Abstract
With the rising incidence of type II diabetes mellitus (DM II) worldwide, methods to identify high-risk geographical areas have become increasingly important. In this comprehensive review following Cochrane Collaboration guidelines, we outline spatial methods, outcomes and covariates used in all spatial studies involving outcomes of DM II. A total of 1894 potentially relevant citations were identified. Studies were included if spatial methods were used to explore outcomes of DM II or type I and 2 diabetes combined. Descriptive tables were used to summarize information from included studies. Ten spatial studies conducted in the USA, UK and Europe met selection criteria. Three studies used Bayesian generalized linear mixed modelling (GLMM), three used classic generalized linear modelling, one used classic GLMM, two used geographic information systems mapping tools and one compared case:provider ratios across regions. Spatial studies have been effective in identifying high-risk areas and spatial factors associated with DM II outcomes in the USA, UK and Europe, and would be useful in other parts of the world for allocation of additional services to detect and manage DM II early.
Keywords: diabetes, spatial, geographic, mapping, systematic review
1. Background
Type II diabetes mellitus (DM II) is on the rise worldwide and is reported to be increasing in every country of the world [1]. Costly sequelae of DM II, including hospital admission, can be prevented with quality primary care [2]; however, many areas suffer from insufficient primary care services to meet the growing epidemic of DM II. Indeed, diabetes care has been reported to be ‘in a state of crisis’ in a recent report from Diabetes UK [3]. An estimated 50% of DM II cases are undiagnosed [1]. Spatial studies that identify high-risk areas for DM II outcomes can highlight regions that would most benefit from additional primary care services to detect, manage and monitor DM II early. In addition, spatial studies have the potential to identify geographical factors that are important to DM II aetiology. We define spatial studies as studies involving aggregate or point-level spatial information. This broad definition includes ecological and multi-level studies, and models with correlated and uncorrelated spatial effects.
A spatial approach to disease modelling is relevant to any chronic disease with elements of environmental causation and is especially relevant to DM II as recent research has demonstrated associations between DM II prevalence and geographical factors such as green space, walkability, increased fast-food availability, car-dominated transport and reduced opportunities for exercise [4,5]. Many of these factors are amenable to health promotion programmes, and spatial analysis can provide useful information to inform resource allocation and public policy decisions [6]. As yet, there are few spatial studies in the literature examining DM II outcomes, and a review of the findings and methodologies used in these studies would be useful as a basis for further studies in other parts of the world to identify areas in need for DM II management, and associated geographical factors amenable to modification.
Currently, 11.3% of the USA and 4.45% of the UK adult population are estimated to have diabetes, and DM II accounts for 90–95% of these cases [7,8]. Diabetes is the leading cause of renal failure, non-traumatic lower-limb amputation, and new cases of blindness, the major cause of heart disease and stroke, and the seventh leading cause of death in the USA [7]. The direct and indirect costs of diabetes are estimated to exceed USD 612 billion in the USA in 2014, £23.7 billion in the UK in 2011 and AUD 14.6 billion in Australia in 2010 [9–11].
The management of DM II is complex and time-consuming and may involve regular health consultations, lifestyle modification, frequent blood glucose and podiatry checks and complex medication regimes [12]. Fortunately, there is evidence that around 60% of DM II cases are preventable with lifestyle change and/or medications [13]. Early detection and management of glycaemic control and cardiovascular risk factors should lead to more effective treatment while reducing the risk of diabetic complications [14]. Screening for undiagnosed cases using a fasting plasma glucose test thus has the potential to significantly reduce the healthcare burden of DM II [14]. Effective placement of screening services can be determined using spatial analysis of DM II outcomes to identify areas of high risk.
Numerous studies, including a systematic review, suggest that a number of demographic and clinical factors and metabolic markers are associated with increased risk of developing DM II [15–19]. In addition, there is some evidence of geographical factors associated with DM II prevalence [4,5,17]. These are summarized in table 1. Several of these factors are modifiable, including lifestyle choices and associated cardiovascular risk, and neighbourhood factors are amenable to health promotion programmes. Owing to spatial clustering of lifestyle factors, identification of areas with higher prevalence of lifestyle-related risk factors would allow provision of targeted health promotion programmes.
Table 1.
Risk factors associated with increased risk of developing type II diabetes mellitus.
| demographic factors | metabolic markers |
|---|---|
| male gender | elevated fasting plasma glucose |
| increasing age | elevated 2-h post-prandial glucose |
| increasing BMI | elevated random glucose |
| indicators of low socio-economic status (education, income, occupation) | elevated triglyceride:high-density lipoprotein ratio |
| increasing waist:hip ratio | white cell count |
| increasing waist:height ratio | elevated HbA1c |
| black/hispanic ethnicity | elevated interleukin-2 receptor A |
| sedentary lifestyle/physical inactivity | elevated adiponectin |
| smoking history | elevated C-reactive protein |
| excessive alcohol use | elevated ferritin |
| low levels of fruit and vegetable consumption | elevated Ga-glutamyl transpeptidase |
| — | elevated insulin level |
| clinical factors | environmental factors |
|---|---|
| hypertension | reduced green space/walkability |
| cardiovascular disease | increased fast-food availability |
| tachycardia | decreased access to healthy food |
| family history of diabetes in first degree relative | car-dominated transport |
| history of gestational diabetes | reduced opportunities for exercise |
| corticosteroid use | lower SES |
| — | higher proportion of daily smokers |
Diagnosis of DM II appears to be associated with diagnosis of several other disorders, including hypertension [20], coronary arterial disease [21–23], congestive heart failure [24,25], chronic obstructive pulmonary disease [26,27], colorectal cancer [28–31], pancreatic cancer [32–34], endometrial cancer [35], acute pancreatitis [36], biliary disease [36], psoriasis [37], urinary tract calculi [38] and diagnosis with high-grade prostate cancer [39]. Spatial analyses allow examination of joint spatial correlations between multiple diseases, and describing these methodologies would be useful for future research into geographical associations between these diseases.
Historically, geographical studies have been effective in finding associations between incidence and mortality of disease and exposure to risk factors, such as lifestyle and environmental factors [40]. Software packages, such as BUGS, R, MapInfo are available for performing spatial mapping of disease outcomes and their relationship with exposure to lifestyle and environmental factors [41–43]. Based on a small range of standard algorithms, these software packages provide smoothed estimates and colour-coded geographical maps. Maps provide a powerful visual tool for identification of geographical patterns of occurrence of disease and are potentially useful in the formulation of hypotheses of DM II aetiology.
Both purely ecological and multilevel studies are useful for ascertaining risk factors underlying spatial variation in DM II outcomes. Purely ecological studies using aggregate spatial data have the advantage that they avoid ethical and confidentiality considerations associated with the identifiability of individuals. Survey data for DM II outcomes and geographical factors aggregated to small area level are readily available, and by aggregating measurements over multiple persons, data may be associated with less measurement error than individual data [44]. Ecological studies are thus able to identify associations between disease outcomes and geographical factors at a small area level.
By contrast, multilevel studies that account for individual-level factors nested within geographical factors have the advantage of being able to identify the residual effects of geographical factors on DM II outcomes after accounting for individual factors, and to measure the relative importance of each. However, obtaining data at both an individual and geographical level requires more effort and ethical consideration.
Bayesian models are particularly well suited to spatial modelling as the information specific to each region can naturally be represented as priors, and both correlated and uncorrelated spatial effects can be examined [45]. The Bayesian framework accounts for different sources of uncertainty and compensates for sparse and missing data. For sparse data, incorporation of spatially correlated priors for residual error allows ‘borrowing of strength’ across neighbouring regions allowing for more robust inferences. Use of spatially correlated priors can also be a good method for imputation of missing outcome or covariate information [46]. In addition, uncertainty around notification rates and measurement error can be incorporated into a Bayesian model. Furthermore, hierarchical Bayesian models allow the exploration of individual-level risk factors nested within correlated and uncorrelated spatial effects.
DM I and DM II differ in underlying aetiological factors; however, many spatial studies analyse both in combination. As the vast majority of diabetic cases (90–95%) are accounted for by DM II, inclusion of these studies is still useful for examination of DM II outcomes, as the remaining small proportion of DM I cases is unlikely to bias results very much. Thus, inferences from spatial studies combining DM I and DM II are also useful for the purpose of this review.
This systematic review aims to perform a comprehensive search of the literature in accordance with Cochrane Collaboration guidelines to identify all spatial studies available involving aggregate or point-level spatial information, and examining outcomes of DM II, or DM I and DM II combined. This review aims to summarize: (i) risk factors for DM II identified by spatial studies, (ii) general spatial methods used, and (iii) to describe in detail statistical analyses used in these studies.
2. Material and methods
2.1. Search strategy
A systematic review was conducted to identify all articles published between January 1950 and June 2013 involving spatial methodology to examine outcomes of DM II, or DM I and DM II combined. The eight steps of the Cochrane Collaboration guidelines for a systematic review below were followed [47]:
step 1: defining the review questions and developing criteria for including studies;
step 2: searching for studies;
step 3: selecting studies and collecting data;
step 4: assessing risk of bias in included studies;
step 5: analysing data and undertaking meta-analyses;
step 6: addressing reporting biases;
step 7: presenting results and ‘summary of findings’ tables; and
step 8: interpreting results and drawing conclusions.
The review questions (step 1) were:
— what health outcomes and covariates have been examined in spatial studies involving outcomes of type II diabetes mellitus?
— what spatial methods have been used in these studies?
Systematic searches (step 2) were performed using MEDLINE, Science Direct, Web of Science, CINAHL and Cochrane Library of Systematic Reviews. A combination of Medical Subject Headings (MeSH) and keyword searches were used. For MEDLINE, which allowed the most tailored search strategy, two subsets of citations were generated, to identify studies using spatial or Bayesian methodology. The MeSH term ‘diabetes mellitus type 2’ was used for participant type, combined with (i) keywords ‘map*’, ‘geographic’, ‘spatial’, ‘areal’ or ‘belt’ to identify spatial studies and (ii) truncated keyword ‘bayes*’ to identify Bayesian studies (* indicates truncation). Searches were limited to English literature and human studies. The search terms ‘type 2 diabetes mellitus’ combined with ‘map*’, ‘geographic’, ‘spatial’, ‘areal’ or ‘belt’ were also used to search Science Direct, Web of Science and CINAHL. Searches were restricted to abstract, title or keywords for Science Direct, topics for Web of Science and abstract for CINAHL. The Cochrane Library of Systematic Reviews was searched using the term ‘type 2 diabetes mellitus’. Reference lists of relevant articles identified by this method were scanned for other studies not identified through the electronic search.
2.2. Selection of studies
Predefined inclusion and exclusion criteria were set for study selection prior to conducting the literature search (step 3). Studies were included if they used spatial methods to explore aggregate or point-level spatial data examining outcomes of DM II, or DM I and DM II combined, and excluded if only non-spatial methods were used or only involved participants with DM I. A two-stage process was used to select relevant studies for the review (figure 1). One author (J.B.) independently examined abstracts of all articles identified through electronic searches and excluded those not meeting the selection criteria. Following this, three authors (J.B., N.W. and K.M.) independently examined full manuscripts obtained and made decisions about inclusion and exclusion of studies. Any disagreement was resolved through discussion. Where more than one article was found describing the same study, only the most recent or most complete publication was included unless methodology used differed significantly.
Figure 1.
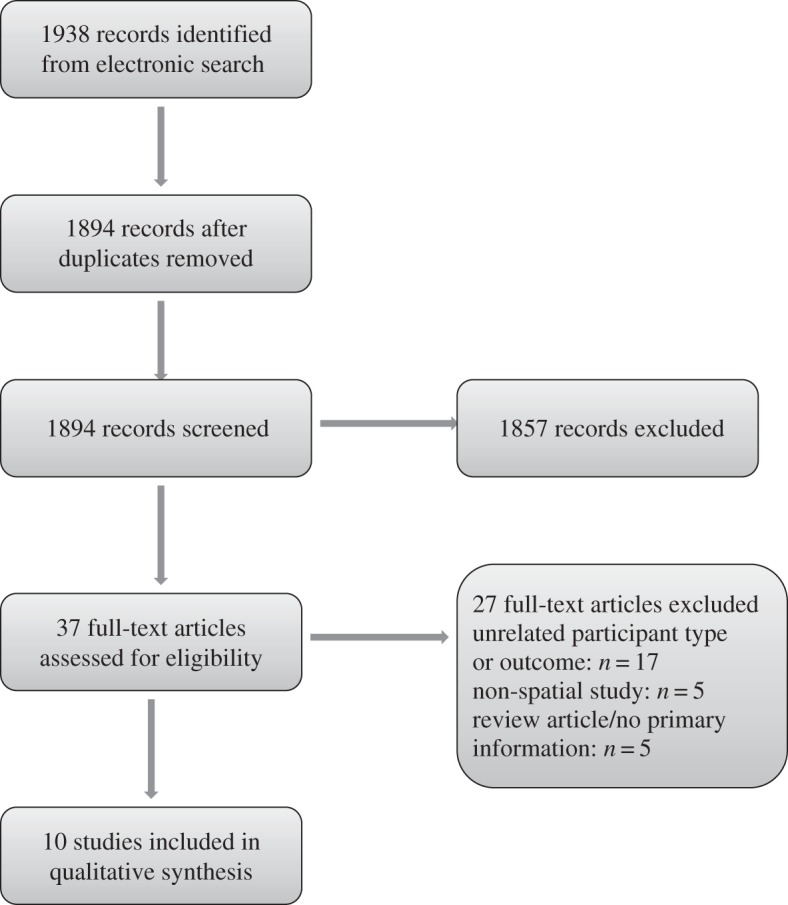
Study selection and exclusion process.
2.3. Data extraction
Data extraction was performed by one author (J.B.) and entered into a predesigned spreadsheet. For each included study, information was extracted about geographical location, sample size, outcome measures examined, statistical methods used, covariates included and results found. Characteristics and results of each study are summarized into a table.
3. Results
The electronic search identified a total of 1938 potentially relevant citations from all databases searched, and after removal of 44 duplicates, abstracts of 1894 citations were screened. Overall, 1857 citations were excluded on the basis of abstract information (figure 1). Full manuscripts were obtained and examined for 37 articles, and 10 studies met selection criteria, of which three used Bayesian spatial methodology and seven used classical modelling techniques. Risk of bias was assessed (step 4), and coverage bias was a possibility with all studies included but difficult to assess. Other types of bias described in the Cochrane Handbook, including selection, performance, detection, attrition and reporting bias, relevant to studies comparing two arms, were less relevant to the spatial studies included.
A descriptive analysis was performed (step 5). Owing to large differences in outcomes measured and methodology used between studies, meta-analysis was not possible, nor was publication bias able to be assessed via funnel plots and sensitivity analyses (step 6). Table 2 outlines characteristics of studies included in this systematic review, including details of study location, sample size, outcome measures, methodology used, covariates included and results found (step 7).
Table 2.
Characteristics of included studies. (US, United States; b/w, between; DM, diabetes mellitus; DM I, type I diabetes mellitus; DM II, type II diabetes mellitus; CAR, conditional autoregressive; MCAR, multivariate conditional autoregressive; HbA1c, glycated haemoglobin; GIS, geographical information science; SES, socio-economic status; +ve, positive; hx, history; CVD, cardiovascular disease; HT, hypertension.)
| primary author, year | country | sample size | outcome measures | methods | covariates in model | results |
|---|---|---|---|---|---|---|
| Liese 2010 [48] | US | four US regions | geographical variation, joint spatial correlation b/w DM I and DM II, smoothed risk estimates | sparse Poisson CAR, MCAR | age, gender, ethnicity | evidence for small area variation in incidence of and joint correlation between DM I and DM II |
| Geraghty 2010 [49] | US | 7288 DM (I or II) pts | DM prevalence, distance to primary care provider, glycaemic control (HbA1c) | regression, geographical information software (GIS) mapping | demographic and laboratory characteristics | SES barrier to optimal glycaemic control |
| Lee 2008 [50] | US | nine US regions | disparities between estimated paediatric DM prevalence and endocrinologist supply | mapping of DM prevalence:paediatric endocrinologist ratio | — | up to 19-fold difference in case:provider ratio across regions |
| Green 2003 [17] | US | 230 Manitoba areas | DM prevalence estimation | spatial scan statistic, spatial autoregressive linear regression | sociodemographic, environmental and lifestyle factors | low SES, poor environmental quality, poor lifestyles +ve correlated with DM prevalence |
| Noble 2012 [51] | England | 130 areas in London | small-area mapping of 10-year risk of developing DM II | GIS mapping | age, gender, ethnicity, deprivation, family hx, CVD, smoking, HT, steroid use, height, weight | small-area geospatial mapping feasible |
| Congdon 2006 [52] | England | 8000 electoral wards | DM prevalence estimation | regression, aggregate data | age, gender, ethnicity, area deprivation, adverse hospitalization indicators | DKA and coma +ve correlated with DM prevalence |
| Weng 2000 [19] | England | 332 DM (type I or II) pts | metabolic control, access to healthcare, clinical outcomes (neuropathy, retinopathy, proteinuria) and mortality | GIS mapping | age, gender, ethnicity, BMI, smoking, glycaemic control (HbA1c), Underprivileged Area Score | ↑ morbidity and mortality in DM pts related to SES and ethnicity |
| Bocquier 2011 [16] | France | 16 Marseilles cantons | prevalence estimation of treated DM | multilevel Poisson regression | area deprivation, population density, adjusted for individual-level factors (age, gender, low SES) | DM prevalence higher in more deprived and population-dense areas |
| Chaix 2011 [53] | France | 2218 Paris census blocks | DM II prevalence, joint spatial correlation with study participation | multilevel logistic modelling | — | DM prevalence highest in areas with low educational attainment |
| Kravchenko 1996 [54] | Ukraine | 27 admin regions | spatio-temporal estimation of DM I and DM II prevalence | regression, aggregate data | — | small area variation and general increase in prevalence over time |
The statistical methods used in the 10 included papers are examined in detail in this section. Three studies used Bayesian generalized linear mixed modelling (GLMM), three used classic generalized linear modelling (GLM), one used classic GLMM, two used geographic information systems (GIS) mapping tools and one compared case provider ratios across regions. For Bayesian analyses involving Normal prior distributions, these were parametrized in terms of variances, unless otherwise specified.
3.1. Bayesian generalized linear models
3.1.1. Multilevel logistic modelling
The paper by Chaix et al. used Bayesian multilevel logistic modelling to perform separate and joint modelling of neighbourhood determinants of both DM II prevalence and study participation, in 2218 census-block groups in the Paris metropolitan area [53]. Data from the RECORD Cohort Study were used. A GLMM was used to model the outcome of diagnosis with diabetes for each individual nested within their area of residence as a Bernoulli distribution with probability parameter pik. The logit(pik) was modelled as a linear function of individual and neighbourhood sociodemographic explanatory variables. Formulae for this multilevel logistic model are provided in the electronic supplementary material, appendix A.
The authors also performed coupled modelling of DM II and study participation, in order to identify selective participation related to neighbourhood, and account for potential bias on the associations with diabetes. A Markov chain Monte Carlo (MCMC) approach was used to simultaneously model study participation and diabetes prevalence using a Poisson model.
Separate modelling of DM II showed evidence that a low neighbourhood education was associated with higher odds of diabetes, controlling for individual education and self-reported financial strain. Joint modelling showed evidence that the odds of diabetes were slightly higher in high-participation areas.
3.1.2. Sparse Poisson convolution conditional autoregression
Liese et al. used a variation of a Bayesian conditional autoregressive (CAR) and multivariate conditional autoregressive (MCAR) methods to separately evaluate geographical variation in DM I and DM II incidence, and to estimate joint spatial correlation between DM I and DM II, in youths aged 10–19 years in four US states [48]. Data from the SEARCH for Diabetes in Youth Study were used. CAR priors can be defined as a spatial structure in which the correlated random error of each region on a map of interest is described by a lattice of neighbouring regions, with i–j denoting that regions i and j are neighbours [45,55].
Sparse Poisson convolution model. A challenge with the data used in this study was the presence of sparse count data in some areas, violating assumptions of traditional Poisson models due to an excessive amount of zeros. The authors selected a sparse Poisson convolution (SPC) model to account for the sparseness of data. The SPC model is a variation of the CAR model with an added indicator variable denoting zero or non-zero count in any area. The SPC models used in this study to model DM I and DM II prevalence are described by the authors.
Joint spatial correlation between DM I and DM II was evaluated using a sparse Poisson MCAR model, which is a variation of the model with MCAR priors described by Gelfand & Vounatsu [56]. This type of model simultaneously models joint correlation between multiple outcomes while accounting for correlated error between spatial neighbours. In the model adapted by Liese et al. DM I and DM II were considered components of a vector of outcomes and a multivariate model applied. Joint spatial correlation between DM I and DM II was examined by calculating an empirical correlation between the RR estimates obtained for the SPC models using the Pearson correlation coefficient. Formulae for the SPC model and sparse Poisson MCAR model are provided in the electronic supplementary material, appendix A.
The study found evidence of geographical variation in DM I and DM II incidence, and evidence for joint spatial correlation between the two types of DM.
3.1.3. Stratified generalized linear modelling
Congdon reported Bayesian GLM using MCMC in WINBUGS to estimate prevalence and mortality of clinically diagnosed diabetes (DM I and DM II combined) based on individual and neighbourhood sociodemographic factors in a small area prevalence study in England [52]. Data from 354 local authority areas from the Health Survey for England study were used. Furthermore, variations between areas in adverse hospitalisation indicators were compared to estimated diabetic prevalence rates. The authors considered three models, briefly described here.
In the first model, the stratified observed counts of diagnosed diabetes cases, stratified by gender, eighteen 5-year age bands and seven ethnic groups were modelled assuming a Poisson distribution.
Two GLMs were modelled, one with and one without age-ethnic group interactions. The coefficient for age was modelled using a random walk prior that assumes diabetes rates for successive age groups will tend to be similar [57].
In the second model, the prevalence gradient of diabetes (DM I and DM II combined) over neighbourhood deprivation quintiles was modelled using logistic regression. For each gender separately, the impact of age, ethnicity in four categories and neighbourhood deprivation quintile were assessed using a Bernoulli trial model for the presence of diabetes in each individual.
In the third model, diabetes mortality was modelled separately for each gender using Poisson regression. Formulae for the three models mentioned earlier are provided in the electronic supplementary material, appendix A.
The results showed evidence that diabetes prevalence rates varied from 2% to 5% across local authority areas. Male and female prevalence was comparable. For males, the area mortality rate from diabetes rose as area prevalence increased but was more regular over prevalence quintiles for females.
Further analysis was undertaken to assess health performance indicators of DM. Rankings were compared between 28 strategic health authority areas based on age-standardized rates of diabetic-ketoacidosis (DKA) coma and amputations, ratios of DKA coma episodes and amputation operations to estimated prevalent populations, and correlation of these indicators with prevalence rates. Results showed evidence that DKA and coma were positively correlated with prevalence, while diabetic amputation was not.
3.2. Classic generalized linear models and generalized linear mixed models
3.2.1. Multilevel Poisson regression
Bocquier et al. used an age- and gender-adjusted multilevel Poisson GLM to model neighbourhood characteristics associated with prevalence of treated diabetes among beneficiaries, using data from drug reimbursement data from the General Health Insurance Scheme in southeastern France [16]. Individual gender and age characteristics were nested within geographical characteristics. Patients were classified as treated diabetic cases if they had oral antidiabetic medication or insulin dispensed three times or more within the year.
Results from this study found a crude prevalence of treated diabetes of 5.4%, and evidence that prevalence was significantly higher in more deprived and population-dense cantons independent of individual-level factors (age, gender, low socio-economic status (SES)).
3.2.2. Generalized linear mixed modelling
Geraghty et al. used a GLMM approach to model HbA1c and low-density lipoprotein (LDL) cholesterol based on individual demographic and laboratory characteristics and neighbourhood SES quintile [49]. Registry data from 13 primary care clinics in Sacramento, California were used, and Euclidean distance from patients' homes to their primary care clinic calculated. GIS tools (ArcInfo) were used to analyse outcome disparities in a population of patients with DM II [58].
The first regression model assessed HbA1c level as a linear mixed model with random intercept and slope based on individual sociodemographic and laboratory characteristics, with practice characteristics or their primary care physician and clinic specialty as fixed effects and neighbourhood SES quintile as a random effect.
The second model dichotomized LDL cholesterol using a cutpoint of 100 mg dl−1, using mixed logistic regression. The same fixed and random effects were included as in the HbA1c model, with the addition of statin prescription. Formulae for both models are provided in the electronic supplementary material, appendix A.
Results of this study showed evidence of an association between neighbourhood SES quintile and HbA1c level. SES was not found to be associated with LDL control.
3.2.3. Linear regression
Green et al. used analysis of variance and linear regression to identify sociodemographic, environmental and lifestyle factors associated with geographical variation in DM prevalence (DM I and DM II combined) in Winnipeg, Manitoba, using census, Health Epidemiology Unit, and hospital and physician claims data [17]. The authors used two methods to aggregate predictor and outcome data into high-risk areas for diabetes prevalence, firstly by aggregating to existing health administrative areas, and secondly by using a spatial scan statistic (SaTScan software [59]), both methods generating very similar results.
The spatial scan statistic places a circular window of varying size on a map surface, allowing its centre to move so that the window includes different sets of neighbouring areas at any given position and circle size. The window is placed alternatively at the centroid of each area and the radius varied continuously from zero, up to a maximum size including 50% of the population, using MCMC simulation to test for elevated risk of DM prevalence. The statistic assumes the number of cases in each geographical region to be Poisson distributed and tests the null hypothesis that within each age/gender group, the risk of DM is the same as in all regions combined [60].
With both aggregation methods, linear regression was used to model standardized DM prevalence as a function of socio-economic, environmental and lifestyle factors ecologically associated with the variability in prevalence, including self-reported Aboriginal status, education, income, family structure, unemployment, housing conditions, crime and smoking rates. Variables were log-transformed when necessary to meet assumptions of normality and homoscedascity (specific variables that were transformed were not given).
Results from this study showed evidence that higher DM prevalence was strongly associated with indicators of low SE status, poor environmental quality and poor lifestyles.
3.2.4. Linear regression including temporal component
Kravchenko et al. [54] used mixed linear regression to separately model time trends in DM I and DM II prevalence, and the effect of smoking on prevalence of DM complications, in various administrative regions of the Ukraine. Statistical reports collected in 1990–1993 by specialized endocrinologic institutions were used in this study. For each region, the time trends for prevalence of DM I and DM II were separately interpolated by mixed linear regression. Formulae for this model are provided in the electronic supplementary material, appendix A.
Student's t-tests were used to test for differences in DM complications between two groups: smokers (10–30 cigarettes day−1 over 10 years) and non-smokers who had received preventative efforts for 5 years to correct nutrition, promote a healthy lifestyle, normalize body weight, metabolism and arterial pressure.
Results showed significant variation in the prevalence of DM II across regions, an overall increase in prevalence of both DM I and DM II over time, and evidence that prophylactic measures directed at a decrease in patient weight, the normalization of metabolism, arterial pressure and the elimination of pernicious habits promoted a decrease in diabetic complications.
3.3. Studies using geographic information system mapping
Weng et al. [19] investigated differences in metabolic control, access to healthcare, clinical outcomes (neuropathy, retinopathy, proteinuria) and mortality rates in a cohort of 610 diabetics living in different geographical areas of central London. Patients were clustered into prosperous, intermediate or deprived areas by electoral ward using Underprivileged Area Score (UPA score). GIS software (MapInfo) was used to analyse the geographical distribution of UPA of a sample of 332 patients [43].
Results showed evidence that patients in deprived areas were older, had higher BMI and worse glycaemic control than those in prosperous areas. Smoking was more prevalent in deprived areas. Prevalence of microvascular complications was related to geographical location, and age–gender-adjusted mortality rate was significantly higher in deprived than prosperous areas.
Noble et al. performed a feasibility study of geospatial mapping in Tower Hamlets, London, using ArcGIS, to examine 10 year risk of developing DM II as measured by QDScore [51,58,61]. Data from general practice electronic records on all non-diabetic individuals were used, and for each individual the QDScore instrument was used to calculate 10 year risk of developing DM II, and data were geocoded into areas.
Basic and smoothed visual maps were produced to identify areas of high and low 10 year risk. A ring map also visually illustrated availability of fast-food outlets, population density and percentage of green spaces for each area. A basic map of index of area multiple deprivation scores visually corresponded very closely with the basic map of 10 year DM risk.
The authors concluded that producing geospatial maps of DM risk from general practice electronic records was feasible and useful for public health and urban planning, but challenging due to data governance issues and technical challenges.
3.4. Study comparing case:provider ratio
3.4.1. Mapping of child diabetes mellitus prevalence:paediatric endocrinologist ratio
Lee et al. [50] examined variations across US regions in the ratio of paediatric DM cases (types I and II, aged less than 18 years) to number of paediatric endocrinologists, and similarly, the ratio of obese children to paediatric endocrinologists. Data from the American Board of Pediatrics were used to estimate the number of board-certified paediatric endocrinologists by state, and data from the National Survey of Children's Health were used to estimate the number of children with diabetes and obesity by state.
Results showed evidence of geographical disparities in DM cases:endocrinologist supply, with a twofold difference between states.
4. Discussion
This review has shown that spatial studies have successfully been used to identify areas of high risk for DM II prevalence or incidence, to show disparities in case:provider ratio, metabolic control, access to healthcare, clinical outcomes and mortality rates across areas, and to compare temporal trends between areas. The two hierarchical models included, found evidence that spatial information influenced outcomes after adjusting for individual-level risk factors. Each included study found evidence for small area variation, and in addition, five found area-level indicators of lower SES, and two found area deprivation level to be positively correlated with outcomes, building on findings from several studies that find low area SES and deprivation to be risk factors for diabetes at an individual level [62–67]. One study found the clinical outcomes of ketoacidosis and coma to be positively correlated with area-level DM prevalence, indicating that areas of high prevalence are also prone to higher complication rates [52].
Joint modelling of multiple outcomes was shown to be useful in finding correlations between DM I and DM II, and between diagnosis with DM and study participation. These methods could usefully be extended to jointly model and examine correlations between incidence of DM II and DM II complications, or DM II and other chronic conditions, at either a mixed level (individual and area-level factors) or purely aggregate level. A full meta-analysis was not possible, owing to large differences in methodology, covariate inclusion and outcomes between the included studies.
In interpreting results and drawing conclusions from this review (step 8), a variety of area-level risk factors were confirmed by the included studies, the usefulness of including spatial information to describe geographical variation and identify regions of high excess risk was highlighted, and joint modelling of conditions was shown to be useful.
Three of the included studies used Bayesian methodology, which allowed several distinctive advantages over classical statistical methods. Individual effects nested within spatial effects were able to be described using hierarchical multilevel modelling and nested random effects [48,52,53]. Non-standard distributions were able to be fitted to these complex models, and the methods were suitable for situations with sparse or missing data. Different sources of uncertainty, such as spatial and non-spatial random error, were able to be incorporated and described within these types of models.
The study by Kravchenko et al. [54] included a temporal component in their model, allowing these authors to compare changes in DM prevalence over time between areas, identifying areas at higher risk for increases in prevalence. Information from spatio-temporal studies of this type may provide useful results influencing health policy and resource allocation decisions.
Standard linear models were used by Kravchenko et al. [54] to model DM prevalence as a function of year, and Geraghty et al. [49] to model HbA1c level as a function of multiple covariates. However, a GLM approach may be more appropriate in situations where the outcome variable is not normally distributed.
Our review methodology is based on the Cochrane collaboration guidelines for review methodology; however, the Cochrane model is implicitly based on the conventional medical model to exclusively identify patient-level risks from randomized clinical trials. Our review provides a template for a more holistic evidence review approach that also includes environmental risk factors, which is immediately applicable to other diseases.
The strengths of this systematic review include that Cochrane collaboration guidelines were followed in setting predefined aims, inclusion and exclusion criteria, selection of studies and assessment of bias. Selection of included studies was based on careful examination of modelling approaches used, and non-spatial studies were excluded. Transparent descriptions of models used in included studies are included in this review, with a view to outline methods that have been useful in examining spatial associations with DM II. Weaknesses of this review are that it does not account for bias from possible coverage and measurement errors, and that many of the included studies reported outcomes on both DM I and DM II, affecting the reliability of estimated outcomes purely for DM II. Other weaknesses are that an examination of reporting bias was not possible, and that there were insufficient studies similar enough to perform a meta-analysis.
5. Conclusion
Findings of this review show that incorporation of spatial information is useful and effective in modelling DM II and can identify spatial risk factors associated with DM II, and areas at high risk for DM outcomes and increasing DM burden over time. Several of the geographical risk factors associated with DM II outcomes, including green space, availability of healthy food, car-dominated transport and opportunities for exercise are amenable to modification. Bayesian methods allow joint modelling and examination of correlations between multiple outcomes. Although several spatial studies have been conducted examining DM II in the USA, UK and Europe, there is a lack of similar studies in other parts of the world. Spatial models conducted in these regions would be useful for identifying spatial risk factors associated with DM II and areas at high risk for DM outcomes, which would be beneficial in guiding public policy and management decisions.
Supplementary Material
Authors' contributions
J.B., N.W. and K.M. contributed to the conception and design of this study. J.B. was involved in the acquisition, analysis and interpretation of the data and drafting of the manuscript. All authors contributed to revision of the manuscript and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This work has been supported by the Cooperative Research Centre for Spatial Information, whose activities are funded by the Australian Commonwealth's Cooperative Research Centres Programme.
References
- 1.International Diabetes Federation. 2014. IDF diabetes atlas: sixth edition. See https://www.idf.org/diabetesatlas/update-2014.
- 2.Billings J, Teicholz N. 1990. Uninsured patients in District of Columbia hospitals. Health Affairs (Millwood) 9, 158–165. (doi:10.1377/hlthaff.9.4.158) [DOI] [PubMed] [Google Scholar]
- 3.Diabetes UK. 2012. State of the Nation 2012 England. Diabetes. See http://www.diabetes.org.uk/Documents/Reports/State-of-the-Nation-2012.pdf.
- 4.Kent J, Thompson S, Jalaludin B. 2011. Healthy built environments: a review of the literature, Sydney Sydney, Australia: Healthy Built Environments Program, City Futures Research Centre, UNSW. [Google Scholar]
- 5.Astell-Burt T, Feng X, Kolt G. 2014. Is neighborhood green space associated with a lower risk of Type 2 diabetes?: Evidence from 267,072 Australians. Diabetes Care 37, 197–201. (doi:10.2337/dc13-1325) [DOI] [PubMed] [Google Scholar]
- 6.Earnest A, Morgan G, Mengersen KL, Ryan L, Summerhayes R, Beard J. 2007. Evaluating the effect of neighbourhood weight matrices on smoothing properties of Conditional Autoregressive (CAR) models. Int. J. Health Geogr. 6, 54 (doi:10.1186/1476-072X-6-54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holden SH, Barnett AH, Peters JR, Jenkins-Jones S, Poole CD, Morgan CL, Currie CJ. 2013. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes. Metab. 15, 844–852. (doi:10.1111/dom.12123) [DOI] [PubMed] [Google Scholar]
- 8.Diabetes UK. 2012. Diabetes in the UK 2012. Diabetes UK. See http://www.diabetes.org.uk/Documents/Reports/Diabetes-in-the-UK-2012.pdf.
- 9.National Diabetes Information Clearinghouse. 2011. National Diabetes Statistics, 2011. National Diabetes Information Clearinghouse. See http://diabetes.niddk.nih.gov/dm/pubs/statistics/dm_statistics.pdf.
- 10.American Diabetes Association. 2013. Economic cost of diabetes in the U.S. in 2012. Diabetes Care 36, 1033–1046. (doi:10.2337/dc12-2625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CM, Colagiuri R, Magliano DJ, Cameron AJ, Shaw J, Zimmet P, Colagiuri S. 2013. The cost of diabetes in adults in Australia. Diabetes Res. Clin. Pract. 99, 385–390. (doi:10.1016/j.diabres.2012.12.002) [DOI] [PubMed] [Google Scholar]
- 12.Australian Bureau of Statistics. 2010. National health survey: summary of results, 2007–2008 (reissue). Canberra, Diabetes: medications and actions taken. See http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4364.02007-2008%20(Reissue)?OpenDocument.
- 13.Palmer AJ, Tucker DM. 2012. Cost and clinical implications of diabetes prevention in an Australian setting: a long-term modeling analysis. Prim. Care Diabetes 6, 109–121. (doi:10.1016/j.pcd.2011.10.006) [DOI] [PubMed] [Google Scholar]
- 14.Harris MI, Eastman RC. 2000. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab. Res. Rev. 16, 230–236. (doi:10.1002/1520-7560(2000)9999:9999<::AID-DMRR122>3.0.CO;2-W) [DOI] [PubMed] [Google Scholar]
- 15.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. 2011. Risk models and scores for type 2 diabetes: systematic review. BMJ 343, 7163 (doi:10.1136/bmj.d7163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bocquier A, Cortaredona S, Nauleau S, Jardin M, Verger P. 2011. Prevalence of treated diabetes: geographical variations at the small-area level and their association with area-level characteristics. A multilevel analysis in southeastern France. Diabetes Metab. 37, 39–46. (doi:10.1016/j.diabet.2010.07.004) [DOI] [PubMed] [Google Scholar]
- 17.Green C, Hoppa RD, Young TK, Blanchard JF. 2003. Geographic analysis of diabetes prevalence in an urban area. Soc. Sci. Med. 57, 551–560. (doi:10.1016/S0277-9536(02)00380-5) [DOI] [PubMed] [Google Scholar]
- 18.Egede LE, Gebregziabher M, Hunt KJ, Axon RN, Echols C, Gilbert GE, Mauldin PD. 2011. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care 34, 938–943. (doi:10.2337/dc10-1504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng C, Coppini DV, Sonksen PH. 2000. Geographic and social factors are related to increased morbidity and mortality rates in diabetic patients. Diabetes Med. 17, 612–617. (doi:10.1046/j.1464-5491.2000.00352.x) [DOI] [PubMed] [Google Scholar]
- 20.Sowers JR, Epstein M, Frohlich ED. 2001. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension 37, 1053–1059. (doi:10.1161/01.HYP.37.4.1053) [DOI] [PubMed] [Google Scholar]
- 21.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. 1998. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316, 823–828. (doi:10.1136/bmj.316.7134.823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyorala K, Simoons M, Standl E, Soler-Soler J, Ohrvik J. 2004. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The Euro Heart Survey on diabetes and the heart. Eur. Heart J. 25, 1880–1890. (doi:10.1016/j.ehj.2004.07.027) [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, McGee DL. 1979. Diabetes and cardiovascular disease. The Framingham study. J. Am. Med. Assoc. 241, 2035–2038. (doi:10.1001/jama.1979.03290450033020) [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Hjortland M, Castelli WP. 1974. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34, 29–34. (doi:10.1016/0002-9149(74)90089-7) [DOI] [PubMed] [Google Scholar]
- 25.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. 2004. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27, 1879–1884. (doi:10.2337/diacare.27.8.1879) [DOI] [PubMed] [Google Scholar]
- 26.Mannino DM, Thorn D, Swensen A, Holguin F. 2008. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 32, 962–969. (doi:10.1183/09031936.00012408) [DOI] [PubMed] [Google Scholar]
- 27.Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, Speizer FE, Barr RG, Camargo CA Jr. 2004. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care 27, 2478–2484. (doi:10.2337/diacare.27.10.2478) [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. 2011. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 26, 863–876. (doi:10.1007/s10654-011-9617-y) [DOI] [PubMed] [Google Scholar]
- 29.Kuriki K, Tokudome S, Tajima K. 2004. Association between type II diabetes and colon cancer among Japanese with reference to changes in food intake. APJCP 5, 28–35 [PubMed] [Google Scholar]
- 30.Cheng I, et al. 2011. Type 2 diabetes risk variants and colorectal cancer risk: the Multiethnic Cohort and PAGE studies. Gut 60, 1703–1711. (doi:10.1136/gut.2011.237727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. 2011. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 106, 1911–1921. (doi:10.1038/ajg.2011.301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben Q, Xu M, Ning X, Liu J, Hong S, Huang W, Zhang H, Li Z. 2011. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur. J. Cancer 47, 1928–1937. (doi:10.1016/j.ejca.2011.03.003) [DOI] [PubMed] [Google Scholar]
- 33.Magruder JT, Elahi D, Andersen DK. 2011. Diabetes and pancreatic cancer: chicken or egg? Pancreas 40, 339–351. (doi:10.1097/MPA.0b013e318209e05d) [DOI] [PubMed] [Google Scholar]
- 34.Li D. 2012. Diabetes and pancreatic cancer. Mol. Carcinog. 51, 64–74. (doi:10.1002/mc.20771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu Z, Yu X, Zhang X, Lu S, Chen X, Lu B. 2010. The association between metabolic abnormality and endometrial cancer: a large case-control study in China. Gynecol. Oncol. 117, 41–46. (doi:10.1016/j.ygyno.2009.12.029) [DOI] [PubMed] [Google Scholar]
- 36.Noel RA, Braun DK, Patterson RE, Bloomgren GL. 2009. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 32, 834–838. (doi:10.2337/dc08-1755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Han J, Hu FB, Curhan GC, Qureshi AA. 2012. Psoriasis and risk of type 2 diabetes among women and men in the United States: a population-based cohort study. J. Invest. Dermatol. 132, 291–298. (doi:10.1038/jid.2011.319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen HS, Su LT, Lin SZ, Sung FC, Ko MC, Li CY. 2012. Increased risk of urinary tract calculi among patients with diabetes mellitus: a population-based cohort study. Urology 79, 86–92. (doi:10.1016/j.urology.2011.07.1431) [DOI] [PubMed] [Google Scholar]
- 39.Kang J, Chen MH, Zhang Y, Moran BJ, Dosoretz DE, Katin MJ, Braccioforte MH, Salenius SA, D'Amico AV. 2012. Type of diabetes mellitus and the odds of Gleason score 8 to 10 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 82, 463 (doi:10.1016/j.ijrobp.2011.07.003) [DOI] [PubMed] [Google Scholar]
- 40.Elliot P, Cuzick J, English D, Stern R. 1992. Geographical and environmental epidemiology New York, NY: Oxford University Press. [Google Scholar]
- 41.Institute for Statistics and Mathematics. 2012. The comprehensive R archive network. See http://cran.r-project.org/.
- 42.The BUGS Project. 2008. 1996–2008 BUGS. See http://www.mrc-bsu.cam.ac.uk/bugs/.
- 43.MapInfo Professional. 2012. InsightGIS. See http://www.insightgis.com.au/mapinfo-professional.aspx.
- 44.Banerjee S, Carlin B, Gelfand A. 2004. Hierarchical modeling and analysis for spatial data London, UK: Chapman & Hall. [Google Scholar]
- 45.Besag J, York J, Mollie A. 1991. Bayesian image restoration with two application in spatial statistics. Ann. Inst. Statist. Math. 43, 1–59. (doi:10.1007/BF00116466) [Google Scholar]
- 46.Baker J, White N, Mengersen K. 2014. Missing in space: an evaluation of imputation methods for missing data in spatial analysis of risk factors for type II diabetes. Int. J. Health Geogr. 13, 47 (doi:10.1186/1476-072X-13-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Cochrane Collaboration. 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0. See http://www.cochrane-handbook.org/.
- 48.Liese AD, et al. 2010. Evaluating geographic variation in type 1 and type 2 diabetes mellitus incidence in youth in four US regions. Health Place 16, 547–556. (doi:10.1016/j.healthplace.2009.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geraghty EM, Balsbaugh T, Nuovo J, Tandon S. 2010. Using geographic information systems (GIS) to assess outcome disparities in patients with type 2 diabetes and hyperlipidemia. J. Am. Board Fam. Med. 23, 88–96. (doi:10.3122/jabfm.2010.01.090149) [DOI] [PubMed] [Google Scholar]
- 50.Lee JM, Davis MM, Menon RK, Freed GL. 2008. Geographic distribution of childhood diabetes and obesity relative to the supply of pediatric endocrinologists in the United States. J. Pediatr. 152, 331–336. (doi:10.1016/j.jpeds.2007.08.037) [DOI] [PubMed] [Google Scholar]
- 51.Noble D, Smith D, Mathur R, Robson J, Greenhalgh T. 2012. Feasibility study of geospatial mapping of chronic disease risk to inform public health commissioning. BMJ Open 2, 000711 (doi:10.1136/bmjopen-2011-000711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Congdon P. 2006. Estimating diabetes prevalence by small area in England. J. Public Health 28, 71–81. (doi:10.1093/pubmed/fdi068) [DOI] [PubMed] [Google Scholar]
- 53.Chaix B, Billaudeau N, Thomas F, Havard S, Evans D, Kestens Y, Bean K. 2011. Neighborhood effects on health: correcting bias from neighborhood effects on participation. Epidemiology 22, 18–26. (doi:10.1097/EDE.0b013e3181fd2961) [DOI] [PubMed] [Google Scholar]
- 54.Kravchenko VI, Tronko ND, Pankiv VI, Venzilovich Yu M, Prudius FG. 1996. Prevalence of diabetes mellitus and its complications in the Ukraine. Diabetes Res. Clin. Pract. 34, 73 (doi:10.1016/S0168-8227(96)90011-X) [DOI] [PubMed] [Google Scholar]
- 55.Hodges JS. 2003. On the precision of the conditionally autoregressive prior in spatial models. Biometrics 59, 317 (doi:10.1111/1541-0420.00038) [DOI] [PubMed] [Google Scholar]
- 56.Gelfand A, Vounatsou P. 2003. Proper multivariate conditional autoregressive models for spatial data analysis. Biostatistics 4, 11–25. (doi:10.1093/biostatistics/4.1.11) [DOI] [PubMed] [Google Scholar]
- 57.Spiegelhalter D, Best NG, Carlin B, Van Der Linde A. 2002. Bayesian measures of model complexity and fit. J. R. Stat. Soc. 64, 583–639. (doi:10.1111/1467-9868.00353) [Google Scholar]
- 58.ESRI. 2012. ArcGIS for Desktop. See http://www.esri.com/software/arcgis/arcgis-for-desktop.
- 59.SaTScan TM. SaTScan TM: software for the spatial, temporal, and space-time scan statistics. 2012 See http://www.satscan.org/ .
- 60.Kuldorff M, Feuer E, Miller B, Freedman L. 1997. Breast cancer clusters in the northeast United States: a geographic analysis. Am. J. Epidemiol. 146, 161–170. (doi:10.1093/oxfordjournals.aje.a009247) [DOI] [PubMed] [Google Scholar]
- 61.Hippisley-Cox J, Coupland C, Robson J, Sheikh A, Brindle P. 2009. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ 338, 880 (doi:10.1136/bmj.b880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayeed MA, Ali L, Hussain MZ, Rumi MAK, Banu A, Khan AKA. 1997. Effect of socioeconomic risk factors on the difference in prevalence of diabetes between rural and urban populations in Bangladesh. Diabetes Care 20, 551–555. (doi:10.2337/diacare.20.4.551) [DOI] [PubMed] [Google Scholar]
- 63.Brancati FL, Whelton PK, Kuller LH, Klag MJ. 1996. Diabetes mellitus, race, and socioeconomic status. A population-based study. Ann. Epidemiol. 6, 67–73. (doi:10.1016/1047-2797(95)00095-X) [DOI] [PubMed] [Google Scholar]
- 64.Middelkoop BJ, Kesarlal-Sadhoeram SM, Ramsaransing GN, Struben HW. 1999. Diabetes mellitus among South Asian inhabitants of The Hague: high prevalence and an age-specific socioeconomic gradient. Int. J. Epidemiol. 28, 1119–1123. (doi:10.1093/ije/28.6.1119) [DOI] [PubMed] [Google Scholar]
- 65.Tang M, Chen Y, Krewski D. 2003. Gender-related differences in the association between socioeconomic status and self-reported diabetes. Int. J. Epidemiol. 32, 381–385. (doi:10.1093/ije/dyg075) [DOI] [PubMed] [Google Scholar]
- 66.Robbins JM, Vaccarino V, Zhang H, Kasl SV. 2005. Socioeconomic status and diagnosed diabetes incidence. Diabetes Res. Clin. Pract. 68, 230–236. (doi:10.1016/j.diabres.2004.09.007) [DOI] [PubMed] [Google Scholar]
- 67.Robbins JM, Vaccarino V, Zhang H, Kasl SV. 2001. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am. J. Public Health 91, 76–83. (doi:10.2105/AJPH.91.1.76) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


