Abstract
Recently, an innovative, nonsurgical regenerative endodontic treatment protocol “SealBio” was introduced to manage mature nonvital permanent teeth with periapical lesions. This paper explains the management of an unusual case of dens evaginatus and an attached supernumerary tooth/an odontome associated with two distinct radiolucencies in a mandibular premolar with “SealBio” technique and discusses the various hypotheses on the pathogenesis of unusual malformation and associated pericervical cyst-like radiolucency in the involved tooth.
Keywords: Dens evaginatus, odontome, pericervical radiolucency, photo-activated disinfection, SealBio, supernumerary premolars
Introduction
Dens evaginatus (DE) is a developmental aberration of a tooth resulting in the formation of an accessory cusp projecting above the adjacent tooth surface. It is known by various names such as odontome, odontoma of the axial core type, evaginatus odontoma, occlusal enamel pearl, occlusal tubercle, tuberculum anomalous, interstitial cusp, tuberculated cusp, Leong's premolar, talon cusp (specifically for anterior teeth), etc.[1] It presents itself as an abnormal tubercle, or elevation with enamel, dentin, and varying amounts of pulp tissue. It is found on the occlusal surface of premolars and the lingual surface of anterior teeth, in 0.5–4.3% of population, predominantly in people of Asian descent.[2]
Supernumerary teeth (ST) are any teeth in excess of the usual complement of teeth in permanent or deciduous dentitions. One or two ST are most commonly seen in anterior maxillary followed by the mandibular premolar region, whereas mandibular premolar region shows a predilection for multiple ST (>5). They are classified according to morphology as rudimentary (smaller and tuberculate in shape), supplemental (resemble the teeth of a group with which they are associated), and odontomes (tumors of odontogenic origin).[3] Odontomes result from growth of completely differentiated epithelial and mesenchymal cells, which differentiate into ameloblasts and odontoblasts that form variable amounts of enamel, dentin, and pulp.[4] It is classified as compound odontome (odontogenic tissues are arranged in an orderly pattern) and complex odontome (odontogenic tissues are arranged in an irregular mass).[5] The prevalence of supernumerary premolars (SP) (or para-premolar) is 0.075–0.26%, accounting for only 8–10% of all supernumerary cases.[6] The SP occur more commonly in the mandible.[7] Around 2% of para-premolars show various complications such as cystic changes (dentigerous cyst, eruption cyst, or primordial cyst), root resorption, etc.[8]
Fusion (union of two adjacent teeth) and germination (an attempt of a single tooth germ to divide resulting in a large single tooth with bifid crown and common root and root canal) are developmental anomalies in size, shape, and structure of teeth. In fusion, crowns are united by enamel and/or dentine with two roots or a single root with two canals. On the contrary, in germination, two crowns are totally or partially separated with a single root and root canal. Fusion can be differentiated from germination by counting the number of teeth. However, these definitions also make a differentiation between fusion and germination difficult when fusion involves a normal and a supernumerary tooth.
Recently, Shah and Logani introduced an innovative, nonsurgical regenerative endodontic treatment protocol “SealBio” to manage mature, nonvital permanent teeth with periapical lesions.[9] It helps in achieving a definite cemental/fibrous barrier at the root apex.[9] This paper describes the management of DE and an attached supernumerary tooth/an odontome associated with two distinct radiolucencies in a mandibular premolar with “SealBio” technique and discusses the various hypotheses on the pathogenesis of unusual malformation and associated pericervical cyst-like radiolucency in the involved tooth.
Case Report
An 18-year-old healthy Asian female presented to the postgraduate endodontic clinic with the chief complaint of intermittent pain and fluid discharge from the lingual sulcus of a mandibular left first premolar (tooth #34) for the past 1½ years. On intraoral clinical examination, the tooth #34 appeared to be caries-free with a striking occlusal anatomy. There was a protuberant mass in the mid-occlusal surface, identified as DE [Figure 1]. On the lingual surface, a small 2 cm × 2 cm bluish, fluctuant swelling was noticed [Figure 1]. On probing through gingival margin, typical oily, straw colored fluid escaped out, denoting a cystic pathology with a probing depth of 5 mm. The lingual mucosa over the fluctuant swelling was compressed in an attempt to completely drain out the cystic fluid. The patient had an intraoral periapical X-ray done from outside, which showed two distinct radiolucencies - One periapical, 2 cm × 2 cm well-circumscribed and another a large cyst-like radiolucency extending from distal aspect of canine to mesial aspect of second premolar tooth around the entire length of the root except the apical 3 mm of the involved first premolar [Figure 2A]. Pulp vitality test (cold tests) was negative for tooth #34. A diagnosis of pulp necrosis with large periapical pathology was made. The patient was explained regarding the aberrant corono-radicular morphology, periapical, and pericervical lesions present in relation to tooth #34 and the treatment plan. Though nonsurgical treatment with “SealBio” was planned to be attempted first, the patient was also informed that surgical enucleation may be required later for the large periapical or periradicular cystic lesions, to which she agreed. The risks, complications, and possible outcome of SealBio were explained, and written informed consent was taken. Endodontic access cavity was prepared, cutting through the DE on the occlusal surface. A large oval canal was debrided along with copious irrigation with 2.5% sodium hypochlorite solution. Working length determined using apex locator (Root ZX; Morita, Tokyo, Japan) was 22 mm with initial apical file size of #20 K-file and master apical file (MAF) size #40 K-file cleaning and shaping of the single, large, and central canal was done by crown-down technique, maintaining the apical patency throughout the process. For rapid disinfection, 60 s of photo-activated disinfection (PAD) with toluidine blue dye in the canal was done. The canal was finally rinsed with normal saline, and an intracanal dressing of calcium hydroxide paste was given. The patient was recalled after 1-week. At the next sitting, all her clinical symptoms were resolved. On re-entry, the canal was dry. It was planned to complete the case by SealBio in this sitting. However, to ensure adequate disinfection of the canal, 60 s of PAD application was repeated and the canal was finally rinsed with 2 ml of 15% ethylenediaminetetraacetic acid (EDTA) (Largal Ultra, Septodont, Saint Maur des Fosses, France) and dried. Following this, the recommended protocol for “SealBio” was performed. It consisted of “apical clearing,” “apical widening,” and “intentional over-instrumentation.” In “apical clearing,” the apical third of the canal was debrided by enlarging it with 2–4 file sizes larger than the MAF at predetermined working length subsequent to this, “apical foramen widening” was done with the larger H-files used sequentially till size 30 to clean the cemental part of the canal. The canal was dried. Two inter appointment intracanal dressings with PAD and calcium hydroxide (Prime Dental Products Pvt., Ltd., Mumbai, Maharashtra, India) were given at 3 weeks’ interval. The tooth was temporarily restored with the intermediate restorative material (IRM, Caulk DENTSPLY, Milford, DE).
Figure 1.
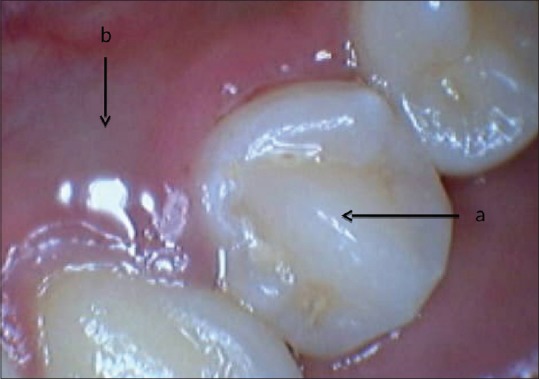
Tooth #34 showing dens evaginatus (a) and a small 2×2 cm2 bluish, fluctuant swelling on the lingual surface (b)
Figure 2.
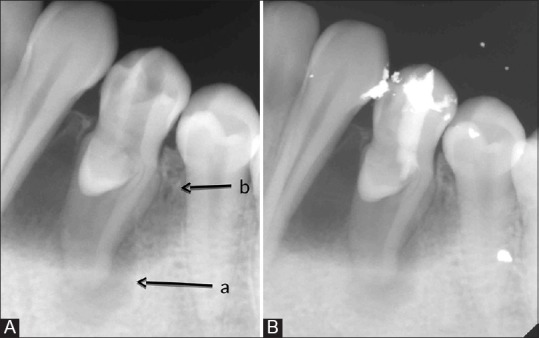
(A) Tooth #34 showing well-circumscribed, periapical(a) and large cyst-like peri-radicular radiolucencies (b). (B) SealBio was done and coronal seal was completed using calcium sulphate-based cement (Cavit) and silver amalgam restoration
Subsequent to the resolution of clinical signs and symptoms, the tooth was re-opened. A final wash with 15% EDTA and normal saline was done and the canals were dried. After checking the patency by the smooth passage of #15 K-file, intentional over-instrumentation into the periapical region was done with #20 K-file to induce bleeding near the apical foramen. The file was gently given 2–3 clockwise turns and then withdrawn by giving counterclockwise rotation. A calcium sulfate-based cement (Cavit) was introduced in the access cavity. It was condensed into the cervical third of root canal with a hand plugger. The coronal seal was done with a silver amalgam restoration [Figure 2B]. The patient was recalled subsequently after 12 and 18 months for clinical and radiographic evaluation [Figure 3].
Figure 3.

Post operative radiographs at 12 months (a) and 18 months (b) exhibited complete resolution of both peri-radicular and peri-apical radiolucencies
Discussion
DE develops due to an abnormal proliferation and folding of a portion of the inner enamel epithelium and subjacent ectomesenchymal cells of the dental papilla into the stellate reticulum of the enamel organ during the bell stage of tooth formation.[10] It causes occlusal interference and/or devitalization of the involved tooth and shows a sexual predilection for females. There are different classifications given for the morphological variations of DE and its effect on the involved tooth. According to the Schulge classification, the presented case was Type V in which a tubercle arising from the occlusal surface obliterated the central groove.[2] According to the classification given by Levitan and Himel, it was Type V because of necrotic pulp and mature apex.[1] Tooth #34 became nonvital because microscopic pulp exposure in the tubercle area facilitated chronic bacterial invasion.
The exact diagnosis of the extra tooth-like structure attached to a cervical third of root and associated cyst-like radiolucency was difficult. One of the following two possibilities was considered for the differential diagnosis of cervical radiopacity.
Rudimentary type of supernumerary tooth fused with #34
Cystic compound composite odontome associated with #34.
Moreover, the following conditions were considered for the differential diagnosis of pericervical radiolucency.
Eruption cyst associated with a supernumerary tooth
Lateral periodontal cysts associated with tooth #34
Cystic odontome.
As the patient had presented with a bluish-tinged, soft, fluctuant swelling on lingual aspect of the tooth which drained readily through the gingival sulcus at the first sitting, it was decided to open the root canal and debride it to facilitate further drainage, if any, through the canal. The access cavity was opened and a single, centrally located large canal was negotiated. The canal was copiously debrided after working length determination. At the second visit, surprisingly, the patient's symptoms had subsided completely, and no swelling was visible on the lingual side. Cleaning and shaping were again performed. At the third visit, it was treated by the innovative, regenerative technique of “SealBio.” It involved “apical clearing,” that is, widening the apical third of the canal 2–4 sizes larger than the MAF without transportation of the canal or apical foramen,[11] “apical foramen widening,” that is, after maintaining the patency of the foramen, widening it to size 20–25, to help clean the cemental part of the canal and, thus, reduce the number of remaining bacteria, and finally, “intentional over-instrumentation” into the periapical region to induce bleeding and allow blood clot formation near the apical foramen. The formed blood clot provides a scaffold where various stem cells from apical region, such as bone marrow mesenchymal stem cells, stem cells from apical papilla (SCAP), stem cells from periodontal ligament (SCPL), etc., can get implanted. They proliferate, differentiate, and form a mineralized barrier, “SealBio” (a biological seal) to seal the apical foramen.[9] The coronal access cavity was restored with silver amalgam after application of cavity varnish.
Recently, some concerns regarding the toxic effect of intracanal disinfectants on stem cell viability have appeared in the regenerative endodontic literature.[12] Effect of intracanal calcium hydroxide dressing in regenerative procedures is being debated. One view suggests that it may reduce the viability and regenerative capacity of the apical vital cells due to periapical coagulation necrosis.[13] However, in a recent study calcium hydroxide was found conducive with SCAP survival and proliferation.[12] In fact, it has been found to stimulate Hertwig's epithelial root sheath, and hence, useful in apexogenesis.[14] Current consensus dictates the confinement of calcium hydroxide in the root canal to avoid its detrimental effect on the periapical stem cells viability.[15]
In the presented case, a combination of calcium hydroxide dressing and PAD was used, which was found to be effective without any adverse impact on healing response. PAD is a new antimicrobial strategy that involves the combination of a nontoxic photo-sensitizer dye toluidine blue and low-intensity visible light.[16] It works on the principle of “lethal photo-sensitization.” When photo-sensitizer is irradiated with light at a specific wavelength, it absorbs energy from the light. This energy is released to oxygen (O2), which is transformed into highly reactive oxygen species such as singlet oxygen.[17] These oxygen ions and radicals are cytotoxic, causing bacterial cell-wall rupture and death of microorganisms. Photo-sensitizer is highly selective for microorganisms, and therefore, probably it did not affect any cells in the periapical region.[18] PAD can be considered an important adjunct in the disinfection protocol of regenerative endodontics, where other disinfectants such as chlorhexidine and sodium hypochlorite may adversely affect the survival of stem cells. Gambarini et al. showed that PAD produced a significantly less cytotoxic effect than 2% chlorhexidine.[19] The intracanal medicament of calcium hydroxide improved the efficacy of PAD because it produces an alkaline environment that improved the production of reactive oxygen species, in particular, singlet oxygen.[20] EDTA acts as a disrupter of biofilm, improving the penetration of the photo-sensitizer to the root canal system.[21] In addition, dentin surrounding the root canal promoted the light backscattering, thus, increasing the number of photons available to the photo-reaction.[22] PAD is capable of killing 97–99.9% of the bacteria in the culture.[23]
The resolution of large cyst-like cervical and periapical radiolucencies was intriguing in the present case. An orthograde, nonobturation, nonsurgical endodontic treatment combined with simple drainage of the lesion through gingival sulcus resulted in marked healing of both the lesions. In SealBio triad of thorough disinfection, intentional over-instrumentation (to achieve a biological barrier of hard and soft tissues), and bacteria tight coronal seal is required. Shah and Logani confirmed the validity of SealBio, which is novel, nonobturation, and nonsurgical endodontic treatment protocol based on “regenerative concept” using cone-beam computed tomography (CBCT) evaluation that showed the deposition of mineralized tissue at the root apex. Moreover, SealBio has obtained an Australian patent (number - AU2010355508), while an application with the US patents office (number - WO2011158245 A1) is under process.
It can be inferred that the cyst-like lesion could have been an eruption cyst associated with the attached supernumerary tooth/compound odontome. Many cases of spontaneous healing of eruption cyst associated with the eruption of unerupted permanent premolars have been reported in the literature.[24]
Such anomalies, as was found in the present case, require in-depth investigation to determine its nature (correct diagnosis), its relation with the normal series tooth (in this case, the first mandibular premolar), whether the pulp space was common between the two. The modern method of diagnosis of such cases is by three-dimensional imaging such as CBCT. In the present case, on the very first sitting after probing the gingival sulcus during the course of the diagnosis itself, the cystic fluid got drained out. It provided symptomatic relief. Compressing and draining the remaining fluid and root canal debridement led to resolution of the cyst; as on the next visit, no sign or symptoms of a cyst was found. Moreover, the patient also had symptomatic relief of apical periodontitis and, therefore, the nonsurgical treatment was continued. On immediate posttreatment clinical and radiographic evaluation, evidence of progressive healing was found, and hence, it was decided to keep the case under regular follow-up. Six months, 1 and 2 years follow-ups showed marked healing of periapical radiolucency and reduced lesion size of cyst-like radiolucency on the lateral aspect.
The case-presented here highlighted very significant points:
Always prefer the most simple and minimally-invasive approach in the treatment of any disease or the lesion. Though on X-ray, large cyst-like lesion associated with an odontome/supernumerary tooth would have led to indication for surgical intervention on first impression, simple intervention of drainage, and orthograde novel endodontic treatment gave excellent result
Newer, more sophisticated radiographic investigation such as CBCT should be reserved for complex diagnostic dilemmas and not resorted to for any and every aberrant anatomy. The cost-benefit ratio in terms of both monetary and X-ray exposure, should be taken into account before advising such investigation
The large cyst-like radiolucency could not have arisen due to endodontic infection resulting from DE, as there was no possible pathway for it to involve lateral aspect. This is also further validated by the fact that the periapical and lateral radiolucencies were separate and were not merging with each other
Use of PAD as an adjunct for disinfection of root canal space was found to be compatible with regeneration-based “SealBio” technique.
Conclusion
An interesting case of a mandibular premolar with pulp and periapical infection resulting from DE and an attached supernumerary tooth/compound odontome is presented for its unique findings, innovative treatment approach, and excellent treatment outcome for both, the large lateral cyst-like lesion, as well as periapical lesion. In the documented case, “SealBio” proved to be an effective treatment modality, which otherwise would have required surgical intervention. With advances in regenerative techniques in Endodontics, the emphasis is now shifting more toward biologically-based procedures. “Sealbio” is an innovation where effective disinfection and utilization of indigenous, locally available stem cells in the periapical region is found to be effective in healing of the pathology and achieving a biological seal of fibrous/cemental barrier over the apical foramen, provided an effective coronal seal is provided. Effect of PAD on the survival of stem cells of apical papilla and its use as an adjunct to disinfect the root canal system in regenerative endodontic procedures need to be further investigated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levitan ME, Himel VT. Dens evaginatus: Literature review, pathophysiology, and comprehensive treatment regimen. J Endod. 2006;32:1–9. doi: 10.1016/j.joen.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Kocsis G, Marcsik A, Kokai E, Kocsis K. Supernumerary occlusal cusps on permanent human teeth. Acta Biol Szeged. 2002;46:71–82. [Google Scholar]
- 3.Mitchell L. Supernumerary teeth. Dent Update. 1989;16:65. [PubMed] [Google Scholar]
- 4.Shafer GW, Hine MK, Levy BM. A Text Book of Oral Pathology. 4th ed. Philadelphia: WB Saunders; 1983. pp. 308–11. [Google Scholar]
- 5.Kramer IR, Pindborg JJ, Shear M. WHO International Histological Classification of Tumors. 2nd ed. Berlin: Springer; 1992. Histological typing of odontogenic tumor; pp. 16–21. [Google Scholar]
- 6.Hyun HK, Lee SJ, Ahn BD, Lee ZH, Heo MS, Seo BM, et al. Nonsyndromic multiple mandibular supernumerary premolars. J Oral Maxillofac Surg. 2008;66:1366–9. doi: 10.1016/j.joms.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Yusof WZ. Non-syndrome multiple supernumerary teeth: Literature review. J Can Dent Assoc. 1990;56:147–9. [PubMed] [Google Scholar]
- 8.Bodin I, Julin P, Thomsson M. Hyperodontia. I. Frequency and distribution of supernumerary teeth among 21,609 patients. Dentomaxillofac Radiol. 1978;7:15–7. doi: 10.1259/dmfr.1978.0002. [DOI] [PubMed] [Google Scholar]
- 9.Shah N, Logani A. SealBio: A novel, non-obturation endodontic treatment based on concept of regeneration. J Conserv Dent. 2012;15:328–32. doi: 10.4103/0972-0707.101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngeow WC, Chai WL. Dens evaginatus on a wisdom tooth: A diagnostic dilemma. Case report. Aust Dent J. 1998;43:328–30. [PubMed] [Google Scholar]
- 11.Card SJ, Sigurdsson A, Orstavik D, Trope M. The effectiveness of increased apical enlargement in reducing intracanal bacteria. J Endod. 2002;28:779–83. doi: 10.1097/00004770-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Spangberg L. Biological effects of root canal filling materials 4. Effect in vitro of solubilised root canal filling materials on HeLa cells. Odontol Revy. 1969;20:289–99. [PubMed] [Google Scholar]
- 14.Saad AY. Calcium hydroxide and apexogenesis. Oral Surg Oral Med Oral Pathol. 1988;66:499–501. doi: 10.1016/0030-4220(88)90277-0. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: Biological basis of regenerative endodontic procedures. J Endod. 2013;39(3 Suppl):S30–43. doi: 10.1016/j.joen.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study) Br Dent J. 2006;200:337–41. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 18.Maisch T, Bosl C, Szeimies RM, Lehn N, Abels C. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob Agents Chemother. 2005;49:1542–52. doi: 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gambarini G, Plotino G, Grande NM, Nocca G, Lupi A, Giardina B, et al. In vitro evaluation of the cytotoxicity of FotoSan™ light-activated disinfection on human fibroblasts. Med Sci Monit. 2011;17:MT21–5. doi: 10.12659/MSM.881435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRosa MC, Crutchley RJ. Photosensitized singlet oxygen and its applications. Coord Chem Rev. 2002;233-234:351–71. [Google Scholar]
- 21.Bonsor SJ, Nichol R, Reid TM, Pearson GJ. An alternative regimen for root canal disinfection. Br Dent J. 2006;201:101–5. doi: 10.1038/sj.bdj.4813819. [DOI] [PubMed] [Google Scholar]
- 22.Garcez AS, Nuñez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod. 2008;34:138–42. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silbert T, Bird PS, Milbum GJ, Walsh LJ. Disinfection of root canals by laser dye photosensitisation. J Dent Res. 2000;79:569–72. [Google Scholar]
- 24.Martínez-Pérez D, Varela-Morales M. Conservative treatment of dentigerous cysts in children: A report of 4 cases. J Oral Maxillofac Surg. 2001;59:331–3. doi: 10.1053/joms.2001.21006. [DOI] [PubMed] [Google Scholar]


