Abstract
Heat shock protein 90α (Hsp90α) immobilized on aminopropyl silica gels was prepared via the N- or C-terminal, which was termed Hsp90α-NT or Hsp90α-CT, respectively. Binding interactions of biscoclaurine alkaloids (cepharanthine (CEP), berbamine (BBM), isotetrandrine (ITD), and cycleanine (CCN)) with Hsp90α were examined using the Hsp90α-NT or -CT columns by frontal and zonal chromatography studies. The dissociation constants of CEP, BBM, ITD, and CCN to Hsp90α-NT were estimated to be 5.3, 18.6, 46.3, and 159 µM, respectively, by frontal chromatography techniques. Similar results were obtained with the Hsp90α-CT column. These data suggest that these biscoclaurine alkaloids interact with the middle domain of Hsp90α. This was confirmed by demonstrating that CEP competed with endothelial nitric oxide synthase at the middle domain of Hsp90α, where it was shown to have a dissociation constant of 15 nM. Furthermore, the Hsp90α-NT column was applied for preliminary screening of natural Hsp90α inhibitors by zonal chromatography studies.
Keywords: Heat shock protein, Frontal affinity chromatography, Cepharanthine, Berbamine, Isotetrandrine, Cycleanine
Heat shock proteins (Hsp’s)1 act as molecular chaperones that guide the normal folding, intracellular disposition, and proteolytic turnover of many key regulators of cell growth and survival [1,2]. Their increased expression in tissues was observed with proteotoxic stressors such as heat, heavy metals, hypoxia, and acidosis [1,2]. Furthermore, increased expression and activity of Hsp90, a family of cellular proteins including Hsp90α, Hsp90β, Grp94, and Trap 1, have been observed with human cancers, compared to normal tissues [1–5]. Recently, it has been reported that Hsp90 exists in an activated form in tumor cells but in a latent form in normal tissues [5]. This means that Hsp90 inhibitors could represent a new and selective class of anticancer agents [6,7].
Structural and functional studies reveal that Hsp90 has three conserved domains, namely the N-terminal, middle, and C-terminal domains, and displays an intrinsic ATPase activity necessary for its function [1,2]. The N-terminal domain contains an adenosine nucleotide binding pocket, which is associated with the ATPase activity [1,2]. The middle domain plays an important role in modulating the ATPase activity, and the C-terminal domain also contains an additional binding pocket for ATP, albeit with lower activity [5]. Several natural Hsp90 inhibitors in cancer treatment were discovered and developed [6,7]: geldanamycin and its derivatives (17-demethoxy geldanamycin and 17-N,N-dimethylaminoethylamino-17-demethoxy geldanamycin) [8] and radicicol and its analogues [9] bind to the N-terminal ATP-binding domain, while novobiocin [10] and (−)-epigallocatechin-3-gallate [11,12] bind at or adjacent to the C-terminal ATP-binding site. However, natural inhibitors that bind to the middle domain of Hsp90 have not been reported yet.
Cepharanthin is extracted from Stephania cepharantha Hayata and includes cepharanthine (CEP), berbamine (BBM), isotetrandrine (ITD), and cycleanine (CCN), which are biscoclaurine alkaloids (BCAs), as the primary components [13]. It is reported that these BCAs have antiproliferative and proapoptotic effects against a diverse range of tumors both in vitro and in vivo [14,15].
In a previous study [16], Hsp90α was covalently immobilized onto the surface of aminopropyl silica gels (APS). The protein was immobilized via the N-terminal to create Hsp90α-NT or via the C-terminal to create Hsp90α-CT. The immobilization was accomplished utilizing glutaraldehyde or 1-ethyl-3-(3-methylaminopropyl) carbodiimide (EDC), respectively, as a coupling reagent for the N- and C-terminals. Furthermore, it was reported that immobilization did not affect ATPase activity or sensitivity to inhibition [16]. In this study, binding interactions of BCAs with Hsp90α, including determination of dissociation constants and elucidation of a binding domain, were examined using Hsp90α-NT and -CT columns by frontal and zonal chromatography studies. Furthermore, the Hsp90α-NT column was applied for preliminary screening of natural Hsp90α inhibitors.
Experimental procedures
Materials
Recombinant human Hsp90α (~90% pure) was purchased from Stressgen Bioreagents (Ann Arbor, MI, USA). Bovine serum albumin (BSA), glutaraldehyde, glutamic acid, pyridine (99.8%), sodium azide, and EDC were obtained from Sigma–Aldrich (St. Louis, MO, USA). Purified recombinant Homo sapiens endothelial nitric oxide synthase (eNOS) was purchased from OriGene Technologies (Rockville, MD, USA). BCAs (CEP, BBM, ITD, and CCN) were kindly provided by Kaken Shoyaku Co. (Osaka, Japan). The water used in the study was prepared using Purelab Ultra (Organo, Tokyo, Japan). The APS gel (Nucleosil 300-7 NH2) was purchased from Macherey–Nagel (Duren, Germany). Other reagents and solvents were of analytical- reagent grade and were used without further purification. The structures of the BCAs used in this study are illustrated in Fig. 1.
Fig. 1.
Structures of CEP, BBM, ITD, and CCN.
Preparation of Hsp90α-NT column
The Hsp90α-NT silica gels were prepared according to a previously reported protocol [16]. Briefly, a 50-mg portion of APS gel was added to 10 ml of pyridine (10 mM, pH adjusted to 6.0 with 100 mM HCl), the mixture was vortex-mixed for 15 min and centrifuged at 1500g for 10 min, and then the supernatant was discarded. The APS gel was suspended in 10 ml of 5% glutaraldehyde, rotated at 200 rpm for 3 h, and then centrifuged at 1500g for 10 min. The supernatant was discarded, and the activated APS gel was washed three times with 10-ml portions of pyridine (10 mM, pH 6.0) as described above. A suspension of 200 µg human Hsp90α protein in 300 µl of pyridine (10 mM, pH 6.0) was added to the activated APS gel and then allowed to stand for 24 h at 4 °c. After the mixture had warmed to room temperature, 5 ml of glutamic acid (1 M, pH 8.0) was added, the resulting mixture was rotated at 200 rpm for 30 min and centrifuged at 1500g for 10 min, and then the supernatant was discarded. The obtained Hsp90-NT silica gel was rinsed three times with 5-ml portions of Tris–HCl buffer (10 mM, pH 7.4) containing 150 mM NaCl, 0.1 w/v% BSA, 1 mM EDTA, and 0.1% sodium azide. The suspension containing the Hsp90α-NT silica gel was packed in a Tricorn 5/20 glass column (50 × 5 mm i.d., GE Healthcare Biosciences, Uppsala, Sweden). The column was washed with Tris–HCl buffer (10 mM, pH 7.4) for 2 h using a chromatographic pump with a flow rate of 0.2 ml/min at 25 °c. The Hsp90α-NT column could be stored at 4 °c until use.
The control-NT silica gels were prepared similar to the procedure above, with the exception of the addition of Hsp90α.
Preparation of Hsp90α-CT column
The Hsp90α-CT silica gel was prepared according to a previously reported protocol [16]. Briefly, a 100-mg portion of APS gel was rinsed with 10 ml of potassium phosphate buffer (10 mM, pH 5.5) containing 150 mM NaCl. Four hundred micrograms of human Hsp90α protein was added to 400 µl of potassium phosphate buffer (10 mM, pH 5.5) containing 150 mM NaCl, and the suspension was added to the APS gel. The mixture was vortex-mixed for 5 min, followed by the addition of 200 µl of a 10-mg/ml solution of EDC. The pH of the reaction mixture was adjusted to 5.0 using 0.1 M HCl, and the mixture was then rotated at 200 rpm for 24 h at 4 °c. The mixture was allowed to warm to room temperature and centrifuged at 1500g for 10 min, and then the supernatant was discarded. The Hsp90α-CT silica gel was rinsed three times with 5-ml portions of Tris–HCl buffer (10 mM, pH 7.4) containing 150 mM NaCl, 0.1 w/v% BSA, 1 mM EDTA, and 0.1% sodium azide. The suspension containing the Hsp90α-CT silica gel was packed in a Tricorn 5/20 glass column. The column was washed with Tris–HCl buffer (10 mM, pH 7.4) for 2 h using a chromatographic pump with a flow rate of 0.2 ml/min at 25 °c. The Hsp90-CT column could be stored at 4 °c until use.
Chromatographic systems used for frontal and zonal chromatography studies
The LC system used was composed of an LC-10ADvp pump, an SPD-10Avp spectrophotometer, a C-R6A integrator (all from Shimadzu, Kyoto, Japan), and a CO-1093C column oven (Uniflows, Tokyo, Japan). A Rheodyne 7125 injector (Rheodyne, Cotati, CA, USA) with 5-ml and 20-µl loops was used in frontal and zonal chromatography studies, respectively. The LC conditions used are specified in the figure legends.
Frontal chromatographic studies
Serial concentrations of CEP (1, 1.5, 2, 3, 4, and 5 µM for Hsp90α-NT and 4, 6, 8, 10, and 20 µM for Hsp90α-CT), BBM (2, 10, 20, 40, and 200 µM for Hsp90α-NT and 1, 5, 10, 20, and 200 µM for Hsp90α-CT), ITD (20, 50, 100, 150, and 200 µM for Hsp90α-NT and 10, 20, 40, 80, and 200 µM for Hsp90α-CT), and CCN (60, 140, 200, 300, and 400 µM for Hsp90α-NT and -CT) were prepared in Tris–HCl buffer (10 mM, pH 7.4). A 5-ml aliquot of each solution was placed in the loop and delivered at 0.2 ml/min at 25 °c to the Hsp90 columns. Detection was performed at 282 nm.
The observed retention volumes were used to calculate binding affinities (Kd values) of the studied BCAs using a previously described approach [16,17],
| (1) |
where V is the retention volume of BCA measured at the midpoint of the breakthrough curve, Vmin is the retention volume of BCA at the highest concentration applied of the displacer ligand, and Bmax is the number of active binding sites of the immobilized protein (Hsp90α). The Kd,BCA values were obtained by plotting [BCA](V − Vmin) versus [BCA], and the data were analyzed by nonlinear regression with the sigmoidal response curve using Prism 4 software (GraphPad Software, San Diego, CA, USA).
Competition studies of eNOS and CEP on Hsp90α-NT column
As described previously [18,19], the case in which a solute (CEP) and competing agent (eNOS) undergo simple, single-site competition on an Hsp90α-NT column is described by the equation
| (2) |
where kCEP is the retention factor of solute CEP, Vm is the void volume of the column, Bmax represents the number of moles of binding sites of the immobilized protein within the column, and Kd,eNOS and Kd,CEP, respectively, represent the dissociation constants for the binding of eNOS and CEP to Hsp90α. The Kd,eNOS and Kd,CEP values were obtained by plotting 1/kCEP versus [eNOS], and the data were analyzed by linear regression analysis using Excel (Microsoft Japan, Tokyo, Japan).
Zonal chromatography studies for preliminary screening of natural Hsp90α inhibitors
The retention factor (k) was calculated from the equation k = (tR − t0)/t0, where tR and t0 are the retention times of retained and unretained solutes, respectively. The retention time of an unretained solute, t0, was measured by injecting a solution of sodium nitrate. The retention times or retention factors of solutes were measured on the Hsp90α-NT, -CT, and control-NT columns under the following conditions: mobile phase, Tris–HCl buffer (10 mM, pH 7.4); flow rate, 0.2 ml/min; column temperature, 25 °c; amount loaded, 500 ng; detection wavelength, 210 nm.
Results and discussion
Frontal chromatography studies
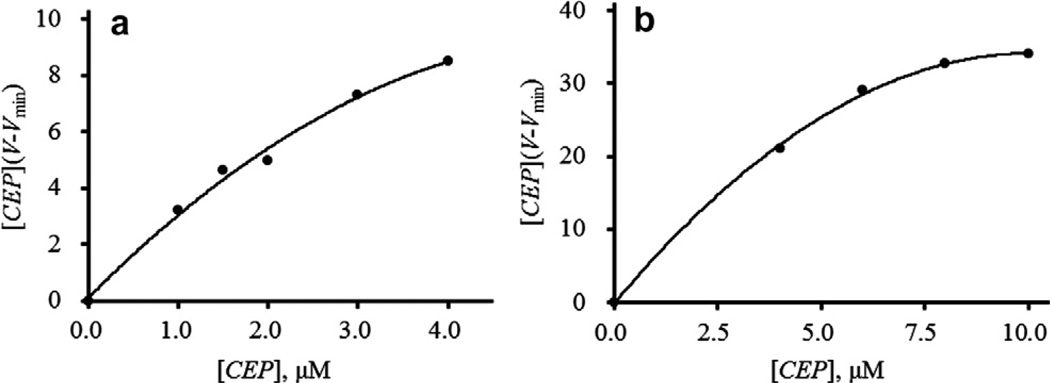
To determine whether the BCAs had affinity for Hsp90α, frontal chromatography techniques were carried out. Initially, the dissociation constants, Kd, of the BCAs were evaluated for Hsp90α on both the Hsp90α-NT and Hsp90α-CT columns. Fig. 2a and b show the sigmoidal plots of [CEP](V − Vmin) versus [CEP] obtained with the Hsp90-NT and -CT columns, respectively. Similar sigmoidal plots were obtained with other BCAs (BBM, ITD, and CCN). Table 1 shows the Kd and Bmax values for the binding of BCAs to the Hsp90α-NT and -CT columns. The Kd values of CEP, BBM, ITD, and CCN to the Hsp90α-NT column were 5.3, 18.6, 46.3, and 159 µM, respectively. The Kd values of BCAs to the Hsp90α-CT column were similar, with binding affinities of 6.3, 18.0, 29.9, and 143 µM, respectively. This means that the binding affinities are in the order CEP > BBM > ITD > CCN for both Hsp90α columns, suggesting that BCAs are most likely interacting with the middle domain of Hsp90α. To confirm that it was not binding to either the N-terminal or the C-terminal domain of Hsp90α, the addition of geldanamycin, an N-terminal binder, or novobiocin, a C-terminal binder, in the mobile phase did not affect the Kd value of CEP, further supporting the possibility that the BCAs could be interacting with the middle domain of Hsp90α.
Fig. 2.
Determination of the dissociation constants of CEP to (a) Hsp90α-NT and (b) Hsp90α-CT columns by frontal chromatography techniques. LC conditions: column, Hsp90α-NT or Hsp90α-CT (20 × 5.0 mm i.d.); mobile phase, 10 mM Tris–HCl buffer (pH 7.4) containing various concentrations of CEP; flow rate, 0.2 ml/min; column temperature, 25 °c; detection, 282 nm; injection volume, 5 ml.
Table 1.
Kd and Bmax values for the binding of BCAs to Hsp90α-NT and -CT columns.
| Hsp90α-NT column | Hsp90α-CT column | |||
|---|---|---|---|---|
| Kd (µM) | Bmax (nmol) | Kd (µM) | Bmax (nmol) | |
| Cepharanthine | 5.3 ± 1.3 | 19.8 ± 3.1 | 6.3 ± 1.7 | 56.9 ± 7.2 |
| Berbamine | 18.6 ± 3.8 | 36.5 ± 3.3 | 18.0 ± 4.9 | 89.7 ± 17.1 |
| Isotetrandrine | 46.3 ± 14.8 | 54.7 ± 6.3 | 29.9 ± 8.4 | 250 ± 29.7 |
| Cycleanine | 159 ± 23.2 | 37.3 ± 2.5 | 143 ± 36.6 | 578 ± 110 |
Kd and Bmax values are given ±SE.
Competition studies of eNOS and CEP on Hsp90α-NT column
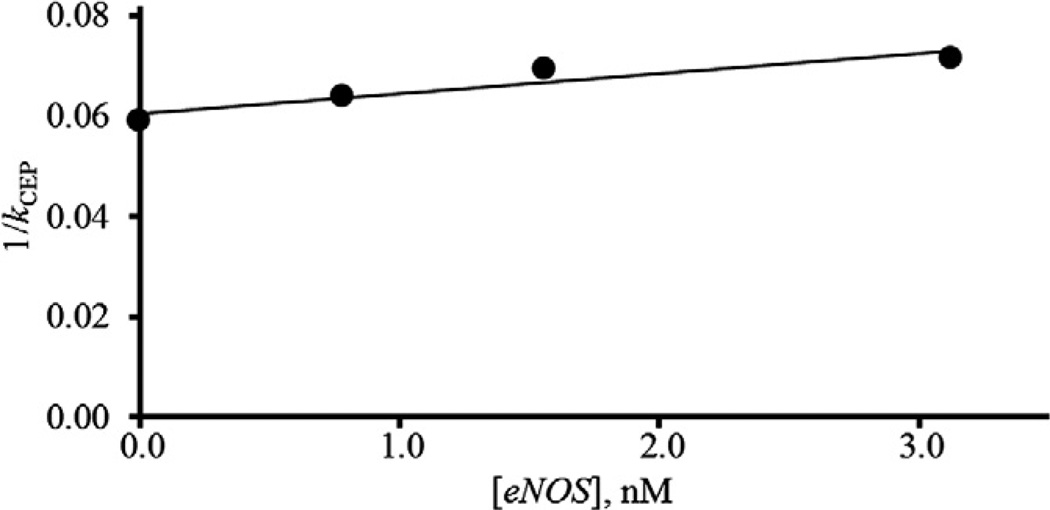
It is reported that the interaction between Hsp90 and eNOS occurs through binding of eNOS to the middle domain of Hsp90 [20]. eNOS was added to the mobile phase and the retention factor of CEP on the Hsp90α-NT column was measured. The retention factors of CEP decreased with an increase in eNOS concentrations. Plots of the reciprocal of k for CEP, kCEP, against a concentration of eNOS gave a linear relationship as shown in Fig. 3. Therefore, CEP competed with eNOS at a single binding site of the middle domain of Hsp90α. The Kd,eNOS, Kd,CEP, and Bmax values were estimated from the slope and intercept of a linear regression line and were estimated to be 15 nM, 5.0 µM, and 26 nmol, respectively. The Kd,CEP and Bmax values estimated by frontal chromatography studies using the Hsp90α-NT column were 5.3 µM and 20 nmol, respectively, as shown in Table 1. Those values estimated by competition and frontal chromatography studies are in good agreement. These results also indicate that CEP competes with eNOS at the middle domain of Hsp90α. Furthermore, it was found that the binding affinity of eNOS to Hsp90α, Kd,eNOS, was 15 nM. In addition, demonstrating that CEP is binding to the middle domain, this is the first report of the dissociation constant for the binding of eNOS to Hsp90α.
Fig. 3.
Relationship for competitive displacement of CEP by eNOS. LC conditions: column, Hsp90α-NT (20 × 5.0 mm i.d.); mobile phase, 10 mM Tris–HCl buffer (pH 7.4) containing various concentrations of eNOS; flow rate, 0.2 ml/min; column temperature, 25 °c; detection, 210 nm; amount loaded, 500 ng.
Zonal chromatographic studies
Table 2 shows the retention times and retention factors of BCAs on Hsp90α-NT and -CT columns. From the results shown in Tables 1 and 2, it seems to be a negative correlation between the Kd values of BCAs for Hsp90α and the retention times (or factors) of BCAs. The correlation coefficient of the Kd values of BCAs versus retention times of BCAs on an Hsp90α-NT column was −0.9419, while that on an Hsp90α-CT column was −0.8075. Furthermore, the correlation coefficients plotted against retention factors instead of retention times gave almost the same values for Hsp90α-NT and -CT columns. These results indicate that Hsp90α columns could be utilized for screening natural Hsp90α inhibitors using zonal chromatography studies. In the following studies, Hsp90α-NT and control-NT columns were used for preliminary screening of natural Hsp90α inhibitors.
Table 2.
Retention times and retention factors of BCAs on Hsp90α-NT and -CT columns.
| Hsp90α-NT column | Hsp90α-CT column | |||
|---|---|---|---|---|
| tR (min) | k | tR (min) | k | |
| Cepharanthine | 34.5 | 18.6 | 44.0 | 22.9 |
| Berbamine | 33.0 | 17.5 | 30.0 | 15.3 |
| Isotetrandrine | 22.6 | 11.7 | 25.7 | 13.0 |
| Cycleanine | 13.9 | 6.80 | 17.3 | 8.37 |
Preliminary screening of natural Hsp90α inhibitors
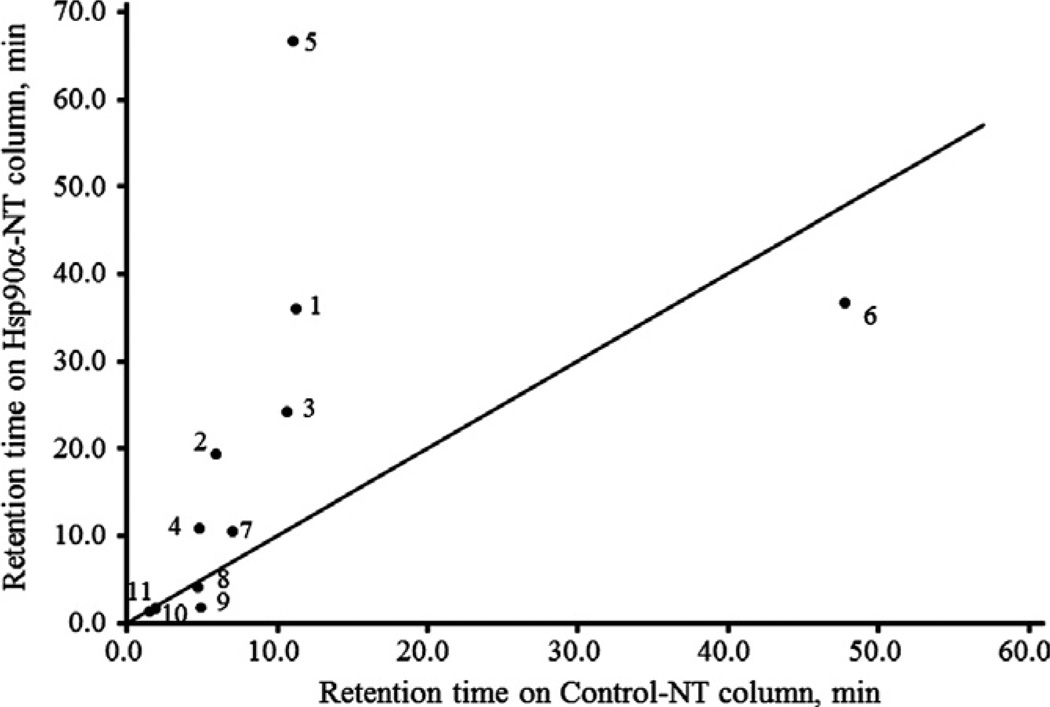
Fig. 4 shows the plots of the retention times of natural products for which anticancer activities are reported on Hsp90α-NT and control-NT columns. The slope of the straight line in Fig. 4 is 1. The natural products that were more retained on the Hsp90α-NT column than the control-NT column are possible Hsp90α inhibitors, upper left corner. However, the change in retention time of the compounds does not necessarily correlate with the binding activity, but rather reflects only whether specific retention on the Hsp90α-NT column is observed. The natural products whose plots are at the bottom right or on the straight line are either N-terminal binders or nonbinders and therefore may have other anticancer mechanisms. The plots of BCAs (CEP, BBM, ITD, and CCN), quercetin, and wogonin are at the upper left, while curcumin, glycyrrhizin, baicalin, baicalein, and ginsenoside are at the bottom right or on the straight line. These results suggest that BCAs (CEP, BBM, ITD, and CCN), quercetin, and wogonin could be Hsp90α inhibitors, which interact with the C-terminal or middle domain of Hsp90α.
Fig. 4.
Plots of the retention times of natural products on Hsp90α-NT and control-NT columns. LC conditions: column, Hsp90α-NT or control-NT (20 × 5.0 mm i.d.); mobile phase, 10 mM Tris–HCl buffer (pH 7.4); flow rate, 0.2 ml/min; column temperature, 25 °c; detection, 210 nm; amount loaded, 500 ng. Symbols: 1, CEP; 2, BBM; 3, ITD; 4, CCN; 5, quercetin; 6, curcumin; 7, wogonin; 8, glycyrrhizin; 9, baicalin; 10, baicalein; 11, ginsenoside.
Further studies are ongoing in our laboratory for screening natural Hsp90α inhibitors in herbal medicines.
Conclusions
Hsp90α immobilized on APS gels was prepared via the N- or C-terminal, which was termed Hsp90α-NT or Hsp90α-CT, respectively. Binding interactions of CEP, BBM, ITD, and CCN with Hsp90α were examined using the Hsp90α-NT and -CT columns by frontal and zonal chromatography studies. The dissociation constants of CEP, BBM, ITD, and CCN to Hsp90α-NT were estimated to be 5.3, 18.6, 46.3, and 159 µM, respectively, by frontal chromatography studies, with similar results obtained for Hsp90α-CT. These data support that these alkaloids interact with the middle domain of Hsp90α. It was found that CEP competed with eNOS at the middle domain of Hsp90α and that the dissociation constant of eNOS to Hsp90α was 15 nM. Furthermore, the Hsp90α-NT column could be applied for preliminary screening of natural Hsp90α inhibitors by zonal chromatography techniques.
Acknowledgment
This research was supported in part by the Intramural Research Program at the National Institute on Aging, NIH (R.M.).
Footnotes
Abbreviations used: Hsp, heat shock protein; CEP, cepharanthine; BBM, berbamine; ITD, isotetrandrine; CCN, cycleanine; BCA, biscoclaurine alkaloid; EDC, 1-ethyl-3-(3-methylaminopropyl)carbodiimide; BSA, bovine serum albumin; eNOS, endothelial nitric oxide synthase; APS, aminopropyl silica.
References
- 1.Whitesell L, Lidquist SL. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 2.Shames DS, Minna JD. IP6K2 is a client for HSP90 and a target for cancer therapeutics development. Proc. Natl. Acad. Sci. USA. 2008;105:1389–1390. doi: 10.1073/pnas.0711993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Mol. Med. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Kasibhatla SR, Hong K, Biamonte MA, Busch DJ, Karjian PL, Sensintaffar JL, Kamal A, Lough RE, Brekken J, Lundgren K, Grecko R, Timony GA, Mansfield RR, Fritz LC, Ulm E, Burrows FJ, Boehm MF. Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J. Med. Chem. 2007;50:2767–2778. doi: 10.1021/jm050752+. [DOI] [PubMed] [Google Scholar]
- 6.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta. 2012;1823:742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhang D, Xu J, Shi J, Jiang L, Yao N, Ye W. Discovery and development of natural heat shock protein 90 inhibitors in cancer treatment. Acta Pharm. Sin. B. 2012;2:238–245. [Google Scholar]
- 8.Smith V, Sausville EA, Camalier RF, Fiebig HH, Burqer AM. Comparison of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother. Pharmacol. 2005;56:126–137. doi: 10.1007/s00280-004-0947-2. [DOI] [PubMed] [Google Scholar]
- 9.Oikawa T, Onozawa C, Kuranuki S, Igarashi Y, Sato M, Ashino H, Shimamura M, Toi M, Kurakata S. Dipalmitoylation of radicicol results in improved efficacy against tumor growth and angiogenesis in vivo. Cancer Sci. 2007;98:219–225. doi: 10.1111/j.1349-7006.2006.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellert M, Odea MH, Itoh T, Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc. Natl. Acad. Sci. USA. 1976;73:4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palermo CM, Westlake CA, Gasiewicz TA. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–5052. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang T, Jiang Y, Lee HF, Schwartz SJ, SunD D. (−)-Epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca-2. Mol. Pharm. 2009;6:1152–1159. doi: 10.1021/mp900037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomita M, Kozuka M. Studies on the alkaloids of menispermaceous plants. CCXLV. Alkaloids of Stephania cepharantha Hayata (7): structure of cepharamine. Yakugaku Zasshi. 1967;87:1203–1208. doi: 10.1248/yakushi1947.87.10_1203. [DOI] [PubMed] [Google Scholar]
- 14.Harada K, Bando T, Yoshida H, Sato M. Characteristics of antitumour activity of cepharanthin against human adenosquamous cell carcinoma cell line. Oral Oncol. 2001;37:643–651. doi: 10.1016/s1368-8375(00)00125-1. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi-Makise N, Suzu S, Hiyoshi M, Ohsugi T, Katano H, Umezawa K, Okada S. Biscoclaurine alkaloid cepharanthine inhibits the growth of primary effusion lymphoma in vitro and in vivo and induces apoptosis via suppression of the NF-κB pathway. Int. J. Cancer. 2009;125:1464–1472. doi: 10.1002/ijc.24521. [DOI] [PubMed] [Google Scholar]
- 16.Marszałł MP, Moaddel R, Jozwiak K, Bernier M, Wainer IW. Initial synthesis and characterization of an immobilized heat shock protein 90 column for online determination of binding affinities. Anal. Biochem. 2008;373:313–321. doi: 10.1016/j.ab.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moaddel R, Jozwiak K, Wainer IW. Allosteric modifiers of neuronal nicotinic acetylcholine receptors: new methods, new opportunities. Med. Res. Rev. 2007;27:723–753. doi: 10.1002/med.20091. [DOI] [PubMed] [Google Scholar]
- 18.Noctor TAG, Wainer IW, Hage DS. Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J. Chromatogr B. 1992;577:305–315. doi: 10.1016/0378-4347(92)80252-l. [DOI] [PubMed] [Google Scholar]
- 19.Matsunaga H, Haginaka J. Investigation of chiral recognition mechanism on chicken α1-acid glycoprotein using separation system. J. Chromatogr. A. 2006;1106:124–130. doi: 10.1016/j.chroma.2005.07.106. [DOI] [PubMed] [Google Scholar]
- 20.Harris MB, Ju H, Venema VJ, Blackstone M, Venema RC. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen. Pharmacol. 2000;35:165–170. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]