Abstract
DJ-1 mutation induces early-onset Parkinson's disease, and conversely over-expression of DJ-1 is associated with cancer in numerous tissues. A gene-trap screening library conducted in embryonic stem cells was utilized for generation of a DJ-1 mutant mouse. Real-time PCR and immunoblotting were utilized to confirm functional mutation of the DJ-1 gene. Normal DJ-1 protein expression in adult mouse tissue was characterized and demonstrates high expression in brain tissue with wide systemic distribution. Primary astrocytes isolated from DJ-1−/− mice reveal a decreased nuclear localization of DJ-1 protein in response to rotenone or LPS, with a concomitant increase in mitochondrial localization of DJ-1 found only in the rotenone exposure. Resting mitochondrial membrane potential was significantly lower in DJ-1−/− astrocytes, as compared to controls. Our DJ-1 knockout mouse provides an exciting tool for exploring the molecular and physiological roles of DJ-1 to further explicate its functions in neurodegeneration.
Keywords: DJ-1, Parkinson's disease, Gene-trap, Mitochondria
1. Introduction
Mutations in DJ-1 are associated with the development of early-onset Parkinson's disease (PD) (Bonifati et al., 2003a). Numerous cellular functions have been proposed for DJ-1, including responding to the cellular redox environment (Yokota et al., 2003; Martinat et al., 2004; Taira et al., 2004; Takahashi-Niki et al., 2004; Kim et al., 2005), a variety of protein–protein interactions (Takahashi et al., 2001; Meulener et al., 2005; Xu et al., 2005; Bretaud et al., 2007; Fan et al., 2008), and plausible chaperone activity (Martinat et al., 2004). However, the precise role DJ-1 plays in cellular homeostasis and how its dysregulation contributes to PD has not been ascertained.
Although DJ-1 mutation induces early onset PD, DJ-1 knockout mice fail to consistently recapitulate the hallmarks of PD including loss of dopaminergic neurons, decrease in dopamine and formation of Lewy bodies, (Chen et al., 2005; Goldberg et al., 2005; Kim et al., 2005; Yamaguchi and Shen, 2007). Kim and colleagues found increased sensitivity of DJ-1−/− neurons to hydrogen peroxide and rotenone, and increased sensitivity of the mutant mouse to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) (Kim et al., 2005). Several independent studies have noted locomotor impairment in DJ-1−/− mice, though other parkinsonian hallmarks were not observed (Chen et al., 2005; Goldberg et al., 2005; Yamaguchi and Shen, 2007). Therefore, mutation of DJ-1 alone is likely not sufficient to induce parkinsonism in the mouse, however these mice provide an excellent platform for investigating Parkinson's as a multi-factorial disease and the role of DJ-1 in this disease process.
An essential element in unraveling the pathogenesis of PD is defining how astroglial dysregulation is involved in the progression of neuronal injury. Reactive gliosis and concomitant neuroinflammation are implicated in the pathogenesis and progression of idiopathic PD (Wu et al., 2002) and neurodegeneration induced by the mitochondrial toxins MPTP (Kohutnicka et al., 1998) and rotenone (Sherer et al., 2003). Furthermore, high DJ-1 immunore-activity is observed in reactive astrocytes (Neumann et al., 2004), suggesting it may play a role in modulating gliosis. Collectively, numerous studies have suggested that compromising astrocytic function could be a significant mechanism in PD neurodegeneration.
Lipopolysaccharide (LPS) is a component of the outer membrane of Gram-negative bacteria, and may potently activate mammalian cells via the NF-κB pathway, resulting in production of proinflammatory cytokines (Chow et al., 1999). In PD, elevated levels of cytokines are observed in the brains of patients (Boka et al., 1994; Mogi et al., 2000), as well as concomitant oxidative stress, which are both thought to be significant in disease development. LPS is well known to induce expression of proinflammatory genes in astrocytes, which produce various inflammatory mediators including cytokines, nitric oxide, and prostanoids (Lieberman et al., 1989; Kong et al., 1997). Animals exposed to LPS provide excellent evidence that neuroinflammation is significant in PD, as progressive loss of dopaminergic neurons is observed (Qin et al., 2007) and maternal exposure to bacterial LPS during pregnancy decreases the number of dopaminergic neurons in offspring (Ling et al., 2002; Carvey et al., 2003). Moreover, expression of DJ-1 increases and DJ-1's isoelectric point shifts following exposure to LPS (Ejima et al., 2000; Mitsumoto and Nakagawa, 2001) indicating DJ-1 may modulate the cellular response to inflammatory stimuli.
A number of epidemiological studies demonstrate a strong correlation between pesticide exposure and an increased incidence of PD (Gorell et al., 1998; Vanacore et al., 2002). Additionally, exposure to agriculture pesticides such as rotenone in rodents has recapitulated parkinsonism (Betarbet et al., 2000). When treated in vitro with rotenone, DJ-1−/− neurons had significantly decreased survival compared to DJ-1+/+ neurons (Kim et al., 2005). Additionally, chronic rotenone exposure induces oxidative modification of DJ-1 and redistribution of DJ-1 to the mitochondria (Betarbet et al., 2006).
Taking into consideration the complex etiology of PD, our experiments coupled DJ-1 mutation with exposure to rotenone or LPS, focusing upon a possible mitochondrial phenotype. Our hypothesis is that DJ-1 mutation induces dysregulation of cellular function in astrocytes.
2. Materials and methods
2.1. DJ-1 knockout mouse
A gene-trap screening library conducted in embryonic stem cells was utilized for creation of our knockout mouse. The DJ-1 gene was mutated and chimeric mice were generated by BayGenomics (San Francisco, CA). Utilizing 5′ rapid amplification of cDNA ends, the spliced sequence upstream of the gene-trap vector was ascertained and included 6 of 7 exons, thus positioning the gene-trap vector within the 6th intron, an approximately 3.5 kbp region. Genotyping was accomplished via amplifying a sequence of neomycin resistance gene within the inserted gene-trap vector (Neo primers, 5′-CTT GGG TGG AGA GGC TAT TC-3′, 5′-GTG AGA TGA CAG GAG ATC-3′) combined with amplification of a sequence of DNA spanning the region of the gene-trap vector insertion (DJ-1 primers, 5′-ACC CTT GCA GTC ACT TTA CC-3′, 5′-TAG CTG GCA GGA GCT TGG-3). Heterozygous mice were bred to establish a colony containing all three genotypes. Primary cortical astrocytes were isolated as previously described (Allen et al., 2000), and maintained at 37 ° and 5% CO2 in MEM with EBSS and l-glutamine (Hyclone) supplemented with 10% fetal bovine serum and 1% penicillin streptomycin neomycin (Invitrogen).
2.2. Reagents
Unless otherwise specified, all reagents were purchased from Sigma–Aldrich.
2.3. Real-time PCR
RNA was isolated from snap frozen tissue samples using the Qiagen RNeasy kit, subjected to DNase I treatment, then reverse-transcribed via iScript kit (BioRad). cDNA was analyzed via real-time PCR by assessing SYBR green (BioRad) incorporation over time on a BioRad iCycler. Expression of DJ-1 (5′-ATC TGA GTC GCC TAT GGT GAA G-3′, 5′-ACC TAC TTC GTG AGC CAA CAG-3′) or VEGF (5′-AGG CTG CTG TAA CGA TGA AG-3′, 5′-TTC TGG CTT TGT TCT GTC TTT C-3′ was normalized to β-actin (5′-GAC AGG ATG CAG AAG GAG ATT ACT G-3′, 5′-GCT GAT CCA CAT CTG CTG GAA-3′) via the delta–delta CT method (Livak and Schmittgen, 2001). Samples without reverse transcriptase were also analyzed to assure no DNA contamination was present.
2.4. Western blotting
Tissues were snap frozen immediately after harvest and protein was isolated by homogenizing tissue in lysis buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 1% nonidet P-40, 0.5% Na deoxycholate, 2 mM Na orthovanadate) supplemented with complete mini protease cocktail inhibitors (Roche). Samples were sonicated for 10 s at 30% output, placed on ice for 20 min, then centrifuged at 14,000 rpm for 10 min at 4 °. Astrocyte protein from primary cultures was harvested in lysis buffer (see above), placed on ice for 5 min, and then centrifuged as above. Protein content of the supernatant was determined by BCA protein assay (Pierce). Protein was subjected to SDS-PAGE, electrotransferred onto PVDF (VWR) and probed with anti-Bax antibody (Cell Signaling, 1:1000), anti-DJ-1 antibody (Neuromics, 1:2000) alone or with β-actin (Sigma, 1:1000) and detected via chemiluminescence.
2.5. Subcellular localization
Images were obtained using a Zeiss Axiovert 200 M microscope equipped with a Hammatsu ORCA-ER cooled charge-coupled device camera. Primary cortical astrocytes were isolated from individual day 1–3 old mice as previously described (Aschner et al., 1992); mating of heterozygous mice was maintained in order to provide littermate controls. LPS (Salmonella enterica serotype typhimurium) was reconstituted in PBS and stored at −80 ° until use. Rotenone was reconstituted in DMSO the day of treatment. Astrocytes were treated with 10 μg/mL LPS + DMSO, 1 μM rotenone + PBS, or DMSO + PBS (control), for 24 h. The control group was exposed to both vehicles concurrently (i.e., DMSO + PBS) because astrocytes were exposed to LPS (in PBS) in combination with rotenone (in DMSO, data not shown), thus yielding coexposure to each vehicle. Following treatment, cells were loaded for 30 min with 500 nM MitoTracker Red (Invitrogen, excitation at λ579, emission at λ599), fixed in 4% paraformaldehyde, permeabilized with 0.01% Triton X-100, and DJ-1 primary antibody was applied (1:2000, Neuromics). AlexaFluor 488-conjugated secondary antibody (Invitrogen, excitation at λ495, emission at λ519) was added, and cells were mounted in DAPI-containing mounting media (Vector Laboratories, excitation at λ360, emission at λ460). Pearson's correlation coefficients, used to assess colocalization of AlexaFluor 488-conjugated DJ-1 with either the nucleus or the MitoTracker-labeled mitochondria, were calculated using SlideBook software.
2.6. Mitochondrial membrane potential
To assess mitochondrial membrane potential, the ratiometric dye JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide) was employed. JC-1 is a cationic dye exhibiting potential-dependent accumulation in the mitochondria, and accumulation is indicated by a shift in fluorescence emission from green to red. A decrease in the ratio of λ568:λ488 indicates formation of fewer J-aggregates, or increased mitochondrial depolarization (Reers et al., 1991). Astrocytes were loaded with 5 μM JC-1 (Invitrogen) for 20 min, washed once with MEM without phenol red supplemented with 10 mM HEPES, and images were collected every 2 min. After 4 images were collected (8 min), 1 μM rotenone or DMSO was added, and images were collected for 52 additional min for a total of 1 h sampling. Fluorescence intensity at λ568 and λ488 was measured using SlideBook software.
2.7. Statistical analyses
All statistical analyses were performed using GraphPad Prism software. Figures with three or more means were analyzed using ANOVA, and when means significantly differed (p < 0.05), Tukey's post hoc test was employed. Figures containing two means were analyzed with two-tailed t-tests; when variances significantly differed, Welch's correction was applied to correct for unequal variances.
3. Results
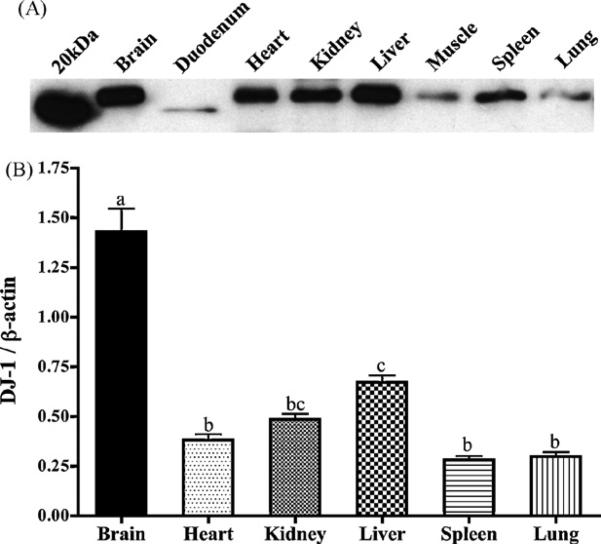
Western blot analysis was utilized to determine relative expression of DJ-1 in numerous tissues in the mouse (Fig. 1). Relative to expression of β-actin, brain tissue had the highest DJ-1 protein expression followed by liver, though DJ-1 was detected in all tissues assayed. While expression of β-actin can vary dramatically in differing tissue samples, standardizing DJ-1 protein to this housekeeping protein allows a relative quantitation of tissue distribution. Fig. 1A depicts expression of DJ-1 in 25 μg of protein from each tissue, and follows the overall pattern of distribution presented in Fig. 1B. Interestingly, DJ-1 in all duodenal samples assayed (n = 9) appeared at a lower molecular weight (approximately 19 kDa) than the protein in all other tissues. Curiously, the theoretical molecular weight of DJ-1 due to its 189 amino acid structure is 20 kDa, closer to the value observed in the duodenum than in the rest of the body. Therefore, it is feasible that modification(s) of non-gastrointestinal DJ-1 protein (i.e., glycosylation), result in a slightly higher molecular weight, while these are cleaved in the highly acidic gastrointestinal environment. Regardless, this lower molecular weight band is consistently present only in the duodenal samples, and thus is unlikely to be a non-specific band. Notably, DJ-1 is widely and systemically distributed, implicating perhaps a critical role in cell homeostasis for DJ-1.
Fig. 1.
DJ-1 protein is widely distributed in the mouse. (A) A representative blot depicts relative expression of DJ-1 in 25 μg of protein from each respective tissue. (B) DJ-1 protein levels were assayed and are expressed relative to β-actin in each tissue (n ≥ 4), and demonstrate high expression in brain tissue. Bars with differing letters are significantly different from one another (p < 0.05).
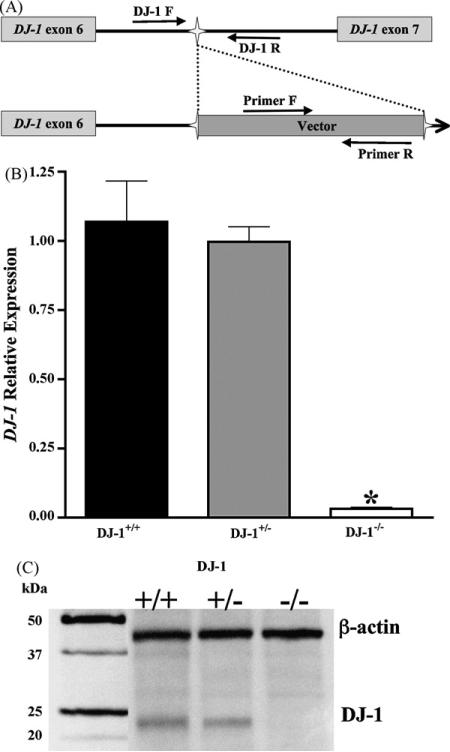
The precise location of the gene-trap vector insertion was determined and genotyping primers spanning the insertion site were created to assay for the normal DJ-1 genomic sequence (Fig. 2A). Additional genotyping primers specific to the neomycin-resistance gene were utilized to detect the inserted exogenous DNA, and allowed differentiation between all three genotypes. Functional mutation of the DJ-1 gene via insertion of the gene-trap vector was assessed in DJ-1+/+, DJ-1+/−, and DJ-1−/− brain tissue (Fig. 2). Real-time PCR of whole brain demonstrated that RNA expression of DJ-1 was greatly diminished in DJ-1−/− tissue compared to DJ-1+/+ (p < 0.001, Fig. 2B). Moreover DJ-1 protein is not detected in DJ-1−/− mice brain (Fig. 2C), therefore our mutant mouse model does indeed represent a functional knockout of DJ-1.
Fig. 2.
Gene-trap insertion into DJ-1 induces a functional mutation. (A) Schematic representation of the gene-trap in the endogenous DJ-1 gene. Following identification of the gene-trap insertion site, primers encompassing this sequence were designed, and, in combination with primers in the exogenous DNA, differentiation of DJ-1+/+, DJ-1+/−, or DJ-1−/− animals was accomplished. (B) DJ-1 mRNA expression was measured via real-time PCR in whole brain tissue. DJ-1−/− brain tissue has significantly decreased expression of DJ-1 compared to DJ-1+/+ or DJ-1+/− (n ≥ 4, p < 0.001). (C) DJ-1 protein expression is evident in DJ-1+/+ and DJ-1+/− animals, but absent in DJ-1−/−.
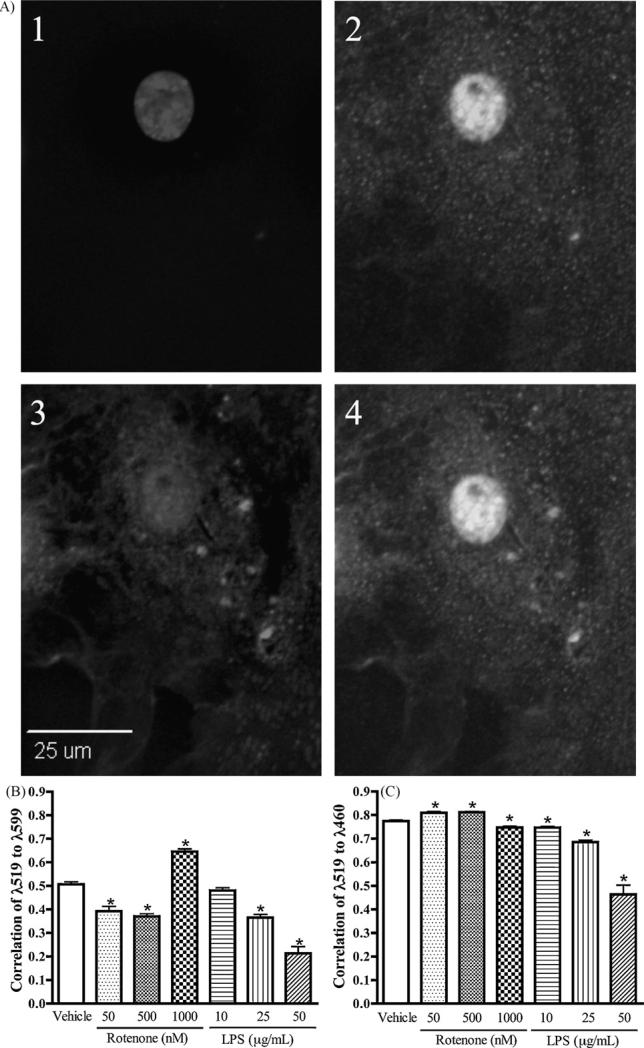
To further investigate the role of DJ-1 in neurodegenerative disease, subcellular localization of the protein in primary astrocytes was analyzed. DJ-1 is normally expressed in three subcellular pools, the cytosol, mitochondria, and nucleus. Therefore, to assess its localization following chemical exposure, the mitochondria were labeled with MitoTracker Red, the nucleus with DAPI, and DJ-1 with AlexaFluor 488. All DJ-1 not localized to the mitochondria or nucleus was considered to be in the cytoplasmic pool, as DJ-1 has not been demonstrated to localize to other subcellular organelles. Astrocytes were treated with vehicle only, LPS (10, 25 or 50 μg/mL), or the rotenone (50, 500, or 1000 nM) and subcellular localization to the nucleus and mitochondria was analyzed. Mitochondrial localization was lower than control in 50 and 500 nM doses, however 1000 nM of rotenone induced a significant increase in mitochondrial localization (p < 0.001, Fig. 3B). Surprisingly, LPS treatment did not increase mitochondrial localization of DJ-1, rather increasing concentrations decreased mitochondrial localization. In the lower rotenone doses, decreased mitochondrial localization was coupled with increased nuclear localization (Fig. 3C). Concomitant decreases in nuclear localization were observed in both 1000 nM rotenone and all LPS stimulated cells (p < 0.001, Fig. 3C). Possibly, the nuclear pool of DJ-1 translocates to the mitochondria under higher doses of rotenone-induced complex I inhibition, but neuroinflammatory stress induced by LPS results instead in an increase of DJ-1 in the cytosol, indicating perhaps different environmental chemicals may result in different functional pathways for DJ-1 within the cell.
Fig. 3.
Rotenone increases mitochondrial localization of DJ-1, and both rotenone and LPS decrease nuclear localization. Primary cortical astrocytes were exposed to 50, 500 or 1000 nM rotenone + PBS, 10, 25, or 50 μg/mL LPS + DMSO, or control (PBS + DMSO) for 24 h, then loaded with MitoTracker Red and probed for DJ-1 immunofluorescence. To allow nuclear visualization, cells were mounted in DAPI-containing media. Pearson's correlation coefficients were calculated using SlideBook software image (A) A representative image used for analysis: (1) DAPI-stained nucleus, (2) DJ-1 immunofluorescence, (3) MitoTracker, and (4) merged image, for color image please see Supplementary Fig. 1. (B) DJ-1 correlation with the mitochondria (λ519:λ599) increased upon exposure to rotenone but not LPS (n ≥ 71, p < 0.001). (C) Nuclear correlation DJ-1 (λ519:λ460) decreased following exposure to either rotenone or LPS (n ≥ 71, p < 0.001).
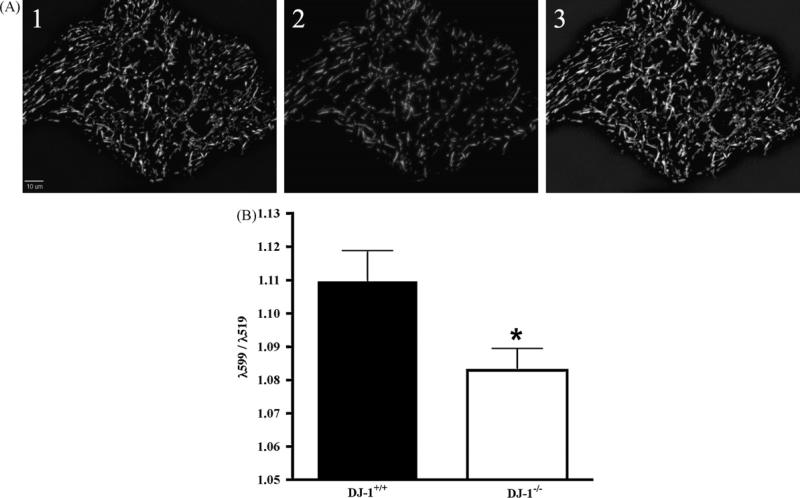
Because DJ-1 may respond to mitochondrial stress, we analyzed mitochondrial membrane potential in primary cultured astrocytes using the ratiometric dye JC-1. In a preliminary experiment, differing concentrations of JC-1 were monitored for sensitivity to the uncoupling agent CCCP (carbonyl cyanide 3-chlorophenylhydrazone). Based upon a robust depolarization following CCCP administration, 5 μM JC-1 was selected for monitoring alterations in mitochondrial membrane potential (data not shown). Interestingly, basal mitochondrial membrane potential was decreased (p < 0.03) in DJ-1−/− cells compared to DJ-1+/+ during the first 8 min of imaging (Fig. 4). However, following exposure to 1 μM rotenone or DMSO, neither DJ-1+/+ nor DJ-1−/− cells had a significant decrease in mitochondrial membrane potential, and were no longer significantly different from one another (data not shown).
Fig. 4.
Mutation of DJ-1 decreases resting mitochondrial membrane potential. Astrocytes were loaded with the ratiometric mitochondrial dye JC-1 (5 μM) for 30 min, then rinsed once in MEM with 10 mM HEPES without phenol red, and images were collected every 2 min for 8 min. Mitochondrial membrane potential, expressed as the ratio of λ599:λ519 was measured using SlideBook image analysis software. (A) Representative image of the mitochondrial dye JC-1, (1) fluorescence emitted at λ519, (2) fluorescence emitted at λ599, and (3) merged images of 1 and 2, for color image please see Supplementary Fig. 2. (B) DJ-1−/− astrocytes have decreased basal membrane potential compared to DJ-1+/+ (n = 48, p = 0.02).
4. Discussion
DJ-1 was initially identified as an oncogene (Nagakubo et al., 1997), and then gained additional distinction as a PD gene (Bonifati et al., 2003b), however the precise role(s) of DJ-1 remain elusive. The DJ-1 distribution data presented here complements data published elsewhere (Olzmann et al., 2004; Zhang et al., 2005), suggesting DJ-1 is very widely expressed systemically.
Due to the propensity of mitochondrial dysfunction contributing to neurodegeneration and the localization of DJ-1 to this organelle, increased localization of DJ-1 to the mitochondria following exposure to rotenone was expected. Betarbet et al. (2006) report increased DJ-1 localization to the mitochondria following chronic (4 week) exposure to 5 nM rotenone in SK-N-MC neuroblastoma cells. At the lower doses of utilized (i.e., 50 and 500 nM), mitochondrial localization actually decreased, whereas at the 1000 nM dose, a significant increase in mitochondrial localization was observed. In contrast to the chronic dosing study published by Betarbet et al., astrocytes in these studies were only exposed for 24 h. Thus, a direct comparison is difficult, however it is possible that the immediate localization of DJ-1 away from the mitochondria occurs in early response to low doses of rotenone, with mitochondrial localization increasing as complex I inhibition become a chronic condition. Further doses and temporal responses of DJ-1 in cells are necessary to resolve this question. Given the highest dose of rotenone induced an immediate localization to the mitochondria, the data is in agreement with the previously published information. Notably, this study is the first to report quantitative analysis on altered localization to either the mitochondria or the nucleus due to rotenone treatment, as well as LPS-induced decline in nuclear localization. While DJ-1 in the nucleus likely translocates to the mitochondria to protect against rotenone-induced toxicity, it is unknown why DJ-1 nuclear and mitochondrial localization declines in response to increasing doses of LPS. Other reports suggest DJ-1 protein expression increases following exposure to LPS (Ejima et al., 2000; Mitsumoto and Nakagawa, 2001), so it is likely DJ-1 may function in a protective manner to combat LPS-induced toxicity, and altering its subcellular location may be critical to this role. Additionally, a decrease in resting mitochondrial membrane potential in DJ-1−/− astrocytes suggests DJ-1 may modulate mitochondrial energy dynamics, dysfunction of which is thought to contribute to PD pathogenesis. In support of this, it has been shown that DJ-1−/− neurons are reportedly more vulnerable to alterations in energy metabolism due to impaired Na+/K+ ATPase function (Pisani et al., 2006).
Interestingly, our DJ-1−/− cells express Bax at a similar level to DJ-1+/+ astrocytes (Supplementary Fig. 3) in contrast to an elegant study by Fan et al. (2008) in a neuroblastoma line demonstrated an increase in Bax expression in DJ-1 knockdown. The mechanisms regulating DJ-1-mediated alterations of Bax expression have not yet been investigated in astrocytes, so comparison of our data with this study is problematic. However, the notion that DJ-1 differentially regulates protein expression in different tissues is quite feasible, and may explicate differences observed in our data and that of Fan and coworkers.
Although many questions remain regarding the role of DJ-1 in physiology, a protein expressed so ubiquitously and involved neurological disorders, reproduction and carcinogenesis requires further investigation. In neurodegenerative research, most studies to date have focused on DJ-1 in neurons, however its role in astrocytes remains largely unknown. As astrocytes are critical in maintaining neuronal viability, especially through maintenance of the shared extracellular space and supplying antioxidants and nutrients, compromised astrocyte function could deleteriously affect neuronal function and/or survival. Further, pathological reactive astrocytosis is shared by all neurological disorders listed above that DJ-1 is implicated currently. Thus, elucidating the role of DJ-1 in astrocytes under normal conditions and during gliosis is vitally important to better understand these diseases, and may provide new targets for therapeutic interdiction. Mutant DJ-1 mice are a useful means for elucidating the role(s) of this protein in a myriad of diseases as well as normal cellular homeostasis.
Supplementary Material
Acknowledgements
The authors would like to thank Courtney Amerin for assistance with various technical aspects of this project. Funding was provided by the American Parkinson's Disease Association and the College of Veterinary and Biomedical Sciences College Research Council at Colorado State University.
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxlet.2008.11.008.
References
- Allen JW, Mutkus LA, Aschner M. Isolation of neonatal rat cortical astrocytes for primary cultures. Curr. Protoc. Toxicol. Suppl. 2000;4:12.14.11–12.14.15. doi: 10.1002/0471140856.tx1204s04. [DOI] [PubMed] [Google Scholar]
- Aschner M, Gannon M, Kimelberg HK. Manganese uptake and efflux in cultured rat astrocytes. J. Neurochem. 1992;58:730–735. doi: 10.1111/j.1471-4159.1992.tb09778.x. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Canet-Aviles RM, Sherer TB, Mastroberardino PG, McLendon C, Kim JH, Lund S, Na HM, Taylor G, Bence NF, Kopito R, Seo BB, Yagi T, Yagi A, Klinefelter G, Cookson MR, Greenamyre JT. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol. Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci. Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Squitieri F, Krieger E, Vanacore N, van Swieten JC, Brice A, van Duijn CM, Oostra B, Meco G, Heutink P. DJ-1(PARK7), a novel gene for autosomal recessive, early onset parkinsonism. Neurol. Sci. 2003a;24:159–160. doi: 10.1007/s10072-003-0108-0. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003b;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Allen C, Ingham PW, Bandmann O. p53-dependent neuronal cell death in a DJ-1-deficient zebrafish model of Parkinson's disease. J. Neurochem. 2007;100:1626–1635. doi: 10.1111/j.1471-4159.2006.04291.x. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front. Biosci. 2003;8:s826–837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, Carvey PM, Ling Z, Kang UJ, Zhuang X. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J. Biol. Chem. 2005;280:21418–21426. doi: 10.1074/jbc.M413955200. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Ejima K, Nanri H, Araki M, Koji T, Shibata E, Kashimura M, Ikeda M. Expression of mitochondrial thioredoxin-dependent antioxidant protein, SP-22, in normal human and inflammatory mouse placentae. Placenta. 2000;21:847–852. doi: 10.1053/plac.2000.0569. [DOI] [PubMed] [Google Scholar]
- Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases bax expression through repressing p53 transcriptional activity. J. Biol. Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, Westaway D, Lozano AM, Anisman H, Park DS, Mak TW. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kong LY, McMillian MK, Hudson PM, Jin L, Hong JS. Inhibition of lipopolysaccharide-induced nitric oxide and cytokine production by ultralow concentrations of dynorphins in mixed glia cultures. J. Pharmacol. Exp. Ther. 1997;280:61–66. [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. U.S.A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov. Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. Sensitivity to oxidative stress in DJ-1-deficient dopamine neurons: an ES-derived cell model of primary parkinsonism. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulener MC, Graves CL, Sampathu DM, Armstrong-Gold CE, Bonini NM, Giasson BI. DJ-1 is present in a large molecular complex in human brain tissue and interacts with alpha-synuclein. J. Neurochem. 2005;93:1524–1532. doi: 10.1111/j.1471-4159.2005.03145.x. [DOI] [PubMed] [Google Scholar]
- Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic. Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor-alpha in 6-hydroxydopamine-lesioned striatum in rats is suppressed by immunosuppressant FK506. Neurosci. Lett. 2000;289:165–168. doi: 10.1016/s0304-3940(00)01275-1. [DOI] [PubMed] [Google Scholar]
- Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem. Biophys. Res. Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- Neumann M, Muller V, Gorner K, Kretzschmar HA, Haass C, Kahle PJ. Pathological properties of the Parkinson's disease-associated protein DJ-1 in alpha-synucleinopathies and tauopathies: relevance for multiple system atrophy and Pick's disease. Acta Neuropathol. (Berl.) 2004;107:489–496. doi: 10.1007/s00401-004-0834-2. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS. Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J. Biol. Chem. 2004;279:8506–8515. doi: 10.1074/jbc.M311017200. [DOI] [PubMed] [Google Scholar]
- Pisani A, Martella G, Tscherter A, Costa C, Mercuri NB, Bernardi G, Shen J, Calabresi P. Enhanced sensitivity of DJ-1-deficient dopaminergic neurons to energy metabolism impairment: role of Na+/K+ ATPase. Neurobiol. Dis. 2006;23:54–60. doi: 10.1016/j.nbd.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Taira T, Niki T, Seino C, Iguchi-Ariga SM, Ariga H. DJ-1 positively regulates the androgen receptor by impairing the binding of PIASx alpha to the receptor. J. Biol. Chem. 2001;276:37556–37563. doi: 10.1074/jbc.M101730200. [DOI] [PubMed] [Google Scholar]
- Takahashi-Niki K, Niki T, Taira T, Iguchi-Ariga SM, Ariga H. Reduced anti-oxidative stress activities of DJ-1 mutants found in Parkinson's disease patients. Biochem. Biophys. Res. Commun. 2004;320:389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]
- Vanacore N, Nappo A, Gentile M, Brustolin A, Palange S, Liberati A, Di Rezze S, Caldora G, Gasparini M, Benedetti F, Bonifati V, Forastiere F, Quercia A, Meco G. Evaluation of risk of Parkinson's disease in a cohort of licensed pesticide users. Neurol. Sci. 2002;23(Suppl. 2):S119–120. doi: 10.1007/s100720200098. [DOI] [PubMed] [Google Scholar]
- Wu DC, Tieu K, Cohen O, Choi DK, Vila M, Jackson-Lewis V, Teismann P, Przedborski S. Glial cell response: a pathogenic factor in Parkinson's disease. J. Neurovirol. 2002;8:551–558. doi: 10.1080/13550280290100905. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhong N, Wang H, Elias JE, Kim CY, Woldman I, Pifl C, Gygi SP, Geula C, Yankner BA. The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum. Mol. Genet. 2005;14:1231–1241. doi: 10.1093/hmg/ddi134. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Mol. Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H. Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem. Biophys. Res. Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.