Abstract
Stereochemistry is an important dimension in pharmacology and plays a role in every aspect of the pharmacological fate of chiral xenobiotics. This includes small molecule–drug transporter binding.
This paper reviews the reported stereoselectivities of substrate and inhibitor interactions with P-glycoprotein and the organic cation transporter obtained using standard functional and binding studies, as well as data obtained from online cellular membrane affinity chromatography studies.
The use of stereochemical data in quantitative structure–activity relationship (QSAR) and pharmacophore modelling is also addressed as is the effect of ignoring the fact that small molecule–drug transporter interactions take place in three-dimensional and asymmetric space.
Keywords: P-glycoprotein, organic cation transporter, cellular membrane affinity chromatography, quantitative structure–activity relationship (QSAR), pharmacophore modelling, enantioselectivity, chirality
Introduction
Drug transporters play a key role in the disposition and function of endogenous and exogenous substances (Ekins et al. 2007). In drug development, the determination of the interaction of lead compounds with these transporters is a key component of the absorption, distribution, metabolism and excretion (ADME) stage of this process. The growing importance of the study of drug transporters is reflected in the publication of this special issue.
While the identification and quantification of interactions with drug transporters is an important component of ADME screens, these studies are primarily accomplished using tedious, inexact and expensive in vitro methods. This has resulted in an increased effort to produce more efficient screening techniques such as in silico approaches using quantitative structure–activity relationships (QSAR) and pharmacophore modelling. These computational techniques and their application in drug transporter modelling have been recently reviewed (Chang and Swaan 2006; Ekins et al. 2007).
QSAR and computational models are only as good as the functional and structural data used in their development. Since drug transporters are proteins, and are therefore chiral entities, it is vital that there is an understanding of the stereochemical aspects of substrate–transporter and inhibitor–transporter interactions. These interactions provide a three-dimensional view of the transporter by using the three-dimensional configurations of the substrate/inhibitor. This review addresses this issue.
Background
Molecular chirality
In 1848, Louis Pasteur used a hand lens and a pair of tweezers to separate the sodium ammonium salts of paratartaric acid into its left-handed (levo-tartaric acid) and right-handed (dextro-tartaric acid) forms. This experiment established the existence of enantiomeric molecules, compounds which contain at least one chiral centre and that are related to each other as non-superimposable mirror images. Ten years after he resolved levo- and dextro-tartaric acid, Pasteur demonstrated that the mould Penicillium glaucum destroyed the dextro-ammonium tartrate faster than levo-ammonium tartrate, thereby establishing the existence of biological enantioselectivity, i.e. the ability of proteins to recognize molecular asymmetry. These studies and the related historical events have been extensively discussed (cf. Drayer 1993).
Enantiomeric compounds are stereoisomers, that is, molecules which are composed of the same constituents and have the same structural formulas, but differ with respect to their three-dimensional structure. Other forms of stereoisomers include diastereoisomers, compounds which have two or more chiral centres and which are not related to each other as non-superimposable mirror images.
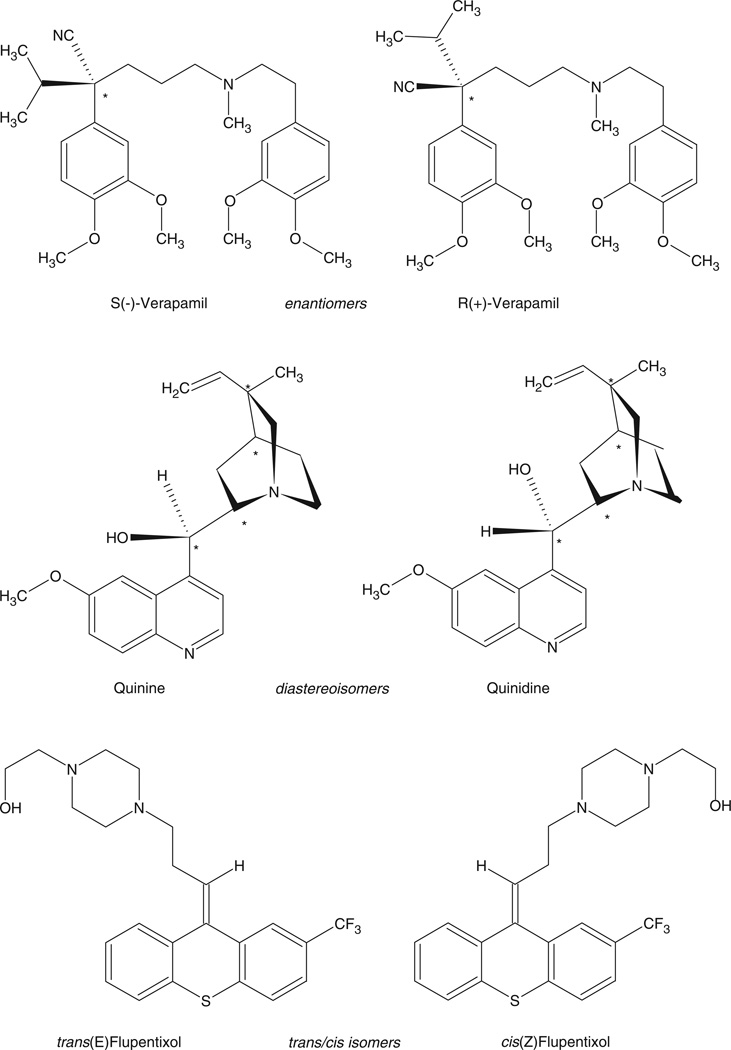
The current accepted method of describing the distribution of substituents around chiral centres contained in enantiomers and diastereomers is the Cahn–Ingold–Prelog Convention, which uses R- and S-designations. This method will be used in this review, except in cases where previously published work has described the stereoisomers by their rotation of plane-polarized light designated as dextro-, d- or (+) and levo-, l- or (−), or by the Fisher Convention using L- and D-designations. Stereoisomers also include geometric isomers, compounds containing carbon–carbon double bonds (or similar double-bonded systems) in which substituents are found on the same side (cis- or Z-isomer) or opposite side (trans- or E-isomer). Representative enantiomers, diastereomers and geometrical isomers are presented in Figure 1 and a review of stereochemical terms and concepts can be found in a number of publications (cf. Wainer and Marcotte 1993).
Figure 1.
Molecular structures of representative enantiomers, diastereomers and geometric isomers.
The initial observation of enantioselective differences in pharmacological properties are attributed to Abderhalde and Muller, who in 1908 described the differential pressor effects of (−)-epinephrine and (+)-epinephrine (Casy 1970). Since that time, the pharmacological interactions of the separate enantiomers of a chiral compound with receptors, enzymes and carrier proteins have been extensively studied (cf. Wainer 1993; Aboul-Enein and Wainer 1997). Indeed, the therapeutic consequences of chirality have been integrated into drug-discovery programmes and regulatory agency guidelines (cf. Langaniere 1997).
Chirality and drug transporters
Receptors and enzymes are key drug-discovery targets. Since the pharmacological activity of the enantiomers of a chiral lead compound will direct further synthesis and testing, the enantioselective interaction of the compound with the target is a component of early drug discovery. However, unlike receptors and enzymes, the investigation of the interactions of a drug candidate with drug transporters, particularly polyspecific transporters, are carried out as part of ADME studies, which usually take place after the preferred chirality of an asymmetric lead compound has been established. Thus, enantioselective differences in candidate–transporter interactions have not been a key issue, except in the case of P-glycoprotein (Pgp), which has also been viewed as a target for new therapies to treat multidrug-resistant tumours (Ford et al. 1989; Hollt et al. 1992; Wigler and Patterson 1994).
In addition, the substrates for many ‘specific’ transporters are endogenous compounds. Since these compounds are predominately single isomers, the enantioselectivity of the transport has been assumed. For example, the glucose transporter (GLUT1) stereospecifically transports D-glucose, which has been demonstrated by studies using d- and l-glucose (Hagglund and Lundahl 2003).
Stereoselective binding to Pgp
Pgp is a member of the ATP-binding cassette (ABC) transporter family that includes the multidrug resistance proteins (MRP1, MRP2) and breast cancer resistance protein (BCRP) (Leslie et al. 2005; see also the papers in this issue). The expression and functional activity of these proteins in tumour cell lines has been associated with the reduced sensitivity of these cells to treatment by anticancer agents such as anthracycline antibiotics and vinca alkaloids (cf. Spiegl-Kreinecker et al. 2002; Leslie et al. 2005). Thus, the identification and development of inhibitors of ABC-transporter mediated drug efflux has been an important part of oncology research programs (cf. Hollt et al. 1992; Wigler and Patterson 1994).
One of the initial candidates for use in the reversal of ABC-transporter associated multidrug resistance was the calcium channel blocker verapamil (VER), which also inhibits Pgp-mediated drug transport (Gruber et al. 1988). However, initial in vitro studies indicated that the serum concentration of VER required to reverse multidrug resistance were associated with significant in vivo cardiotoxicity, and this drug has not been recommended for clinical use as a Pgp inhibitor (Fisher and Sikic 1995).
VER is an enantiomeric compound (Figure 1) and is administered as a racemic (50:50) mixture of R-VER and S-VER. Clinical and in vitro studies have established that the cardiovascular effects of the S-VER are ten times greater than R-VER (Longstreth 1993). Therefore, one possible approach would be to reduce cardiovascular effects by administering only R-VER. Initial studies demonstrated that R-VER and S-VER had the equivalent Pgp-inhibitory activity (Hollt et al. 1992), but the use of R-VER in the treatment of multidrug-resistant tumours has not been pursued.
The initial study of the effect of VER on Pgp transport was carried out using a doxorubicin-resistant cell line (F4-6RADR) and measured the effect of the test drugs on intracellular accumulation of vinblastine (VBL) (Hollt et al. 1992). In this study, the calculated EC50 of (+)-VER, which is the R-enantiomer, appeared to be less than the EC50 of (−)-VER, which is S-VER, 2.6 and 2.9 µM, respectively, but the difference was not statistically significant. The enantioselectivity (α) of the interaction is the ratio of the two EC50 and can be defined as EC50(S)/EC50(R). The calculated α for VER was 1.11.
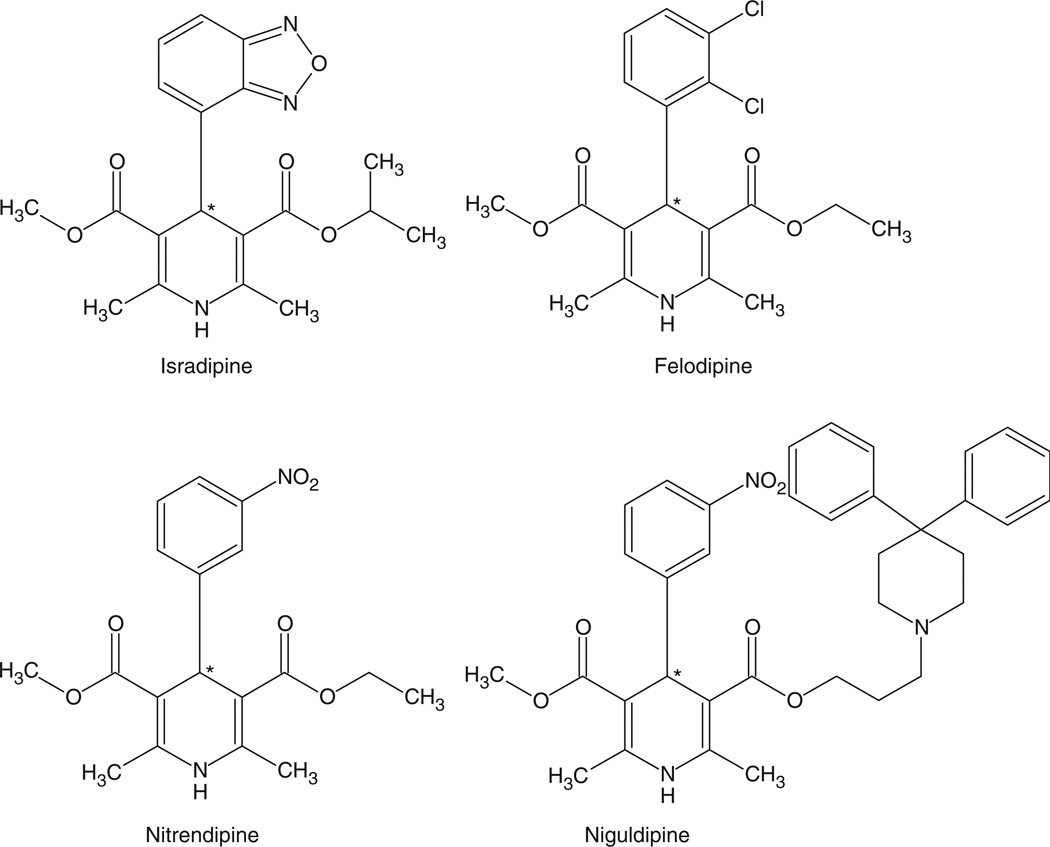
The latter study also included two additional calcium channel blockers (devapamil and emopamil) and four dihydropyridines (niguldipine, nitrendipine, felodipine and isradipine). Significant enantioselective differences in the EC50 values were observed with four of the six compounds (Table I). An examination of the structures of the four dihydropryridines (Figure 2) indicates that the data contain some significant SAR information. For example, increased hydrophobicity in the 3-position of the piperdine ring decreases the EC50 value, niguldipine, while an m-nitrophenyl substituent in the 4-position decreases the enantioselective interactions with Pgp, niguldipine and nitrendipine versus felodipine and isradipine. In addition, the R-configuration at the chiral centre increases the interaction with Pgp (Table I).
Table I.
Reported enantioselective interactions with P-glycoprotein.
| Compound | EC50 (µM) | IC50 (µM) | α (S/R) | Marker | Reference |
|---|---|---|---|---|---|
| (−)-S-VER | 2.9 | VBL | Hollt et al. (1992) | ||
| (+)-R-VER | 2.6 | 1.11 | VBL | Hollt et al. (1992) | |
| S-VER | 1.93 | VBL | Neuhoff et al. (2000) | ||
| R-VER | 2.29 | 0.84 | VBL | Neuhoff et al. (2000) | |
| S-VER | 2.09 | VER | Neuhoff et al. (2000) | ||
| R-VER | 2.37 | 0.88 | VER | Neuhoff et al. (2000) | |
| (−)-S-devapamil | 1.0 | VBL | Hollt et al. (1992) | ||
| (+)-R-devapamil | 2.1 | 0.48 | VBL | Hollt et al. (1992) | |
| (−)-S-emopamil | 3.0 | VBL | Hollt et al. (1992) | ||
| (+)-R-emopamil | 2.4 | 1.25 | VBL | Hollt et al. (1992) | |
| (−)-R-niguldipine | 1.3 | VBL | Hollt et al. (1992) | ||
| (+)-S-niguldipine | 1.2 | 0.92 | VBL | Hollt et al. (1992) | |
| (−)-S-nitrendipine | 9.4 | VBL | Hollt et al. (1992) | ||
| (+)-R-nitrendipine | 10.3 | 0.91 | VBL | Hollt et al. (1992) | |
| (−)-S-felodipine | 8.6 | VBL | Hollt et al. (1992) | ||
| (+)-R-felodipine | 4.7 | 1.83 | VBL | Hollt et al. (1992) | |
| (−)-R-isradipine | 4.7 | VBL | Hollt et al. (1992) | ||
| (+)-S-isradipine | 9.2 | 1.96 | VBL | Hollt et al. (1992) | |
| S-talinolol | 2290 | VER | Neuhoff et al. (2000) | ||
| R-talinolol | 2340 | 0.98 | VER | Neuhoff et al. (2000) | |
| S-propranolol | 574 | VER | Neuhoff et al. (2000) | ||
| R-propranolol | 583 | 0.98 | VER | Neuhoff et al. (2000) | |
| S-carvedilol | 18.40 | VER | Neuhoff et al. (2000) | ||
| R-carvedilol | 9.77 | 1.89 | VER | Neuhoff et al. (2000) | |
| S-omeprazole | 91.1 | VER | Neuhoff et al. (2000) | ||
| R-omeprazole | 97.2 | 0.94 | VER | Neuhoff et al. (2000) | |
| S-LY335977 | 0.290 | VBL | Ekins et al. (2002a) | ||
| R-LY335979 | 0.059 | 4.92 | VBL | Ekins et al. (2002a) | |
| S-LY335990 | 0.330 | VBL | Ekins et al. (2002a) | ||
| R-LY335981 | 0.250 | 1.32 | VBL | Ekins et al. (2002a) | |
| S-LY335983 | 0.120 | VBL | Ekins et al. (2002a) | ||
| R-LY335984 | 0.053 | 2.26 | VBL | Ekins et al. (2002a) |
Note: VBL, vinblastine; VER, verapamil.
Figure 2.
Molecular structures of dihydropyridines that inhibit P-glycoprotein-mediated transport of vinblastine (see Table I) (Hottl et al. 1992).
Enantioselective interactions with Pgp were also observed in a study that determined the IC50 values associated with the Pgp binding affinities of R- and S-VER, the enantiomers of three β-adrenergic receptor antagonists, and R- and S-omeprazole, a proton pump inhibitor (Neuhoff et al. 2000). In these studies, small enantioselective differences was observed between the IC50 values for R- and S-VER when [3H]-VBL or [3H]-R,S-VER was used as the marker ligand, α{IC50(S)/IC50(R)}=0.84 and 0.88, respectively (Table I). It is of interest to note that while the enantioselective differences in the EC50 and IC50 values for VER may not be significant, there were opposite trends, i.e. EC50(S)>EC50(R) and IC50(R) >IC50(S). In this study, the only enantioselective interaction was observed with carvedilol where the IC50 value for R-carvedilol was significantly lower than the value for S-carvedilol (Table I). Enantioselective interactions with Pgp have been recently reported (Table I) (Ekins et al. 2002a, 2002b). In these studies, significant enantioselectivities were observed for two of the compounds, α=2.26 and 4.92.
In addition to enantioselective interactions with Pgp, stereoselective differences have been reported for diastereomers and geometrical isomers. Transport and inhibition studies using the diastereomeric compounds quinidine and quinine (Figure 1), and their N-methyl monoquaternary derivatives demonstrated that the quinidine-based compounds were better substrates and inhibitors than the quinine-based compounds (Hooiveld et al. 2002). Both cis- and trans-flupenthixol (Figure 1) inhibit Pgp-mediated transport, which was first observed in the effect of these compounds on the potency of doxorubicin in MCF-7/DOX-resistant cells (Ford et al. 1989). In these studies the trans-isomer increased the effect of doxorubicin by 15-fold relative to the cis-isomer. Later studies demonstrated that these stereoisomers have opposite effects on Pgp-mediated ATP hydrolysis and Pgp substrate recognition (Dey et al. 1999). In these studies, cis-flupenthixol stimulated both of these activities while trans-flupenthixol acted as an inhibitor.
Stereoselective binding to cation transporters from the human SLC22 family
The SLC22 family of drug transporters has a number of transporters that facilitate the transport of cationic compounds including the polyspecific organic cation transporters (OCT) subtypes hOCT1, hOCT2 and hOCT3 and the carnitine transporters hOCTN1, hOCTN2, hOCTN3 and CT2. This family has been recently reviewed (Koepsell et al. 2003) and is addressed in this special issue. These transporters interact with a broad range of substrates and inhibitors and, as in the case of Pgp, stereoselective interactions have been reported. For example, the transport of L-carnitine by hOCTN2 is three-fold greater that d-carnitine and l-carnitine has a 20-fold greater inhibitory effect on co-transported substrates (Wagner et al. 2000).
Determination of stereoselective ligand interactions with drug transporters using cellular membrane affinity chromatography
The ability to identify definitively and measure quantitatively small molecule interactions with proteins is an important component of the determination and description of enantioselective interactions. In the study of Pgp interactions, transport and competitive binding techniques have been validated and used (cf. Table I), and the identification and characterization of interactions with the hOCT have been primarily accomplished using competitive binding, cellular uptake and trans-stimulation studies (cf. Zhang et al. 1998; Koepsell et al. 2003; Moaddel et al. 2005a).
The present authors have developed an alternative approach to the study of small molecule–protein interactions using an online liquid chromatographic technique, cellular membrane affinity chromatography (CMAC), which has been recently reviewed (Moaddel and Wainer 2006; Moaddel et al. 2007a). In this method, solubilized membrane fragments are immobilized onto the surface of the immobilized artificial membrane (IAM) stationary phase or the surface of a glass capillary and the resulting chromatographic columns placed in liquid chromatographic systems. CMAC columns containing membranes from cell lines expressing ligand gated ion channels, G-protein coupled receptors and drug transporters have been prepared and characterized (cf. Beigi et al. 2004; Moaddel and Wainer 2006; Moaddel et al. 2007b).
The interactions between small molecules and a target protein are studied using frontal affinity chromatography and non-linear chromatography techniques. A series of typical frontal displacement chromatography curves are presented in Figure 3 where the initial flat portion of the chromatographic trace represents specific and non-specific interactions of the marker ligand with the immobilized membrane and target protein, the vertical rise in the trace represents the saturation of the target and the re-established flat trace as the saturated target. Chromatographic retention is measured at the half-height of the frontal curve. The effect of increasing displacer concentration on the chromatographic retention of the marker can be correlated with the affinity of the displacer ligand for the site at which the marker ligand binds, similar to standard competitive membrane binding studies.
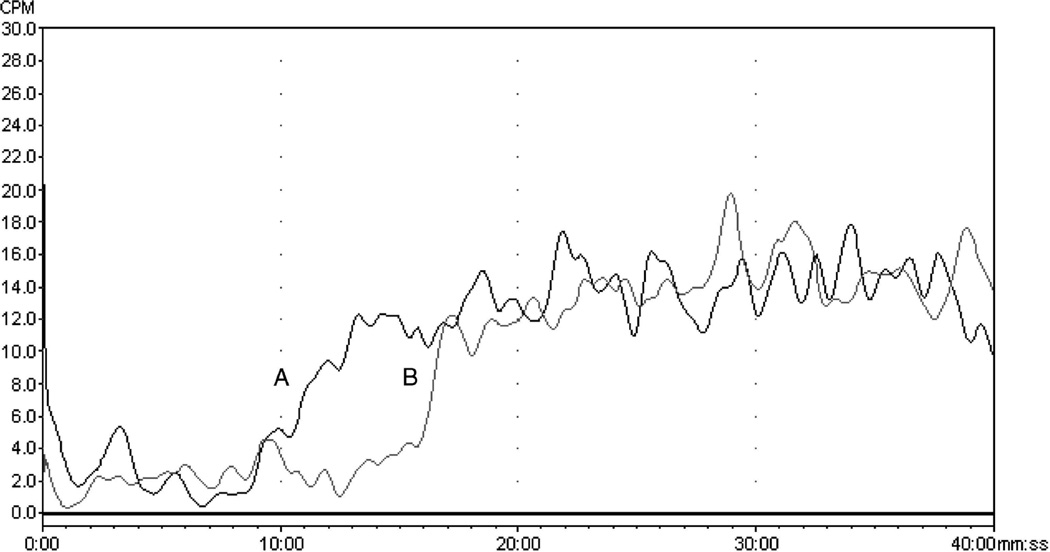
Figure 3.
Comparison of the frontal chromatograms of the human organic cation transporter-1 (hOCT1) marker [3H]-MPP+ obtained on: (A) a cellular membrane affinity column (CMAC) containing cellular membrane fragments from a stably transfected MDCK cell line expressing hOCT1 CMAC(hOCT1(+)) column; and (B) a cellular membrane affinity column (CMAC) containing cellular membrane fragments from the non-transfected MDCK cell line CMAC(hOCT1(−)) column. Reprinted from Moaddel et al. (2005b).
CMAC studies can be utilized to identify ligand–target interactions and to characterize these interactions through the determination of binding affinities (Kd values), functional parameters (IC50 and EC50 values) as well as the kinetics and the thermodynamics of the binding process (Moaddel et al. 2007a). Since enantiomeric compounds have essentially the same physicochemical properties, the non-specific interactions between the enantiomers of a test ligand and the immobilized target will be equivalent. Therefore, any difference in the chromatographic properties will reflect the enantioselectivity of the ligand-target interaction. In this manner, chiral structure–activity data can be collected and used in computational modelling studies. This approach has been used in the study of binding in the internal lumen of nicotinic acetylcholine receptors (Jozwiak et al. 2004, 2007) and hOCT1 (Moaddel et al. 2007c).
In the study of small molecule–drug transporter interactions, CMAC columns have been created from cell lines that stably express Pgp (Zhang et al. 2000; Moaddel et al. 2006), hOCT1 (Moaddel et al. 2005b) and hOAT1 and 2 (Kimura et al. 2007). These columns have been used in a variety of studies that are described below.
CMAC studies of Pgp binding and transport
Solubilized membrane fragments were prepared from the Pgp-positive MDA435/LCC6MDR1 (Pgp(+)) cell line obtained by transduction of Pgp-negative MDA435/LCC6 breast cancer cells (Pgp(−)) with a retroviral vector carrying MDR1 cDNA (Leonessa et al. 1996). Pgp(+)- and Pgp(−)-CMAC columns were prepared by the immobilization of the Pgp(+) and Pgp(−) membranes on the surface of a silica-based liquid chromatography support to create CMAC(Pgp(+)) and CMAC(Pgp(−)) columns (Zhang et al. 2000) or within a glass capillary column (Moaddel et al. 2006). The latter columns were designated as Pgp open tubular columns, Pgp(+)-OT and Pgp(−)-OT.
The ability of the immobilized Pgp to identify Pgp substrates was established using the Pgp(+)-OT and Pgp(−)-OT (Moaddel et al. 2006). In these studies an LC-MS system was used with a parallel screening approach. In this technique, test compounds were chromatographed on the Pgp(−)-OT, and Pgp(+)-OT and the differential retention, t(Pgp(+)-OT – t(Pgp(−)-OT (Δt), was used to rank compounds according to their relative Kd values for the immobilized Pgp.
The Caco-2 cell monolayer model was used to classify 13 compounds as Pgp substrates or non-substrates based upon a permeability ratio >2 (Table II). The same compounds were chromatographed on the Pgp(+)-OT and Pgp(−)-OT columns and the Δt values determined (Table II). A statistically significant correlation was observed between the Δt values and the permeability ratios, r2=0.7749 (p=0.0063). The results indicate that Δt ≥ 0.5 min was a reliable measure of a permeability ratio > 2 and could be used as a rapid qualitative determination of whether a test compound was a Pgp substrate.
Table II.
Results of chromatographic and Caco-2 permeability assays reported as the mean ± standard deviation (SD) where n = 3.
| Pgp-OT | Caco-2 assay Permeability ratio |
|||||
|---|---|---|---|---|---|---|
| Compound | Pgp(+)-OT (min) |
Pgp(−)-OT (min) |
Δt (min) | Pgp substrate (Pgp-OT) |
Pgp substrate (Caco-2) |
|
| Domperidone | 23.5 ± 0.7 | 2.0 ± 0.0 | 21.5 | Y | Y | 45.0 ± 27.0 |
| Ritonavir | 13.2 ± 0.3 | 2.1 ± 0.0 | 11.1 | Y | Y | 36.5 ± 12.1 |
| Vinblastine | 14.1 ± 0.5 | 1.6 ± 0.2 | 12.5 | Y | Y | 31.1 ± 18.5 |
| Labetalol | 14.8 ± 0.4 | 1.9 ± 0.1 | 12.9 | Y | Y | 8.9 ± 6.9 |
| Doxorubicin | 10.3 ± 0.2 | 9.7 ± 0.2 | 0.6 | Y | Y | 8.6 ± 5.4 |
| Prazocin | 10.4 ± 0.1 | 3.1 ± 0.4 | 7.3 | Y | Y | 7.1 ± 6.2 |
| Verapamil | 4.1 ± 0.4 | 2.9 ± 0.0 | 1.2 | Y | Y | 3.4 ± 1.0 |
| Loratadine | 7.4 ± 0.9 | 2.1 ± 0.1 | 5.3 | Y | Y | 2.0 ± 0.5 |
| Ketoconazole | 1.7 ± 0.0 | 1.6 ± 0.0 | 0.1 | N | Y | 2.1 ± 1.2 |
| Imipramine | 2.1 ± 0.1 | 1.2 ± 0.2 | 0.9 | Y | N | 1.8 ± 0.7 |
| Nicardipine | 1.9 ± 0.1 | 1.8 ± 0.0 | 0.1 | N | N | 1.4 ± 0.7 |
| Atenolol | 1.6 ± 0.3 | 1.2 ± 0.0 | 0.4 | N | N | 1.4 ± 0.5 |
| Fexofenadine | 1.8 ± 0.0 | 1.8 ± 0.0 | 0.0 | N | N | 1.0 ± 0.9 |
Notes: Y, assessment that the compound is a Pgp substrate; N, assessment that the compound is not a Pgp substrate. For experimental details, see Moaddel et al. 2006).
Binding sites and conformational mobility of the immobilized Pgp
Initial studies with the CMAC(Pgp(+)) columns demonstrated that the immobilized Pgp could be used to determine Kd values for known Pgp substrates and inhibitors. These studies were conducted using a running buffer of Tris-HCl [50 mM, pH 7.4] and under these conditions retention the volumes of [3H]-VBL and [3H]-VER were >30 ml and the retention volume of [3H]-cyclosporin A (CSA) was 7.8 ml (Table III) (Lu et al. 2001a). When 60 nM VBL was added to the running buffer the retention volume of [3H]-VBL was reduced to 11.0, there was no effect on the retention volume of [3H]-VER and the retention of [3H]-CSA increased to 15.7 ml (Table III). The results indicate that VBL and VER do not bind at the same site on the Pgp molecule and that the binding of VBL produced a change in Pgp protein conformation resulting in a cooperative allosteric effect on CSA binding.
Table III.
Effect of the addition of vinblastine (VIN) and adenosine triphosphate (ATP) to the running buffer on the chromatographic retention (ml) of [3H]-VIN, [3H]-verapamil (VER) and [3H]-cyclosporin A (CSA) on the CMAC(Pgp(+)) column, where the control running buffer was Tris-HCl [50 mM, pH 7.4].
| Compound | Retention (ml), control |
Retention (ml), control +100 nM VIN |
Retention volume (ml), control +3 mM ATP |
|---|---|---|---|
| VIN | 32.1 | 9.5 | 8.4 |
| VER | 34.2 | 34.0 | 5.9 |
| CSA | 7.8 | 18.8 | 17.5 |
Note: For experimental details, see Lu et al. (2001a).
The addition of 3 mM ATP to the running buffer reduced the retention volumes of [3H]-VBL and [3H]-VER to 8.4 and 5.9 ml, respectively, and increased the retention volume of [3H]-CSA to 17.5 ml (Table III). These effects were reversed when the ATP was removed from the running buffer. The results indicate that the binding of ATP to the Pgp transporter produced a conformational change in the Pgp transporter that altered the VBL and VER binding sites reducing the affinity of these two compounds, anticooperative allosteric effect, and increased the binding affinity of CSA, cooperative allosteric effect. This is consistent with the data that the binding of ATP to Pgp produces conformational changes in the tertiary structure of the protein (Sonveaux et al. 1996).
Enantioselective Pgp binding of mefloquine enantiomers determined using CMAC
Mefloquine (MQ) is an antimalarial agent administered as a racemic mixture of its (+)-[11R, 2́S], (+)-MQ, and (−)-[11S, 2′R], (−)-MQ, isomers. In an immortalized rat brain capillary endothelial cell line, GPNT cells, (+)-MQ produced an eight-fold greater inhibition of VBL transport than (−)-MQ (Pham et al. 2000). However, the MQ inhibition of VBL transport was not enantioselective in Caco-2 cells.
In order to determine if the observed enantioselective inhibition by MQ was due, in part, to interactions with Pgp, the effect of (+)-MQ and (−)-MQ on the chromatographic retention of VBL and CSA were investigated using the CMAC(Pgp(+)) column (Lu et al. 2001b). When (+)-MQ and (−)-MQ were added to a running buffer that did not contain ATP, both isomers completely suppressed the binding of [3H]-VBL to the CMAC(Pgp(+)) column in an anticooperative allosteric manner, and CSA was not specifically retained on the column. When 3 mM ATP was added to the running buffer, [3H]-CSA was specifically retained on the column, while VBL was not. The addition of (+)-MQ to the running buffer competitively displaced [3H]-CSA while (−)-MQ had no effect on the retention volume of this marker.
The results of this study indicate that observed enantioselectivity of MQ induced VBL transport is due to the binding of MQ to multiple sites on the Pgp molecule. Both (+)-MQ and (−)-MQ bind equally to at least one of these sites, while only (+)-MQ binds to a site at which CSA also binds. The key issue is that the binding of substrates and inhibitors to Pgp is a complex, multi-site process that can be enantioselective at some sites and not at others.
CMAC studies of the stereoselective binding to the hOCT1
The identification and characterization of interactions with the hOCT1 has been primarily accomplished using cellular uptake and trans-stimulation studies (Zhang et al. 1999; Bednarczyk et al. 2003), which are complicated and variable. The development of a CMAC(hOCT1) column has recently been reported as an alternative approach to the determination of hOCT1 binding interactions (Moaddel et al. 2005b). The CMAC(hOCT1) column was created from cellular membrane fragments obtained from a stably transfected MDCK cell line that expresses hOCT1. The Ki values obtained using the CMAC(hOCT(+)) column correlated with previously reported Ki values obtained using cellular uptake techniques, r2=0.9363; p=0.0016 (Moaddel et al. 2005b).
During the initial chromatographic studies, it was determined that R-VER had an 69-fold lower Ki than S-VER. The observed enantioselectivity was consistent with a previous study in which it was demonstrated that the IC50 value associated with (R)-disopyramide inhibition of hOCT1-mediated uptake of TEA was two-fold lower than that of (S)-disopyramide (Zhang et al. 1998) and with a more recent study that demonstrated that the IC50 value associated with (S)-propranolol inhibition of hOCT1-mediated uptake of TEA was 2.75-fold lower than that of (R)-propranolol (Moaddel et al. 2005a). Subsequently the Ki values of eight pairs of enantiomers and three pairs of diastereomers were determined and the results demonstrated that this binding was stereoselective and that the CMAC approach could be used to probe this enantioselectivity (Table IV) (Moaddel et al. 2007b).
Table IV.
Experimentally determined Ki value {Ki (Exp)} and enantioselectivity determined using the CMAC(hOCT1) column, where the stereoselectivity factor (α) is defined as the ratio of the Ki of the compound with the experimentally determined lowest affinity for the hOCT1 divided by the Ki of the compound with the highest experimentally determined affinity.
| Compound | Ki (Exp) (µM) | α |
|---|---|---|
| (R)-verapamil | 0.05 | |
| (S)-verapamil | 3.46 | 69.2 |
| (S)-atenolol | 0.46 | |
| (R)-atenolol | 0.98 | 2.13 |
| (S)-propranolol | 2.85 | |
| (R)-propranolol | 0.95 | 3.04 |
| (1R,2R)-pseudoephedrine | 1.12 | |
| (1S,2S)-pseudoephedrine | 1.71 | 1.53 |
| Quinidine | 6.33 | |
| Quinine | 10.18 | 1.61 |
| (S,S)-fenoterol | 3.73 | |
| (R,R)-fenoterol | 12.6 | 3.38 |
| (S,R)-fenoterol | 6.18 | |
| (R,S)-fenoterol | 13.2 | 2.14 |
| (S)-isoproterenol | 180 | |
| (R)-isoproterenol | 120 | 1.5 |
| (R)-disopyramide | 15.0 | |
| (S)-disopyramide | 30.0 | 2 |
Note: For experimental details, see Moaddel et al. (2007b).
Pharmacophore modelling of the interactions of small molecules with drug transporters
The application of QSAR and pharmacophore modelling techniques to the determination of small molecule interactions with drug transporters have been recently reviewed (Chang and Swaan 2006; Ekins et al. 2007). However, the stereochemical aspects of substrate–transporter and inhibitor–transporter interactions were not addressed in these papers. Indeed, chiral compounds were used in the development of the Pgp models (Ekins et al. 2002a, 2002b) and hOCT1 (Bednarczyk et al. 2003) but the chirality of the compounds was ignored and/or racemic mixtures were used instead of the single enantiomer. The result is that the models were unable to provide an accurate three-dimensional view of the transporter and the binding interactions.
The modelling of Pgp interactions
Five three-dimensional QSAR models describing the binding of various substrates to Pgp have been recently described (Ekins et al. 2002a, 2002b). The necessity for the development of multiple models reflects the fact that Pgp contains multiple binding sites as was observed by the authors (Ekins et al. 2002b) and that these sites are affected by the conformational mobility of the protein (cf. Sonveaux et al. 1996; Lu et al. 2001a).
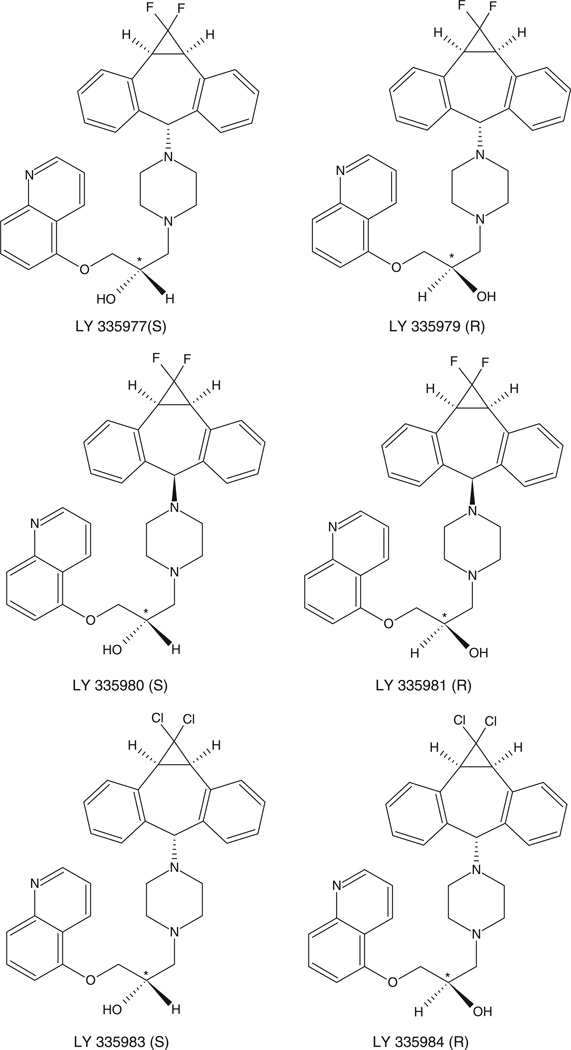
These models were constructed using IC50 and Ki data obtained for digoxin transport, VBL and calcein accumulation and VBL binding (Ekins et al. 2002a) and the inhibition of VER binding (Ekins et al. 2002b). In both of these studies chiral molecules were used in the training and test sets, but the stereochemistry was not considered. It appears that in most cases racemic mixtures of enantiomers were used, except in the case of one series of compounds designated as LY3359-77, -79, -81, -83, -84 and -90 (Figure 4). These compounds represent three sets of enantiomers and displayed significant enantioselectivity in the inhibition of VBL binding (Table V). Although LY335979 was used in the training sets, none of the models was able to predict the activity of this compound or the other LY3359 compounds nor the enantioselectivity (cf. Table V) (Ekins et al. 2002a, 2002b).
Figure 4.
Molecular structures of the enantiomeric LY series of compounds reported in Ekins et al. (2002a, 2002b).
Table V.
Observed and predicted inhibition of vinblastine binding where the predicted values were determined using a binding model created using data from the inhibition of verapamil binding (Ekins et al. 2002b).
In these latter studies, the authors identified at least four distinct features common to the pharmacophores consisting of two hydrophobic domains, a hydrogen bond acceptor region and a ring aromatic region (Ekins et al. 2002b). However, since the derived pharmacophore models did not contain information about the three-dimensional distribution of these sites, which can be obtained by studying the enantioselectivity of the interactions, these models cannot reflect the actual spatial relationships between the proposed binding sites. This opinion is supported by the results of pharmacophore modelling studies involving the hOCT1 described below.
The modelling of stereoselective binding to the hOCT1
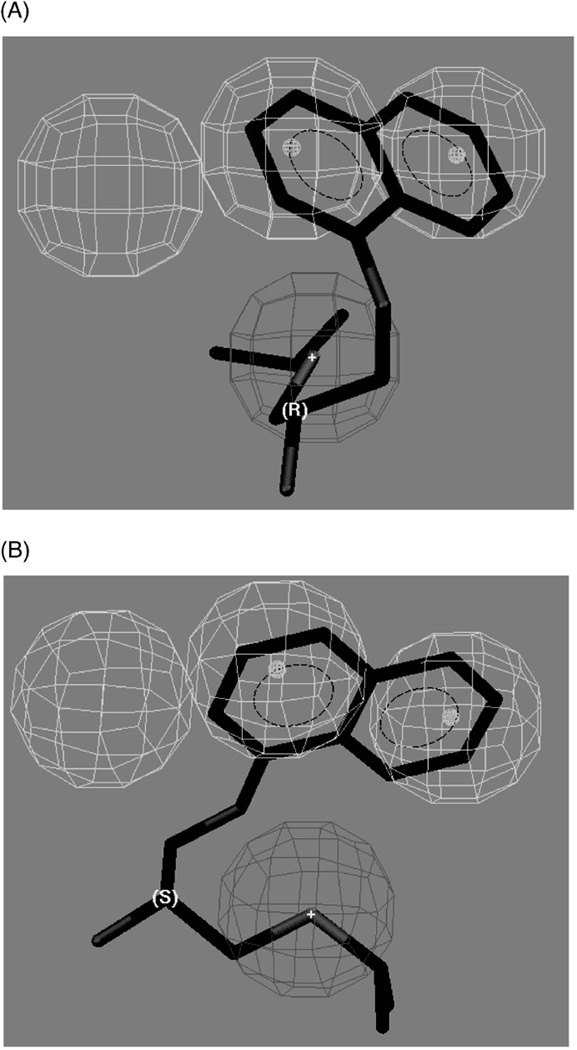
An hOCT1 pharmacophore model containing three hydrophobic pockets and one positive ionizable site has been recently reported (Bednarczyk et al. 2003). The model was generated using a training set composed of structurally diverse organic cations but did not include sets of stereoisomers and, therefore, did not explore the stereochemical relationship between these sites. Consequently, when the model was reproduced it was unable to predict the experimentally observed enantioselectivity of a set of chiral compounds (Table IV). The problem is illustrated by the predicted interactions of R- and S-propranolol when the compounds were placed in the model (Figure 5A and B). Both enantiomers make the same interactions with two of the three hydrophobic pockets and the positive ionizable site, but there are no additional interactions that can differentiate between the spatial configurations at the chiral centre.
Figure 5.
Mapping of R- and S-propranolol to the human organic cation transporter pharmacophore reported by Bednarczyk et al. (2003), where: (A) the mapping of R-propranolol, and (B) the mapping of S-propranolol. Reprinted from Moaddel et al. (2007b).
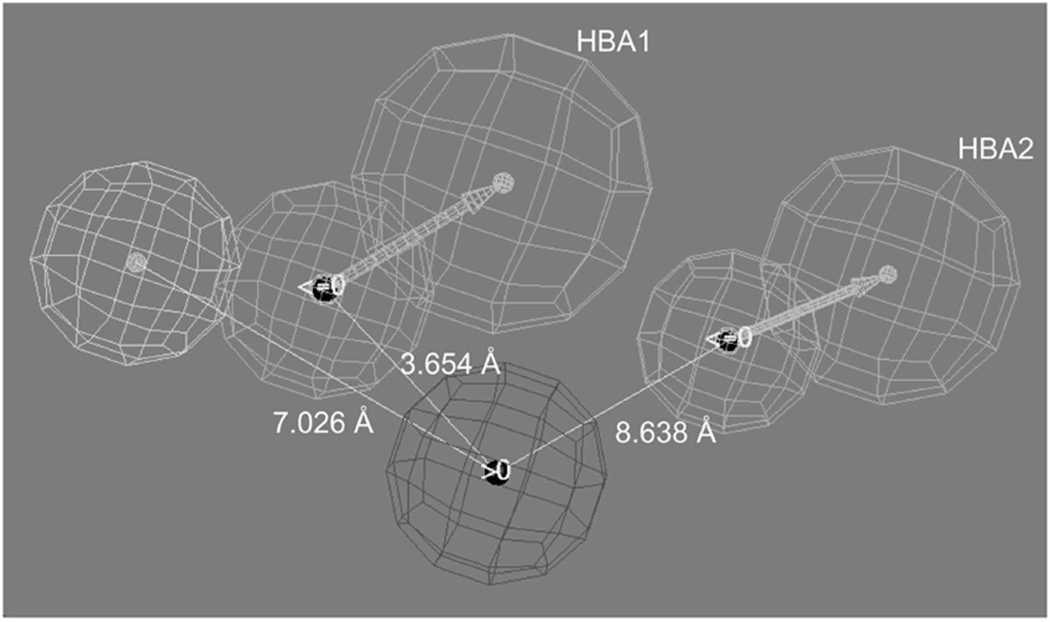
As a result, a new pharmacophore model was developed using a set of 22 compounds that included the eight pairs of enantiomers and three pairs of diastereomers included in Table IV (Moaddel et al. 2007c). The pharmacophore modelling was carried out using Catalyst version 4.11 and HypoGen and was based upon the correlation of chiral structures and experimentally determined Ki values determined using 1-methyl-4-phenylpyridinium (MPP+) as the marker ligand (Moaddel et al. 2007c). The resulting pharmacophore contained a positive-ion interaction site, a hydrophobic interaction site and two hydrogen-bond acceptor sites (Figure 6). Using the centre of the positive-ion interaction site as the origin, the distances to the centre the hydrogen-bond acceptor sites are approximately 3.7 Å (HBA1) and approximately 8.6 Å (HBA2). Using the same approach, the distance to the centre of the hydrophobic site is approximately 7 Å.
Figure 6.
Human organic cation transporter pharmacophore model developed using stereoselective binding data in which the red sphere represents a positive-ion interaction site, the blue sphere represents a hydrophobic interaction site, and the green spheres represent two hydrogen-bond acceptor sites, HBA1 and HBA2. Reprinted from Moaddel et al. (2007b).
It is of interest to note that the model developed in this latter study is similar to the previously reported pharmacophore which contained three hydrophobic sites and a positive ionizable site where the calculated distances between the positive ion site and the three hydrophobic sites were 4.2, 5.1 and 5.3 Å (Bednarczyk et al. 2003). If the three hydrophobic sites postulated in the earlier work are considered to be a single hydrophobic area, then the major differences between the two models are the added hydrogen bonding areas.
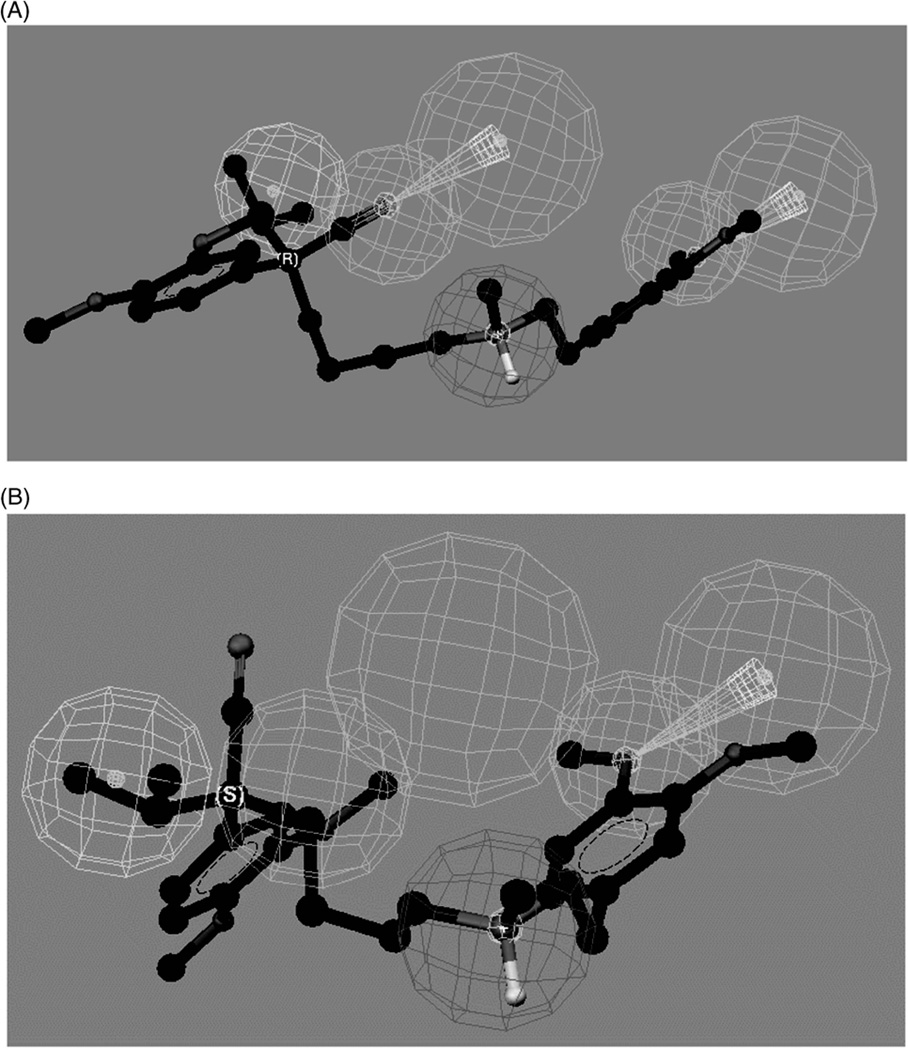
The data indicate that the mapping of compounds to three or more of these sites is related to stereoselective binding to the hOCT1. The fitting of R- and S-VER into the proposed pharmacophore demonstrate the source of the observed enantioselectivity (Figure 7). When R-VER was fitted to the proposed pharmacophore, all the relevant functional groups of the molecule matched the hypothesis (Figure 7A), while S-VER could be mapped to only three of the model feature sites (Figure 7B). The difference, and therefore the source of the enantioselectivity, was the mapping of the nitrile moiety present on the chiral carbon, where the R-configuration permitted this interaction with HBA1, while the S-configuration did not. This analysis would not be possible using the previously reported pharmacophore.
Figure 7.
Fit of verapamil enantiomers in the human organic cation transporter pharmacophore model developed using stereoselective binding data, where: (A) the mapping of (R)-verapamil; and (B) the mapping of (S)-verapamil. Reprinted from Moaddel et al. (2007b).
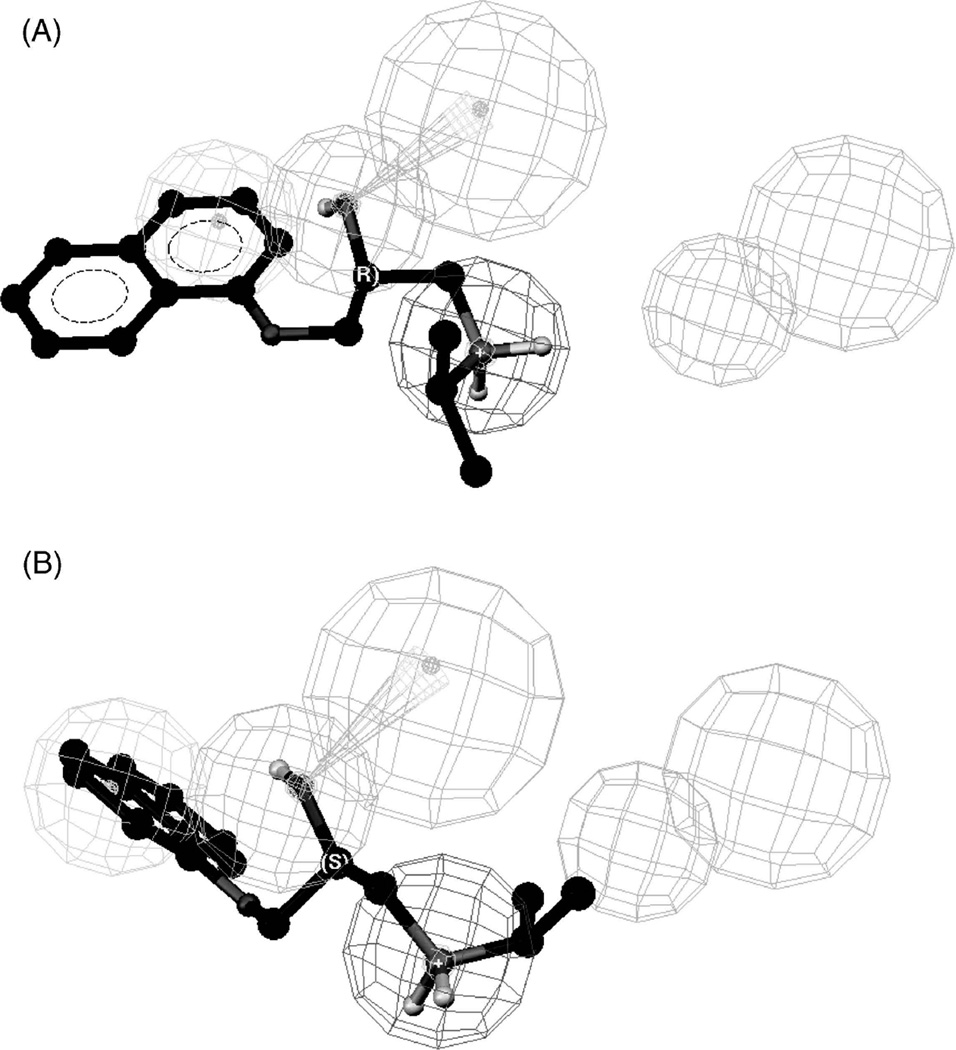
When (R)- and (S)-propranolol were mapped to the pharmacophore, both enantiomers interacted with the positive ion interaction and hydrophobic sites (Figure 8), which was similar to the interaction of these isomers with the previously reported pharmacophore model (Figure 5). However, both enantiomers also bound to a third site HBA1, which was not present in the earlier pharmacophore. While both propranolol enantiomers bind to the same sites on the pharmacophore model, the source of the enantioselectivity is the relative fits which were 6.45 for (R)-propranolol and 6.31 for (S)-propranolol.
Figure 8.
Mapping of R- and S-propranolol to a human organic cation transporter pharmacophore reported by Moaddel et al. (2007b) in which the red sphere represents a positive-ion interaction site, the blue sphere represents a hydrophobic interaction site, and the green spheres represent two hydrogen-bond acceptor sites, HBA1 and HBA2; where: (A) the mapping of R-propranolol, and (B) the mapping of S-propranolol.
The ability of the proposed pharmacophore to identify differences in the relative fit between enantiomeric and diastereomeric pairs suggests that the three-dimensional relationship between the identified interaction sites reflects the spatial distribution of similar binding sites within hOCT1. The fact that these differences in relative fit produced the experimentally observed stereoselectivities is also consistent with previously identified chiral recognition mechanisms (cf. Booth and Wainer 1996). In these mechanisms, each enantiomer of a chiral compound interacts with the same sites on the chiral selector and the differential stabilities of the resulting diastereomeric complexes is a function of the relative conformational energies required to create the complexes.
The model was able to predict experimentally determined Ki values (r2=0.6489, p< 0.0001) and the experimentally determined stereoselectivites of the 13 sets of enantiomers/diastereomers (r2=0.9992, p< 0.0001). The data and the pharmacophore model suggest that the initial binding to the immobilized hOCT1 occurs via an ionic interaction between an ammonium moiety on the ligand and an anionic site on the extracellular portion of the hOCT1, which is followed by the second interaction between a hydrophobic moiety on the ligand and a hydrophobic pocket within the lumen of the hOCT1. Both interactions are necessary for significant binding to occur between the ligand and the hOCT1, and they position the ligand for the enantioselective hydrogen-bonding tertiary interactions.
Conclusions
Quantitative structure–activity relationship (QSAR) and modelling studies of substrate and inhibitor binding to drug transporters have demonstrated that these interactions are complex, multi-site and conformationally sensitive processes. Since these interactions take place in three-dimensional and asymmetric space, ignoring the stereoselectivity associated with small molecule–transporter binding only complicates and reduces the accuracy of the QSAR and modelling analyses. The problems associated with an achiral approach have been demonstrated in the modelling of P-glycoprotein (Pgp) and hOCT1 interactions as, in the case of hOCT1, have the results when chirality is taken into consideration. Since the pioneering work of Louis Pasteur, it has been obvious that stereochemistry is an important dimension in chemistry, biology and pharmacology, and stereoselectivity should become a key aspect of the modelling of small molecule– transporter interactions.
Acknowledgements
This work was supported by funding from the National Institute on Aging Intramural Research Program (I. W. W.) and from the Foundation for Polish Science (FOCUS 4/2006 programme) (K. J.). The authors would also like to thank Sarangan Ravichandran (Advanced Biomedical Computing Center, National Cancer Institute, Frederick/SAIC) for his valuable help in the development of the manuscript.
reference
- Aboul-Enein HY, Wainer IW. The Impact of stereochemistry on drug development and use. New York, NY: Wiley; 1997. [Google Scholar]
- Bednarczyk D, Ekins S, Wikel JH, Wright SH. Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Molecular Pharmacology. 2003;63:489–498. doi: 10.1124/mol.63.3.489. [DOI] [PubMed] [Google Scholar]
- Beigi F, Chakir K, Xiao R-P, Wainer IW. G-protein coupled receptor chromatographic stationary phases II: Ligand-induced conformational mobility in an immobilized β2-adrenergic receptor. Analysis of Chemistry. 2004;76:7187–7194. doi: 10.1021/ac048910c. [DOI] [PubMed] [Google Scholar]
- Booth TD, Wainer IW. Investigation of the enantioselective separations of α-arylcarboxylic acids on an amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase using quantitative structure–enantioselective retention relationships: Identification of a conformationally driven chiral recognition mechanism. Journal of Chromatography A. 1996;737:1657–1169. [Google Scholar]
- Casy AF. Stereochemistry and biological activity. In: Burger A, editor. Medicinal chemistry. 3rd ed. New York, NY: Wiley; 1970. pp. 81–107. [Google Scholar]
- Chang C, Swaan PW. Computational approaches to modeling drug transporters. European Journal of Pharmaceutical Science. 2006;27:411–424. doi: 10.1016/j.ejps.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Dey S, Hafkemeyer P, Pastan I, Gottesman MM. A single amino acid residue contributes to distinct mechanisms of inhibition of the human multidrug transporter by stereoisomers of the dopaine receptor antagonist flupentixol. Biochemistry. 1999;38:6630–6639. doi: 10.1021/bi983038l. [DOI] [PubMed] [Google Scholar]
- Drayer DE. The early history of stereochemistry: From the discovery of molecular asymmetry and the first resolution of a racemate to the asymmetrical chiral carbon of van’t Hoff and Le Bel. In: Wainer IW, editor. Drug stereochemistry: Analytical methods and pharmacology. 2nd ed. New York, NY: Marcel Dekker; 1993. pp. 1–24. [Google Scholar]
- Ekins S, Ecker GF, Chiba P, Swann PW. Future directions for drug transporter modelling. Xenobiotica. 2007;37:1152–1170. doi: 10.1080/00498250701646341. [DOI] [PubMed] [Google Scholar]
- Ekins S, Kim RB, Leake BF, Dantzig AH, Schuetz EG, Lan L, Yasuda K, Shepard RL, Winter MA, Schuetz JD, et al. Three-dimensional quantitative structure–activity relationships of inhibitors of P-glycoprotein. Molecular Pharmacology. 2002a;61:964–973. doi: 10.1124/mol.61.5.964. [DOI] [PubMed] [Google Scholar]
- Ekins S, Kim RB, Leake BF, Dantzig AH, Schuetz EG, Lan L, Yasuda K, Shepard RL, Winter MA, Schuetz JD, et al. Application of three-dimensional quantitative structure–activity relationships of P-glycoprotein inhibitors and substrates. Molecular Pharmacology. 2002b;61:974–981. doi: 10.1124/mol.61.5.974. [DOI] [PubMed] [Google Scholar]
- Fisher GA, Sikic BI. Clinical studies with modulators of multidrug resistance. Hematology and Oncology Clinics of North America. 1995;9:363–382. [PubMed] [Google Scholar]
- Ford JM, Prozialeck WC, Hait WN. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Molecular Pharmacology. 1989;35:105–115. [PubMed] [Google Scholar]
- Gruber A, Peterson C, Reizenstein P. D-verapamil and L-verapamil are equally effective in increasing vincristine accumulation in leukemic cells in vitro. International Journal of Cancer. 1988;41:224–226. doi: 10.1002/ijc.2910410211. [DOI] [PubMed] [Google Scholar]
- Hagglund CL, Lundahl P. Centrifugal and chromatographic analyses of tryptophan and tyrosine uptake by red blood cells and LGUT1 proteoliposomes with permeability estimates and observations on dihydrocytochalasin. British Journal of Biochemical and Biophysical Methods. 2003;55:127–140. doi: 10.1016/s0165-022x(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Hollt V, Kouba M, Dietel M, Vogt G. Stereoisomers of calcium antagonists which differ markedly in their potencies as calcium blockers are equally effective in modulating drug transport by p-glycoprotein. Biochemistry and Pharmacology. 1992;43:2601–2608. doi: 10.1016/0006-2952(92)90149-d. [DOI] [PubMed] [Google Scholar]
- Hooiveld GJEJ, Heegsma J, Van Montfoort JE, Jansen PLM, Meijer DKF, Muller M. Stereoselective transport of hydrophilic quaternary drugs by human MDR1 and rat Mdr1b P-glycoproteins. British Journal of Pharmacology. 2002;135:1685–1694. doi: 10.1038/sj.bjp.0704620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak J, Ravichandran R, Collins JR, Wainer IW. The interaction of non-competitive inhibitors alpha3beta4 nicotinic acetylcholine receptor investigated by affinity chromatography, QSAR and molecular docking. Journal of Medicine and Chemistry. 2004;47:4008–4021. doi: 10.1021/jm0400707. [DOI] [PubMed] [Google Scholar]
- Jozwiak K, Ravichandran S, Collins JR, Moaddel R, Wainer IW. Interaction of noncompetitive inhibitors the α3β2 nicotinic acetylcholine receptor investigated by affinity chromatography and molecular docking. Journal of Medicine and Chemistry. 2007;50:6279–6283. doi: 10.1021/jm070784s. [DOI] [PubMed] [Google Scholar]
- Kimura T, Perry J, Anzai N, Pritchard J, Moaddel R. Development and characterization of immobilized human organic anion transporter based liquid chromatographic stationary phase: hOAT1 and hOAT2. Journal of Chromatography B. 2007;859:267–271. doi: 10.1016/j.jchromb.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Schmitt BM, Gorboulev V. Organic cation transporters. Reviews in Physics, Biochemistry and Pharmacology. 2003;150:36–90. doi: 10.1007/s10254-003-0017-x. [DOI] [PubMed] [Google Scholar]
- Langaniere S. Current regulatory guidelines of stereoisomeric drugs: North American, European and Japanese point of view. In: Aboul-Enein HY, Wainer IW, editors. The impact of stereochemistry on drug development and use. New York, NY: Wiley; 1997. pp. 545–564. [Google Scholar]
- Leonessa F, Green D, Licht T, Wright A, Wingate-Legette K, Lippman J, Gottesman MM, Clarke R. MDA435/LCC6 and MDA435/LCC435MDR1: Ascities models of human breast cancer. British Journal of Cancer. 1996;73:154–161. doi: 10.1038/bjc.1996.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicology and Applied Pharmacology. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Longstreth JA. Verapamil: A chiral challenge to the pharmacokinetic and pharmacodynamic assessment of bioavailability and bioequivalence. In: Wainer IW, editor. Drug stereochemistry: Analytical methods and pharmacology. 2nd ed. New York, NY: Marcel Dekker; 1993. pp. 315–335. [Google Scholar]
- Lu L, Leonessa F, Baynham MT, Clarke R, Gimenez F, Pham YT, Roux F, Wainer IW. The enantioselective binding of mefloquine enantiomers to P-glycoprotein determined using an immobilized P-glycoprotein liquid chromatographic stationary phase. Pharmacology Research. 2001b;18:1327–1329. doi: 10.1023/a:1013098213770. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonessa F, Clarke R, Wainer IW. Competitive and allosteric interactions in ligand binding to P-glycoprotein as observed on an immobilized P-glycoprotein liquid chromatographic stationary phase. Molecular Pharmacology. 2001a;59:62–68. doi: 10.1124/mol.59.1.62. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Calleri E, Massolini G, Frazier CR, Wainer IW. The synthesis and initial characterization of an immobilized purinergic receptor (P2Y1) liquid chromatography stationary phase for online screening. Annals of Biochemistry. 2007b;364:216–218. doi: 10.1016/j.ab.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Jozwiak K, Wainer IW. Allosteric modifiers of neuronal nicotinic acetylcholine receptors: New methods, new opportunities. Medicine Research Review. 2007a;27:713–753. doi: 10.1002/med.20091. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Patel S, Jozwiak K, Yamaguchi R, Ho PC, Wainer IW. Enantioselective binding to the human organic cation transporter-1 (hOCT1) determined using an immobilized hOCT1 liquid chromatographic stationary phase. Chiralty. 2005a;17:501–506. doi: 10.1002/chir.20195. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Ravichandran S, Bighi F, Yamaguchi R, Wainer IW. Pharmacophore modelling of stereoselective binding to the human organic cation transporter (hOCT1) British Journal of Pharmacology. 2007c;151:1305–1314. doi: 10.1038/sj.bjp.0707341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel R, Wainer IW. Development of immobilized membrane-based affinity columns for use in the online characterization of membrane bound proteins and for targeted affinity isolations. Analytica Chimica Acta. 2006;564:97–105. doi: 10.1016/j.aca.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Yamaguchi R, Ho PC, Patel S, Hsu CP, Subrahmanyam V, Wainer IW. Development and characterization of an immobilized human organic cation transporter based liquid chromatographic stationary phase. Journal of Chromatography B. 2005b;818:263–268. doi: 10.1016/j.jchromb.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Hamid R, Patel S, Bullock P, Wainer IW. Identification of P-glycoprotein substrates using open tubular chromatography on an immobilized P-glycoprotein column: Comparison of chromatographic results with Caco-2 permeability. Analytica Chimica Acta. 2006;578:25–30. doi: 10.1016/j.aca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Neuhoff S, Langguth P, Dressler C, Andersson TB, Regardh CG, Spahn-Langguth H. Affinities at the verpamil binding site of MDR1-encoded P-glycoprotein: Drugs and analogs, stereoisomers and metabolites. International Journal of Clinical Pharmacology Therapy. 2000;38:168–179. doi: 10.5414/cpp38168. [DOI] [PubMed] [Google Scholar]
- Pham YT, Regina A, Farinotti R, Couraud PO, Wainer IW, Roux F, Gimenez F. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochimica et Biophysica Acta. 2000;1524:212–219. doi: 10.1016/s0304-4165(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Sonveaux N, Shapiro AB, Goormaghtigh E, Ling V, Ruysschaert JM. Secondary and tertiary structure changes of reconstituted P-glycoprotein. A Fourier transform attenuated total reflection infrared spectroscopy analysis. Journal of Biology and Chemistry. 1996;271:24617–24624. doi: 10.1074/jbc.271.40.24617. [DOI] [PubMed] [Google Scholar]
- Spiegl-Kreinecker S, Buchroithner J, Elbling L, Steiner E, Wurm G, Bodenteich A, Fischer J, Mickshe M, Berger W. Expression and functional activity of the ABC-transporter proteins P-glycoprotein and multidrug-resistance protein 1 in human brain tumor cells and astrocytes. Journal of Neuro-Oncology. 2002;57:27–36. doi: 10.1023/a:1015735815111. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Lukewille U, Kaltenbach S, Moschen I, Broer A, Risler T, Broer S, Lang F. Functional and pharmacological characterization of the human Na+/carnitine cotransporter hOCTN2. American Journal of Physiology and Renal Physiology. 2000;279:F584–F591. doi: 10.1152/ajprenal.2000.279.3.F584. [DOI] [PubMed] [Google Scholar]
- Wainer IW. Drug stereochemistry: Analytical methods and pharmacology. 2nd ed. New York, NY: Marcel Dekker; 1993. [Google Scholar]
- Wainer IW, Marcotte AA. Stereochemical terms and concepts: An overview. In: Wainer IW, editor. Drug stereochemistry: Analytical methods and pharmacology. 2nd ed. New York, NY: Marcel Dekker; 1993. pp. 25–34. [Google Scholar]
- Wigler PW, Patterson FK. Reversal agent inhibition of the multidrug resistance pump in human leukemic lymphoblasts. Biochimica et Biophysica Acta. 1994;1189:1–6. doi: 10.1016/0005-2736(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gorset W, Dresser MJ, Giacomini KM. The interaction of n-tetraalkylammonium compounds with a human organic cation transporter, hOCT1. Journal of Pharmacology and Experimental Therapy. 1999;288:1192–1198. [PubMed] [Google Scholar]
- Zhang L, Schaner ME, Giacomini KM. Functional characterization of an organic cation transporter (hOCT1) in a transiently transfected human cell line (HeLa) Journal of Pharmacology and Experimental Therapy. 1998;286:354–361. [PubMed] [Google Scholar]
- Zhang Y, Leonessa R, Clarke R, Wainer IW. Development of an immobilized P-glycoprotein stationary phase for on-line liquid chromatographic determination of drug binding affinities. Journal of Chromatography B. 2000;739:33–37. doi: 10.1016/s0378-4347(99)00384-9. [DOI] [PubMed] [Google Scholar]