Abstract
Background/Aims:
Tenofovir disoproxil fumarate (TDF) is a nucleotide analog used in the treatment of chronic hepatitis B (CHB) infection. This study evaluated the efficacy of TDF in achieving undetectable HBV DNA after 48 weeks of treatment in a Saudi cohort of CHB patients.
Patients and Methods:
This retrospective study included patients treated at a tertiary care center in Saudi Arabia from January 2009 to December 2012. Of the 68 eligible patients, 51 were treatment naïve and 17 were treatment-refractory. Twenty-three patients tested positive for HBeAg. The remaining 45 patients were HBeAg-negative.
Results:
The mean HBV DNA viral load decreased from 95 million IU/mL at baseline to 263 IU/mL after 48 weeks of treatment (P < 0.001). Overall, 62% of patients achieved a complete virological response (CVR) and 37% a partial virological response (PVR). Respective CVR and PVR rates according to subgroup were: HBeAg-positive (21.7% and 78.3%) and HBeAg-negative (84.4% and 15.6%). At 48 weeks, HBV DNA was undetectable in 66.7% of treatment-naïve and 53% of treatment-refractory patients (P = 0.3). Seroconversion occurred in 13 (57%) of HBeAg-positive patients. Two (3%) of the HBeAg-negative patients lost HBsAg at follow up. Mean alanine aminotransferase decreased significantly from 134 U/L before treatment to 37 U/L at 48 weeks (P < 0.001). Significant adverse events were not encountered during the study period.
Conclusion:
Forty-eight weeks of treatment with TDF reduced HBV DNA to undetectable levels in more than half of our patients regardless of whether they were treatment-naïve or refractory. HBeAg-negative (vs positive) patients experienced a better response rate.
Keywords: Hepatitis B, Saudi population, tenofovir
An estimated 400 million people worldwide are currently infected with the hepatitis B virus (HBV), and approximately 600,000 die annually from this disease.[1,2] Left untreated, chronic hepatitis B (CHB) accompanied by high DNA viral load associates with an increased risk of cirrhosis and hepatocellular carcinoma (HCC).[3,4,5] In Saudi Arabia, HBV was once considered endemic, where infection was acquired mainly through horizontal transmission in early life. The first large-scale community-based epidemiologic study conducted in Saudi children showed a hepatitis B surface antigen (HBsAg) seroprevalence of 6.7%. The introduction of a mass vaccination program against HBV in 1989 has resulted in an almost complete absence of HBsAg or antihepatitis B core antigen (HBc) detection among those born after 1989.[6]
Achieving sustained suppression of HBV replication and remission of the ongoing liver disease are two important therapeutic goals for antiviral agents in the prevention of cirrhosis and HCC. Currently, seven drugs are licensed for the treatment of CHB, including standard interferon, pegylated interferon-alfa,[7,8] lamivudine (3TC),[9] adefovir (ADF), telbivudine (LdT), entecavir (ETV), and tenofovir disoproxil fumarate (TDF). TDF is an oral nucleotide analog that blocks HBV DNA synthesis activity by competitively binding to the active site of the viral polymerase. In 2008, TDF was approved for the treatment of CHB in the United States and approved for the treatment of HBV infection in Europe, Canada, Australia, and the United States. Approvals were granted based on results from two prospective randomized double-blind trials that showed superior efficacy of TDF 300 mg/day compared with adefovir dipivoxil 10 mg/day in suppressing viral replication in both HBeAg-positive and HBeAg-negative patients.[10]
There are no data from Saudi Arabia addressing the efficacy of TDF in a Saudi population. We aimed to determine the rate of achieving a CVR after 48 weeks of TDF in a cohort of Saudi patients with CHB.
PATIENTS AND METHODS
Our study retrospectively evaluated CHB patients treated with TDF (300 mg/day) in a Saudi tertiary care center (Department of Medicine, King Faisal Specialist Hospital and Research Centre) from January 2009 to December 2012. Sixty-eight patients deemed eligible for analysis were diagnosed with CHB based on the following criteria: HBsAg-positive >6 months, serum HBV DNA >2000 IU/mL, persistent or intermittent elevation in alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels and/or histologic analysis showing chronic hepatitis with moderate or severe necroinflammation. We excluded patients younger than 14 years, and those with co-infection (HCV or HIV), hepatocellular carcinoma, a decompensated liver cirrhosis or viral load <2000 IU/mL. Refractory patients were those defined as having persistent viremia despite previous treatment with any antiviral agents for a minimum of 6 months before shifting to TDF. Treatment-naïve patients did not receive any antiviral treatment prior to starting TDF.
The primary outcome was complete viral response (CVR) defined as undetectable HBV DNA by real-time PCR within 48 weeks of start of TDF therapy. A secondary objective was to find the predictors of virologic response.[11,12] Partial virologic response (PVR) was defined as a decrease in HBV DNA of more than 1 log10 IU/mL, but detectable HBV DNA by real-time PCR technology. Primary nonresponse was defined as <1 log10 IU/mL decrease in HBV DNA from baseline to 3 months of treatment. A confirmed rise in HBV DNA while on antiviral therapy indicated virologic breakthrough. The real-time PCR assay (Abbott Diagnostic, M2000RT) used to quantify serum HBV DNA provided a detection limit (analytical measurement range) from 15 to 1 000 000 000 IU/mL, where one IU/mL of HBV DNA equals 3.41 copies/mL.
Patient statements of compliance with medication, regular clinic visits, and regular pharmacy encounters served as the source of assessment for treatment compliance.
Statistical analysis
Relevant patient data were extracted from both paper and ICIS electronic medical records and then transferred to SPSS version 16 (Chicago, IL, USA) for analysis. Descriptive statistics were used to summarize continuous variables. Categorical variables were expressed as proportions while continuous variables were expressed as medians and/or means. The Pearson's Chi-square test was used to compare categorical variables, and t-test was used to compare continuous variables. A two-tailed P value of < 0.05 was considered statistically significant.
RESULTS
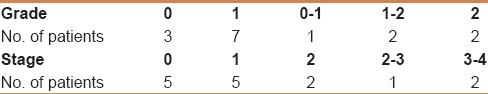
Table 1 summarizes the baseline characteristics of the study population of which 25 (36.8%) were male and 23 (33.8%) were HBeAg-positive. Genotype D, the most common genotype in our study population was present in 32 patients. The features of cirrhosis seen in 6 patients were based on radiologic evidence. Fifty-one patients (75%) were treatment naïve, whereas 17 (25%) had prior resistance or failure to therapy. Antiviral agents used previously in the treatment of refractory patients included interferon (4 patients), ETV (2 patients), 3TC (8 patients), ADF (6 patients), and a combination of 3TC and ADF (5 patients). HBV DNA sequencing detected tyrosine- methionine-aspartate-aspartate (YMDD) mutations in two of 17 tested patients. Of 15 patients who underwent liver biopsy; 11 patients had mild activity (grade 0–1) and two patients had stage 3–4 fibrosis. The degree of inflammation and fibrosis per tissue sample was graded according to the Metavir scoring system [Table 2]. Histopathological assessment (as per Metavir scoring system) verified mild grade necroinflammatory activity in 10 patients, a moderate degree of necroinflammatory activity in 4 patients, and severe necroinflammatory activity in one patient.
Table 1.
Baseline clinical characteristics of patients treated with tenofovir (n=68)
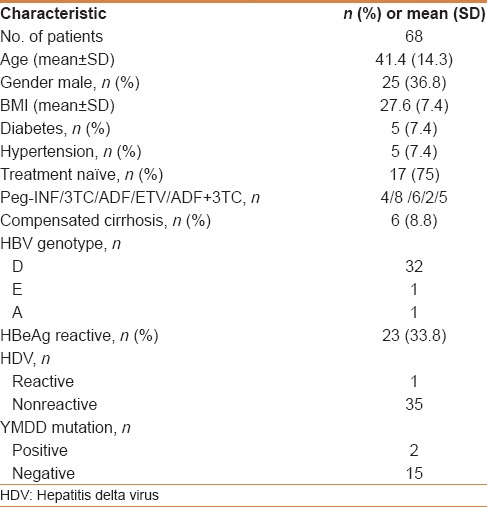
Table 2.
The degree of inflammation and fibrosis per tissue sample as per the Metavir scoring system
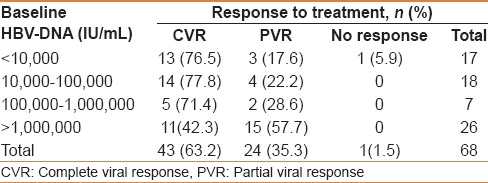
The mean HBV DNA viral load decreased from 95 million IU/mL at baseline to 263 IU/mL at 48 weeks of treatment with TDF. Application of the Student's paired t-test verified a significant mean difference between HBV DNA at baseline and HBV DNA at the end of 48 weeks (P = 0.0006). The mean log10 IU/mL HBV-DNA was 7.98 before initiating TDF and decreased to 2.42 after 48 weeks of treatment (P < 0.001). Overall, 62% of patients achieved a CVR, 37% achieved a PVR and 1% had no response [Figure 1]. Among those characterized as HBeAg positive, 5 (21.7%) and 18 (78.3%) patients achieved CVR and PVR, respectively, as compared to 38 (84.4%) and 6 (13.3%) patients in the HBeAg-negative subgroup (P < 0.0001). The binary logistic regression analysis determined HBeAg-negative status as a predictor of undetectable HBV DNA at 48 weeks (P < 0.01). The patient labeled as a “nonresponder” was HBeAg negative and was noncompliant to TDF therapy. There was no virologic breakthrough detected. Applying the Fisher's exact test determined a significant association between HBV DNA level at baseline and CVR (P = 0.0312) [Table 3].
Figure 1.
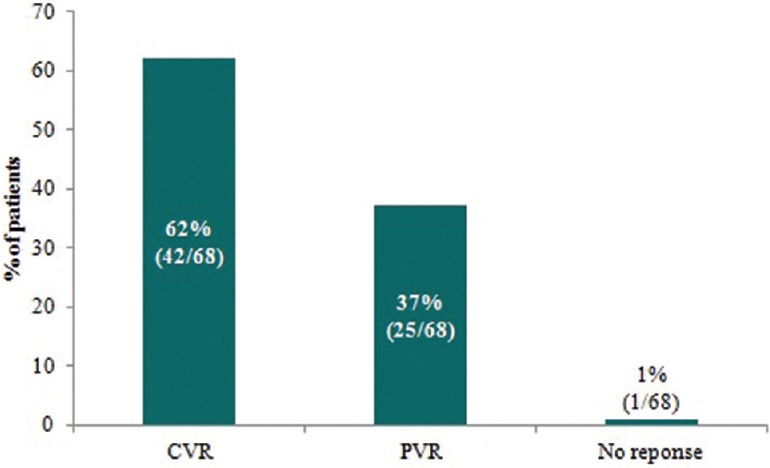
Proportion of patients overall achieving a complete virological response (CVR), partial virological response (PVR), or no response to TDF after 48 weeks of treatment
Table 3.
HBV DNA level at baseline and after 48 weeks of treatment
After 48 weeks, the HBV DNA was undetectable in 34/51 (66.7%) of treatment-naïve patients and in 9/17 (53%) of treatment-refractory patients (P = 0.3) [Figure 2]. Undetectable HBV DNA at 48 weeks occurred in 3 out of 8 patients previously treated with 3TC monotherapy, 1 out of 6 patients treated with ADF monotherapy, 4 out of 5 patients treated with a combination of 3TC and ADF and neither of the 2 patients with prior ETV monotherapy. Three (13%) of HBeAg-positive patients converted to HBeAg-negative status and two HBeAg-negative patients (3%), both treatment-naïve, lost their HBsAg and developed anti-HBs antibodies.
Figure 2.
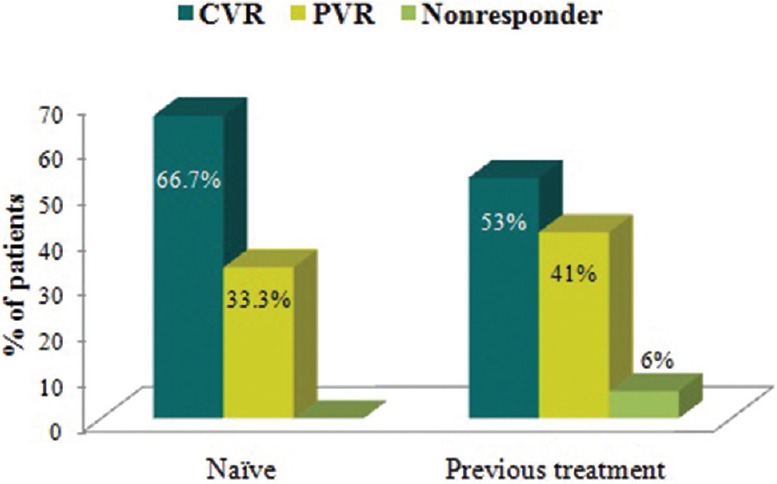
Rate of achieving a complete virological response (CVR) (P = 0.3), partial virological response (PVR), or no response stratified according to treatment naïve (left) and treatment refractory (right) patients
ALT significantly decreased from 134 U/L before treatment to 37 U/L at study end (P < 0.001), regardless of HBeAg status. In HBeAg-positive patients, the mean ALT decreased from 72 ± 35 U/L to 34 ± 2 U/L after 48 weeks of treatment, whereas in HbeAg-negative patients ALT decreased from 156 ± 270 U/L to 39 ± 30 U/L (P = 0.43). There was no significant influence of gender or body mass index (BMI) on virologic response. However, with regard to age, fewer patients ≤30 years achieved a CVR compared with patients older than 30 years; 5/18 (27.8%) versus 37/50 (74%) patients, respectively (P = 0.001).
One patient developed significant hypophosphatemia (<0.4 mmol/L) and 1 patient had a rise in serum creatinine from 50 μmol/L at baseline to 117 μmol/L that was attributed to medical comorbidities and treatment. There was no significant change in international normalized ratio (INR), lactic acid, alfa-fetoprotein (AFP), and bilirubin before and after therapy [Table 4].
Table 4.
Laboratory parameters for participants at baseline and after 48 weeks of treatment

DISCUSSION
Data from the 48-week study reported here provides clear evidence of TDF efficacy in a Saudi Arabian population of patients with CHB. Most importantly, we demonstrated a CVR in 62% of patients and a PVR in 37% of patients overall. After 48 weeks of TDF, the HBV DNA was undetectable in 66.7% of treatment-naïve patients and 53% of treatment-refractory patients.
The higher frequency of patients achieving a CVR in the HBeAg-negative (84.4%) versus HBeAg-positive (21.7%) group is consistent with results from two large scale studies that prospectively compared the efficacy of TDF 300 mg with ADF 10 mg in CHB patients.[10] In these two studies, 48 weeks of treatment with TDF resulted in undetectable virus in 93% of HBeAg-negative and 76% of HBeAg-positive patients. Patients in both trials were allowed to have prior 3TC, but not ADF. The comparatively lower rate of treatment response among patients in our study likely reflects the inclusion of ADF experienced patients because ADF resistance associates with TDF efficacy reduction.[13] The retrospective nature of this study may also account for the comparatively lower treatment response reported here due to less control over subject variables.
Rates of achieving undetectable HBV with TDF in retrospective studies of treatment-refractory patients consistently exceeded the rate reported here (62%), ranging from 79% to 100%.[13,14,15] Several factors may have contributed to the more modest response in our patients including genotype, previous exposure to antiviral agents, a high number of HBeAg-positive patients, and perhaps low compliance in some patients. Duration of treatment and HBV assay detection limits also vary between studies, which may explain differences in reported values. One large scale retrospective analysis that investigated the efficacy of TDF monotherapy in treatment-experienced patients reported an undetectable viral load (<15 IU/mL) in 79% of patients overall. However, when analyzed for a subgroup of patients with ADF genotypic resistance, only 33% achieved a CVR after 12 months of therapy.[13] At least half of our treatment-refractory patients had ADF failure, which could account for the lower rate of CVR in this subset of patients.
Several studies have reported a lower propensity of developing drug resistance when administering ADF and 3TC in combination versus either agent alone.[16,17,18] In our study, a higher percentage of patients with prior 3TC/ADF combination therapy had undetectable HBV DNA at 48 weeks compared with those having received either therapy alone. Another prospective study evaluated whether adding ADF to 3TC provides a better treatment option than ADF monotherapy in HBeAg-negative patients with 3TC resistance. Over 36 months of treatment, 16% of patients in the monotherapy group developed ADF resistance versus none in the combination group.[16,17] Another clinical trial prospectively compared the treatment effect of 3TC compared with an ADF/3TC combination in 115 HBeAg-positive patients. The data demonstrated a much lower 3TC resistance rate at 2 years in the combination versus monotherapy group (15% vs 43%, respectively)[18] Therefore, combining antivirals from different classes may reduce the subsequent rate of resistance to either drug.
In the subgroup of patients with prior 3TC/ADF combination therapy, 80% (4/5) of patients achieved a CVR, which is higher than that reported by Patterson et al. The Patterson study prospectively evaluated a similar population and determined a complete TDF response in two-thirds of patients after 96 weeks of treatment.[19] Patients in this trial had confirmed 3TC resistance and ADF failure at baseline. The extent of prior treatment exposure may explain the differences in response to TDF between the Patterson study and ours, as patients in the former were heavily pretreated and had a high rate of genotypic resistance to ADF.[19]
The data in the literature supporting a switch to TDF after a suboptimal response to ETV are conflicting. One multicenter, retrospective analysis conducted in the United States identified 14 of 482 CHB patients with a suboptimal response to ETV.[14] The 14 patients, all of them Chinese, were infected with either HBV genotype C (71%) or B (29%), and the entire subgroup (14/14) achieved a CVR after switching to TDF (median 30 weeks of TDF therapy).[14] TDF used as a rescue therapy in a cohort of Korean patients after multiple treatment failures demonstrated an 86.2% cumulative probability of achieving a CVR (decrease of serum HBV DNA ≤60 IU/mL) after 12 months of treatment. Within this cohort, 62% of patients had been exposed to ETV and a large proportion likely carried the genotype C virus as this genotype predominates in Korea.[20,21] On the contrary, none of the patients with patient with prior ETV failure in our study attained undetectable HBV DNA after 48 weeks of treatment (both genotype D). A distinction in genotype predominance between this study and others may explain why our patients experienced an inferior response after ETV. Whether this result translates to the larger HBV-infected population in Saudi Arabia known to have a high prevalence of genotype D, warrants further exploration.[22] A lack of consistency in TDF response among the different ETV refractory populations reported in retrospective studies suggests that genotype, ethnicity, as well as number of prior antivirals may influence TDF efficacy after ETV. A meta-analysis recently addressed this question for a number of antivirals and concluded a need for larger multicenter clinical trials for further elucidating the relationship between genotype and response to CHB therapies.[23]
Additionally, the subgroup of refractory patients represents a difficult-to-treat population and local data on these patients can help determine the most appropriate treatment strategies for the management of CHB in our patients.
The main limitation of our study is the small number of patients. However, the current study is relevant considering the lack of data from the region regarding the role of TDF in treating CHB patients.
CONCLUSION
TDF given as monotherapy suppressed HBV DNA in both treatment-naïve and treatment-refractory CHB patients. The HBeAg-negative (vs positive) patients had a better response. Our data builds on conclusions from other studies supporting the collection of nationwide data to better define the most appropriate treatment for patients with CHB based on distinctions in HBV genotype, geographical location, and prior therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25(Suppl 1):3–8. doi: 10.1055/s-2005-915644. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. (8.e1-2).Gastroenterology. 2011;141:1240–8. doi: 10.1053/j.gastro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 4.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: Are we off the hook? Saudi J Gastroenterol. 2012;18:349–57. doi: 10.4103/1319-3767.103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrillo RP, Schiff ER, Davis GL, Bodenheimer HC, Jr, Lindsay K, Payne J, et al. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B.The Hepatitis Interventional Therapy Group. N Engl J Med. 1990;323:295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- 8.Hoofnagle JH, Peters M, Mullen KD, Jones DB, Rustgi V, Di Bisceglie A, et al. Randomized, controlled trial of recombinant human alpha-interferon in patients with chronic hepatitis B. Gastroenterology. 1988;95:1318–25. doi: 10.1016/0016-5085(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–63. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 11.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Al-Ashqar HI, Al-Quaiz M, Dahab ST, Peedikayil MC. Entecavir for the treatment of real-life chronic hepatitis B patients: A study from Saudi Arabia. Ann Saudi Med. 2013;33:119–23. doi: 10.5144/0256-4947.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73–80. doi: 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 14.Pan CQ, Hu KQ, Yu AS, Chen W, Bunchorntavakul C, Reddy KR. Response to tenofovir monotherapy in chronic hepatitis B patients with prior suboptimal response to entecavir. J Viral Hepat. 2012;19:213–9. doi: 10.1111/j.1365-2893.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ, Sinn DH, Gwak GY, Choi MS, Koh KC, Paik SW, et al. Tenofovir rescue therapy for chronic hepatitis B patients after multiple treatment failures. World J Gastroenterol. 2012;18:6996–7002. doi: 10.3748/wjg.v18.i47.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lampertico P, Marzano A, Levrero M, Santantonio T, Di Marco V, Brunetto M, et al. Adefovir and lamivudine combination therapy is superior to adefovir monotherapy for lamivudine-resistant patients with HBeAg-negative chronic hepatitis B [EASL abstract 502] J Hepatol. 2007;46(Suppl 1):S191. [Google Scholar]
- 17.Gish RG. Hepatitis B treatment: Current best practices, avoiding resistance. Cleve Clin J Med. 2009;76(Suppl 3):S14–9. doi: 10.3949/ccjm.76.s3.04. [DOI] [PubMed] [Google Scholar]
- 18.Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48:728–35. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60:247–54. doi: 10.1136/gut.2010.223206. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Yoon KH, Lee KE, Park DS, Lee YJ, Moon HB, et al. Distribution of hepatitis B virus genotypes in Korea. Korean J Hepatol. 2009;15:140–7. doi: 10.3350/kjhep.2009.15.2.140. [DOI] [PubMed] [Google Scholar]
- 21.Song BC, Cui XJ, Kim H. Hepatitis B virus genotypes in Korea: An endemic area of hepatitis B virus infection. Intervirology. 2005;48:133–7. doi: 10.1159/000081740. [DOI] [PubMed] [Google Scholar]
- 22.Abdo AA, Al-Jarallah BM, Sanai FM, Hersi AS, Al-Swat K, Azzam NA, et al. Hepatitis B genotypes: Relation to clinical outcome in patients with chronic hepatitis B in Saudi Arabia. World J Gastroenterol. 2006;12:7019–24. doi: 10.3748/wjg.v12.i43.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raimondi S, Maisonneuve P, Bruno S, Mondelli MU. Is response to antiviral treatment influenced by hepatitis B virus genotype? J Hepatol. 2010;52:441–9. doi: 10.1016/j.jhep.2009.12.014. [DOI] [PubMed] [Google Scholar]


