Abstract
Background/Aims:
The association between platelet–lymphocyte ratio (PLR), neutrophil–lymphocyte ratio (NLR), and survival with response rates were evaluated in metastatic gastric cancer (MGC).
Patients and Methods:
MGC patients on firstline modified docetaxel/cisplatinum/5-fluorourasil [mDCF; docetaxel 60 mg/m2 (days 1–5), cisplatin 60 mg/m2 (day 1), 5FU 600 mg/m2 (days 1–5), q3w] were evaluated retrospectively. The cutoff values were 160 for PLR and 2.5 for NLR. Progression-free survival (PFS) and overall survival (OS) were estimated for group I (PLR >160), group II (PLR ≤ 160), group III (NLR ≥ 2.5), group IV (NLR < 2.5), group V (PLR > 160 and NLR ≥ 2.5), group VI (PLR ≤ 160 and NLR <2.5), and group VII [VIIa (PLR > 160 and NLR < 2.5) and VIIb (PLR ≤160 and NLR ≥ 2.5)].
Results:
One hundred and nine MGC patients were evaluated for basal hematological parameters and survival analysis, retrospectively. Most of the patients were male in their fifties with grade III adenocarcinoma (62.9%) and liver metastasis (46.7%). Patients with PLR > 160 and/or NLR ≥ 2.5 had significantly shorter PFS and OS (P = 0.04, 0.01, 0.019, and P = 0.003, 0.002, 0.000, respectively).
Conclusion:
High PLR (> 160) and/or NLR (≥ 2.5) seem to be poor prognostic factors in MGC.
Keywords: Metastatic gastric cancer, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio
Gastric cancer is one of the most common causes of cancer-related deaths despite improvements in treatment modalities. Diagnosis at advanced stage, especially in developing countries, makes it more significant despite the annual death rate declining in recent years. Most of the patients with gastric cancer are often diagnosed at an extensive stage of the disease.[1] The median overall survival (OS) of metastatic gastric cancer (MGC) has been reported to improve to approximately a year with palliative chemotherapy in a recent meta-analysis (HR: 0.37).[2] Combination chemotherapy regimens such as DCF (docetaxel, cisplatinum, 5-fluorouracil [5FU]) have been demonstrated to have higher response rates with longer survival at the expense of increased toxicity.[3,4] Therefore, modified-DCF (mDCF) regimen should be preferred to DCF to decrease toxicity rates with similar efficacy.[5]
The prognostic and predictive factors for gastric cancer are still cotroversial despite promising recent reports.[6,7] The role of the systemic inflammatory response in cancer has been emphasized in previous reports.[8,9,10] Hematological parameters with estimated ratios, such as the neutrophil–lymphocyte ratio (NLR) and/or platelet–lymphocyte ratio (PLR) were reported to have significance in prognosis of cancer patients.[8,11,12,13,14,15,16] However, their predictive role is not so clear. In this retrospective study, the association between PLR and/or NLR and survival with response rates was aimed to be evaluated in MCG receiving firstline mDCF.
PATIENTS AND METHODS
MGC patients followed at our center between March 2007 and July 2012 were evaluated retrospectively. All patients had a firstline mDCF [docetaxel 60 mg/m2 (days 1–5), cisplatin 60 mg/m2 (day 1), 5FU 600 mg/m2 (days 1–5), q3w] regimen. In this study, patients characteristics, basal hematological parameters with NLR and/or PLR, and survival rates. The patients with bone marrow involvement were excluded because basal hematological parameters might have been affected by involvement leading to false PLR or NLR calculations as predictors of cancer-related inflammatory response. We aimed to calculate these ratios as systemic inflammation-based scores rather than bone marrow involvement.
Hematological parameters
Venous blood samples collected in the ethylenediaminete traacetic acid (EDTA) containing tubes at MGC diagnosis were analyzed for hematological parameters (neutrophil, platelet, lymphocyte). The ratios of these parameters (PLR, NLR) were calculated retrospectively. The division of neutrophil count by lymphocyte count was defined as NLR, whereas the division of platelet count by lymphocyte count was defined as PLR. The cutoff values were estimated as 160 for PLR (>160 vs ≤160) and 2.5 for NLR (≥2.5 vs <2.5) according to previous reports.[7,8,9]
Statistical analysis
Statistical analyses were performed with SPSS for Windows version 18.0 (SPSS, Chicago, IL, USA). Chi-square or Fisher's exact test were used for comparative analysis of categorical variables. Patients with missing values were omitted. Survival analyses were estimated according to Kaplan–Meier Method. Progression-free survival (PFS) was defined as the duration from the date of mDCF initiation to the objective tumor progression while OS was defined as the interval between MGC diagnosis and death or the last date the patient was known to be survival. Clinical benefit rate was defined as the sum of complete response, partial response, and stable disease rates. Subgroup suvival analysis was performed for group I (PLR >160), group II (PLR ≤160), group III (NLR ≥2.5), group IV (NLR <2.5), group V (PLR >160 and NLR ≥2.5), group VI (PLR ≤160 and NLR <2.5), and group VII [VIIa (PLR >160 and NLR <2.5) and VIIb (PLR ≤160 and NLR ≥2.5)]. Subgroup survival rates were compared by log-rank test and P ≤ 0.05 was considered as statistically significant.
RESULTS
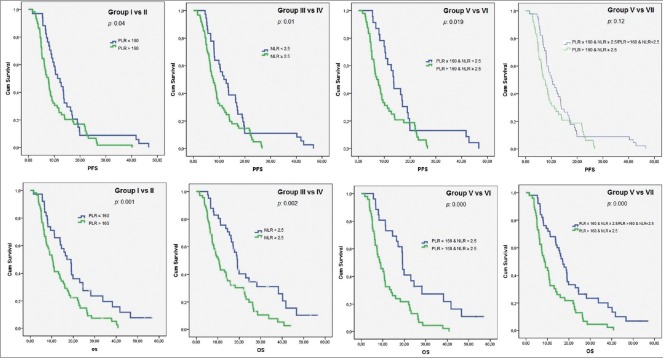
A total of 109 MGC patients between 2007 and 2012 were included in the study. Median follow-up was 12.2 (range: 1.51–50.4) months. Patient characteristics with subgroup analysis are summarized in Table 1. Most of the patients were male, in their fifties, in all subgroups except group VIIa (PLR >160 and NLR <2.5). However, median age of the patients who had AFP secreting MGC was 43 (range: 32–66). All the of the patients had adenocarcinoma and most of them had grade III tumor with corpus localization, lymphovascular invasion, and/or perineural invasion [Table 2]. Most of our MGC patients had better ECOG-PS (78.9% for 0–1). None of our patients had ECOG-PS >2 since all the of the patients enrolled to the study were candidates for palliative chemotherapy. Two-third of the patients had a single metastatic site. The liver was the most common site [Table 2]. All patients received mDCF and there was no toxicity-related death with managable toxicity [Table 1]. The median number of mDCF cycles was 6 (range: 2–8). Response rates are shown in Table 3. One third of the patients progressed after first line mDCF and most of these progressed patients had second line chemotherapy. Partial remission was only achieved by EOX as a second line chemotherapy. The clinical response rate with first line mDCF was 73.4%, whereas it was 21.2% with second line chemotherapy. There were no toxicity-related deaths. However, Grade III/IV neutropenia was highest in group VII [30.7% in group VIIa (PLR >160 and NLR <2.5) and 38.4% in group VIIb (PLR ≤160 and NLR ≥2.5), respectively]. However, none of them had neutropenic fever in contrast to group V (1.8%) and group VI (1.9%) [Table 1]. Median OS was 13.1 months (CI 95%: 11.09–15.2), whereas median PFS was 9 months (CI 95%: 7.67–10.34) in all patients [Figure 1]. Median values for PLR and NLR were 188 and 3. respectively [Table 4]. The patients with high PLR (>160) and/or NLR (≥2.5) had lower PFS and OS, whereas the others with low PLR (≤160) and NLR (<2.5) had higher PFS and OS [Figure 2]. Basal hematological parameters with median values are shown in Table 4. Most of the patients had anemia with a median hemoglobin level of 11.3 g/dL.
Table 1.
Patient characteristics with survival analysis
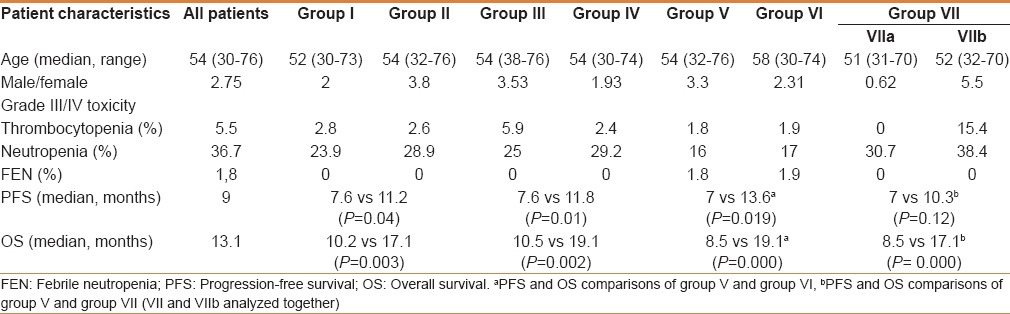
Table 2.
Clinicopathological features of all metastatic gastric cancer patients receiving mDCF
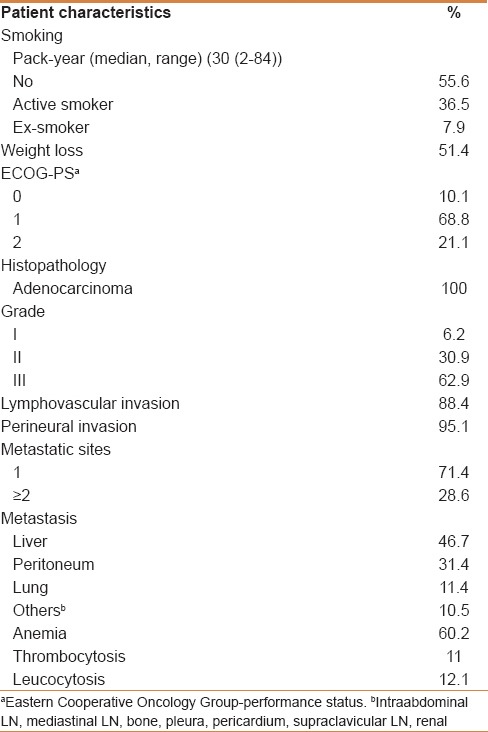
Table 3.
Response rates of firstline and secondline chemotherapy
Figure 1.
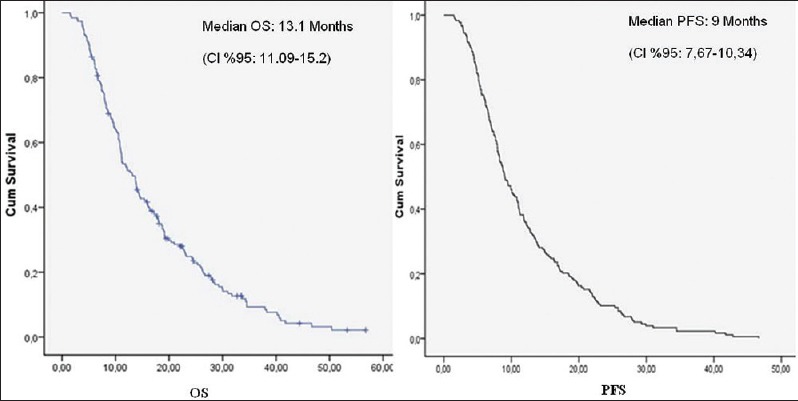
Clinicopathological features with overall survival and progression-free survival analysis
Table 4.
Basal laboratory values with normal ranges
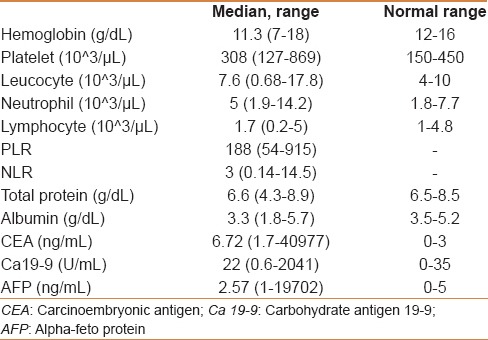
Figure 2.
Subgroup survival analysis according to neutrophil–lymphocyte ratio and/or platelet–lymphocyte ratio
DISCUSSION
More than half of all gastric cancer patients are at an advanced stage of the disease at diagnosis.[1] The mDCF regimen was preferred as firstline chemotherapy in MGC according to the previous results similar to DCF regimen with less toxicity.[5] All of our MGC patients had firstline chemotherapy as mDCF, which makes the study group more homogenous for all subgroup analysis. All subgroups except group VIIa (PLR >160 and NLR <2.5) had male predominance in concordance with the literature.[8] In addition, our patients were younger.
The systemic inflammation-based scores were reported to have prognostic value in cancer.[10,12,14,17,18,19] We believe that the homogenous firstline chemotherapy regimen (ie, mDCF) in our study seems to increase the prognostic significance of these parameters because it provides a more uniform study group as mentioned above.
Basal NLR was emphasized as a negative prognostic factor in gastric cancer previously.[8,17,18] Pretreatment NLR was reported to be a better predictor than PLR in breast cancer.[16] However, other studies in other types of cancer such as esophageal carcinoma claimed that PLR might have had a higher prognostic value than NLR.[19] Therefore, we evaluated the clinical significance of both ratios with other clinicopathological characteristics in MGC. Median PFS and OS of the patients in high-risk groups with high PLR (>160) and/or NLR (≥2.5) were significantly lower than those in low-risk groups with low PLR (≤160) and/or NLR (<2.5) [Table 1]. When the patients were subanalyzed according to NLR and/or PLR, the patients with low PLR (≤160) and NLR (<2.5) in group VI had highest PFS and OS similar to the results of neodjuvant chemotherapy study reported by Jin et al. [Figure 2].[8] The patients with “low NLR (<2.5) and low PLR (≤160)” had both highest PFS (13.6 months) and OS (19.1 months), whereas the patients with only “low NLR (<2.5)” had highest OS (19.1 months) but not the highest PFS (11.8 months). In this case, we consider that NLR might have more clinical significance as a prognostic factor.
The serum biomarkers or pathology-based prognostic and predictive factors in daily clinical practice are generally expensive and cumbersome. Therefore, we need cost-effective and easier-to-validate parameters for the optimal treatment modality. The parameters such as NLR and/or PLR can be calculated easily from basal pretreatment peripheral venous blood samples without additional cost. Our findings appear to coincide with multiple published studies.
The inflammation within the tumor and microenviroment may contribute to the antitumor response by cellular interactions and cytokines such as interleukin-18 and vascular endothelial growth factor.[20] On the other hand, antitumor inflammatory response might also be suppressed by recruiting T cells and it might lead to the promotion of tumor growth and metastasis.[21] However, the neutrophilic activity in the microenviroment might inhibit the antitumor cellular immune response via T lymhocytes and natural killer cells.[8] Platelets were also reported to have a role in cancer-related inflammatory response.[22] So, neutrophilia, thrombocytosis, and/or lymphopenia leading to high NLR and PLR might contribute to a decrease in the antitumor response. Our results supported this hypothesis in concordance with existing data.[11,12,13,14,15,16] However, most of the reports in the literature seem to have heterogenous treatment modalities, which might have affected the outcomes in terms of survival and response rates. Additionally, this heterogenity might have contributed to the prognostic and/or predictive value of the parameters, such as NLR and/or PLR. Our more homogenous study group demonstrated the effect of NLR and PLR on survival of MGC patients on firstline mDCF more clearly.
Cancer treatment is not only arduous but also expensive worldwide. Therefore, we need clinically significant prognostic and predictive factors to plan the optimal treatment modality for the patients. Calculation of clinically significant, easier, and cheaper basal ratios, such as PLR and NLR seem to contribute to better outcomes in oncology.
CONCLUSION
We consider that MGC patients with high PLR (>160) and/or NLR (≥2.5) have shorter PFS and OS. The homogenous firstline chemotherapy regimen (ie, mDCF) has demonstrated the clinical significance of these parameters as prognostic and predicitive factors. However, furtherer prospective trials with larger number of patients to substantiate our findings are required.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Ca Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AD, Unverzagt S, Grothe W, Klebere G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD004064.pub3. CD004064. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–7. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorourasil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 5.Inal A, Kaplan MA, Kucukoner M, Isikdogan A. Docetaxel and cisplatin plus fluorourasil compared with modified docetaxel, cisplatin and 5-fluorourasil as first-line therapy for advanced gastric cancer: A retrospective analysis of single institution. Neoplasma. 2012;59:233–6. doi: 10.4149/neo_2012_030. [DOI] [PubMed] [Google Scholar]
- 6.Kim KH, Kwon HC, Oh SY, Kim SH, Lee S, Kwon KA, et al. Clinicopathological significance of ERCC1, thymidylate synthase and glutathione S-transferase P1 expression for advanced gastric cancer patients receiving adjuvant 5-FU and cisplatin chemotherapy. Biomarkers. 2011;16:74–82. doi: 10.3109/1354750X.2010.533284. [DOI] [PubMed] [Google Scholar]
- 7.Lim JB, Kim DK, Chung HW. Clinical significance of serum thymus and activation-regulated chemokine in gastric cancer: Potential as a serum biomarker. Cancer Sci. 2014;105:1327–33. doi: 10.1111/cas.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H, Zhang G, Liu X, Liu X, Chen C, Yu H, et al. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol. 2013;11:112. doi: 10.1186/1477-7819-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 11.Aliustaoglu M, Bilici A, Seker M, Dane F, Gocun M, Konya V, et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology. 2010;57:640–5. [PubMed] [Google Scholar]
- 12.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–6. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–9. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol. 2014;110:333–40. doi: 10.1002/jso.23651. [DOI] [PubMed] [Google Scholar]
- 15.He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439. doi: 10.1007/s12032-012-0439-x. [DOI] [PubMed] [Google Scholar]
- 16.Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol. 2013;30:432. doi: 10.1007/s12032-012-0432-4. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JE, Kim HN, Kim DE, Choi HJ, Jung SH, Shim HJ, et al. Prognostic significance of a systemic inflammatory response in patients receiving first-line palliative chemotherapy for recurred or metastatic gastric cancer. BMC Cancer. 2011;11:489. doi: 10.1186/1471-2407-11-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong JH, Lim SM, Yun JY, Rhee GW, Lim JY, Cho JY, et al. Comparison of two inflammation-based prognostic scores in patients with unresectable advanced gastric cancer. Oncology. 2012;83:292–9. doi: 10.1159/000342376. [DOI] [PubMed] [Google Scholar]
- 19.Kilincalp S, Coban S, Akinci H, Hamamc M, Karaahmet F, Coşkun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2014 doi: 10.1097/CEJ.0000000000000092. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Jablonska E, Puzewska W, Grabowska Z, Jablonski J, Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30:93–9. doi: 10.1016/j.cyto.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]