Abstract
Background/Aim:
In the present study, we aimed to compare the potential protective effects of thymoquinone and melatonin by using equivalent dose, on oxidative stress-induced ischemia–reperfusion (IR) injury in the intestinal tissue of rats.
Materials and Methods:
The study was performed using 32 male Wistar–Albino rats (weighing 180–200 g) randomly divided into four groups: Group I, sham group; Group II, IR group; Group III, IR with melatonin group; and Group IV, IR with thymoquinone group. After laparotomy, ischemia and reperfusion were performed for 60 and 120 min, respectively, on all the groups. Intestinal tissue sections were stained using routine histological methods and examined under the light microscope. In addition, the sections were immunohistochemically stained using the TUNEL method for determination of apoptosis. Superoxide dismutase (SOD) activity, glutathione peroxidase (GSH-Px) activity, and malondialdehyde (MDA) levels in the intestinal tissue were also measured.
Results:
The IR group had significantly elevated tissue SOD activity, GSH-Px activity, and MDA levels compared with the sham group. Administration of thymoquinone and melatonin efficiently reduced these increases. Statistically significant number of apoptotic cells was observed in the intestinal tissue of IR group rats compared with the sham group. Treatment with thymoquinone and melatonin markedly reduced the number of apoptotic cells.
Conclusion
The effects of melatonin and thymoquinone on IR-induced oxidative stress in rat intestines were similar. Our findings suggest that melatonin and thymoquinone protect against IR-induced injury to intestinal tissues.
Keywords: Intestine, ischemia–reperfusion injury, melatonin, oxidative stress, thymoquinone
Intestinal ischemia–reperfusion (IR) injury is an important problem in patients with circulatory failure and inadequate perfusion of the intestines due to necrotizing enterocolitis, midgut volvulus, incarcerated hernia, cardiopulmonary bypass, multiple traumas, shock, and sepsis.[1,2] The mortality rate among patients with acute mesenteric ischemia varies between 60 and 100%.[3] Tissue damage largely occurs due to IR injury, rather than due to the initial ischemic insult or the oxygen free radicals, which initiate reperfusion injury. Neutrophils, platelets, endothelial factors, and cytokines are also believed to be important pathogenic mechanisms of intestinal IR injury.[4] Previous experimental studies showed the antioxidant agents’ protective effects on many IR-injured tissues.[5,6]
Nigella sativa, or blackseed, is an annual herb used to treat various diseases. Thymoquinone, the major bioactive constituent of N. sativa seed oil, suppresses oxidative stress and has diverse pharmacological properties, including antioxidative, antidiabetic, analgesic, and anti-inflammatory effects.[7,8,9] Melatonin is a lipophilic and hydrophilic neuropeptide produced by the pineal gland,[10] which easily penetrates all biological membranes.[11] Melatonin scavenges for hydroxyl and peroxyl radicals, and so, functions effectively as an antioxidant and protects tissue from oxidative stress.[12,13] Although several antioxidant enzymes and drugs have been used to decrease intestinal IR injury to date, no research has compared the effectiveness of both melatonin and thymoquinone in intestinal IR injury.
In the present study, we aimed to compare the potential protective effects of melatonin and thymoquinone, which have antioxidant and anti-inflammatory properties, on oxidative stress-induced IR injury in the intestinal tissue of rats.
MATERIALS AND METHODS
Animals
This study was conducted at Gaziosmanpasa University Biomedical Research Unit after permission was obtained from the local ethics committee. The study was conducted on 32 male Wistar–Albino rats (weighing 180–200 g) randomly divided into four groups (n = 8 in each group):
Groups
Sham group (n = 8). Rats were administered 5% ethanol only. Laparotomy was performed, and the superior mesenteric artery (SMA) was identified.
IR group (n = 8). Rats were administered 5% ethanol 30 min before the ischemic period.
Thymoquinone group (n = 8). Laparotomy was performed. Rats were administered 50 mg/kg thymoquinone (TQ, Sigma, USA) intraperitoneally 30 min before the ischemic period
Melatonin group (n = 8). Laparotomy was performed. Rats were administered 50 mg/kg melatonin (Sigma–Aldrich, St Louis, MO, USA) intraperitoneally 30 min before the ischemic period.
After laparotomy, ischemia and reperfusion were performed for 60 and 120 min, respectively, for all the groups except the sham group.
Surgical procedure
Food was removed, and the animals received only water, 12 h prior to the start of the intestinal ischemia reperfusion procedure. The rats were anesthetized with an intramuscular injection of ketamine (50 mg/kg; Ketalar; Parke Davis, Turkey) and xylazine (10 mg/kg; Rompun; Bayer AG, Germany) under aseptic conditions. The abdomen was opened with a midline incision. The intestines were exteriorized, and the superior mesenteric artery (SMA) was occluded with an atraumatic microvascular clamp as described by Terzi et al.[14] Thus, intestinal ischemia was created in 60 minutes. Pale coloring of the intestines and the absence of a pulse indicated ischemia. During ischemia–reperfusion, the abdominal organs were covered with gauze moistened with warm (38°C) saline solution. Following ischemia, the clamp was removed, and 120 min reperfusion was induced. The re-establishment of a pulse and of pink coloring indicated reperfusion of the intestines. At the end of reperfusion, the ileum segment was removed, and the animals were euthanized by exsanguination.
Sample collection
At the end of reperfusion, all rats were sacrificed, and their small intestines were removed. The intestines were divided into two parts. The first part was used for immunohistochemical and histopathological evaluations. The second part was used to evaluate antioxidant enzyme activity and malondialdehyde (MDA) levels.
Biochemical analysis of intestinal tissues
Homogenate, supernatant, and extracted samples were prepared after weighing the tissue. Protein assays were developed using the method described by Lowry et al.[15] The tissue homogenate was used to determine MDA levels, and the supernatant was used for analysis of GSH-Px. Superoxide dismutase activity was assessed in the ethanol phase of the supernatant from the intestinal tissue after 1.0 mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume supernatant and centrifuged. The following determinations were made on the samples using commercial chemicals supplied by Sigma (St. Louis, USA):
-
Determination of SOD levels
Total (Cu/Zn and Mn) SOD activity was determined according to the method described by Sun et al.[16] The method is based on the inhibition of nitroblue tetrazolium (NBT) reduction, using the xanthine–xanthine oxidase system as a superoxide generator. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the NBT reduction rate. SOD activity in the intestinal tissues was expressed as units per gram protein (U/g protein).
-
Determination of GSH-Px activity
Glutathione peroxidase activity was measured using the method described by Paglia and Valentine.[17] The enzymatic reaction in the tube, which contained NADPH, reduced glutathione, sodium azide, and glutathione reductase, was initiated by addition of H2O2, and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was expressed as U/g protein.
-
Determination of MDA levels
MDA levels were determined based on the reaction of MDA with thiobarbituric acid (TBA) at 90°C–100°C.[18] MDA and MDA-like substances react with TBA to produce a pink pigment with an absorption maximum at 532 nm. The results were expressed as nanomoles per gram protein.
Microscopic examination of intestinal tissue
Tissues from the small intestines were excised and placed in 10% formaldehyde. After routine histological examination, the tissues were embedded in paraffin. Five micrometer thick sections were taken from the paraffin-embedded tissues and stained using the hematoxylin and eosin (H and E) method. Histologic evaluation was performed by two observers, blinded to the specimen source, on two separate occasions. The entire cross-section for each specimen was visually split into quarters and graded 1–5, using the Philip M. Tatum, 2010, modification of the original grading system. Grade 0 represented normal mucosal villi and no injury. Grade 1 represented subepithelial edema and partial separation of apical cells. Grade 2 represented the development of mucosal slough at the villous tips. Grade 3 represented the progression of slough to the base of the villi. Grade 4 represented denuded villi with partial mucosal necrosis of the lamina propria. Grade 5 represented digestion and disintegration of the lamina propria in villi and the presence of total mucosal necrosis. The histologic injury grade for each quarter was averaged to represent the grade for each section, and grades for each of the five sections were averaged in each animal. Stained specimens were studied under an Olympus BH2 light microscope.
TUNEL assay
Apoptotic cells were detected using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Cat no: S7101, USA) according to the manufacturer's instructions. Sections (5 μm) taken from the paraffin blocks were placed onto polylysine-coated slides, deparaffinized using xylene, dehydrated with a series of alcohol rinses, and then washed with phosphate-buffered saline (PBS). Tissues were incubated with a proteinase K solution (0.05%), and then with 3% hydrogen peroxide for 5 min to prevent endogenous peroxidase activity. After washing with PBS, the tissues were incubated with Equilibration Buffer for 6 min and in working solution (% 70 μL Reaction Buffer + 30% TdT Enzyme) at 37°C under moist conditions for 60 min. Tissues were then incubated in stop/wash buffer for 10 min and incubated in Anti-Digoxigenin-Peroxidase for 30 min. Apoptotic cells were observed using the Diaminobenzidine substrate. Cross-sections contrast-stained with methyl green were sealed using a proper covering solution. Mamma tissue was used as a positive control. PBS was used instead of the TdT enzyme in the negative control tissue. Preparations were observed and evaluated using a research microscope (Olympus BH2 light microscope) and then photographed.
To evaluate the TUNEL staining, after staining with methyl green, cells with green nuclei were considered normal, whereas cells with brown nuclei were considered apoptotic. Apoptotic (TUNEL positive) cells were counted and statistically evaluated.
This analysis was performed in at least eight areas of each small intestine section (two sections/animal), and the sections were examined at 400× magnification.
Statistical analysis
All groups were compared based on apoptotic index using the Kruskal–Wallis test. P < 0.01 was considered statistically significant. Differences among groups were determined using the Mann–Whitney U test and the independent samples t-test. For biochemical analysis, the distribution of the groups was analyzed with the one sample Kolmogorov–Smirnov test. Since the biochemical results were normally distributed, comparison of the results from the different experimental groups was performed using one-way ANOVA followed by an independent samples t-test. All analyses were completed using SPSS version 15.0 software (SPSS Inc. Chicago, IL, USA). Results are presented as mean ± SD. A P value less than 0.05 was considered statistically significant.
RESULTS
Biochemical findings
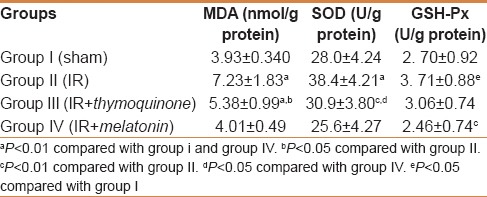
Tissue levels of oxidative stress markers and antioxidant enzymes in each group are shown in Table 1. MDA was measured as a biomarker of lipid peroxidation. Higher tissue MDA levels were found in the IR group compared with the sham group (P = 0.001). Thymoquinone and melatonin administration reduced tissue MDA production. This reduction was more pronounced in the melatonin group (P = 0.024 and P = 0.001, respectively, compared with the IR group), with levels close to those of the sham-operated control group. Otherwise, IR caused significant elevation of tissue SOD activity (P < 0.0001) compared with the sham group. Conversely, there was no significant tissue SOD elevation in the thymoquinone- and melatonin-administered groups compared with the sham group. Tissue GSH-Px levels were also found to be significantly elevated in the IR group (P = 0.043), but no difference was found in the thymoquinone- and melatonin-administered groups compared with the sham group.
Table 1.
Tissue MDA, SOD, and GSH-Px levels in each group
Macroscopic findings
Except for the sham group, all IR animals had obvious edema, dilatation, and venous congestion of the intestinal loops. Sham group intestines showed no evidence of lesions.
Histopathologic findings
To assess the degree of ischemia-reperfusion intestinal injury, and the effects of melatonin and thymoquinone on this injury, the small intestines from each group were analyzed using H&E staining [Figure 1a-d]. Figure 1e represents the histological findings used for injury scoring (on a scale of 0-5). The mean histological injury scores were significantly higher in the ischemic groups compared to the sham-operated and treatment groups. Additionally, these data show that rats treated with melatonin and thymoquinone after IR injury had lower injury scores [Figure 1e].
Figure 1.
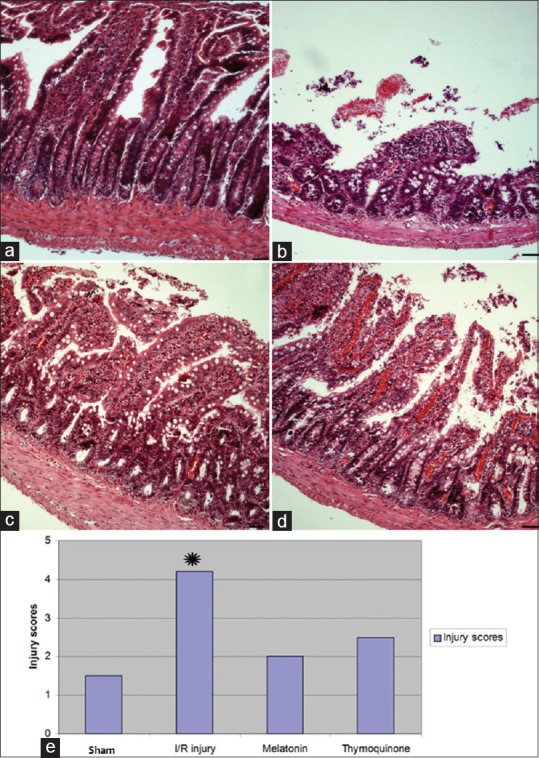
Representative photomicrographs of histology of small bowel demonstrated by hematoxylin and eosin staining. (a) Sham-operated animal tissue. (b) Ischemia–reperfusion (IR) injury with saline perfusion. (c) IR injury followed by melatonin. (d) IR injury followed by thymoquinone. (e) Histologic injury scores after IR injury. The scoring range was 0–5 using a modified scoring system reported by Philip M. Tatum (2010) based on depth of injury. The median score for each strain was analyzed by analysis of variance. Scale bars: 50 μm. *P < 0.05
TUNEL findings
TUNEL staining of the intestinal tissues of sham group revealed few TUNEL-positive cells [Figure 2]. Higher numbers of apoptotic cells were observed in the intestinal tissues of the IR group compared to the control group. Treatment with thymoquinone and melatonin markedly reduced the number of apoptotic cells [Table 2].
Figure 2.
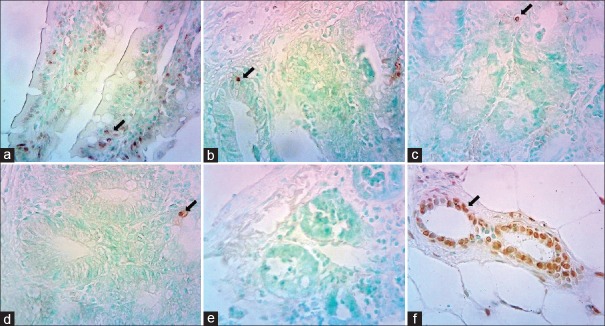
Representative photomicrographs of histology of small bowel demonstrated by TUNEL staining. (a) Ischemia–reperfusion (IR) injury with saline perfusion. (b) IR injury followed by melatonin. (c) IR injury followed by thymoquinone. (d) Sham- operated animal tissue. (e) Negative control tissue. (f) Positive control of mamma tissue
Table 2.
Apoptotic index of each group

DISCUSSION
Oxidative stress has been described as a disturbance in the equilibrium status of pro-oxidant/antioxidant systems in undamaged cells.[5] Generation of large amounts of free radicals impairs this equilibration between oxidants and antioxidants. Reperfusion in the ischemic tissue also triggers a chain of events, which, in turn, cause tissue damage; ischemia-induced tissue damage is milder than the damage occurring after reperfusion.[19,20] In physiological conditions, the activity of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and others, increases.[21] Significantly higher antioxidant activity and malondialdehyde (MDA) levels were reported by some researcher in the intestines of IR-injured rats compared to the sham control group.[22,23] On the contrary, some researchers have reported lower levels of antioxidant enzymes depending on the consumption in IR injury of intestine tissue.[14,19] Previous studies have reported increased, decreased, or unchanged antioxidant enzyme levels after ischemia-reperfusion injury; however, MDA levels increased in all studies.
Many experimental studies have evaluated the protective and healing effects of pharmacological agents, such as resveratrol,[24] melatonin,[19] and Cape,[22,23] on intestinal ischemia-reperfusion injury. Improved antioxidant capacity and MDA levels were demonstrated in Nigella sativa-[14] and melatonin[25]- administered groups of rats with IR-injured ileum tissue.
In our study, we observed increased antioxidant enzyme (SOD, GSH-Px) activity and elevated levels of lipid peroxidation markers (MDA) in the intestinal tissues of ischemic rats. Increased SOD and GSH-Px enzyme activity in the intestinal tissues may reflect preceding cellular oxidative stress or may be involved in compensatory mechanisms. Disorganization of cell structure and function may be observed due to lipid peroxidation caused by reactive oxygen species. In contrast, melatonin and thymoquinone led to a decrease in MDA, SOD, and GSH-Px levels in IR-injured intestinal tissue. It has been suggested that the protective effects of melatonin and thymoquinone depend mainly on antioxidant properties.
In this study, melatonin seems partially more effective more than TQ. Melatonin is a direct free radical scavenger and indirect antioxidant and works with other antioxidants to improve the overall effectiveness of each antioxidant.[11,12] This activity may be due to this feature of melatonin.
Intestinal IR causes an acute inflammatory response and neutrophil infiltration that augments ischemic injury by releasing cytotoxic reactive oxygen species (ROS) and proteolytic enzymes.[26] ROS cause apoptosis and necrosis resulting in lipid peroxidation and sequentially structural and metabolic alterations.[27] Apoptosis is a form of programmed cell death without lysis or damage to neighboring cells.[28] It has been demonstrated that occlusion of the SMA followed by reperfusion can cause apoptosis in rat intestinal tissue.[29,30] Kojima et al.[6] claimed that oxidative stress after ischemia reperfusion plays an important role in induction of apoptosis in the intestinal mucosa. They further determined that antioxidative agents diminish increases in intestinal apoptosis. Pergel et al.[31] demonstrated that antiapoptotic treatment could be effective in preventing intestinal IR injury. Previous studies have demonstrated histopathological improvement in injured intestinal rat tissue treated with antioxidants such as Aminoguanidine,[32] Ebselen,[33] CAPE,[22] and Leflunomide.[23] Al Mofleh et al. histopathologically demonstrated that black seed significantly prevented gastric ulcer formation induced by necrotizing agents.[9]
In the present study, histological examination of intestinal tissues from IR-injured rats demonstrated loss of intestinal villi, edema, and increased numbers of TUNEL-positive (apoptotic) cells. In contrast, treatment with thymoquinone and melatonin markedly reduced the number of apoptotic cells and improved pathology.
Our study demonstrated that TQ exerts antioxidant effects similar to melatonin that is known to be among the most powerful antioxidants. However, melatonin is a hormone that is not suitable for continuous use, but in many areas N. sativa (TQ) is used continuously. In Turkey, India, Arabia, and many Middle Eastern countries N. sativa is widely used. People in these areas regularly use N. sativa (black seed) for the treatment of numerous diseases. Due to its antioxidant properties, we believe that N. sativa, when regularly used, could have protective effects in ischemic events such as mesenteric ischemia and testis torsion. However, some studies have reported that thymoquinone may have a therapeutic effect against cancer.[34,35]
As a result of this study, we propose that treatment with TQ may be a novel approach to therapy for mesenteric ischemia. However, further studies are required to elucidate the mechanisms by which TQ offers these protective functions. Additionally the protective effect of combination of melatonin and thymoquinone on mesenteric ischemia can be planned in future studies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sizlan A, Guven A, Uysal B, Yanarates O, Atim A, Oztas E, et al. Proanthocyanidin protects intestine and remote organs against mesenteric ischemia/reperfusion injury. World J Surg. 2009;33:1384–91. doi: 10.1007/s00268-009-0011-9. [DOI] [PubMed] [Google Scholar]
- 2.Al Salamah SM, El Keyali AY. Ileo-caecal volvulus post-cesarean section: A case report. Saudi J Gastroenterol. 2000;6:163–4. [PubMed] [Google Scholar]
- 3.Chang JB, Stein TA. Mesenteric ischemia: Acute and chronic. Ann Vasc Surg. 2003;17:323–8. doi: 10.1007/s10016-001-0249-7. [DOI] [PubMed] [Google Scholar]
- 4.Daniel RA, Cardoso VK, Gois E, Jr, Parra RS, Garcia SB, Rocha JJ, et al. Effect of hyperbaric oxygen therapy on the intestinal ischemia reperfusion injury. Acta Cir Bras. 2011;26:463–9. doi: 10.1590/s0102-86502011000600010. [DOI] [PubMed] [Google Scholar]
- 5.Ozyurt H, Ozyurt B, Koca K, Ozgocmen S. Caffeic acid phenethyl ester (CAPE) protects rat skeletal muscle against ischemia-reperfusion-induced oxidative stress. Vascul Pharmacol. 2007;47:108–12. doi: 10.1016/j.vph.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Iwakiri R, Wu B, Fujise T, Watanabe K, Lin T, et al. Effects of antioxidative agents on apoptosis induced by ischaemia-reperfusion in rat intestinal mucosa. Aliment Pharmacol Ther. 2003;18(Suppl 1):139–45. doi: 10.1046/j.1365-2036.18.s1.16.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilhotra N, Dhingra D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol Rep. 2011;63:660–9. doi: 10.1016/s1734-1140(11)70577-1. [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan C, Pari L. Thymoquinone ameliorates chemical induced oxidative stress and beta-cell damage in experimental hyperglycemic rats. Chem Biol Interact. 2011;190:148–54. doi: 10.1016/j.cbi.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Al Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Al-Yahya MA, Rafatullah S, et al. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J Gastroenterol. 2008;14:128–34. doi: 10.4103/1319-3767.41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy BA, Martin AM, Furney P, Elliott JA. Absence of a serum melatonin rhythm under acutely extended darkness in the horse. J Circadian Rhythms. 2011;9:3. doi: 10.1186/1740-3391-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y. Melatonin reduces oxidative damage of neural lipids and proteins in senescence-accelerated mouse. Neurobiol Aging. 2002;23:639–44. doi: 10.1016/s0197-4580(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 12.Tas U, Ogeturk M, Meydan S, Kus I, Kuloglu T, Ilhan N, et al. Hepatotoxic activity of toluene inhalation and protective role of melatonin. Toxicol Ind Health. 2011;27:465–73. doi: 10.1177/0748233710389853. [DOI] [PubMed] [Google Scholar]
- 13.Guclu M, Demirogullari B, Barun S, Ozen IO, Karakus SC, Poyraz A, et al. The effects of melatonin on intestinal adaptation in a rat model of short bowel syndrome. Eur J Pediatr Surg. 2014;24:150–7. doi: 10.1055/s-0033-1343081. [DOI] [PubMed] [Google Scholar]
- 14.Terzi A, Coban S, Yildiz F, Ates M, Bitiren M, Taskin A, et al. Protective effects of Nigella sativa on intestinal ischemia-reperfusion injury in rats. J Invest Surg. 2010;23:21–7. doi: 10.3109/08941930903469375. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 17.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 18.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 19.Ates B, Yilmaz I, Geckil H, Iraz M, Birincioglu M, Fiskin K. Protective role of melatonin given either before ischemia or prior to reperfusion on intestinal ischemia-reperfusion damage. J Pineal Res. 2004;37:149–52. doi: 10.1111/j.1600-079X.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 20.Kazez A, Demirbag M, Ustundag B, Ozercan IH, Sağlam M. The role of melatonin in prevention of intestinal ischemia-reperfusion injury in rats. J Pediatr Surg. 2000;35:1444–8. doi: 10.1053/jpsu.2000.16410. [DOI] [PubMed] [Google Scholar]
- 21.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009;2:259–69. doi: 10.4161/oxim.2.5.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koltuksuz U, Ozen S, Uz E, Aydinç M, Karaman A, Gültek A, et al. Caffeic acid phenethyl ester prevents intestinal reperfusion injury in rats. J Pediatr Surg. 1999;34:1458–62. doi: 10.1016/s0022-3468(99)90103-3. [DOI] [PubMed] [Google Scholar]
- 23.Yildiz Y, Serter M, Ek RO, Ergin K, Cecen S, Demir EM, et al. Protective effects of caffeic acid phenethyl ester on intestinal ischemia-reperfusion injury. Dig Dis Sci. 2009;54:738–44. doi: 10.1007/s10620-008-0405-9. [DOI] [PubMed] [Google Scholar]
- 24.Ozkan OV, Yuzbasioglu MF, Ciralik H, Kurutas EB, Yonden Z, Aydin M, et al. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J Exp Med. 2009;218:251–8. doi: 10.1620/tjem.218.251. [DOI] [PubMed] [Google Scholar]
- 25.Gokce A, Oktar S, Koc A, Gonenci R, Yalcinkaya F, Yonden Z, et al. Protective effect of thymoquinone in experimental testicular torsion. Urol Int. 2010;85:461–5. doi: 10.1159/000318890. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi A, Tomomasa T, Kaneko H, Watanabe T, Tabata M, Morikawa H, et al. Intestinal motility in an in vivo rat model of intestinal ischemia-reperfusion with special reference to the effects of nitric oxide on the motility changes. J Pediatr Gastroenterol Nutr. 2001;33:283–8. doi: 10.1097/00005176-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 27.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 28.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–37. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 29.Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol. 1998;274:G270–6. doi: 10.1152/ajpgi.1998.274.2.G270. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–7. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pergel A, Kanter M, Yucel AF, Aydin I, Erboga M, Guzel A. Anti-inflammatory and antioxidant effects of infliximab in a rat model of intestinal ischemia/reperfusion injury. Toxicol Ind Health. 2012;28:923–32. doi: 10.1177/0748233711427056. [DOI] [PubMed] [Google Scholar]
- 32.Soliman MM. Effects of aminoguanidine, a potent nitric oxide synthase inhibitor, on myocardial and organ structure in a rat model of hemorrhagic shock. J Emerg Trauma Shock. 2014;7:190–5. doi: 10.4103/0974-2700.136864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guven A, Tunc T, Topal T, Kul M, Korkmaz A, Gundogdu G, et al. Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–35. doi: 10.1007/s00595-007-3752-9. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee S, Azmi AS, Padhye S, Singh MW, Baruah JB, Philip PA, et al. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer. Pharm Res. 2010;27:1146–58. doi: 10.1007/s11095-010-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee S, Kaseb AO, Wang Z, Kong D, Mohammad M, Padhye S, et al. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009;69:5575–83. doi: 10.1158/0008-5472.CAN-08-4235. [DOI] [PubMed] [Google Scholar]


