Abstract
In the contemporary era, use of drugs is the dominant paradigm of health care. The most quotidian drug used for fever and pain is paracetamol. Although adverse reactions to paracetamol in India are rare, at times they can cause life-threatening situations. Stevens-Johnson syndrome (SJS) is one such potentially lethal adverse drug reaction. The most reported cases of analgesic-induced SJS were due to oxicams or propionic acid derivatives. There are very few detailed reports of SJS due to the use of paracetamol. We report a case of SJS, which occurred due to the use of paracetamol. The clinical features of this condition and multidisciplinary management of the patient are described in brief.
Keywords: Adverse drug reaction, paracetamol, Stevens-Johnson syndrome
Introduction
Steven-Johnson syndrome (SJS) is an infrequent and a severe form of erythema multiforme (EM). It can occur due to an adverse hypersensitivity reaction to drugs which results in skin and mucosal eruptions that can be potentially fatal. It is considered to be a less severe form of toxic epidermal necrolysis (TEN). The only difference being the extent of epidermal detachment; that is, 30% of the total body surface area; while 10–30% is known as SJS-TEN overlap.[1]
“A new eruptive fever with stomatitis and ophthalmia” was described as a severe variant of EM and was termed by Steven and Johnson in 1922.[2]
SJS may present as a nonspecific febrile illness leading to malaise, headache, cough, rhinorrhea with polymorphic lesions of the skin and mucous membrane characterized by acute blisters and erosions.
The incidence of SJS has been estimated to be around 1–6/1,000,000 persons per year with a mortality rate of 1–5% which rises up to 30% in TEN. Multiple drugs have been identified to cause SJS and TEN, antibiotics (sulfonamides) being the most common.[3]
The drugs that cause SJS commonly are antibacterials (sulfonamides), anticonvulsants (phenytoin, phenobarbital, and carbamazepine), nonsteroidal anti-inflammatory drugs (oxicam derivatives), and oxide inhibitors (allopurinol).[4]
Paracetamol is among the most extensively used analgesic and anti-pyretic because of easy availability and cost-effectiveness. Despite being considered relatively safe, adverse reactions including cutaneous hypersensitivity reactions have been reported.[5]
Very few cases of EM or SJS have been reported with the ingestion of paracetamol. Publications from 1995 to 2011 describing SJS and TEN in Indian population were searched by Patel et al. in PubMed, Medline, Embase, and UK PubMed Central Electronic Databases showed 6.17% of cases of SJS and TEN were due to the ingestion of paracetamol. Hence, we present a rare case of SJS occurred due to the ingestion of paracetamol.[6]
Case Report
A 14-year-old male patient reported to Department of Oral Medicine and Radiology with a chief complaint of painful ulceration of lips and oral cavity leading to difficulty in opening mouth and eating food since 5 days. History of present illness revealed that there was burning sensation followed by ulcers which appeared first in oral cavity, lips, and other parts of body including chest, arms, legs and thighs, and genital organs [Figure 1a and b]. The redness of eye was evident, and there was a history of watery discharge. Numerous healed lesions were also seen on the chest, axilla which gave the typical appearance of “target lesions” or “bull's eye “appearance” [Figure 1c].
Figure 1.
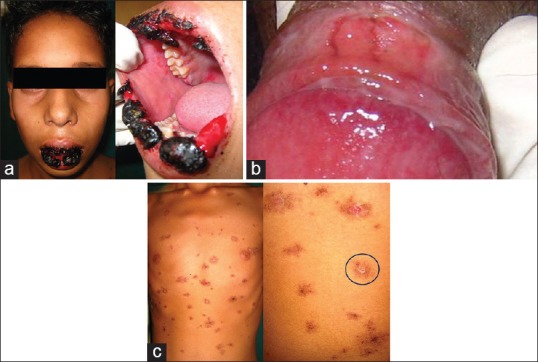
(a) Initial presentation of the case showing encrusted lesion on lips and intra-oral erythema. (b) Hemorrhagic erosion on mucous membrane of glans penis, (c) targetoid rashes over chest
The past medical history revealed that the patient was suffering from fever and pain since 2 weeks. The patient was prescribed tablet crocin for fever and pain for 7 days by a local medical practitioner. The patient was relieved from fever and pain but later he had burning sensation followed by ulcers in the oral cavity and extra-oral surface.
Intra-oral examination revealed a solitary mixed red and white lesion present on both right and left buccal mucosa. On left buccal mucosa it measured 10 mm × 8 mm in diameter and right side about 16 mm × 9 mm approximately.
The lesions have well-defined borders and on palpation it was non scrapable and nontender with a rough texture. The upper and lower lips were swollen, and hemorrhagic crusts were present along with profuse bleeding. Laboratory investigations revealed leukocytosis (white blood cells, 15101/l, reference value, 4000−11,000/l) and raised C-reactive protein 59.87 g/ml; reference range, 0−5 g/Ml. We had subjected the patient to only the hematological investigation as the lesion being acute, and the patient was under severe discomfort. Based on clinical examination, medical history, and physical examination our diagnosis was SJS.
The patient was treated under an expert guidance of dermatologist with systemic steroids; tablet prednisolone 30 mg twice daily daily for 7 days. Further reduced to 20 mg twice daily for next 7 days. Gradually, 10 mg and 5 mg for consecutive 7 days were administered. Gentian violet application 3 times daily for lips and skin lesions was advocated.
Application of Kenacort (triamcinolone) ointment thrice a day was advised for ulcers in the oral cavity. The patient was reviewed after a week. Lesions had healed significantly in the oral cavity and on the skin. Recall after 2 weeks revealed almost resolved lesions on all the surfaces and completely recovered approximately in 40 days [Figure 2a and b].
Figure 2.
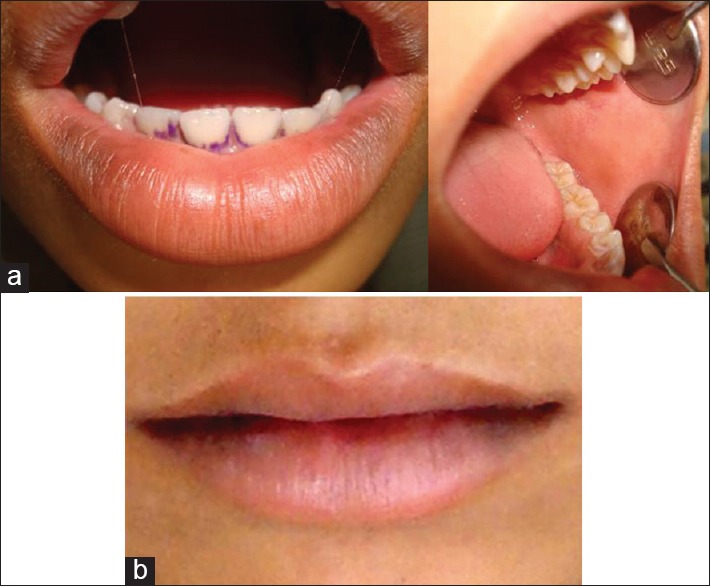
(a) Healing of crusting of lips and healing lesion of buccal mucosa, (b) Healed lesion of upper and lower lips
Discussion
SJS is an uncommon, severe, mucocutaneous blistering disorder with an acute and unpredictable onset causing considerable morbidity. Its more severe form is called TEN. Previously, SJS was considered as EM major, but now is considered distinct from EM on the basis of severity, presence of constitutional signs, atypical target lesions with tendency to confluence, positive Nikolsky's sign, more than one mucosal site involvement, and residual sequelae.
In the oral cavity, SJS causes widespread ulcerative lesions. Prodromal symptoms seen in about 30% of cases and may possibly initiate within 1–3 weeks of starting a new drug, and lasts for 1–2 weeks, presenting with flu-like symptoms, sore throat, headache, arthralgias, myalgias, fever, and other rashes.
Ocular changes such as dry eyes that resemble those of mucous membrane pemphigoid may be noted in few cases. Urethritis and vulval ulcers may occur.[7] Our patient did not report any prodrome, but the eye and genital ulcerations were present, along with skin and mouth ulcers.
Although many factors have been proposed as risk factors of SJS, including drug-induced, infections, malignant disorders, and graft rejection, most of them were due to the adverse effect of drugs. The most common drugs are a nonsteroidal anti-inflammatory drug (NSAIDs), antipsychotics, antibiotics, allopurinol, and anticonvulsants.[4]
SJS can be differentiated from other skin conditions on three clinical criteria, (i) the pattern of individual skin lesions, (ii) distribution of lesions, and (iii) extent of epidermal detachment.
The characteristic findings in SJS are widespread erythematous or purpuric macules which form flat atypical target lesions as the disease progresses to cause full thickness epithelial necrosis.[3]
Our case showed ulceration of oral cavity, crusting of lips and profuse bleeding, involvement of eye with redness and watery discharge, ulceration of genital region along with numerous healed lesions on chest, axilla which showed typical appearance of “target lesions” or “bull's eye “appearance. The lesions were widespread as compared to EM, which is localized.
In a study done among children in the hospital, it was found that anticonvulsant drugs were reported the highest risk for SJS and paracetamol lest and vaccines presented no risk at all. Among the NSAIDs, paracetamol and nimesulide are most common reported. The Severe cutaneous adverse reactions (SCARs) study has found an overall risk of SJS with oxicam derivatives. It reports the increased risk with paracetamol from Germany, Italy, and Portugal except France but very few cases from India.[6,8]
However, paracetamol is found to be a potential risk factor in children according to survey data from pediatric patients from the SCAR.[6] The present case was diagnosed as paracetamol induced SJS based upon the fact that a sequential relationship with the drug was established, and correlation with exposure with signs and symptoms was made.
Khawaja et al.[1] reported a case of Acetaminophen induced SJS and TEN with widespread macula-papular rash, stinging in the eyes, oral mucosal ulcerations, and high-grade fever. Similar features were seen in our case, but there was the absence of high-grade fever and epithelial detachment.
The first step in the management was an immediate withdrawal of the offending agent followed by supportive care. Garcia-Doval et al., report that earlier the drug is withdrawn, better the prognosis while exposure to drugs with longer half-lives increases the risk of death. Supportive care must include the management of fluid and electrolyte requirements.[9]
Routine antibiotics are not indicated unless there is the evidence of infection as fever may be part of the disease process. Debridement of necrotic skin should not be performed before disease activity ceases. However, in our case, there were lesions on axilla, abdomen, thighs and trunk region in the healing phase, so debridement was not a necessary step.
Topical antiseptics (0.5% silver nitrate or 0.05% chlorhexidine) are used to paint, bathe, or dress the patients. Dressings may be gauzes with petrolatum, silver nitrate, povidone-iodine, and hydrogels. Some authors use biologic skin covers after epidermal stripping cadaveric allografts, cultured human allogeneic or autologous epidermal sheets. In our case, gentian violet application for lips and skin lesions were advocated.
Dramatic improvement in both SJS-TEN has been reported with the use of intravenous immunoglobulin, 0.2–0.75 g/kg body weight. Alternative systemic treatment methods for the acute phase of SJS-TEN include hemodialysis, plasmapheresis, cyclophosphamide, and cyclosporine.[10]
Use of corticosteroid in the management of SJS is controversial. According to some, their use can lead to delayed wound healing, increased chances of infection, masking of early signs of sepsis, gastrointestinal bleeding, and increased mortality. If steroids are to be used, it should be initiated during initial stage and rapidly tapered off.[11,12] Antibiotics with intravenous corticosteroid shown a remarkable improvement in a similar case.[13] Hence, we prescribed tablet predinsolone 30 mg three times daily daily for 7 days. Further tapered to 20 mg twice daily for next 7 days. Gradually, 10 mg and 5 mg for consecutive 7 days. His condition improved no sequelae were found during 35–40 days of follow-up.
Conclusion
This case report reports the fact that severe hypersensitivity reactions can occur with paracetamol, which can be possibly dangerous and life-threatening. Therefore, clinicians must be more cautious while prescribing. Patients should also be educated regarding the adverse effects of NSAIDs.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have obtained the necessary patient consent forms where the patients have given their approval for participation in the investigation, followed by representation in the concerned article. The patients do understand that the authors will ensure that their identities won't be revealed, however anonymity cannot be guaranteed.
References
- 1.Khawaja A, Shahab A, Hussain SA. Acetaminophen induced Steven Johnson syndrome-toxic epidermal necrolysis overlap. J Pak Med Assoc. 2012;62:524–7. [PubMed] [Google Scholar]
- 2.Ramineni HB, Eluri P, Vipparla K, Suryadevara V. Phenobarbital induced Stevens-Johnson syndrome: A case report. Int J Res Med Sci. 2015;3:492–3. [Google Scholar]
- 3.French LE. Toxic epidermal necrolysis and Stevens Johnson syndrome: Our current understanding. Allergol Int. 2006;55:9–16. doi: 10.2332/allergolint.55.9. [DOI] [PubMed] [Google Scholar]
- 4.Deore SS, Dandekar RC, Mahajan AM, Shiledar VV. Drug induced-Stevens Johnson syndrome: A case report. Int J Sci Stud. 2014;2:84–7. [Google Scholar]
- 5.Kvedariene V, Bencherioua AM, Messaad D, Godard P, Bousquet J, Demoly P. The accuracy of the diagnosis of suspected paracetamol (acetaminophen) hypersensitivity: Results of a single-blinded trial. Clin Exp Allergy. 2002;32:1366–9. doi: 10.1046/j.1365-2222.2002.01476.x. [DOI] [PubMed] [Google Scholar]
- 6.Patel TK, Barvaliya MJ, Sharma D, Tripathi C. A systematic review of the drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Indian population. Indian J Dermatol Venereol Leprol. 2013;79:389–98. doi: 10.4103/0378-6323.110749. [DOI] [PubMed] [Google Scholar]
- 7.Farthing P, Bagan JV, Scully C. Mucosal disease series. Number IV. Erythema multiforme. Oral Dis. 2005;11:261–7. doi: 10.1111/j.1601-0825.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel PP, Gandhi AM, Desai CK, Desai MK, Dikshit RK. An analysis of drug induced Stevens-Johnson syndrome. Indian J Med Res. 2012;136:1051–3. [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: Does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323–7. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 11.Budania RJ, Dakhale GN, Sontakke SD, Patnaik PS. Fatal Stevens-Johnson syndrome induced by phenytoin: A case report. Int J Basic Clin Pharmacol. 2013;2:843–5. [Google Scholar]
- 12.Patterson R, Grammer LC, Greenberger PA, Lawrence ID, Zeiss CR, Detjen PF, et al. Stevens-Johnson syndrome (SJS): Effectiveness of corticosteroids in management and recurrent SJS. Allergy Proc. 1992;13:89–95. doi: 10.2500/108854192778878926. [DOI] [PubMed] [Google Scholar]
- 13.Biswal S, Sahoo SS. Paracetamol induced Stevens-Johnson syndrome – Toxic epidermal necrolysis overlap syndrome. Int J Dermatol. 2014;53:1042–4. doi: 10.1111/ijd.12355. [DOI] [PubMed] [Google Scholar]


