Abstract
Background/Aim:
Proinflammatory markers such as interleukin (IL)-6 have been closely associated with atrial fibrillation (AF). These markers are characteristically elevated in chronic inflammatory bowel disease (IBD) and positively correlate with disease activity. Although IBD and AF have similar pathogenesis, there have been very limited studies looking at their association. The aim of this study is to determine the prevalence of AF in patients with IBD.
Patients and Methods:
Medical records of patients with biopsy proven IBD (n = 203, both in and outpatient) were retrospectively reviewed. One hundred and forty-one IBD patients with documentary evidence of electrocardiograms (ECG's) were included. The “Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA)” study, a large cross-sectional study (n = 1.89 million) done to evaluate the prevalence of AF among the US population, was our control population. All ECGs available till December 2010 for each IBD patient were reviewed carefully for evidence of AF. We studied the prevalence of AF among IBD population and compared it to that of control (ATRIA) population.
Results:
The prevalence of AF was significantly higher among IBD patients compared with the ATRIA study patients (11.3% vs 0.9%, P < 0.0001). Additionally, the IBD patient population were much younger compared with the controls (64.4 ± 10.7 vs 71.2 ± 12.2, P = 0.02).
Conclusion:
AF has an overall higher prevalence across all age groups in IBD compared with the subjects of ATRIA study, which could be due to the chronic inflammatory state of IBD. Further studies are needed to study the association in detail.
Keywords: Atrial fibrillation, Crohn's disease, Inflammatory bowel disease, Ulcerative colitis
Atrial fibrillation (AF) is a complex disease of multifactorial etiology and the most common cardiac rhythm disturbance, affecting 2.3 million people in the United States.[1] It has an overall prevalence of less than 0.5% in the general population aged less than 55 years, steadily increasing to 4%–9% in the age group of 65–80 years and up to 9% or higher in people over the age of 80 years.[2] AF is also a major cause of morbidity with a twofold increase in mortality from stroke and thromboembolism.[2,3,4] A growing body of evidence suggests inflammation to be an independent causative risk factor of AF.[5,6] Presence of systemic inflammation as determined by elevations in C-reactive protein (CRP) and interleukins 6 (IL-6) is not only associated with the prevalence of AF but also predicts increased risk for future development of AF.[7] However, the exact mechanism of association between inflammation and AF is unclear.
Studies have suggested that increased circulating and local CRP may localize in atrial tissue, activating the complement system, and induce inflammation leading to “atrial myocarditis.”[8,9,10,11] In the presence of Ca2+ ions, CRP binds to phosphatidylcholine leading to the generation of long-chain acylcarnitines and lysophosphatidlycholines.[12] These can contribute to cellular membrane dysfunction affecting transmembrane ion transport with subsequent electrical and structural changes in the atrium, resulting in initiation and maintenance of AF.[6,13] Also left atrial dysfunction has been described in patients with increased CRP but without AF, suggesting that inflammation perse affects left atrial function.[14]
IL-6 is a pro-inflammatory cytokine that is involved in the synthesis of acute phase proteins such as CRP.[15] Like CRP, high plasma IL-6 levels have been correlated with the presence and duration of AF and increased left atrial diameter.[16] In patients undergoing cardiac surgery, the development of postoperative AF was related to increased levels of IL-6, and linked to polymorphisms in the promoter region of the IL-6 gene.[17] Furthermore, in a cohort of subjects with CAD, AF was independently associated with IL-6 levels and the CC genotype of −174G/C IL-6 polymorphism.[16] These findings raise the possibility of a genetic susceptibility to an enhanced inflammatory response with subsequent development of AF.
Crohn's disease (CD) and ulcerative colitis (UC) are chronic intestinal inflammatory bowel diseases (IBDs) occurring in genetically susceptible individuals independent of a specific pathogen. The interaction between antigen-presenting cells and the local bacterial flora contributes to an uncontrolled activation of mucosal CD4-T-lymphocytes with the consecutive release of cascade of proinflammatory cytokines such as IL-6.[18] IL-6, a pleiotropic cytokine playing a crucial role in inflammation, immune regulation, hematopoiesis, and oncogenesis is the principal culprit of the uncontrolled intestinal inflammatory process in IBD.[19,20,21,22,23,24]
Given the similarities in pathogenesis between the two conditions, is there an under - appreciated link between inflammatory diseases such as IBD and AF? Our aim was to study the prevalence of AF in patients diagnosed of IBD and evaluate the probable association of AF and IBD.
PATIENTS AND METHODS
Study population
A retrospective review of electronic medical records (EMR) between January 2001 and December 2010 of patients hospitalized or seen in the gastroenterology clinic with a diagnosis of IBD (n = 203, old and new) were identified from MetroHealth Hospital database. The diagnosis of UC and CD was based on clinical and laboratory criteria including upper and lower gastrointestinal endoscopy and histopathologic evaluation. Study design is summarized in Figure 1.
Figure 1.
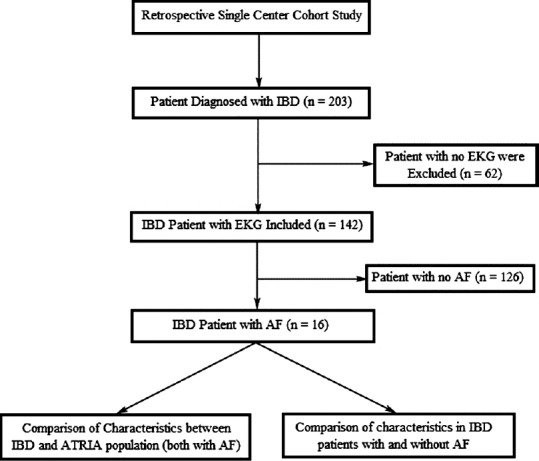
Study design. IBD, Inflammatory bowel disease; AF, Atrial fibrillation; EKG, Electrocardiogram; ATRIA, Anticoagulation and risk factores in atrial fibrillation
Control population
“Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA)” study,[2] a large cross-sectional study (n = 1.89 million), evaluating the prevalence of AF among US general population was our control population.
Electrocardiogram
All electrocardiograms available from electronic medical records till December 2010 for each IBD patient were obtained and reviewed carefully. Patients who did not have ECG (n = 62) were excluded from the study. In our study, patients were considered to have atrial fibrillation only if documentary (ECG) evidence of AF existed. Prevalence of AF among the IBD population was studied and compared with that of ATRIA study (control) population. We then compared the characteristics between the IBD patients with and without atrial fibrillation.
Other clinical and laboratory variables
Clinical and demographic characteristics including age, gender, race, history of hypertension, smoking, diabetes, renal failure, and peripheral arterial disease were abstracted from electronic patient records. Laboratory data included serum creatinine, estimated glomerular filtration rate and serum low-density lipoprotein cholesterol, white cell count, calcium, magnesium, and potassium.
Statistical analysis
Categorical variables are reported as counts and percentages, and continuous variables are presented as mean ± standard deviation (SD). Categorical variable were compared using Chi-square test and continuous data using Student's t-test or Wilcoxon nonparametric statistics (PASW Statistics version 18, SPSS Inc, Chicago, IL, USA). All tests were two-tailed, and P values of less than 0.05 were considered statistically significant.
RESULTS
Patient characteristics
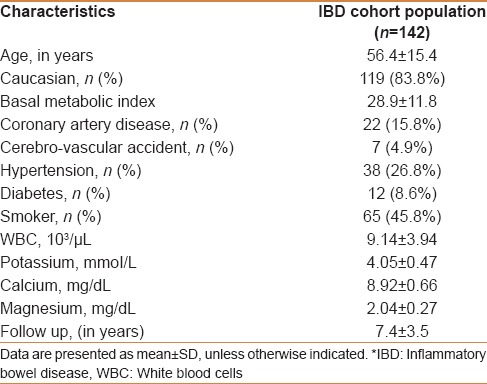
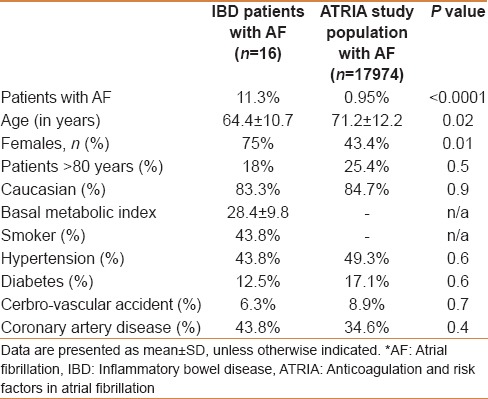
Baseline characteristics of the study population are presented in Table 1. Of the 203 cases with IBD, 141 patients with standard ECGs were included and 62 patients without an ECG were excluded from the study. The mean age of the study population was 56.4 ± 15.4 years with female and Caucasian predominance (63.4% and 83.8%, respectively). The mean follow-up period was 7.4 ± 3.5 years.
Table 1.
Characteristics of inflammatory bowel disease study population
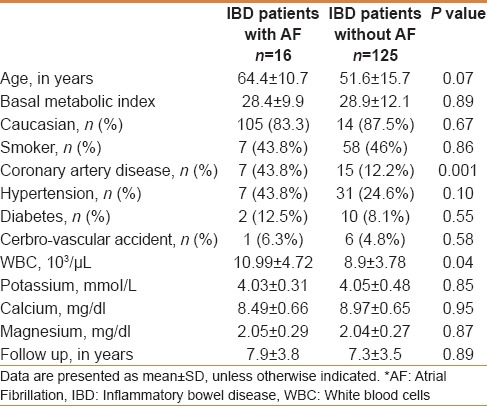
IBD patient characteristics with and without AF are shown in Table 2. Sixteen of the 141 patients (11.3%) were found to have AF. The mean age of a patient with AF was 64.4 ± 10.7 years and those without were 51.6 ± 15.7 years. Of the total patients, 13.3% of women and 7.69% of men were found to have AF. No statistically significant difference was noted among the comorbidities between the two groups. Serum electrolytes including calcium, magnesium, and potassium were similar in both the groups. However, mean white blood cell count was higher among the patients with IBD and AF (10.99 ± 4.72 vs 8.9 ± 3.78, P = 0.046).
Table 2.
Comparison of characteristics of IBD patients with and without AF
Comparison of prevalence of atrial fibrillation to the general population in ATRIA study
There was higher prevalence of AF among IBD population compared with that of control (ATRIA) population (11.3% vs 0.9%, P < 0.0001) [Figure 2]. The detailed comparison between the IBD patient population and ATRIA population is presented in Table 3. Patients in IBD group were significantly younger compared with ATRIA study group (64.4 ± 10.7 vs 71.2 ± 12.2, P = 0.02). Comorbidities such as hypertension, cerebrovascular accidents (CVA), and diabetes though statistically not significant, were much lower in the IBD population compared with ATRIA population.
Figure 2.
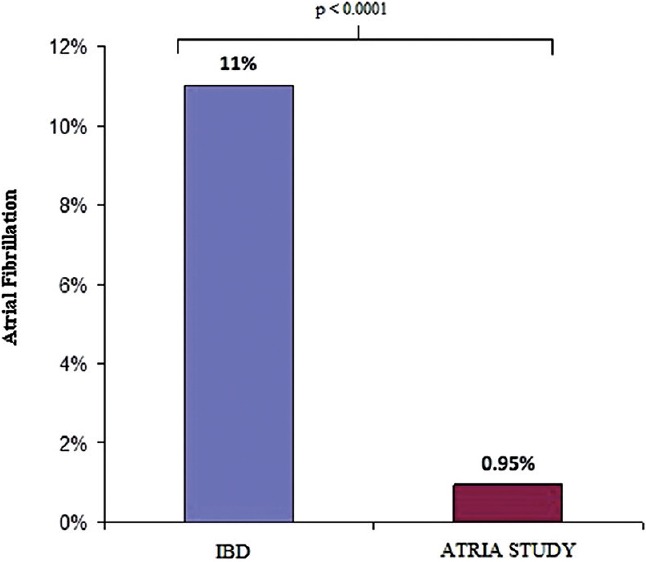
Comparison of prevalence of atrial fibrillation between inflammatory bowel disease and ATRIA study population. AF, Atrial fibrillation; IBD, Inflammatory bowel disease; ATRIA, Anticoagulation and risk factors in atrial fibrillation
Table 3.
Comparison of characteristics of AF patients between IBD and ATRIA study population
Since increasing age is an independent risk factor for AF, we compared the prevalence of AF in different age categories (less than 59 years, 60–64 years and greater than 65 years) between the IBD and ATRIA patients. Prevalence of AF in IBD patients less than 59 years was 5.5% compared with 0.21%; 25% in patients of age 60–64 years compared with 1.35% and 20% in age group greater than 65 years compared with 4.7%, respectively, in ATRIA study [Figure 3].
Figure 3.
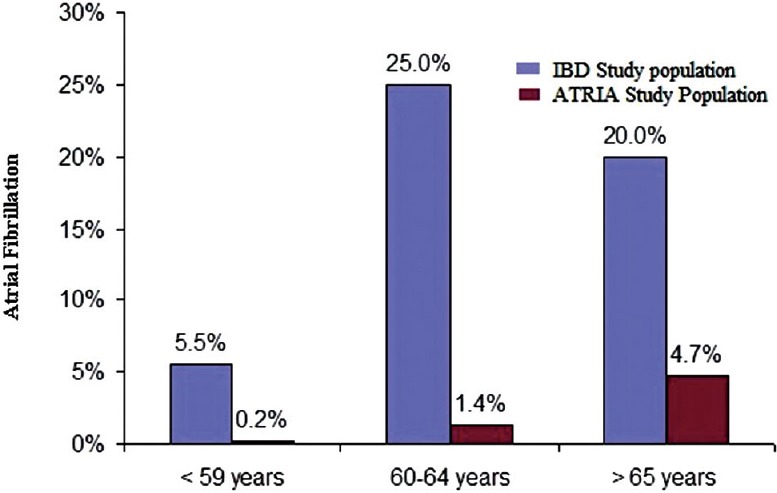
Age distribution comparison of AF in IBD and ATRIA study population. AF, Atrial fibrillation; IBD, Inflammatory bowel disease; ATRIA, Anticoagulation and risk factors in atrial fibrillation
DISCUSSION
Atrial fibrillation is a complex disease with multiple possible mechanisms. Atrial fibrillation is the most common arrhythmia encountered in daily clinical cardiology practice. The prevalence of AF in adult US general population is 0.95% (n = 17,974 in 1.89 million study population) as reported by Go et al. (ATRIA study) in a cross-sectional study of a large health care organization (2). Forty-five percent of the AF population were 75 years or older. Prevalence of AF increased from 0.1% among adults younger than 55 years to 9.0% in persons 80 years or older. Several studies have shown that inflammatory process is one of the mechanisms for the occurrence of AF.[7,25,26] Frustaci et al. demonstrated inflammatory changes in atrial tissues obtained from patients with isolated persistent AF.[25] Gedikli et al. found two- to threefold increase in the presence of serum inflammatory markers in AF patients compared with controls.[27] It is suggested that inflammation contributes to both occurrence and persistence of AF.[28] Inflammation is thought to cause tissue damage by ischemia and oxidative stress, progressively leading to loss of atrial muscle mass with interstitial fibrosis and resulting in structural remodeling.[27] This process also impairs intracellular calcium current resulting in atrial electrical remodeling, which are known determinants of AF.[27]
IBD is characterized by chronic inflammation of the gastrointestinal tract.[2,3] Pro-inflammatory cytokines have been implicated in regulating the intestinal immune response, inducing tissue injury, and mediating complications of IBD.[18,19,20,21,22,23,24] Objective markers such as IL-6 have been reproducibly detected in serum of IBD patients and correlate with disease activity.[18,19,20,21,22,23,24] IL-6 stimulates the proliferation of mature T cells, enhances the differentiation of cytotoxic T-lymphocytes and affects the terminal differentiation and immunoglobulin production of B cells. Furthermore, it also induces acute-phase proteins.[29,30] Despite having less comorbidity, we found a significantly higher prevalence of AF compared with the population in ATRIA study (general population). This higher prevalence of AF in IBD population could be attributed to systemic inflammation. This is the first study demonstrating an association between IBD and AF. The main findings of the study can be summarized as follows:
There is higher prevalence of AF in IBD compared with general population
Prevalence of AF in IBD patients is higher across all age groups compared with the general population
IBD patients with AF are much younger compared with the study subjects in ATRIA.
Although our findings are provocative, our results should be interpreted in light of some potential limitations. The study is a retrospective review of the clinical information recorded in the patient's medical records. Conditions not recorded in the medical records would have been missed. In addition, the retrospective design does not permit an estimate of lifelong inflammatory burden. We could only study an association, but no prospective prediction or causation. Similarly, the influence of confounding factors such as use of corticosteroids, immunomodulators or biologic agents, IBD activity, malnutrition, body mass index, and CRP could not be completely evaluated.
Given the higher incidence of mortality and morbidity in AF, it is important to identify the patients at risk and establish a longitudinal database to further explore these associations. With an increased risk of AF in IBD, patients with IBD should be evaluated for presence of AF and followed up closely, as timely intervention can reduce both the morbidity and mortality occurring due to the arrhythmia complications.
ACKNOWLEDGMENTS
Presented in Part at the Annual Scientific Sessions of Heart Rhythm Society, May 2011, San Francisco, CA, USA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Page RL. Clinical practice. Newly diagnosed atrial fibrillation. N Engl J Med. 2004;351:2408–16. doi: 10.1056/NEJMcp041956. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Falk RH. Atrial fibrillation. N Engl J Med. 2001;344:1067–78. doi: 10.1056/NEJM200104053441407. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4. [PubMed] [Google Scholar]
- 5.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 6.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: Evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–80. doi: 10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 7.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 9.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–5. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 11.Volanakis JE, Wirtz KW. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers. Nature. 1979;281:155–7. doi: 10.1038/281155a0. [DOI] [PubMed] [Google Scholar]
- 12.Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, van den Bosch H. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today. 1997;18:111–5. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- 13.Goggins MG, Goh J, O’Connell MA, Weir DG, Kelleher D, Mahmud N. Soluble adhesion molecules in inflammatory bowel disease. Ir J Med Sci. 2001;170:107–11. doi: 10.1007/BF03168821. [DOI] [PubMed] [Google Scholar]
- 14.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 15.Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–52. doi: 10.1016/j.ahj.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin-6 and atrial fibrillation in patients with coronary artery disease: Data from the Heart and Soul Study. Am Heart J. 2008;155:303–9. doi: 10.1016/j.ahj.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burzotta F, Iacoviello L, Di Castelnuovo A, Glieca F, Luciani N, Zamparelli R, et al. Relation of the -174 G/C polymorphism of interleukin-6 to interleukin- 6 plasma levels and to length of hospitalization after surgical coronary revascularization. Am J Cardiol. 2001;88:1125–8. doi: 10.1016/s0002-9149(01)02046-x. [DOI] [PubMed] [Google Scholar]
- 18.Mudter J, Neurath MF. IL-6 Signaling in Inflammatory Bowel Disease: Pathophysiological Role and Clinical Relevance. Inflamm Bowel Dis. 2007;13:1016–23. doi: 10.1002/ibd.20148. [DOI] [PubMed] [Google Scholar]
- 19.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 20.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 21.Kishimoto T. Interleukin-6: From basic science to medicine-40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 22.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–96. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 24.Ito H. IL-6 and Crohn's disease. Curr Drug Targets Inflamm Allergy. 2003;2:125–30. doi: 10.2174/1568010033484296. [DOI] [PubMed] [Google Scholar]
- 25.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Takeishi Y, Hirono O, Itoh M, Matsui M, Nakamura K, et al. C-reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessels. 2005;20:45–9. doi: 10.1007/s00380-004-0800-x. [DOI] [PubMed] [Google Scholar]
- 27.Gedikli O, Dogan A, Altuntas I, Altinbas A, Ozaydin M, Akturk O, et al. Inflammatory markers according to types of atrial fibrillation. Int J Cardiol. 2007;120:193–7. doi: 10.1016/j.ijcard.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Korantzopoulos P, Kolettis T, Siogas K, Goudevenos J. Atrial fibrillation and electrical remodeling: The potential role of inflammation and oxidative stress. Med Sci Monit. 2003;9:RA225–9. [PubMed] [Google Scholar]
- 29.Uyttenhove C, Coulie PG, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988;167:1417–27. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]


