25. Available at: http://www.fda.gov/RegulatoryInformation/Legislation/ucm180189.htm. Accessed June 5, 2015.
- [23].VAERS Table of Reportable Events Following Vaccination. Available at: https://vaers.hhs.gov/resources/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf. Accessed June 5, 2015.
- [24].Cook KM, Evans G. The National Vaccine Injury Compensation Program. Pediatrics. 2011 May;127(Suppl 1):S74–7. doi: 10.1542/peds.2010-1722K. [DOI] [PubMed] [Google Scholar]
- [25].Vaccine injury table. Available at: http://www.hrsa.gov/vaccinecompensation/vaccineinjurytable.pdf. Accessed June 5, 2015.
- [26].Medical Dictionary for Regulatory Activities (MedDRA) doi: 10.2165/00002018-199920020-00002. Available at: http://www.meddra.org/. Accessed June 5, 2015. [DOI] [PubMed]
- [27].Smith JC. The structure, role, and procedures of the U.S. Advisory Committee on Immunization Practices (ACIP) Vaccine. 2010 Apr 19;28(Suppl 1):A68–75. doi: 10.1016/j.vaccine.2010.02.037. [DOI] [PubMed] [Google Scholar]
- [28].DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat. 1999;53:177–90. [Google Scholar]
- [29].Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf. 2002;25(6):381–92. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- [30].Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–6. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- [31].Ambrose CS, Walker RE, Connor EM. Live attenuated influenza vaccine in children. Semin Pediatr Infect Dis. 2006 Oct;17(4):206–12. doi: 10.1053/j.spid.2006.08.007. [DOI] [PubMed] [Google Scholar]
- [32].Kohl KS, Bonhoeffer J, Braun MM, Chen RT, Duclos P, Heijbel H, Heininger U, Loupi E, Marcy SM. The Brighton Collaboration: Creating a Global Standard for Case Definitions (and Guidelines) for Adverse Events Following Immunization. In: Henriksen K, Battles JB, Marks ES, Lewin DI, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology) Agency for Healthcare Research and Quality (US); Rockville (MD): 2005. [PubMed] [Google Scholar]
- [33].Rosenthal S, Chen R. The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health. 1995;85(12):1706–9. doi: 10.2105/ajph.85.12.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT, Vaccine Adverse Event Reporting System Team Enhancing vaccine safety surveillance: a capture-recapture analysis of intussusception after rotavirus vaccination. Am J Epidemiol. 2001;154(11):1006–12. doi: 10.1093/aje/154.11.1006. [DOI] [PubMed] [Google Scholar]
- [35].Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, Lewis P, McNeil MM, Bryant M, Singleton J, Martin D, DeStefano F. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010;28(45):7248–55. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- [36].Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: reports to the Vaccine Adverse Events Reporting System (VAERS) Vaccine. 2010;28(40):6609–12. doi: 10.1016/j.vaccine.2010.07.039. [DOI] [PubMed] [Google Scholar]
- [37].Loughlin AM, Marchant CD, Adams W, Barnett E, Baxter R, Black S, Casey C, Dekker C, Edwards KM, Klein J, Klein NP, LaRussa P, Sparks R, Jakob K. Causality assessment of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2012;30(50):7253–9. doi: 10.1016/j.vaccine.2012.09.074. [DOI] [PubMed] [Google Scholar]
- [38].VAERS Data: Guide to Interpreting VAERS Case Report Information. Available at: https://vaers.hhs.gov/data/index. Accessed June 5, 2015.
- [39].Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K. Centers for Disease Control and Prevention. Measles outbreak--California, December 2014-February 2015. MMWR Morb Mortal Wkly Rep. 2015 Feb 20;64(6):153–4. [PMC free article] [PubMed] [Google Scholar]
- [40].Shilhavy B. Zero US measles deaths in 10 years, but over 100 measles vaccine deaths reported. Health Impact News. 2015 Feb 12; Available at: http://healthimpactnews.com/2015/zero-u-s-measles-deaths-in-10-years-but-over-100-measles-vaccine-deaths-reported/. Accessed June 5, 2015.
- [41].Huff EA. Measles vaccines kill more people than measles, CDC data proves. Global Research. 2015 Feb 5; Available at http://www.globalresearch.ca/measles-vaccines-kill-more-people-than-measles-cdc-data-proves/5429736. Accessed June 5, 2015.
- [42].Moro PL, Arana J, Cano M, Lewis P, Shimabukuro TT. Deaths reported to the Vaccine Adverse Event Reporting System (VAERS), United States, 1997-2013. Clin Infect Dis. 2015 May;:28. doi: 10.1093/cid/civ423. pii: civ423. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weber JCP. Epidemiology of adverse reactions to nonsteroidal antiinflammatory drugs. In: Rainsford KD, Velo GP, editors. Advances in inflammation research. Vol. 6. Raven Press; New York: 1984. pp. 1–6. [Google Scholar]
- [44].Erickson N. How closely does the CDC monitor HPV vaccine safety? 2014 Jan 5; Available at: http://sanevax.org/closely-cdc-monitor-hpv-vaccine-safety/. Accessed June 5, 2015.
- [45].Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley MF, Klein NP, Weintraub ES. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011 Oct 26;29(46):8279–84. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- [46].Chao C, Klein NP, Velicer CM, Sy LS, Slezak JM, Takhar H, Ackerson B, Cheetham TC, Hansen J, Deosaransingh K, Emery M, Liaw KL, Jacobsen SJ. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012 Feb;271(2):193–203. doi: 10.1111/j.1365-2796.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- [47].Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, Cheetham TC, Liaw KL, Takhar H, Jacobsen SJ. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012 Dec;166(12):1140–8. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- [48].Arnheim-Dahlström L, Pasternak B, Svanström H, Sparén P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013 Oct 9;347:f5906. doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Grimaldi-Bensouda L, Guillemot D, Godeau B, Bénichou J, Lebrun-Frenay C, Papeix C, Labauge P, Berquin P, Penfornis A, Benhamou PY, Nicolino M, Simon A, Viallard JF, Costedoat-Chalumeau N, Courcoux MF, Pondarré C, Hilliquin P, Chatelus E, Foltz V, Guillaume S, Rossignol M, Abenhaim L, PGRx-AID Study Group Autoimmune disorders and quadrivalent human papillomavirus vaccination of young female subjects. J Intern Med. 2014 Apr;275(4):398–408. doi: 10.1111/joim.12155. [DOI] [PubMed] [Google Scholar]
- [50].Scheller NM, Pasternak B, Svanström H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA. 2014 Jul;312(2):187–8. doi: 10.1001/jama.2014.2198. [DOI] [PubMed] [Google Scholar]
- [51].Scheller NM, Svanström H, Pasternak B, Arnheim-Dahlström L, Sundström K, Fink K, Hviid A. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA. 2015 Jan 6;313(1):54–61. doi: 10.1001/jama.2014.16946. [DOI] [PubMed] [Google Scholar]
- [52].Centers for Disease Control and Prevention Intussusception among recipients of rotavirus vaccine–United States, 1998-1999. MMWR Morb Mortal Wkly Rep. 1999;48(27):577–81. [PubMed] [Google Scholar]
- [53].Centers for Disease Control and Prevention Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine--United States, June-July 2005. MMWR Morb Mortal Wkly Rep. 2005 Oct 14;54(40):1023–5. [PubMed] [Google Scholar]
- [54].Centers for Disease Control and Prevention Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine--United States, October 2005-February 2006. MMWR Morb Mortal Wkly Rep. 2006 Apr 7;55(13):364–6. [PubMed] [Google Scholar]
- [55].Centers for Disease Control and Prevention Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine--United States, June 2005-September 2006. MMWR Morb Mortal Wkly Rep. 2006 Oct 20;55(41):1120–4. [PubMed] [Google Scholar]
- [56].Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Pediatr Adolesc Med. 1997 Mar;151(3):255–9. doi: 10.1001/archpedi.1997.02170400041007. [DOI] [PubMed] [Google Scholar]
- [57].Centers for Disease Control and Prevention Syncope after vaccination--United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep. 2008 May 2;57(17):457–60. [PubMed] [Google Scholar]
- [58].Leroy Z, Broder K, Menschik D, Shimabukuro T, Martin D. Febrile seizures after 2010-2011 influenza vaccine in young children, United States: a vaccine safety signal from the vaccine adverse event reporting system. Vaccine. 2012;30(11):2020–3. doi: 10.1016/j.vaccine.2011.12.042. [DOI] [PubMed] [Google Scholar]
- [59].Martin D, Menschik D, Bryant-Genevier M, Ball R. Data mining for prospective early detection of safety signals in the Vaccine Adverse Event Reporting System (VAERS): a case study of febrile seizures after a 2010-2011 seasonal influenza virus vaccine. Drug Saf. 2013;36(7):547–56. doi: 10.1007/s40264-013-0051-9. [DOI] [PubMed] [Google Scholar]
- [60].Braun MM, Mootrey GT, Salive ME, Chen RT, Ellenberg SS. Infant immunization with acellular pertussis vaccines in the United States: assessment of the first two years' data from the Vaccine Adverse Event Reporting System (VAERS) Pediatrics. 2000 Oct;106(4):E51. doi: 10.1542/peds.106.4.e51. [DOI] [PubMed] [Google Scholar]
- [61].Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun MM, Markowitz LE, Iskander J. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302(7):750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- [62].Centers for Disease Control and Prevention Safety of influenza A (H1N1) 2009 monovalent vaccines - United States, October 1-November 24, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(48):1351–6. [PubMed] [Google Scholar]
- [63].Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204(2):146.e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- [64].Centers for Disease Control and Prevention Suspension of rotavirus vaccine after reports of intussusception--United States, 1999. MMWR Morb Mortal Wkly Rep. 2004 Sep 3;53(34):786–9. Erratum in: MMWR Morb Mortal Wkly Rep. 2004 Sep 24;53(37):879. [PubMed] [Google Scholar]
- [65].Centers for Disease Control and Prevention Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999 Nov 5;48(43):1007. [PubMed] [Google Scholar]
- [66].Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM, VSD Rapid Cycle Analysis Influenza Working Group Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine. 2012 Mar 2;30(11):2024–31. doi: 10.1016/j.vaccine.2012.01.027. [DOI] [PubMed] [Google Scholar]
- [67].Centers for Disease Control and Prevention Febrile Seizures in Children Following Vaccination with Influenza Vaccines and Pneumococcal Vaccines — 2010-2011 Influenza Season. Available at: http://www.cdc.gov/vaccinesafety/Concerns/FebrileSeizures-archived.html. Accessed June 5, 2015.
- [68].Shimabukuro T. The Vaccine Adverse Event Reporting System (VAERS) form Version 2.0 (proposed) 2014 Sep 9; September 9, 2014, National Vaccine Program Office/HHS: Presented at the National Vaccine Advisory Committee meeting. [Google Scholar]
- [69].Request for Comment on Draft Vaccine Adverse Event Reporting System (VAERS) 2.0 Form, C.f.D.C.a.P. Department of Health and Human Services, Editor. 2014 Nov 24;:69853–69854. [Google Scholar]
- [70].Postmarketing Safety Reports for Human Drug and Biological Products; Electronic Submission Requirements., F.a.D.A. Department of Health and Human Services. :33072–33092. Editor. June 10, 2014, Department of Health and Human Services, Food and Drug Administration. [PubMed] [Google Scholar]
- [71].Draft Guidance for Industry on Providing Submissions in Electronic Format–Postmarketing Safety Reports, D.o.H.a.H. Services, Editor. :33200–33201. June 10, 2014, Department of Health and Human Services. Food and Drug Administration. [Google Scholar]
- [72].Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, Lewis P, McNeil MM, Bryant M, Singleton J, Martin D, DeStefano F. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010 Oct 21;28(45):7248–55. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- [73].Hinrichsen VL, Kruskal B, O'Brien MA, Lieu TA, Platt R. Using electronic medical records to enhance detection and reporting of vaccine adverse events. J Am Med Inform Assoc. 2007 Nov-Dec;14(6):731–5. doi: 10.1197/jamia.M2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Baker MA, Kaelber DC, Bar-Shain DS, Moro PL, Zambarano B, Mazza M, Garcia C, Henry A, Platt R, Klompas M. Advanced Clinical Decision Support for Vaccine Adverse Event Detection and Reporting. Clin Infect Dis. 2015 Jun 9; doi: 10.1093/cid/civ430. pii: civ430. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lieu TA, Kulldorff M, Davis RL, Lewis EM, Weintraub E, Yih K, Yin R, Brown JS, Platt R, Vaccine Safety Datalink Rapid Cycle Analysis Team Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007 Oct;45(10 Supl 2):S89–95. doi: 10.1097/MLR.0b013e3180616c0a. [DOI] [PubMed] [Google Scholar]
- [76].Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997 Aug 28;337(9):581–8. doi: 10.1056/NEJM199708283370901. Erratum in: N Engl J Med 1997 Dec 11;337(24):1783. [DOI] [PubMed] [Google Scholar]
- [77].Centers for Disease Control and Prevention Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb Mortal Wkly Rep. 1997 Nov 14;46(45):1061–6. [PubMed] [Google Scholar]