Abstract
Background
Accurate information on stroke burden in men and women are important for evidence-based health care planning and resource allocation. Previously, limited research suggested that the absolute number of deaths from stroke in women was greater than in men, but the incidence and mortality rates were greater in men. However, sex differences in various metrics of stroke burden on a global scale have not been a subject of comprehensive and comparable assessment for most regions of the world, nor have sex differences in stroke burden been examined for trends over time.
Methods
Stroke incidence, prevalence, mortality, disability-adjusted life-years (DALYs) and healthy years lost due to disability (YLDs) were estimated as part of the Global Burden of Disease (GBD) 2013 Study. Data inputs included all available information on stroke incidence, prevalence, and death and case fatality rates. Analysis was performed separately by sex and 5-year age categories for 188 countries. Statistical models were employed to produce globally comprehensive results over time. All rates were age-standardized to a global population and 95% uncertainty intervals (UI) were computed.
Findings
In 2013 global ischaemic stroke (IS) and haemorrhagic stroke (HS) incidence (per 100 000) in men (IS 132.77 [95% UI, 125.34-142.77]; HS 64.89 [95% UI 59.82-68.85]) exceeded those of women (IS 98.85 [95%UI, 92.11 - 106.62]; HS 45.48 [95% UI, 42.43-48.53]). IS incidence rates were lower in 2013 compared with 1990 rates for both sexes (1990 male IS incidence 147.40 [95% UI, 137.87-157-66]; 1990 female IS incidence 113.31 [95%UI, 103.52 – 123.40]), but the only significant change in IS incidence was among women. Changes in global HS incidence were not statistically significant for males (1990 = 65.31 [95% UI, 61.63 – 69.0], 2013 = 64.89[95% UI, 59.82-68.85]), but was significant for females (1990= 64.892 [95% UI, 59.82-68.85], 2013= 45.48 [95% UI, 42.427-48.53]). The number of DALYs related to IS rose from 1990 (male = 16.62 [95% UI, 13.27-19.62] female = 17.53 [95% UI, 14.08-20.33]) to 2013 (male = 25.22 [95% UI, 20.57-29.13], female = 22.21 [95% UI, 17.71 – 25.50]). The number of DALYs associated with HS also rose steadily and was higher than DALYs for IS at each time point (male1990 = 29.91[95% UI, 25.66-34.54], male 2013 = 37.27 [95% UI, 32.29-45.12]; female1990= 26.05 [95% UI, 21.70- 30.90], female2013= 28.18 [95% UI, 23.68-33.80]).
Interpretation
Globally, men continue to have a higher incidence of IS then women while significant sex differences in the incidence of HS were not observed. The total health loss due to stroke as measured by DALYs was similar for men and women for both stroke subtypes in 2013, with HS higher than IS. Both IS and HS DALYs show an increasing trend for both men and women since 1990 which is statistically significant only for IS among men. Ongoing monitoring of sex differences in the burden of stroke will be needed to determine if disease rates among men and women continue to diverge. Sex disparities related to stroke will have important clinical and policy implications that can guide funding and resource allocation for national, regional and global health programs.
Keywords: sex differences, stroke, epidemiology, burden, global
Introduction
Evidence before this study
The burden of stroke in women was often underestimated until the early 1980's.[1] Although once considered primarily a disease of men, stroke is now recognized as a major public health problem for women as well.[2] Recent data has shown that 60,000 more women than men have a stroke each year in the US. [3] Globally, more women than men die of stroke.[4]
Although there is also increasing evidence of sex-specific differences in stroke symptoms, diagnosis, peri-procedural risk, treatment and preventive interventions,[5-11] controversies regarding differences in stroke epidemiology between men and women continue, [12-17] making this an area worthy of further investigation.
Knowledge gap
The data on sex-specific stroke burden have remained scarce. [18-20] In the Greater Cincinnati–Northern Kentucky Stroke Study (GCNKSS)[21], apart from higher incidence in women aged ≤34 years, incidence rates were lower for women aged 45–74 years, but higher in those aged ≥ 75 years. This is possibly a reflection of the open-ended age category. Projections of GCNKSS rates to the 2000 US population gave an estimated 82,000 incident stroke events in women and 49,000 events in men, and in 2050, an estimated 198,000 events in women compared with 129,000 events in men.[22] Total number of deaths were higher for women but the cause-specific mortality rates were lower, possibly because women tend to be more affected by multimorbidity.
Other authors have reported lower incidence rates of stroke in women than in men except in older age (75+ years) where women's’ incidence exceeded that of men. [23, 24] Petrea and colleagues’ examination of the Framingham Study data showed that stroke incidence increased with each decade of life in both women and men with no significant sex difference in stroke subtypes, severity or case fatality rates.[25] Data from the Framingham Study further suggested that one in five women and one in six men reaching the age of 55 years free of stroke will develop a stroke event during their remaining lifetime.[26] The major methodological limitations of the literature available are the lack of national representativeness in terms of generalizability, as well as potential bias issues, such as study population selection and study time periods.[7] Epidemiologic studies reveal a clear age-sex interaction in stroke with premenopausal women experiencing fewer strokes than similarly aged men but having higher rates in postmenopausal women than similarly aged men.[27] Generally any data on stroke epidemiology is scarce in developing countries.
Study Aim
While the above provides an indication of sex differences in stroke epidemiology, comprehensive and comparable assessments of stroke incidence, prevalence, mortality, and disability trends over time have not been produced by sex for most regions of the world. Our aim was to estimate the global incidence, prevalence, years lived with disability, disability adjusted life years, and mortality of stroke in men and women as part of the 2013 Global Burden of Disease study (GBD 2013).
Methods
Methods for determining incidence, all-cause mortality, cause-specific mortality, disability, and disease prevalence in the GBD 2013 study have been previously described, [28, 29] including the approach to stroke disease modelling.[30] Briefly, all available mortality data were compiled. Non-specific cause codes were redistributed based on expert opinion and statistical methods. The total for all cause-specific deaths was fit to an envelope for all-cause mortality. Deaths were compiled into 240 causes, including ischaemic stroke (IS) and haemorrhagic stroke (HS). Stroke was defined based on the WHO clinical criteria.[31] For stroke death estimates, GBD defined stroke ICD-10 codes as IS, HS, or nonspecific as to type. The parent category of cerebrovascular disease was based on the mapping of the detailed causes. Deaths coded as due to G45 (transient ischaemic attack) were coded as IS and deaths coded as due to unruptured aneurysms (ICD code I67.0) were coded as HS. Nonspecific codes, including I64, I67.9, I68.8, I69.4-I69.9, were redistributed to IS or HS using a regression model. An ensemble model was used to estimate a continuous time-series for mortality by age, sex, country, and year. Country-level covariates were incorporated into the model and out-of-sample validity testing was used to assess model performance. Uncertainty intervals (UI) were estimated using 1000 draws from the posterior distribution for each age-sex-country group, with the interval taken as the 2.5 and 97.5 percentiles of the resulting distribution. Disease prevalence was estimated using DisMod-MR disease modeling software[32]. All available estimates of stroke incidence, prevalence and case fatality from systematic reviews of the scientific literature, population surveys, and stroke registries were used. IS and HS were modeled separately. Adjustments were made to account for incidence estimates specifying first-ever or any stroke. Disability due to acute stroke was considered to last for up to 28-30 days while chronic stroke lasted from 30 days until death.[33] Years lived with disability (YLDs) were calculated as the product of a disability weight and prevalent cases of stroke. Disability-adjusted life years (DALYs) were calculated as the sum of years of life lost prematurely, based on maximum observed global longevity, and YLDs. Countries were stratified by development status (i.e., developed, developing).
Results
Incidence and Prevalence
Table 1 shows overall trends in the number of women and men with stroke by stroke type in 1990 and 2013. While the number of women and men with IS was similar in 1990 and increased over time, this increase was more marked for men. Non-overlapping 95% uncertainty intervals (UIs) show the increase over time in numbers of incident IS and HS was significant for both women and men. The number of women with HS was lower than that of men, though with a similar proportional increase over time to men.
Table 1.
Global number of women and men with stroke (in millions) by stroke type in 1990 and 2013 (95% uncertainty limits are in brackets).
| Female | Male | ||||
|---|---|---|---|---|---|
| 1990 | 2013 | 1990 | 2013 | ||
| Ischaemic stroke | Incident | 2.14 (1.96- 2.33) | 3.28 (3.06- 3.52) | 2.17 (2.05 – 2.33) | 3.62 (3.43 – 3.85) |
| Prevalent | 4.86 (4.56- 5.19) | 8.66 (8.32- 9.00) | 5.18 (4.93 – 5.46) | 9.65 (9.27 – 10.05) | |
| Haemorrhagic stroke | Incident | 0.86 (0.79 – 0.92) | 1.53 (1.42- 1.63) | 1.03 (0.96 – 1.09) | 1.84 (1.72 – 1.94) |
| Prevalent | 1.78 (1.67 – 1.87) | 3.36 (3.23- 3.51) | 2.11 (2.02 – 2.22) | 4.00 (3.81- 4.17) | |
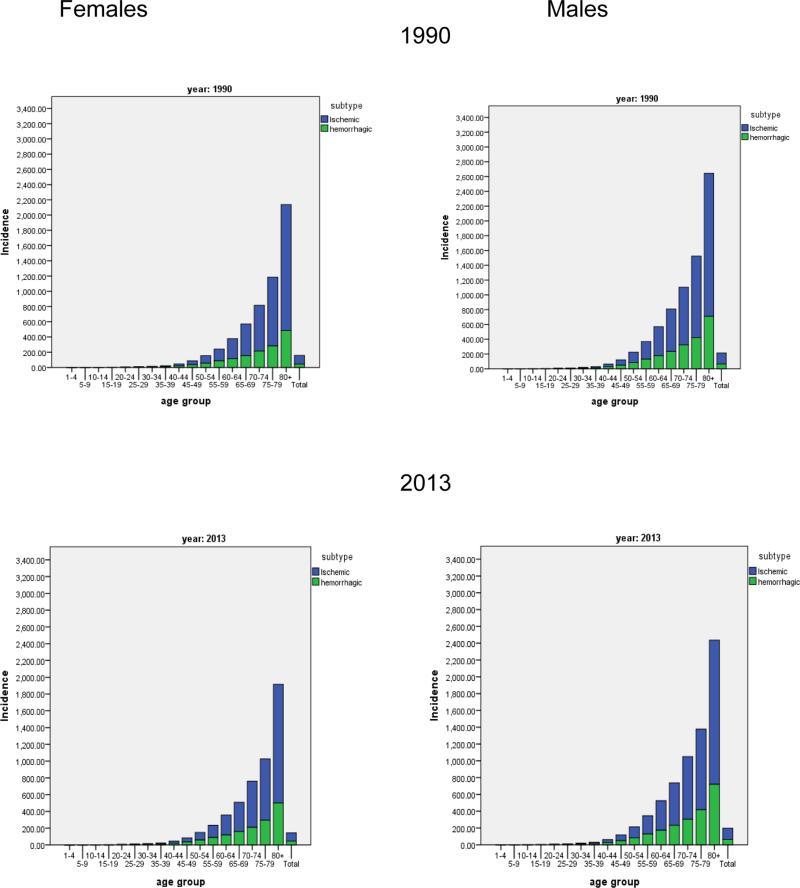
Figure 1 presents the incidence rates of HS and IS broken down into 5 year age bands for men and women in 1990 and 2013. The age related patterns of incidence for men and women remain relatively similar across time and stroke types. The global age-adjusted incidence of IS showed a trend to decline in both men and women, but no significant change was detected as UI overlapped between 1990 and 2013 (table 2). For HS, there was little change detected in incidence over time.
Figure 1.
Incidence per 100,000 of ischemic and haemorrhagic stroke in females and males by 5-year age bands in 1990 and 2013.
Table 2.
Age-standardized incidence and prevalence rates per 100 000 persons by sex in 1990 and 2013 globally (95% uncertainty limits are in brackets), with prevalence rates by country development status
| Females | Males | ||||
|---|---|---|---|---|---|
| 1990 | 2013 | 1990 | 2013 | ||
| Global | |||||
| Ischaemic stroke | Incidence | 113.31 (103.52 – 123.40) | 98.85 (92.11 - 106.62) | 147.40 (137.87-157-66) | 132.77 (125.34-142.77) |
| Prevalence | 253.60 (237.19-271.50) | 260.40 (249.97-271.03) | 339.02 (322.03- 357.88) | 346.08 (332.43-360.57) | |
| Haemorrhagic stroke | Incidence | 44.25 (40.78-47.36) | 45.48 (42.43-48.53) | 65.31 (61.63 – 69.8) | 64.89 (59.82-68.85) |
| Prevalence | 89.18 (83.63-93.97) | 99.90 (95.98- 104.26) | 126.37 (120.51-132.72) | 136.65 (130.41-142.37) | |
| Developed | |||||
| Ischaemic stroke | Prevalence | 396.74 (364.28-437.18) | 500.62 (474.62-526.94) | 585.87 (546.56-629.74) | 678.544 (646.93-713.21) |
| Haemorrhagic stroke | Prevalence | 84.02 (74.60-93.41) | 125.64 (116.01-135.80) | 108.03 (97.18-119.15) | 134.15 (122.03-145.32) |
| Developing | |||||
| Ischaemic stroke | Prevalence | 131.706 (125.238-138.710) | 130.494 (122.616-139.06) | 182.34 (171.30-192.00) | 185.15 (173.51-197.20) |
| Haemorrhagic stroke | Prevalence | 93.51 (88.72-97.53) | 91.57 (87.55-96.13) | 136.65 (130.00-143.40) | 139.87 (133.10- 146.74) |
Stroke prevalence rates for both stroke types were significantly higher for men compared with women in both 1990 and remained so in 2013. The increase in number of prevalent cases of stroke between 1990 and 2013 despite significant declines in incident stroke are consistent with change due to population growth, population ageing, and, for some regions, decreases in stroke-related mortality (table 3). Therefore, there were no detectable change in age-standardized prevalence rates for IS from 1990-2013. In contrast to IS rates, HS age-standardized prevalence rates increased overtime for both sexes.
Table 3.
Global median percent change in number and age-standardized rate per 100 000 persons for deaths, disability adjusted life years (DALYs) and years lived with disability (YLD) from 1990 to 2013 by stroke type and sex.
| DALYs | Deaths | YLD | Prevalence | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Rate | Number | Rate | Number | Rate | Number | Rate | ||||||||||
| % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | % Change | 95% UI | ||
| Ischaemic Stroke | Male | 51.6 | 71.1, 36.8 | −16.3 | −6.5, −24.2 | 64.0 | 84.4, 48.4 | −15.4 | −5.6,−22.8 | 86.1 | 99.5, 73.2 | 2.8 | 9.9, −4.7 | 85.9 | 98.8, 73.9 | 2.2 | 9.3, −5.0 |
| Female | 27.4 | 35.7, 15.3 | −26.0 | −21.5,−32.8 | 39.8 | 49.5, 28.7 | −23.2 | −17.8,−29.3 | 78.0 | 94, 64.1 | 3.3 | 12.7, −4.9 | 77.7 | 93.7, 63.4 | 2.6 | 12.1, −6.0 | |
| Haemorrhagic Stroke | Male | 23.9 | 42.1, 12.3 | −25.6 | −13.8, −32.5 | 38.3 | 63.4, 25.1 | −23.0 | −8.1,−30.6 | 88.7 | 101, 75.5 | 8.5 | 16.4, 0.9 | 89.3 | 101.6, 77.0 | 8.1 | 15.6, 1.1 |
| Female | 7.9 | 20, −0.9 | −33.8 | −26.2, −39.2 | 23.1 | 41.4, 13.3 | −29.1 | −18.5,−34.8 | 88.1 | 103.6, 76.5 | 12.1 | 21.4, 5.1 | 88.4 | 103.4, 77.4 | 11.7 | 20.7, 5.0 | |
Mortality, DALYs, and YLDs
The age-standardized death rate (Table 4) from IS declined at a similar rate for men and women, although absolute values were higher in men. Mortality due to HS also declined over time in both sexes. While mortality in women with HS was slightly lower than that of men in 1990, it was higher than that of men in 2013 with no overlap of UIs.
Table 4.
Age-standardized DALY, YLD and death rates per 100 000 persons with 95% uncertainty intervals by stroke type and sex in 1990 and 2013.
| Female | Male | ||||
|---|---|---|---|---|---|
| 1990 | 2013 | 1990 | 2013 | ||
| Ischemic Stroke | DALYs | 919.3 (740.1 to 1,063.2) | 675.8 (539.2 to 774.9) | 1,101.5 (894.3 to 1,288.4) | 922.1 (758.1 to 1,061.5) |
| YLD | 36.3 (25.3 to 48.4) | 37.5 (26.3 to 49.6) | 48.5 (33.9 to 63.7) | 49.8 (35.4 to 65.3) | |
| Mortality | 69.0 (55.7 to 78.7) | 52.9 (42.5 to 60.3) | 73.4 (60.6 to 85.3) | 62.1 (51.1 to 71.7) | |
| Haemorrhagic Stroke | DALYs | 1,259.7 (1,041.5 to 1,500.3) | 835.8 (703.9 to 1,001.4) | 1,613.8 (1,368.4 to 1,870.7) | 1,212.5 (1,052.7 to 1,477.0) |
| YLD | 13.1 (9.2 to 17.4) | 14.8 (10.3 to 19.5) | 18.6 (12.8 to 24.6) | 61.0 (52.4 to 75.3) | |
| Mortality | 63.9 (52.2 to 76.1) | 45.7 (39.0 to 56.6) | 77.9 (64.9 to 90.6) | 20.2 (13.8 to 26.5) | |
DALYs due to ischemic stroke in men rose significantly from 1990 to 2013 while among women the trend towards an increase was not significant (Table 4). DALYs related to HS were higher in men than women, though increases from 1990 to 2013 were not significant for either sex.
YLDs related to IS remained similar for females and for males over time. While YLDs also remained stable over time for women with HS, there was a significant rise in YLD for men from 1990 to 2013.
The number of deaths DALYs and YLDs increased, while the age-standardized rates of deaths and DALYs per 100, 000 decreased (table 3). The increase in number of IS stroke deaths was significantly greater among men than women. There was a trend towards a greater increase among men for HS though the UI in percentage change overlapped.
Current Country Specific DALYs, YLDs, and Mortality
Web Appendix A shows country-specific stroke burden in 1990 and 2013 for women and men; whilst Web Appendix B presents this information by stroke type (IS and HS). Overall death rates in 2013 ranged from a low of 28.6 per 100,000 (95% UI 19.9, 34.4) in Israel, to 357.9 (95% UI 297.8, 432.0) in Madagascar. Death rates for males exceeded those of females in the majority (n=138, 73.8%) of countries. Overall DALYs for 2013 ranged from a low of 380.7 (95%UI 288.0, 443.8) in Israel to a high of 6559.5per 100,000 (95% UI 5243.0, 8169.3) in Madagascar.
Of those countries where women had higher death rates than men, listed in parentheses, many of which (indicated with *) also had higher DALYs in females than in males (Afghanistan*, Algeria*, Australia, Bahrain*, Bangladesh*, Belize, Brunei, Burundi*, Central African Republic*, Canada, Chad*, Comoros, Democratic Republic of Congo, Djibouti*, Eritrea*, Ethiopia*, Guatemala*, Guinea*, Haiti*, Indonesia, Iran*, Iraq*, Israel, Jamaica*, Jordan, Kuwait*, Laos, Lebanon*, Libya*, Malawi*, Maldives, Mali*, Mauritania*, Morocco*, New Zealand, Niger*, Nigeria*, Pakistan*, United Arab Emirates, United Kingdom, Uganda*, Tunisia*, The Gambia*, Somalia*, Senegal*, Saint Vincent and the Grenadines, Quatar*, Uzbekistan, Yemen*, Zimbabwe*, Zambia*). Aside from Canada, New Zealand, and the United Kingdom where the difference in death rate between males and females was minimal (in the order of 3 to 5 per 100 000), the majority of these countries are developing and/or have recent negative historical events (e.g., warfare, natural disaster). YLDs ranged from a low of 2.3 (895% UI 1.5, 3.3) in Libya to a high of 127.5 (95% UI 90.0, 178.4) in Canada. Males had higher YLD than females in all countries.
Discussion
Main Findings
Over time, the global burden of stroke has been increasing for both men and women but the increases have been greater among men. There is a trend towards a decreased incidence of IS in women from 1990 to 2013 with no significant change detected for men. Improved vascular risk factor control and better health care interventions are likely explanations for reductions in stroke incidence over this period seen for both sexes. It is possible that differences between men and women in the extent of improvement are partly explained by women in some countries being more sensitive to health information, having better health seeking behaviour, and having better access to primary prevention.[34, 35] An alternative explanation is that neuro-vascular risk factors are more frequent and severe in men and have declined faster in women, for example tobacco.[36, 37]
Comparison with previous research
These findings are in contrast to those previously reported in which the reduction in incidence rates was more marked in men than in women. For example, in a review of 56 population-based studies, data from high-income countries revealed a 42% decrease overall in worldwide stroke incidence rates from 163 per 100,000 person-years in the period 1970-79 to 94 per 100,000 person-years in the period 2000-08, [38] a faster decline was observed for men. One explanation for difference between those findings and the present are that they were from a relatively smaller study catchment. Our inclusion of all countries leads to greater uncertainty intervals which blunts our ability to detect trends in some subgroups. Our uncertainty for prevalence and incidence was considerably greater than for mortality because there is data on deaths from almost all countries but data on incidence and prevalence from a limited number of countries. In their 2008 comprehensive review of literature, Reeves et al. [28] showed that women have more stroke events than men but age-specific stroke rates are higher in men and there were little sex differences in stroke subtypes (except subarachnoid haemorrhage). Our findings demonstrated that the risk (rate of stroke) and absolute number of ischaemic and haemorrhagic stroke events (both incident and prevalent strokes) in 2013 was significantly greater in men than in women, suggesting changes in the sex distrubution of stroke burden in the world.
Similarly, sex-specific data from the Framingham Heart Study showed decreases in stroke incidence of 30.3% for men and 17.8% for women from 1950 to 2004,[39] though the study periods are not the same. One possible contributing factor is the increase in the ‘smoking epidemic’ in women which, according to Lopez et al[40], occurs 10 to 20 years later than for men.
The decline in IS incidence and mortality rates over the past decades represents a major improvement in population health and is observed for both sexes and across age groups.[41] These significant improvements are concurrent with cardiovascular risk factor control interventions. An American study concluded that efforts in arterial hypertension control initiated in the 1970s appeared to have had the most substantial influence on the accelerated decline in stroke risk and mortality. Although implemented later, control of diabetes mellitus and dyslipidaemia and smoking cessation programs, particularly in combination with treatment of hypertension, were also implicated in contributing to the decline in stroke incidence and mortality.[41] A history of hypertension, current smoking, waist-to-hip ratio, diet, physical activity, diabetes mellitus, alcohol intake, psychosocial stress and depression, cardiac causes and ratio of apolipoproteins B to A1 was estimated to account for 88·1% of the population attributable risk for all stroke.[42] However, sex interacts with these risk factors. For example, women with diabetes have a higher risk of death from cardiovascular disease than men with diabetes, and after menopause blood pressure and cholesterol levels rise drastically.[43]
In contrast to IS, there were no major changes in HS incidence for women or men. Prior studies suggested the risk for intracerebral haemorrhage (ICH) to be marginally greater in men than in women. This differential risk by sex might be driven by an excess of deep haemorrhage in men, [44, 45] although it is well know that the risk of subarachnoid haemorrhage (about 5% of all HS) is much greater in women than in men. HS incidence rates are reportedly slightly higher in Eastern Asia, where ICH has historically accounted for a larger percentage of all strokes than in Western populations possibly due to the increased prevalence of hypertension.[46-49] Studies of incidence trends for HS in recent decades have produced mixed results. There was a trend toward a reduced ICH incidence in Oxfordshire between 1981 and 2006,[50] and during the 1990s in several Chinese cities.[46] Other studies observed a decline of HS only in women aged < 60 years in the period between 1985 and 2005, [51] or do not report such declines. [52, 53] Also, ICH has a high mortality rate especially with increased age. There are some reports to suggest that withdrawal of aggressive care practices may be different between sexes (this may also vary by region and culture), and this may account for some of the mortality differences in sex in ICH.[54] Women with strokes have also been found to present more often than men with non‐traditional stroke symptoms.[55] Delays in presentation, evaluation, diagnosis, and treatment of women with ICH may contribute to the association between female sex and more severe stroke.
The increasing global population over time, even in the context of declining incident stroke rate, will lead to an increasing number of strokes. Population ageing will also increase the number of strokes, and this has implications for burden faced by health care systems. The number of deaths, DALYs and YLDs all increased for both men and women since 1990 for both stroke subtypes, with these increases being much higher in men. [56]
In 2013, women had higher number of deaths and DALYs, which in examining country level statistics, seems to be driven by developing countries and countries with recent histories of adversity (e.g., war, natural disaster). Otherwise men have greater deaths, YLDs, and DALYs both as absolute numbers and rates. One possible explanation for this is the differential smoking rate, with men having a much greater prevalence of smoking than women.[57] Although the overall number of adult smokers has decreased during the last 20 years, the number of women in their teens and 20s who smoke has increased. This increases the risk of stroke in young women. It must also be noted here that there is no data from some of countries, so the results are purely modelled by the geospatial model. As such any conclusions drawn about these particular data points must be tentative at best. It is also possible that women from some countries might be ‘outside the statistics’ because they are not admitted to hospital, though this is more likely when there is no National healthcare system.
Alternatively, this may have a direct relationship to accessing care. More specifically, it is possible that the global trends in IS for women, especially in the Middle East, where the rates did not mirror global rates may be due to ascertainment and diagnosis patterns that may be different in men compared to women. For example, an Egyptian study of acute myocardial infarction found significant sex differences in presentation and treatment with women less likely to receive aspirin upon admission or aspirin or statins at discharge, and had poorer in-hospital mortality rates.[58] Similarly, stroke mortality rate may also be influenced by access to emergency services, with elderly women possibly living alone and thus potentially having more difficulty accessing emergency medical services.
The World Health Organization (WHO) assessed sex-specific mortality rates across 39 countries in Europe and Central Asia,[59] and reported an excess of total deaths because of stroke among women compared with men, of which 60% occurred in those over 75 years and 4% among those younger than 55 years. The Centers for Disease Control and Prevention WONDER database in the United States, suggests that men and women under 45 years of age show similar stroke mortality rates. While women aged 45 to 74 years have lower stroke mortality compared with age-matched men, this advantage declines with advancing age.[22] A strong sex-by-age interaction was also reported by WHO, as the male/female stroke mortality rate was greater in men than women aged less than 65 years but greater in women than men among those aged ≥ 75 years, possibly reflecting compounding disability in the older age range.
Strengths and Limitations
There are limitations to the GBD methods. These include the extrapolation of data from subnational regions to the whole country and missing data overall, in particular from low-income countries. Strengths include the use of consistent methods to enable comparison between subtypes of stroke and between diseases, the ability to highlight regions where stroke is a particular problem. This highlights the importance of studies being clear on how stroke and its subtypes are defined (e.g., TOAST criteria[60]) and if they were confirmed using gold standard methods such as by CT scans
Implications for research, policy, and practice
While age-adjusted incidence and death rates from stroke have been declining since the late 20th century, the global stroke burden measured as the absolute numbers of people affected by stroke, disabled due to stroke, or deaths from stroke are increasing for both men and women, with larger increases in men. This increasing burden is a reflection of population growth and ageing and lifestyle and environmental changes. That women tend to survive longer and experience stroke at an older age suggests that in the future, alongside the aging of the population, we can expect a dramatic increase of stroke in older women. To combat this it is important that preventive efforts and guidelines for treatment reflect sex differences in the profile of stroke.
Specifically, women have strokes associated with a higher prevalence of arterial hypertension, atrial fibrillation, and pre-stroke disability; but having a lower prevalence of heart disease, peripheral vascular disease, smoking and alcohol use than men, and these will need to be taken into account.[22] In terms of treatment women are less likely to receive intravenous alteplase treatment and lipid testing while in hospital in some countries. [22] These disparities suggest the need to explore whether differential strategies are required to target primary and secondary prevention of stroke in women, and to determine if treatment protocols must also accommodate sex differences. Further strategies are particularly needed to lower high case fatality. These could include investment in pre-hospital and acute care in some regions, and could perhaps include better home care provided in treatment and monitoring of blood pressure. Also, with extension of the average length of life, ratios of atrial fibrillation increase, the prevalence of atrial fibrillation is increasing especially in developed countries, and atrial fibrillation is a strong risk factor for ischemic stroke.[61, 62] The anticoagulant warfarin has been conventionally used for prophylaxis of cerebral infarction with atrial fibrillation. The prescription rate of warfarin increased after 2000 in particular. Recently, new oral anticoagulants have been used as alternatives to warfarin anticoagulation in non-valvular atrial fibrillation. There is a particular need to focus future efforts on obtaining data by sex from undeveloped regions.
Conclusion
Age-adjusted incidence rates for IS appear to be declining worldwide at a faster rate in women than in men. The reasons for this decline may reflect better risk factor control and health care. Despite the reduction in the occurrence of stroke, burden is still increasing. In line with the World Stroke Organization's initiative ‘I am woman-stroke affects me’ that was launched at the World Stroke Congress in Istanbul (22-25 October, 2014) more research on stroke epidemiology, including both indices of occurrence and burden, is required to provide better estimates, particularly for developing countries where the data remains sparse. Countries whose estimates were entirely based on statistical modelling should be a focus of efforts to improve data availability by designing, implementing and reporting population-based studies of chronic disease including stroke. Guidelines for achieving good quality studies in stroke epidemiology are recently available. [63]
Research into factors that potentially contribute to the causes of sex differences in risk and outcomes is also required. In addition, there is adequate evidence of differences in stroke incidence and burden between the sexes to support the need for well-designed stroke intervention trials which are equally powered for men and women to examine effectiveness of primary care, risk factor management strategies, and hospital services.
Supplementary Material
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the U.S. Department of Health and Human Services.
GBD Stroke Expert Writing Group members (in alphabetical order by country)
Argentina (Maria Cecilia Bahit); Australia (Amanda Thrift, Atte Meretoja, Bill Stavreski, Craig Anderson, Edwin Pearse, Geoffrey Donnan, Graeme Hankey, Mark T. Mackay, Stephen Davis, Zanfina Ademi; Austria (Michael Brainin); Azerbaijan (Tural Guliyev); Bahrain (Randah R. Hamadeh); Barbados (Heather Harewood, Karen Springer); Brazil (Iuri da Costa Leite, Jefferson Gomes Fernandes, Norberto Cabral, Paulo Lotufo); Bulgaria (Klara Dokova); Canada (Farshad Pourmalek, Luciano A. Sposato, M. Patrice Lindsay, Patricia Riccio); Chile (Pablo M. Lavados); China (Bin Li, Chuanhua Yu, Guohong Jiang, Jixiang Ma, Maigeng Zhou, Ming Liu, Shankuan Zhu, Wenzhi Wang, Xiaofeng Liang, XXX Deji, Yong Zhang); Colombia (Gabriel Alcalá-Cerra, Gabrielle DeVeber), Denmark (Hanne Christensen, Thomas Truelsen); Egypt (Foad Abd-Allah); Ethiopia (Awoke Temesgen, Berhe Weldearegawi Sahle, Semaw Abera, Yohannes Adama Melaku), Fiji (Devina Nand); France (Maurice Giroud); Germany (Jost B. Jonas, Matthias Endres, Ronny Westerman); Greece (Konstantinos Stroumpoulis); India (Dorairaj Prabhakaran, Jeyaraj Durai Pandian, Man Mohan Mehndiratta, Nobhojit Roy, Panniyammakal Jeemon, Rajeev Gupta, Vasanthan Rajagopalan); Indonesia (Soewarta Kosen, Tati Suryati Warouw); Iran (Reza Malekzadeh); Ireland (Martin O'Donnell); Israel (David Tanne, Natan Bornstein); Italy (Stefano Ricci, Valeria Caso); Japan (Yoshihiro Kokubo, Yukito Shinohara); Jordan (Majed Asad); Kenya (Vitalis Kizito Bwire); Korea (Sun Ha Jee, Young-Ho Khang); Malaysia (Kim Yunjin, Ramesh Sahathevan); Morocco (Fortune Gankpe); Myanmar (Chaw Yin Myint); New Zealand (Priya Parmar, Rita Krishnamurthi, Suzanne Barker-Collo, Valery Feigin); Nigeria (Rufus Olusola Akinyemi); Norway (Ole Norheim); Qatar (Shams Eldin Khalifa); Russia (Michael Kravchenko, Michael Piradov, Nicolay Shalamov, Vasiliy Vlassov, Yuri Varakin); Rwanda (Jean De Dieu Ngirabega, Jean Pierre Nyemazi, Marie Aimee Muhimpundu); Saudi Arabia (Mohammad Saeedi, Neeraj Bedi); Singapore (Narayanaswamy Venketasubramanian); South Africa (Andre Pascal Kengne); Spain (David Rojas-Rueda, Ferrán Catalá-López); Sri Lanka (Samath Dharmaratne); Sweden (Bo Norrving, Rasmus Havmoeller); The Netherlands (Johanna M. Geleijnse); Uganda (Leo Atwine); United Kingdom (Amitava Banerjee, Charles Wolfe, Derrick Bennett, Finbar O'Callaghan, Ivy Shiue, Julia Critchley, Majid Ezzati, Michael Soljak, Myles Connor, Peter Rothwell, Rajiv Chowdhury, Rustam Al-Shahi Salman, William Whiteley, Zhengming Chen); Uruguay (Mercedes Colomar); USA (Adnan Durrani, Anand Dayama, Andrew Moran, Awoke Misganaw, Brett Kissela, Catherine Amlie-Lefond, Catherine O. Johnson, Cheng Huang, Chugh Sumeet, Daniel Kim, David Cundiff, David Lawrence Tirschwell, Dhruv Kazi, Dima Qato, Edmond Kabagambe, Eric Ding, Gene Bukhman, Gene Kwan, George Mensah, George Thurston, Grant Nguyen, Gregory A. Roth, Ismael Campos-Nonato, Josef Coresh, Kate Lefondulq, Kevin Sheth, Matthew Corriere, Mohammad H. Forouzanfar, Mohsen Naghavi, Nana Mainoo, Norman Beauchamp, Ralph Sacco, Richard Gillum, Sanjay Basu, Stephen Schwartz, Sumeet Chugh, Teresa Fung, Tim Byers, Uchechukwu K.A. Sampson, Walter Rocca, Warren Lo).
Conflict of interest
All the authors declare that they have no conflict of interest.
Contributor Information
Suzanne L. Barker-Collo, School of Psychology, level 6, 10 Symonds Street, The University of Auckland, Private Bag 92019, Auckland, New Zealand..
Derrick A. Bennett, Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK
Rita Krishnamurthi, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, AUT University, Private Bag 92006, Auckland, New Zealand.
Priya Parmar, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, AUT University, Private Bag 92006, Auckland, New Zealand.
Valery L Feigin, National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, AUT University, Private Bag 92006, Auckland, New Zealand.
Mohsen Naghavi, Department of Global Health, School of Medicine and Public Health, University of Washington.
Mohammad H. Forouzanfar, Institute for Health metrics and Evaluatrion. University of Washington, Seattle, Washington, USA.
Catherine Johnson, Institute for Health Metrics and Evaluation. University of Washington, Seattle, Washington, USA..
Grant Nguyen, Institute for Health Metrics and Evaluation. University of Washington, Seattle, Washington, USA..
George A. Mensah, Center for Translation Research and Implementation Science (CTRIS), National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, USA.
Theo Vos, Department of Global Health, School of Medicine and Public Health, University of Washington, Seattle, Washington, USA..
Christopher Murray, Department of Global Health, School of Medicine and Public Health, University of Washington, Seattle, Washington, USA.
Gregory A. Roth, Institute for Health Metrics and Evaluation and the Division of Cardiology, School of Medicine, University of Washington, Seattle, Washington, USA.
Foad Abd-Allah, Department of Neurology, Cairo University, Cairo, Egypt..
Semaw Ferede Abera, Department of Epidemiology and Biostatistics, School of Public Health, College of Health Sciences, Mekelle University, Ethiopia.
Rufus, O. Akinyemi, Institute for Advanced Medical Research and Training, College of Medicine, University of Ibadan, Nigeria
Cecilia Bahit, Department of Cardiology INECO Neurociencias Oroño, Rosario, Santa Fe. New Mexico, USA..
Amitava Banerjee, Farr Institute of Health Informatics Research, University College London, London, UK..
Sanjay Basu, School of Medicine, Stanford Universityl, Stanford, California, USA..
Michael Brainin, Department of Clinical Neurology, Danube University Krems and Karl Landsteiner University, Krems, Austria.
Natan M. Bornstein, Tel-Aviv Sourasky Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Valeria Caso, Stroke Unit, University of Perugia, Perugia, Italy.
Ferrán Catalá-López, Department of Medicine, University of Valencia, Valencia, Spain.
Rajiv Chowdhury, Department of Public Health and Primary Care, School of Clinical Medicine, University of Cambridge, Cambridge, UK..
Hanne Christensen, University of Copenhagen and Bispebjerg Hospital, Denmark.
Merceded Colomar, Unidad de Investigacion Clinica y Epidemiologica Montevideo, Uruguay.
Stephen Davis, University of Melbourne Parkville, Australia..
Gabrielle deVeber, Children's Stroke Program, Division of Neurology Hospital for Sick Children Senior Scientist, Research Institute, Hospital for Sick Children, Torontro, Ontario, Canada..
Samath D. Dharmaratne, Department of Community Medicine, Faculty of Medicine, University of Peradeniya and Institute for Health Metrics and Evaluation, Department of Global Health, School of Public Health, University of Washington, USA.
Geoffrey Donnan, The Florey Institute of Neuroscience and Mental Health and School of Neurology. University of Melbourne, Melbourne, Australia..
Prabhakaran Dorairaj, Centre for Chronic Conditions and Injuries and Public Health Foundation of India, Delhi, India..
Klara Dokova, Department of Social Medicine and Health Care Organization Faculty of Public Health, Medical University of Varna, Varna, Bulgaria.
Matthias Endres, Klinik und Hochschulambulanz für Neurologie, Charité-Universitätsmedizin Berlin, Germany..
Jefferson G Fernandes, School of Health Education and Sciences German Hospital Oswaldo Cruz São Paulo, Brazil..
J. Marianne Geleijnse, Division of Human Nutrition / Epidemiology, Wageningen University, The Netherlands..
Richard F. Gillum, Department of Internal Medicine and Department of Community and Family Medicine, Howard University College of Medicine, Washington, DC. USA
Maurice Giroud, Dijon Stroke Registry, Service de Neurologie, Dijon, France..
Jiang Guohong, Tianjin Centers for Diseases Control and Prevention, School of Public Health, Tianjin Medical University, and School of Public Health, Tongji Medical University, Hubei, China..
Randah R. Hamadeh, Department of Family and Community Medicine, College of Medicine and Medical Sciences, Arabian Gulf University, Bahrain.
Graeme J. Hankey, School of Medicine and Pharmacology, The University of Western Australia.
Panniyammakal Jeemon, Centre for Control of Chronic Conditions, Public Health Foundation of India and Centre for Chronic Disease Control, New Delhi..
Kim Yun Jin, Faculty of Chinese Medicine Southern University College, Johor. Malaysia.
Jost B. Jonas, Department of Ophthalmology, Medical Faculty Mannheim of the University of Heidelberg, Mannheim Germany.
Yogesh Kalkonde, Society for Education, Action & Research in Community Health, District of Gadchiroli, India..
Andre P Kengne, South African Medical Research Council, Francie van Zijl Drive, Parrow Valley, Cape Town, South Africa..
Daniel Kim, Department of Health Sciences, Northeastern University, Boston, USA.
Brett M. Kissela, Department of Neurology and Rehabilitation Medicine, University of Cincinnati, USA.
Yoshihiro Kokubo, Departoment of Preventive Cardiology, National Cerebral and Cardiovascular Center, Suita, Japan..
Pablo Lavados, Servicio de Neurología, Departamento de Medicina, Clínica Alemana de Santiago-Universidad del Desarrollo. Santiago, Chile..
Patrice Lindsay, Stroke Heart and Stroke Foundation, Fondation des maladies du cœur et de l'AVC, Ontario, Canada..
Paulo A Lotufo, Centre for Clinical and Epidemiological Research, University of Sao Paulo, Sao Paulo, Brazil..
Mark T. Mackay, Department of Neurology, Royal Children's Hospital Melbourne and Murdoch Childrens Research Institute, Parkville Australia.
Reza Malekzadeh, Digestive Disease Research Institute, Tehran University of Medical Sciences, Shariati Hospital, Tehran, Iran..
Man Mohan Mehndiratta, Janakpuri Super Speciality Hospital, New Delhi, India.
Devina Nand, Health Information Unit, Dinem Hous, Toorak, Suva, Republic of Fiji.
Bo Norrving, Department of Clinical Sciences, Neurology, Lund University, Sweden..
Jeyaraj Durai Pandian, Department of Neurology Christian Medical College, Ludhiana, Punjab, India..
Harry Perkins, Institute of Medical Research, QEII Medical Centre, Perth, Western Australia.
Farshad Pourmalek, School of Population and Public Health, University of British Columbia.
Stefano Ricci, UO Nurologia, Umbria edi di Città di Castello e Branca..
Patricia M. Riccio, Department of Clinical Neurological Sciences. Londonde Health Sciences Centre. Western University. London, ON. Canada.
David Rojas-Rueda, Centre for Research in Environmental Epidemiology, ISGlobal, Barcelona, Spain..
Nobhojit Roy, Environmental health resource hub, School of Habitat studies, Tata Institute of Social sciences, Mumbai, India.
Ralph, L. Sacco, University of Miami Miller School of Medicine, Department of Neurology, Miami, Florida, USA.
Ramesh Sahathevan, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latiff, Bandar Tun RazakKuala Lumpur, Malaysia; and Calvary Healthcare Bruce, ACT, Australia.
Kevin N. Sheth, Division of Neurocritical Care and Emergency Department of Neurology Neurosciences Intensive Care Unit, Yale School of Medicine & Yale New Haven Hospital, New York, USA.
Ivy Shiue, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, UK..
Luciano A. Sposato, Department of Clinical Neurological Sciences. London Health Sciences Centre. Western University. London, Ontario. Canada.
David Tanne, Chaim Sheba Medical Center and Tel-Aviv University, Israel..
Amanda Thrift, Stroke and Ageing Research (STARC), Department of Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, Vic., Australia.
George Thurston, Department of Population Health and Environmental Medicine, Tuxedo, New York, USA..
David Tirschwell, University of Washington School of Medicine, UW Medicine Comprehensive Stroke Center, Harborview Medical Center, Seattle Washington, USA..
Narayanaswamy Venketasubramanian, Raffles Neuroscience Centre, Raffles Hospital, Singapore..
Vasiliy Vlassov, National Research University Higher School of Economics, Moscow, Russia..
Ronny Westerman, Competence Center Mortality-Follow-Up of the German National Cohort, Federal Institute for Population Research, Wiesbaden, Germany.
Charles Wolfe, Division of Health and Social Care Research, King's College London, London, United Kingdom; and National Institute for Health Research Comprehensive Biomedical Research Centre, Guy's & St. Thomas’ NHS Foundation Trust and King's College London, London, UK.
References
- 1.Bousser M. Stroke in women: the 1997 Paul Dudley White International Lecture. Circulation. 1999;99:463–7. doi: 10.1161/01.cir.99.4.463. [DOI] [PubMed] [Google Scholar]
- 2.Bushnell CD, Hurn P, Colton C. Advancing the study of stroke in women—summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke. 2006:37. doi: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 3.Flegal RW, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–90. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. The New England journal of medicine. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 6.Group. SCotPHSR Final report on the aspirin component of the ongoing Physicians’ Health Study. New England Journal of Medicine. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 7.Di CA, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–9. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 8.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, et al. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34:1970–5. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 9.Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke. 2005;36:1447–51. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- 10.Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, et al. A comprehensive view of sex-specific issues related to cardiovascular disease. Canadian Medical Association Journal. 2007;176:S1–S44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto T, Baba T, Ito A, Maekawa K, Koshiji T. Gender differences in stroke risk among the elderly after coronary artery surgery. Anesthesia Analgesia. 2007;104:1016–22. doi: 10.1213/01.ane.0000263279.07361.1f. [DOI] [PubMed] [Google Scholar]
- 12.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. Journal of Stroke and Cerebrovascular Disease. 2003;12:119–26. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 13.Paolucci S, Bragoni M, Coiro P, De AD, Fusco FR, Morelli D, et al. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006;37:2989–94. doi: 10.1161/01.STR.0000248456.41647.3d. [DOI] [PubMed] [Google Scholar]
- 14.Kelly-Hayes M, Wolf PA, Kannel WB, Sytkowski P, D'Agostino RB, Gresham GE. Factors influencing survival and need for institutionalization following stroke: the Framingham Study Archives of Physical and Medical Rehabilitation. 1988;69:415–8. [PubMed] [Google Scholar]
- 15.Gresham GE, Kelly-Hayes M, Wolf PA, Beiser AS, Kase CS, D'Agostino RB. Survival and functional status 20 or more years after first stroke: the Framingham Study. Stroke. 1998;29:793–7. doi: 10.1161/01.str.29.4.793. [DOI] [PubMed] [Google Scholar]
- 16.Chong JY, Lee HS, Boden-Albala B, Paik MC, Sacco RL. Gender differences in self-report of recovery after stroke: the Northern Manhattan Study. Neurology. 2006;67:1282–4. doi: 10.1212/01.wnl.0000238161.71591.e9. [DOI] [PubMed] [Google Scholar]
- 17.Ayala C, Croft JB, Greenlund KJK, N.L., Donehoo RS, Malarcher AM, Mensah GA. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995-1998. Stroke. 2002;33:1197–201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 18.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. Journal of the American Medical Association. 2006;296:2939–46. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 19.Dyall L, Carter K, Bonita R, Anderson C, Feigin V, Kerse N, et al. Incidence of stroke in women in Auckland, New Zealand. Ethnic trends over two decades: 1981-2003. New Zealand Medical Journal. 2006;119:U2309. [PubMed] [Google Scholar]
- 20.Eisenblatter D, Heinemann L, Classen E. Community-based stroke incidence trends from the 1970s through the 1980s in East Germany. Stroke. 1995;26:919–23. doi: 10.1161/01.str.26.6.919. [DOI] [PubMed] [Google Scholar]
- 21.Kissela B, Schneider A, Kleindorfer D. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 22.Reeves MJ, Bushnell CD, Howard G, Warner Gargano J, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurology. 2008;7:915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lofmark U, Hammarstrom A. Evidence for age-dependent education-related differences in men and women with first-ever stroke. Neuroepidemiology. 2007;28:135–41. doi: 10.1159/000102141. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM, Coull AJ, Silver LE. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1776–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 25.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Stroke in women - Gender Differences in Stroke Incidence and Post-stroke Disability in the Framingham Heart Study. Stroke. 2009;40:1032–7. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 27.Haast RAM, Gustafson D, Kiliaan AJ. Sex differences in stroke. Journal of Cerebral Blood Flow & Metabolism. 2012;32:2100–7. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study. The Lancet. 2013;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collaborators GS. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. 2015 doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth GA. Methodology of the GBD 2013 stroke burden estimates. Neuroepidemiology. 2015 [Google Scholar]
- 31.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bulletin of the World Health Organization. 1980;58:113–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Flaxman AD, Vos T, Murray CJL. An Integrative Metaregression Framework for Descriptive Epidemiology. University of Washington Press; 2015. [Google Scholar]
- 33.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study. The Lancet. 2013 doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baghshomali S, Bushnell CD. Reducing Stroke in Women With Risk Factor Management. Women's Health. 2014;10:535–44. doi: 10.2217/whe.14.47. [DOI] [PubMed] [Google Scholar]
- 35.Guarnizo-Herren O, Carol C, Agudelo C. Gender-related equity/inequity in gaining access to health services. Reviewsa Salud publica. 2008;10:44–57. doi: 10.1590/s0124-00642008000600005. [DOI] [PubMed] [Google Scholar]
- 36.Yao X, Lin Y, Geng J, Sun Y, Chen Y, Shi G, et al. Age- and Gender-Specific Prevalence of Risk Factors in Patients with First-Ever Ischemic Stroke in China. Stroke Research and Treatment. 2012;2012:1–6. doi: 10.1155/2012/136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roquer J, Rodríguez Campello A, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–5. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 38.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurology. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 39.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. Journal of the American Medical Association. 2006;296:2939–46. doi: 10.1001/jama.296.24.2939. [DOI] [PubMed] [Google Scholar]
- 40.Lopez PR, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tobacco Control. 1994;3:242–7. [Google Scholar]
- 41.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–53. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin S, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 43.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? Journal of the American College of Cardiology. 2009;54:2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flaherty ML, Woo D, Haverbusch M. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36:934–7. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 45.Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, et al. Incidence of lobar and non-lobar spontaneous intracerebral haemorrhage in a predominantly Hispanic-Mestizo population--the PISCIS stroke project: a community-based prospective study in Iquique, Chile. Neuroepidemiology. 2010;34:214–21. doi: 10.1159/000289353. [DOI] [PubMed] [Google Scholar]
- 46.Jiang B, Wang W, Chen H. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 2006;37:63–5. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- 47.Inagawa T, Ohbayashi N, Takechi A, Shibukawa M, Yahara K. Primary intracerebral hemorrhage in Izumo City, Japan: incidence rates and outcome in relation to the site of hemorrhage. Neurosurgery 2003; 53: 1283–1298. Neurosurgery. 2003;53:1283–98. doi: 10.1227/01.neu.0000093825.04365.f3. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Ueda Y, Date C. Incidence of stroke in Shibata, Japan: 1976–1978. Stroke. 1981;12:460–6. doi: 10.1161/01.str.12.4.460. [DOI] [PubMed] [Google Scholar]
- 49.Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. Journal of Neurology Neurosurgery and Psychiatry. 2014 doi: 10.1136/jnnp-2013-306992. [DOI] [PubMed] [Google Scholar]
- 50.Lovelock CE, Molyneux AJ, Rothwell PM. Study obotOV. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurology. 2007;6:487–93. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 51.Khellaf M, Quantin C, d'Athis P, Fassa M, Jooste V, Hervieu M, et al. Age–Period–Cohort Analysis of Stroke Incidence in Dijon from 1985 to 2005. Stroke. 2010;41:2762–7. doi: 10.1161/STROKEAHA.110.592147. [DOI] [PubMed] [Google Scholar]
- 52.Kleindorfer D, Broderick J, Khoury J. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37:2473–8. doi: 10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 53.Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival:secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–80. [PubMed] [Google Scholar]
- 54.Zahuranec DB, Gonzales NR, Brown DL, Lisabeth LD, Longwell LJ, Eden SV, et al. Presentation of intracerebral haemorrhage in a community. Journal of Neurology Neurosurgery and Psychiatry. 2006;77:340–4. doi: 10.1136/jnnp.2005.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labiche LA, Chan W, Saldin KR. Sex and acute stroke presentation. Annals of Emergency Medicine. 2002;40:453–60. doi: 10.1067/mem.2002.128682. [DOI] [PubMed] [Google Scholar]
- 56.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–5. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 57.Gehani AA. Association of risk factors with acute myocardial infarction in Middle Eastern countries: the INTERHEART Middle East study. European Journal of Preventive Cardiology. 2014;21:400–10. doi: 10.1177/2047487312465525. [DOI] [PubMed] [Google Scholar]
- 58.Butala NM, Desai MM, Linnander EL, Wong YR, Mikhail DG, L.S. O, et al. Gender Differences in Presentation, Management, and In-Hospital Outcomes for Patients with AMI in a Lower-Middle Income Country: Evidence from Egypt. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0025904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redon J, Olsen MH, Cooper RS, Zurriaga O, Martinez-Beneito MA, Laurent S. Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and Central Asia: implications for control of high blood pressure. European Heart Journal. 2011;32:1424–31. doi: 10.1093/eurheartj/ehr045. [DOI] [PubMed] [Google Scholar]
- 60.Adams H, Bendixen B, Kappelle L. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 61.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chugh SS, Roth GA, Gillum RF, Mensah GA. Global burden of atrial fibrillation in developed and developing nations. Glob Heart. 2014;9:113–9. doi: 10.1016/j.gheart.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Bennett D, Brayne C, Feigin V, Barker-Collo S, Brainin M, Davis D, et al. Development of the Standards of Reporting of Neurological Disorders (STROND) checklist: A guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neurology. 2015 doi: 10.1212/WNL.0000000000001866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.