Abstract
Although all weight-loss approaches may improve insulin sensitivity in type 2 diabetes, bariatric surgery is believed to be the only reliable means of achieving diabetes remission. We conducted a retrospective cohort study to compare rates of diabetes remission, relapse and all-cause mortality among severely obese individuals with diabetes who underwent bariatric surgery versus nonsurgically treated individuals. Severely obese adults with uncontrolled or medication-controlled diabetes who underwent bariatric surgery or received usual medical care from 2005 to 2008 in three health care delivery systems in the United States were eligible. Diabetes status was identified using pharmacy, laboratory, and diagnosis information from electronic medical records. A propensity approach and exclusion criteria identified 1395 adults with diabetes who had bariatric surgery and 62322 who did not. Most procedures were Roux-en-Y gastric bypass (72.0% laparoscopic; 8.2% open); 4.4% were gastric banding, 2.4 % sleeve gastrectomy, and 13.2% were other procedures. At two years, bariatric subjects experienced significantly higher diabetes remission rates [73.7% (95% CI: 70.6, 76.5)] compared to nonsurgical subjects [6.9% (95%CI: 6.9, 7.1)]. Age, site, duration of diabetes, hemoglobin A1c level, and intensity of diabetes medication treatment were significantly associated with remission. Bariatric subjects also experienced lower relapse rates than nonsurgical subjects (adjusted HR: 0.19; 95% CI: 0.15 to 0.23) with no higher risk of death (adjusted HR: 0.54; 95% CI: 0.22 to 1.30). We conclude that bariatric surgery can effectively induce remission of diabetes among most severely obese adults, and this treatment approach appears to be superior to nonsurgical treatment in inducing diabetes remission.
Keywords: bariatric surgery, diabetes, remission, comparative effectiveness
Introduction
Severe obesity and type 2 diabetes mellitus (T2DM) are increasingly common chronic medical conditions in the United States. Both are associated with excess mortality among adults, with obesity alone contributing an estimated 300 000 deaths a year in the United States.[1] To combat these twin epidemics, the medical community has increasingly turned to bariatric surgery as a weight-loss and diabetes-control intervention in severely obese adults.[2-4] The American Society for Metabolic and Bariatric Surgery reported that the annual number of bariatric surgeries nearly doubled from 2003 (103000 procedures) to 2007 (205000 procedures) and estimated that by 2020, up to 13% of the United States population will be eligible for bariatric surgery.[5]
Bariatric surgery includes a number of surgical procedures that alter the gastrointestinal tract primarily for the purpose of producing weight loss that in turn, may also induce T2DM remission. Weight loss is believed to be the primary mechanism by which restrictive bariatric procedures improve T2DM; however, it is increasingly clear that the gut plays a role in glucose homeostasis, by influencing insulin secretion and possibly sensitivity; and procedures that include an intestinal bypass probably improve glucose control by influencing multiple gastrointestinal pathways in complementary ways.[6] A recent meta-analysis of 621 randomized trials and observational studies published by Buchwald and colleagues indicates that bariatric surgery has profound and potentially long-lasting effects on T2DM.[7] They reported high rates of T2DM remission across various procedure types, including: biliopancreatic diversion/duodenal switch (BPD/DS) 95.1%, Roux-en-y gastric bypass (RYGB) 80.3%, gastroplasty (including gastric sleeve) 79.7%, and adjustable gastric band (AGB) (56.7%). The Centers for Medicare and Medicaid Service (CMS) independently reviewed this literature and came to a similar conclusion that BPD/DS, RYGB, and AGB were all effective methods for inducing diabetes remission.[8]
Despite mounting evidence that bariatric surgery results in high rates of T2DM remission, many health insurance plans have called for additional research to conclusively establish the superiority of bariatric surgery over medical management.[9] They point out that very few studies in this area have included comparison control groups of T2DM patients who did not have surgery. This conclusion was supported by the CMS literature review and the recent meta-analysis.[7, 8] For example, even though the meta-analysis included more than 12000 diabetic patients, most studies were at single sites (89%) and very few had a comparison group of nonsurgical patients.[7]
To address these gaps in the literature and to inform coverage decisions by health insurance plans, this report presents findings from a large, population-based investigation of the short-term impact of bariatric surgery versus nonsurgical treatment on T2DM remission among severely obese adults using data from 2005-2008 extracted from three integrated health insurance plans and care delivery systems in the United States. We hypothesized that the likelihood of T2DM remission would be significantly higher in severely obese patients who had bariatric surgery compared to those who continued nonsurgical care for T2DM and obesity.
Methods and Procedures
We conducted a retrospective cohort study to determine whether bariatric surgery is superior to nonsurgical treatment in inducing T2DM remission among severely obese patients. Study subjects included adults enrolled during 2005 to 2008 in one of three integrated United States health plan and care delivery systems: HealthPartners (Minnesota), Kaiser Permanente Northern California, and Kaiser Permanente Southern California. Analyses were conducted at the Group Health Research Institute (Seattle, Washington). All procedures were reviewed and approved by the Institutional Review Boards of the four sites.
Primary inclusion criteria were: a) uncontrolled or medication-controlled T2DM; b) measured body mass index (BMI) of at least 35 kg/m2 (extracted from electronic medical records) and c) age at least 18 not yet 80 years old. Initial classification as having diabetes required one or more of the following criteria in a defined 12-month (calendar) period: a) 1+ fills for diabetes-specific medication (oral or insulin); b) HbA1c ≥7.0% on one or more occasions; c) fasting blood glucose ≥126 mg/dL on two or more occasions; d) random blood glucose ≥200 mg/dL on two or more occasions; e) one fasting blood glucose ≥126 mg/dL plus one random glucose ≥200 mg/dL; f) one or more inpatient hospital discharge ICD-9 code related to diabetes; g) two or more outpatient ICD-9 codes related to diabetes.
Uncontrolled T2DM was defined as a hemoglobin A1c (HbA1c) ≥7.0% at the most recent measurement prior to eligibility; medication-controlled T2DM was defined as a current prescription for diabetes medication at the time of eligibility with the most recent HbA1c <7.0%. The 7.0% threshold was chosen prior to release of new American Diabetes Association guidelines suggesting a threshold of 6.5%.[10] Among eligible subjects, we identified all primary bariatric surgery procedures from 2005 to 2008 using ICD-9 and CPT-4 procedure codes from inpatient hospitalizations. We identified adults with a BMI ≥35 using data from electronic medical records (EMRs), which were available at the three participating sites, all of which implemented EMRs in 2005.
Core exclusion criteria included gestational diabetes, current pregnancy, history of malignancy, prior gastrointestinal surgery for cancer or peptic ulcer disease, and peritoneal effusion/ascites. These inclusion and exclusion criteria, while generally accepted in the clinical community,[11] are quite broad and in current practice only a small proportion of patients meeting these criteria actually undergo bariatric surgery. Thus, eliminating patients unlikely to undergo bariatric surgery was critical. Therefore, we also used a propensity score approach to identify and exclude individuals a low probability of undergoing bariatric surgery. Cox proportional hazards modeling estimated the two-year probability of each subject choosing to undergo bariatric surgery as a function of observed covariates at baseline (initial date of eligibility based on the above definitions).[12] Covariates included in our propensity model were based on scientific considerations aimed at characterizing the severity of diabetes and obesity at eligibility. Final covariates included: age at eligibility, sex, site, year of eligibility, time since initial diabetes diagnosis, baseline BMI, baseline HbA1c, current diabetes treatment indicators (insulin, oral agents, none), and number of current diabetes medications (eTable 1). Propensity score distributions were examined graphically (eFigure 1) and in tabular format (eTable 2) among subjects who did and did not choose to undergo bariatric surgery in follow-up. By consensus, a propensity threshold of 0.01 was selected (eFigure 2), so all subjects with a very low probability of undergoing bariatric surgery within the next two years (propensity score below 0.01) were excluded from subsequent analyses. Sensitivity analyses were conducted to explore the impact of varying the propensity score threshold between 0.00 and 0.05. Eligible subjects with a propensity score greater than 0.01 comprised our main analytic sample. Our propensity analytic approach is described in greater detail in our online-only material.
Using standardized data definitions and programming specifications, we extracted a health care dataset for each eligible subject from administrative and clinical databases at participating sites. Data included demographic characteristics (age, sex, insurance type), measures height and weight, survival status, laboratory data related to glycemic control, outpatient diagnoses, and inpatient hospitalizations including diagnoses and procedures. Survival status as of December 31, 2008 was ascertained through medical databases and by linking to state death indices in California and Minnesota.
Our primary outcome of interest was T2DM remission, defined as the co-occurrence of: a) diabetes medication discontinuation (absence of pharmacotherapy for diabetes for ≥90 days after last prescription end date); and b) control of T2DM (fasting glucose <126 and/or HbA1c <7% occurring ≥90 days after last prescription end date).
Of interest were both the occurrence and timing of diabetes remission, so a Cox proportional hazards analytic framework was chosen to investigate the impact of bariatric surgery on remission. Time of entry into the cohort was defined as the date of the first BMI ≥35 in the electronic medical record. Censoring was for: a) disenrollment from the health plan (loss to follow-up of medication and laboratory data); b) disenrollment from pharmacy coverage (loss to follow-up of medication data); c) pregnancy; d) new diagnoses of cancer, ascites, or peritoneal effusion, e) reaching one’s 80th birthday, or f) end of the study period (December 31, 2008). Model specification ensured that the primary exposure, bariatric surgery, was time-dependent at least over the course of observed person-time at risk. This permitted observation of person-time both presurgery and postsurgery.
For all three outcomes, we examined unadjusted and fully adjusted models. Adjustment covariates included study site, calendar year of eligibility date, sex, baseline age, HbA1c, diabetes medication use, and T2DM duration. Propensity scores were not used for model adjustment. Diabetes duration was defined as the time since the subject was first observed to meet one of our seven T2DM criteria. Study site and calendar year of eligibility were adjusted for via stratification of the baseline hazard function. Sensitivity analyses explored the effect of excluding individuals with missing pre-surgical BMI. All analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and R 2.10 (R Foundation for Statistical Computing, Vienna, Austria).
Results
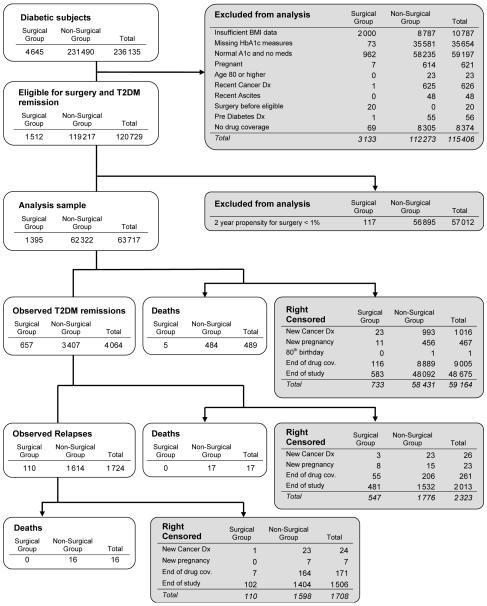
Our core eligibility criteria identified 4645 adults with uncontrolled or medication-controlled T2DM who had bariatric surgery at one of the three participating sites from 2005 to 2008 and 231490 obese (BMI ≥35 kg/m2) diabetic adults that did not undergo surgery (Figure 1). After applying our exclusion criteria, our main cohort was 1512 adults with uncontrolled or medication-controlled T2DM and a BMI ≥35 kg/m2 who had bariatric surgery and 119217 that did not undergo surgery. Our propensity modeling approach was applied to this cohort (see Methods and online-only material), further excluding 7.7% of surgical cases and 47.7% of nonsurgical subjects who had a two-year propensity of undergoing bariatric surgery of <0.01. Thus, our final cohort comprised 1395 adults with uncontrolled or medication-controlled T2DM who had bariatric surgery and 62322 who did not have surgery.
Figure 1. Flow diagram.
Inclusion, exclusion, remission, relapse, death, and censoring for adults with uncontrolled or medication-controlled type 2 diabetes mellitus, with and without bariatric surgery.
The characteristics of our final analytic cohort are presented in Table 1. Nonsurgical subjects, compared to surgical subjects, were slightly older on average (49.1 vs. 48.2 years), less often female (73% vs. 80%), had lower average BMI values (42.6 vs. 47.4), and included a substantially larger proportion with a BMI between 35.0 and 39.9 (43% vs. 15%). A greater proportion of surgical subjects had an HbA1c value below 7% (36% vs. 33%). Current insulin use was found among 24% of surgical subjects and 20% of nonsurgical subjects. Overall, 13% of all subjects were not using either oral agents or insulin at baseline. Among the 1395 surgical subjects in our cohort, 23 (1.6%) were censored prior to surgery for developing an exclusion condition and 72 subjects met T2DM remission criteria prior to undergoing surgery; thus, 1300 subjects in our final analytic model experienced bariatric surgery during follow-up. Most bariatric procedures were RYGB (72.0% laparoscopic; 8.2% open); 4.4% were AGB, 2.4% were sleeve gastrectomy, 0.4% were vertical banded gastroplasty, and 12.7% were coded using more than one different bariatric procedure code; however, among the 12.7% procedures that had more than one procedure code, 97% of those included at least one code indicating either open or laparoscopic RYGB.
Table 1.
Characteristics for subjects with 2-year bariatric surgery propensity of 1% or higher
| At date of eligibility | At surgery | ||
|---|---|---|---|
| Bariatric patients N=1 395 |
Non-surgical controls N=62 322 |
Bariatric patients N=1 300a |
|
| Age, mean (sd) | 48.2 (9.3) | 49.1 (9.3) | 49.2 (9.2) |
|
| |||
| Female, n (%) | 1 116 (80%) | 45 188 (73%) | 1 034 (80%) |
|
| |||
| Site, n (%) | |||
|
| |||
| Site One | 128 (9%) | 2 175 (3%) | 120 (9%) |
|
| |||
| Site Two | 596 (43%) | 27 133 (44%) | 552 (42%) |
|
| |||
| Site Three | 671 (48%) | 33 014 (53%) | 628 (48%) |
|
| |||
| Calendar Year, n (%) | |||
|
| |||
| 2005 | 222 (16%) | 6 626 (11%) | 51 (4%) |
|
| |||
| 2006 | 532 (38%) | 1 284 (28%) | 218 (17%) |
|
| |||
| 2007 | 568 (41%) | 27 242 (44%) | 434 (33%) |
|
| |||
| 2008 | 73 (5%) | 11 170 (18%) | 597 (46%) |
|
| |||
| Body Mass Index, mean (sd) | 47.4 (7.6) | 42.6 (6.6) | 45.9 (7.6) |
|
| |||
| Body Mass Index category, n (%) | |||
|
| |||
| Below 35 | 0 (0%) | 0 (0%) | 27 (2%) |
|
| |||
| 35 to 40 | 211 (15%) | 26 746 (43%) | 260 (20%) |
|
| |||
| 40 to 45 | 393 (28%) | 17 925 (29%) | 382 (29%) |
|
| |||
| 45 to 50 | 371 (27%) | 9 902 (16%) | 324 (25%) |
|
| |||
| 50 and higher | 420 (30%) | 7 749 (12%) | 307 (24%) |
|
| |||
| Years since T2DM diagnosis, mean (sd) | 4.4 (3.8) | 4.3 (3.9) | 5.5 (3.9) |
|
| |||
| HbA1c percentage, n (%) | |||
|
| |||
| Below 7% | 505 (36%) | 20 586 (33%) | 699 (54%) |
|
| |||
| 7% to 8% | 422 (30%) | 18 391 (30%) | 311 (24%) |
|
| |||
| 8% to 9% | 196 (14%) | 9 085 (15%) | 144 (11%) |
|
| |||
| 9% to 10% | 139 (10%) | 6 484 (10%) | 76 (6%) |
|
| |||
| 10% and higher | 133 (10%) | 7 776 (12%) | 70 (5%) |
|
| |||
| Diabetes medication use, n (%) | |||
|
| |||
| Insulin | 336 (24%) | 12 694 (20%) | 303 (23%) |
|
| |||
| Sulfonylurea | 525 (38%) | 23 547 (38%) | 501 (39%) |
|
| |||
| Metformin | 890 (64%) | 39 661 (64%) | 778 (60%) |
|
| |||
| TZD | 223 (16%) | 7 759 (12%) | 198 (15%) |
|
| |||
| Other oral medication | 26 (2%) | 755 (1%) | 34 (3%) |
|
| |||
| Combined medications, n (%) | |||
|
| |||
| None | 186 (13%) | 8 127 (13%) | 218 (17%) |
|
| |||
| Oral medication only | 873 (63%) | 41 501 (67%) | 779 (60%) |
|
| |||
| Insulin only | 87 (6%) | 4 594 (7%) | 93 (7%) |
|
| |||
| Oral and insulin | 249 (18%) | 8 100 (13%) | 210 (16%) |
Among bariatric patients, 23 individuals were censored and 72 remitted diabetes before surgery
b; T2DM diabetes mellitus; HbA1c, hemoglobin A1c; TZD, thiazolidinediones; date of eligibility – first date meeting definition of unremitted T2DM, body mass index ≥35, and age ≥18 years.
Table 2 shows primary T2DM remission analyses, both unadjusted and fully adjusted Cox model results. Our unadjusted Cox model estimated probability of T2DM remission within 2 years was 73.7% (95% CI: 70.6, 76.5) for patients exposed to surgery, and 6.9% (95%CI: 6.9, 7.1) among non-surgical patients. Bariatric subjects experienced a significantly higher hazard of T2DM remission than nonsurgical subjects (unadjusted hazard ratio [HR]: 18.8; 95% CI: 17.2, 20.6), indicating that severely obese subjects with diabetes who received bariatric surgery were 18.8 times more likely to experience T2DM remission during follow-up than those receiving usual care and still at risk. Older subjects were significantly less likely to remit. A longer time since T2DM diagnosis was significantly associated with lower T2DM remission, as was higher HbA1c and more intensive medication treatment. Notably, the unadjusted effect of surgery exposure on T2DM remission differed significantly by health plan site, with two site-specific remission HRs significantly higher than the third; however, all three site-specific remission HRs associated with surgery receipt exceeded 10.
Table 2.
Unadjusted and adjusted diabetes remission hazard ratio estimates
| Unadjusted Estimates |
Adjusted Estimatesa,b | |
|---|---|---|
| Time-Varying | ||
| Bariatric Surgery | ||
|
| ||
| Pooled samplec | 18.79 (17.18, 20.56) | – |
|
| ||
| Site-specific surgery effects | ||
|
| ||
| Site One | 10.25 (7.75, 13.56) | 12.35 (9.24, 16.51) |
|
| ||
| Site Two | 21.07 (18.50, 24.01) | 23.92 (20.92, 27.34) |
|
| ||
| Site Three | 18.37 (16.07, 21.01) | 16.91 (14.70, 19.44) |
|
| ||
| Time-invariant (measured at Index) | ||
|
| ||
| Female | 1.05 (0.97, 1.12) | 1.03 (0.96, 1.10) |
|
| ||
| Age at eligibility (5-year increase) | 0.91 (0.90, 0.93) | 0.97 (0.96, 0.99) |
|
| ||
| Site | ||
|
| ||
| Site One | 1 (referent) | – |
|
| ||
| Site Two | 0.55 (0.49, 0.63) | – |
|
| ||
| Site Three | 0.55 (0.49, 0.62) | – |
|
| ||
| Calendar year of eligibility date | ||
|
| ||
| 2005 | 1 (referent) | – |
|
| ||
| 2006 | 1.03 (0.93, 1.14) | – |
|
| ||
| 2007 | 1.22 (1.11, 1.35) | – |
|
| ||
| 2008 | 1.71 (1.49, 1.97) | – |
|
| ||
| Years since T2DMd diagnosis | 0.85 (0.85, 0.86) | 0.90 (0.89, 0.91) |
|
| ||
| Body Mass Index | ||
|
| ||
| 35 to 40 | 1 (referent) | 1 (referent) |
|
| ||
| 40 to 45 | 1.24 (1.15, 1.34) | 1.14 (1.06, 1.23) |
|
| ||
| 45 to 50 | 1.38 (1.26, 1.51) | 1.21 (1.10, 1.32) |
|
| ||
| 50 and higher | 1.51 (1.38, 1.66) | 1.18 (1.08, 1.30) |
|
| ||
| HbA1c category | ||
|
| ||
| Below 7% | 1 (referent) | 1 (referent) |
|
| ||
| 7% to 8% | 1.32 (1.23, 1.41) | 0.59 (0.54, 0.64) |
|
| ||
| 8% to 9% | 0.51 (0.45, 0.57) | 0.30 (0.26, 0.34) |
|
| ||
| 9% to 10% | 0.44 (0.38, 0.51) | 0.27 (0.24, 0.32) |
|
| ||
| 10% and higher | 0.38 (0.33, 0.44) | 0.22 (0.19, 0.26) |
|
| ||
| Combined diabetes medications | ||
|
| ||
| None | 1 (referent) | 1 (referent) |
|
| ||
| Oral medication only | 0.20 (0.19, 0.22) | 0.15 (0.14, 0.16) |
|
| ||
| Insulin only | 0.18 (0.16, 0.21) | 0.22 (0.19, 0.26) |
|
| ||
| Oral and insulin | 0.07 (0.06, 0.08) | 0.08 (0.07, 0.10) |
A total of 4 064 remissions occurred among 63 717 individuals who contributed person-time to the analysis of time to diabetes remission. All subjects contributed to both unadjusted and adjusted models above.
Adjusted model contained all covariates shown, and stratified the baseline hazard on site and calendar year of eligibility, hence no main effects are estimated for those two factors.
The adjusted model contained a significant interaction between site and bariatric surgery exposure, and so only site-specific surgery effects are presented. Unadjusted site-specific effects were based on a model containing site, surgery exposure, and their interaction only.
; T2DM type 2 diabetes mellitus; HbA1c, hemoglobin A1c; TZD, thiazolidinediones
Table 2 shows multivariable adjusted Cox model results. After adjusting for site, calendar year, sex, age, years since diabetes diagnosis, HbA1c category, and diabetes medication category, subjects who underwent bariatric surgery remained significantly more likely to remit their diabetes than those who continued usual care. Again our results indicated significant variability in T2DM remission by health plan site, so site-specific HRs are reported: Site One (HR 12.4, 95% CI: 9.2, 16.5), Site Two (HR 23.9, 95% CI: 20.9, 27.3), and Site Three (HR 16.9, 95% CI: 14.7, 19.4). Again, age, years since T2DM diagnosis, HbA1c level, and intensity of medication treatment were strongly associated with T2DM remission rate.
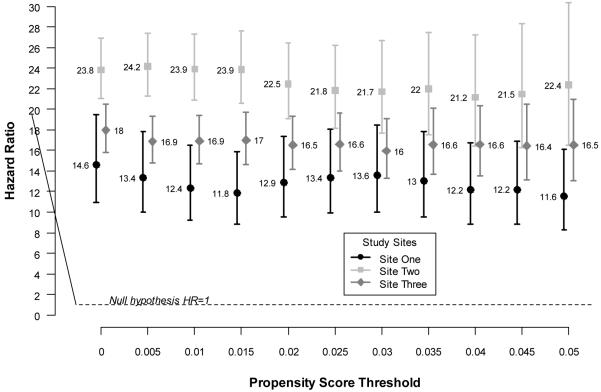
A series of sensitivity analyses examined the impact of choice of propensity score threshold on our main results. Figure 2 shows site-specific HRs and 95% CIs for T2DM remission across a range of propensity thresholds from 0.0 (no surgical or nonsurgical subjects excluded based on propensity) to 0.05 (66.2% of surgical subjects and 94.4% of nonsurgical subjects excluded; see online-only materials for propensity method). The results indicate that our main results were robust to a wide range of propensity threshold choices.
Figure 2. Adjusted site-specific hazard ratios for diabetes remission across a range of propensity thresholds.
Site-specific hazard ratios and confidence intervals for diabetes remission across propensity thresholds. Models were adjusted for age, sex, calendar year, body mass index, HbA1c, diabetes medication category and duration of diabetes at date of eligibility.
We conducted additional analyses to explore the heterogeneity of treatment effect by site. We found that the differences in HRs for remission followed closely with variations in the frequency of fasting glucose measurement during follow-up, with little variation in diabetes medication discontinuation or HbA1c measurement. Furthermore, we conducted sensitivity analyses to assess the potential impact of excluding bariatric subjects who were missing pre-operative BMI values (because their surgery occurred prior to implementation of our electronic medical record systems allowing easy capture of BMI data during clinical care). We used Kaplan-Meyer analyses to compare the time to diabetes remission among bariatric subjects with and without baseline BMI values and found that these plots were not significantly different.
Bariatric surgery and nonsurgical treatment also differed significantly in T2DM relapse rates among the 4064 subjects who experienced an initial T2DM remission event. Unadjusted Cox estimates of the probability of relapse within 2 years following initial remission was 24.4% (95% CI: 19.7, 28.8) among surgical subjects, and 71.9% (95% CI: 69.1, 74.5) among nonsurgical subjects. Our multivariable adjusted analyses indicated that those who underwent bariatric surgery were significantly less likely to experience T2DM relapse (adjusted HR = 0.19; 95% CI: 0.15 to 0.23). Finally, no statistically significant differences were observed between the bariatric surgical and nonsurgical treatment groups for risk of death within 2 years (adjusted HR = 0.54; 95% CI: 0.22 to 1.23).
Discussion
Diabetes is a highly prevalent health condition among adults with severe obesity and is associated with significant morbidity, premature death, and excess health care expenditures. In this large, retrospective multicenter cohort study, we found that severely obese adults with T2DM who choose to undergo bariatric surgery (80.2% RYGB) had superior rates of remission of T2DM than those who chose to continue usual medical care. The effect of bariatric surgery persisted after adjusting for age, sex, site, BMI, and several indicators of T2DM severity, including years since T2DM diagnosis, HbA1c level, and intensity of medication treatment. The main result of our study is consistent with prior research in smaller samples from single clinical centers, and the main strengths of this study are that it extends the prior literature by confirming this finding in a multisite cohort involving more than 40 bariatric surgeons and 20 surgical centers across three integrated health plans while using pragmatic inclusion and exclusion criteria.[7]
Bariatric surgery generally refers to surgical procedures that are performed for the purpose of helping severely obese people lose weight and to treat, as well as prevent, some obesity-related complications. This current study was only designed to examine bariatric surgery that was being done in this context – that is, to induce weight loss among morbidly obese patients who also happen to have T2DM. We cannot generalize our findings to all patients with T2DM, especially those with BMI less than 35 or those with diet controlled diabetes, who were necessarily excluded from our analyses. Indeed, there is good reason to believe that the distinction is important. Preliminary evidence exists that bariatric surgery that has been performed specifically to treat T2DM (i.e., “metabolic surgery”) may be less effective in inducing T2DM remission than what has been seen among more unselected populations of morbidly obese patients.[13] In our study we primarily examined RYGB procedures (80.2%), and we had few patients who underwent AGB (4.4%) or sleeve gastrectomy (2.4%). Thus, we were unable to address the question as to whether RYGB is superior to AGB and sleeve gastrectomy for diabetes remission. In sensitivity analyses that restricted to RYGB only, we found that the hazard ratios for remission increased slightly, albeit not to a statistically significant degree. Further research is needed to examine these comparative effectiveness questions.
Consistent with prior studies, we found that the likelihood of T2DM remission was strongly associated with several indicators of T2DM severity. The longer severely obese subjects had been diagnosed with T2DM, the less likely they were to remit it after bariatric surgery. Similarly, subjects with more poorly controlled T2DM (higher HbA1c levels) were significantly less likely to remit their T2DM than those with lower HbA1c levels. Finally, a higher intensity of medication treatment was associated with lower remission rates. All these indicators suggest that subjects with more advanced T2DM (i.e., less beta cell function) are less likely to achieve euglycemia without medical therapy after bariatric surgery.
While a few other studies have documented significant rates of diabetes relapse after initial remission following bariatric surgery, [14-16] Our study is the first to clearly document that the rate of T2DM relapse is significantly lower among those who underwent bariatric surgery compared to those who initially remitted T2DM using nonsurgical approaches, at least in the short term. While our study indicates that nearly one-quarter of bariatric patients who initially resolve their diabetes will relapse within two years, the UKPDS and DCCT/EDIC studies demonstrated that even a transient period of aggressive glycemic control can induce a beneficial “metabolic memory” effect and reduce incident microvascular events.[17-21] Thus, it is possible that patients who eventually relapse their type 2 diabetes after bariatric surgery will still continue to experience reduced microvascular and macrovascular complications long-term compared to those never experience a relapse of type 2 diabetes.
We did not observe a statistically significant survival benefit in our study; however, given our short follow-up time, the absence of evidence should not be equated to evidence for absence of survival benefit. Prior research suggests that surgical treatment of obesity is likely to yield a significant survival benefit for the average bariatric patient over the long-term,[22, 23] and our point estimate for mortality (HR 0.54) suggests a strong trend toward reduction in mortality that favors surgery. Those prior studies had larger samples of surgical cases and longer-term follow-up than our current study.[22, 23] It is important to consider, however, that some higher-risk patient populations may experience less survival benefit from surgery.[24]
This study represents the largest and most representative study to date on the question of the effect of bariatric surgery on T2DM remission. Because our study was conducted in the setting of three large health insurance plans with subjects that were predominantly enrolled in employer-based commercial products, the outcomes we observed are likely to generalize to other commercial health insurance populations in the United States. Given the substantial morbidity, mortality, and health care cost implications of type 2 diabetes, our finding of the superiority of bariatric surgical treatment in this population – when combined with evidence from recent meta-analyses and randomized trials[7, 25] – should be a strong indicator for more consistent coverage of bariatric surgery for adult patients with type 2 diabetes.
The retrospective nature of our study has several important limitations. Key information used to identify eligible subjects and to indicate our outcomes of interest (e.g., BMI and fasting glucose measures) were collected for the purpose of clinical care, not specifically for the goal of conducting research. Thus, we found extensive missingness among several key data elements – most notably BMI values at baseline. This was due to the late implementation of electronic medical records at each of the study sites – which occurred after receiving bariatric surgery for many patients. Despite these missing values, our sensitivity analyses indicate that surgical patients with and without baseline BMI experienced similar rates and time to diabetes remission. We were also unable to assess the relationship between weight changes and outcomes. Given the important role that the amount of weight loss appears to play in determining diabetes remission,[26] significant weight regain might reasonably return patients to a diabetic state. Two recent small case series found that greater weight regain increased the likelihood of diabetes recurrence.[15, 16] Buchwald and colleagues' review of 621 studies suggested that patients with diabetes actually lose more weight in the first 2 years after bariatric surgery than those without diabetes.[7] Beyond 2 years postsurgery, those with diabetes had weight loss similar to those without. Other studies refute this claim, suggesting that those with diabetes lose less weight in the first 2 years after surgery than patients who do not have diabetes.[27-29] Clearly more work is needed in this area to clarify the relationship between long-term changes in body weight and durable diabetes remission.
Furthermore, our analyses of the heterogeneity of treatment effects by site indicated significant variations in missing data across sites (especially measures of fasting glucose), which could be related to differences in data management (e.g., handling of laboratory data from different labs) or true differences in clinical care (e.g., clinical guidelines that emphasize HbA1c surveillance above fasting glucose). We acknowledge that the observed differences in measurement of fasting glucose after surgery might not fully explain the site-specific variations in hazard ratios, and this issue likely warrants further investigation. However, while the magnitude of the site-to-site variability was seemingly non-negligible on the hazard ratio scale, all of the hazard ratios were extremely large and in the same direction. Thus, the key conclusion at each site remains the same – that bariatric surgery is superior to non-surgical treatment in inducing remission of T2DM among severely obese patients. As a final limitation, given the relatively small sample of patients receiving procedures other that RYGB, we were unable to assess heterogeneity of treatment effects by surgery type in this study.
In conclusion, we found in this large, multicenter study of patients with severe obesity and type 2 diabetes that underwent bariatric surgery that remission of diabetes occurs much more frequently after surgery than under the conditions of usual medical care. These findings are consistent with prior small studies, and taken as a whole, suggest that bariatric surgery is likely to be a superior treatment alternative to usual diabetes care among severely obese patients, especially when the goal of treatment is T2DM remission. The likelihood of remission appears to be strongest among severely obese patients who are earlier in the course of their disease (shorter T2DM duration, lower HbA1c levels, and less intensive medication treatment). Thus, the option of bariatric surgery should be discussed with all severely obese diabetic patients soon after diagnosis to provide them with the greatest opportunity to achieve remission.
Supplementary Material
Acknowledgements
Funding/Support: This research was conducted by Group Health Research Institute, HealthPartners, Kaiser Permanente Northern California, Kaiser Permanente Southern California, and the University of Wisconsin. This project was funded under Contract No. HHSA290-2005-0033-I-TO10-WA1 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services.
Role of the Sponsor: The authors of this article are responsible for its content. No statement may be construed as the official position of the Agency for Healthcare Research and Quality of the U. S. Department of Health and Human Services. The sponsor provided feedback to the authors on the preliminary study design and analysis as well as review of the manuscripts. However, the sponsor had no role in the conduct of the study; collection, management, analysis, and interpretation of the data; or preparation and approval of the manuscript.
Footnotes
Author Contributions: Dr. Arterburn had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Arterburn, Bogart, Coleman, Haneuse, Selby, Sherwood, Sidney, and O’Connor
Acquisition of data: Arterburn, Coleman, Selby, Sherwood, Sidney, Theis, and O’Connor
Analysis and interpretation of data: Arterburn, Bogart, Coleman, Haneuse, Selby, Sherwood, Sidney, Theis, Campos, McCulloch, and O’Connor
Drafting of the manuscript: Arterburn, Bogart, Coleman, and Haneuse
Critical revision of the manuscript for important intellectual content: Arterburn, Bogart, Coleman, Haneuse, Selby, Sherwood, Sidney, Theis, Campos, McCulloch, and O’Connor
Statistical analysis: Arterburn, Bogart, Haneuse, Theis
Obtaining funding: Arterburn, Selby, Campos, and O’Connor Administrative, technical, or material support: Arterburn, Coleman, Selby, Sherwood, Sidney, and O’Connor
Study supervision: Selby, Sidney, and O’Connor
Financial Disclosures: None reported
Additional Contributions: The team would like to gratefully acknowledge the contributions of our project managers (Rene Hawkes, Cathy Chou, and Mark Pierce) and programmers (Mary Becker, Mike Sorel, and Julie Liu). We would also like to acknowledge the scientific and clinical input of our Technical Expert Panel members: Edward H. Livingston, MD, FACS (University of Texas Southwestern); David R. Flum, MD, MPH, FACS (University of Washington); and Melinda Maggard Gibbons, MD (University of California at Los Angeles).
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005 Apr 20;293(15):1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 2.Buchwald H. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005 May-Jun;1(3):371–81. doi: 10.1016/j.soard.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995 Sep;222(3):339–50. doi: 10.1097/00000658-199509000-00011. discussion 50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arterburn D. Bariatric surgery. Bmj. 2008;337:a755. doi: 10.1136/bmj.a755. [DOI] [PubMed] [Google Scholar]
- 5.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Spitz AF, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 2009 Apr;17(Suppl 1):S1–70. doi: 10.1038/oby.2009.28. v. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009 Apr;33(Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009 Mar;122(3):248–56. doi: 10.1016/j.amjmed.2008.09.041. e5. [DOI] [PubMed] [Google Scholar]
- 8.Coverage Decision Memorandum for Surgery for Diabetes. 2008.
- 9.Kessler RM, Eckstein B. Obesity: Health Insurance Plans Respond to a Public Health Challenge. AHIP Coverage [serial on the Internet] 2005 [PubMed] [Google Scholar]
- 10.International Expert C International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009 Jul;32(7):1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yermilov I, McGory ML, Shekelle PW, Ko CY, Maggard MA. Appropriateness criteria for bariatric surgery: beyond the NIH guidelines. Obesity (Silver Spring) 2009 Aug;17(8):1521–7. doi: 10.1038/oby.2009.78. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression Models and Life Tables. Journal of the Royal Statistical Society Series B. 1972;34(2):187–220. [Google Scholar]
- 13.Demaria EJ, Winegar DA, Pate VW, Hutcher NE, Ponce J, Pories WJ. Early postoperative outcomes of metabolic surgery to treat diabetes from sites participating in the ASMBS bariatric surgery center of excellence program as reported in the Bariatric Outcomes Longitudinal Database. Ann Surg. 2010 Sep;252(3):559–66. doi: 10.1097/SLA.0b013e3181f2aed0. discussion 66-7. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 15.Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010 May-Jun;6(3):254–9. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010 May-Jun;6(3):249–53. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Writing Team for the Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Research G Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003 Oct 22;290(16):2159–67. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. Am J Ophthalmol. 2000 May;129(5):704–5. doi: 10.1016/s0002-9394(00)00453-0. [DOI] [PubMed] [Google Scholar]
- 19.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006 Dec;55(12):3556–65. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 21.Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep. 2010 Nov;12(6):432–9. doi: 10.1007/s11883-010-0128-1. [DOI] [PubMed] [Google Scholar]
- 22.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007 Aug 23;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 23.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 24.Maciejewski ML, Livingston EH, Smith VA, Kavee AL, Kahwati LC, Henderson WG, et al. Survival among high-risk patients after bariatric surgery. JAMA. 2011 Jun 15;305(23):2419–26. doi: 10.1001/jama.2011.817. [DOI] [PubMed] [Google Scholar]
- 25.Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Jama. 2008 Jan 23;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 26.Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009 Jan 20;150(2):94–103. doi: 10.7326/0003-4819-150-2-200901200-00007. [DOI] [PubMed] [Google Scholar]
- 27.Campos GM, Rabl C, Mulligan K, Posselt A, Rogers SJ, Westphalen AC, et al. Factors associated with weight loss after gastric bypass. Arch Surg. 2008 Sep;143(9):877–83. doi: 10.1001/archsurg.143.9.877. discussion 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbonell AM, Wolfe LG, Meador JG, Sugerman HJ, Kellum JM, Maher JW. Does diabetes affect weight loss after gastric bypass? Surg Obes Relat Dis. 2008 May-Jun;4(3):441–4. doi: 10.1016/j.soard.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008 Feb;12(2):250–5. doi: 10.1007/s11605-007-0427-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.