Abstract
One of the most important goals of biodiversity studies is to identify which characteristics of local habitats act as filters that determine the diversity of functional traits along environmental gradients. In this study, we investigated the relationship between the environmental variables of ponds and the functional trait diversity distribution of anuran tadpoles in an agricultural area in southeastern Brazil. Our results show that the functional trait diversity of frog tadpoles has a bell-curve-shaped relationship with the depths of ponds inserted in a pasture matrix. Because we are witnessing increasing human pressure on land use, simple acts (e.g. maintaining reproductive habitats with medium depth) can be the first steps towards preserving the diversity of Neotropical frog tadpole traits in agricultural landscapes.
Keywords: anuran, land use, biodiversity loss, pasture matrix, traits
1. Introduction
A fundamental concept in community assembly theory is that when a regional species pool (e.g. the species group likely to colonize a local community [1]) is recognized, it is possible to predict which biotic (e.g. the presence of predators) and abiotic (e.g. climatic variables) characteristics act as filters to select the composition of species in local habitats [2–4]. In this context, habitat characteristics act as one of the selective forces on functional species traits, influencing the species composition of communities on a local scale [2,3,5]. Functional traits are defined as any phenotypic character that indirectly affects the fitness of the organism through biochemical, physiological, morphological, developmental or behavioural mechanisms [6]. The potential of a species to establish itself and persist under any set of environmental conditions is largely influenced by the biological characteristics of the species [2,7,8]. Therefore, identifying how the characteristics of local habitats are related to functional diversity may bring new insight into the mechanisms that determine the assembly of communities in modified landscapes.
In this paper, we investigated the relationship between the functional diversity of frog tadpoles and the environmental descriptors of 38 ponds in an agricultural area in southeastern Brazil that consisted primarily of a matrix of pastures. Although recent studies in this region have shown a strong influence of hydroperiod on the richness of species in adult anuran communities [9,10], the depth of ponds may represent more accurate habitat information for habitat use by tadpoles because they present different swimming traits (i.e. nektonic or benthic). For example, permanent reproductive habitats can be represented by puddles or ponds displaying great depth, as these habitats can be represented by shallow marshes. Furthermore, the increasing complexity of vegetation in ponds is an important factor in explaining the taxonomic diversity in tadpole communities [11,12]. However, with the expansion of agriculture [13], ponds are becoming more homogeneous because of the absence or reduction of vegetation cover in breeding habitats, and its effects on tadpole functional trait diversity are still unknown. Therefore, we are attempting to understand how the characteristics of ponds in an agricultural area are related to the functional diversity in the larval stage of frogs. Our predictions are the following: (i) given that the characteristics of ponds can act as a filter for the occurrence of tadpoles of some species, we predict that shallow reproductive habitats will harbour less functional diversity than deep ones; and/or (ii) because the homogenization of ponds can limit the diversity of functional traits, we predict that reproductive habitats with a higher number of vegetation types (increasing feeding habits and providing refuge from predators) will harbour higher functional diversity than reproductive habitats with fewer vegetation types. We hope that an understanding of how the functional spaces occupied by tadpole communities change along these gradients will be useful in anticipating the potential loss of trait diversity that is associated with biodiversity erosion in altered landscapes.
2. Material and methods
2.1. Data acquisition
To test our hypotheses, we gathered information on the species composition of frog tadpoles in 38 ponds from four previous studies performed by our laboratory [9,14–16]. Because we obtained all data from literature surveys, no specific permission or licence to conduct the fieldwork was required. The four studies used the same tadpoles and pond characteristic sampling methodologies and carried out the surveys of tadpoles during one year. These studies examined the association of environmental descriptors of ponds on species richness and anuran, but we knew nothing of the relationship of these descriptors with frog tadpole traits. The region where the studies were developed was originally covered with semi-deciduous forest and patches of Cerrado biome, which were altered during the establishment of agricultural crops. Currently, this region is considered one of the most deforested and fragmented in the state [13].
Frog tadpoles were collected in 38 ponds with different physiognomic characteristics. All ponds were located in a pasture matrix and were at least 1000 m away from sugarcane, orange and rubber plantations. For each of the 25 frog species recorded, we compiled 11 functional traits of tadpoles (table 1) for five tadpoles between stages 33 and 39 (sensu Gosner 1960) of each species. The tadpoles measured are deposited in DZSJRP—Amphibian Tadpole Collection of Department of Zoology and Botany, UNESP, São José do Rio Preto. The traits were chosen because they have well-known relationships with tadpole feeding and swimming behaviours, habitat use or life-history strategies [17–21]. To test our hypotheses, we compiled two environmental descriptors for the 38 ponds: (i) the maximum depth (DEPTH) of each breeding habitat, which ranged from 0.1 to 2.1 m (an average depth of 0.7 m); and (ii) the number of vegetation types in the interior of ponds (NVI), scored as one of four categories of increasing complexity.
Table 1.
Traits used to measure tadpole functional diversity. To determine the morphometric measurements (i.e. continuous variables), we used the average of five individuals between stages 33 and 39 for each species (sensu Gosner 1960).
| trait type | trait | variables |
|---|---|---|
| position on the water column | benthic (live in the bottom of ponds, either in shallow or deep water), nektonic (live in open water of ponds, often moving through vegetation) or neustonic (move from bottom to surface films of ponds to feed on organisms) | categorical |
| feeding behaviour | scratcher (feeding by rasping substrate or taking in particulate matter), filter (feeding by filtering microscopic particles out of the water) or macrophagous (feeding on large food particles) | categorical |
| position of the eyes | lateral or dorsal | categorical |
| position of oral disc | quantified by the angular orientation of the oral disc of anuran tadpoles relative to a defined longitudinal body axis [15]: terminal (90° angle—extreme one), ventral (0° angle—extreme one) or antero-ventral (angles that fall between the two previous positions) | categorical |
| presence of flagella | presence or absence | binary |
| body form | body length/total length | continuous |
| body form | body width/total length | continuous |
| body form | body height/total length | continuous |
| body form | width of the tail musculature/total length | continuous |
| body form | height of dorsal tail/width of the tail musculature | continuous |
| body form | height of ventral tail/width of the tail musculature | continuous |
2.2. Data analysis
We computed the functional dispersion (FDis), which is a multi-dimensional index based on multi-trait dispersion [22]. It measures the mean distance of an individual species to the centroid of all species in the community [22]. FDis has no upper limit, and small values indicate that communities are composed of species with similar traits, whereas high values indicate that communities are composed of species with distinct traits. According to Laliberté & Legendre [22], this index presents several desirable properties: (i) it is by construction unaffected by species richness; (ii) it can be computed from any distance or dissimilarity measure; (iii) it can handle any number and type of traits (including more traits than species); and (iv) it is not strongly influenced by outliers.
To reduce dimensionality and correlations between continuous variables within our trait database, we performed a principal component analysis (PCA) on measures of tadpole body forms (table 1). The first two principal component axes explained 75% of the variation of these measures (see results in electronic supplementary material, appendix S1). Therefore, for subsequent analysis, we used the first two axes of PCA along with categorical traits (see electronic supplementary material, appendix S1). We used Gower's distance to measure the differences in trait variation across species because it accommodates quantitative, nominal and categorical variables in a single measure [23]. Then, following previous studies [24], we used generalized least-squares models with different combinations of predictor variables taking spatial autocorrelation (i.e. locations close to each other exhibit more similar values than those further apart) into account by fitting an exponential spatial covariance structure [25]. To determine which model best described the FDis values, we used the Akaike information criterion, corrected for the small sample size (AICc) [26]. In addition, to evaluate the uncertainty of the model selection, we used the weight of the model AICc (wAICc) that expressed the weight of the evidence favouring the model as the best among all models compared [26].
To permit comparative analyses among ponds, we tested whether observed FDis values for each pond were higher or lower than simulated values, using a null model in which species richness was fixed and only the identities of the species in the ponds were randomized 999 times. To obtain a significance test, we computed the observed value ranks in the null distribution, and then we calculated a p-value by dividing it by the number of null model interactions plus one [27]. All analyses were performed in R software [28] using the functions in FD [29], Picante [30] and bbmle [31] packages.
3. Results
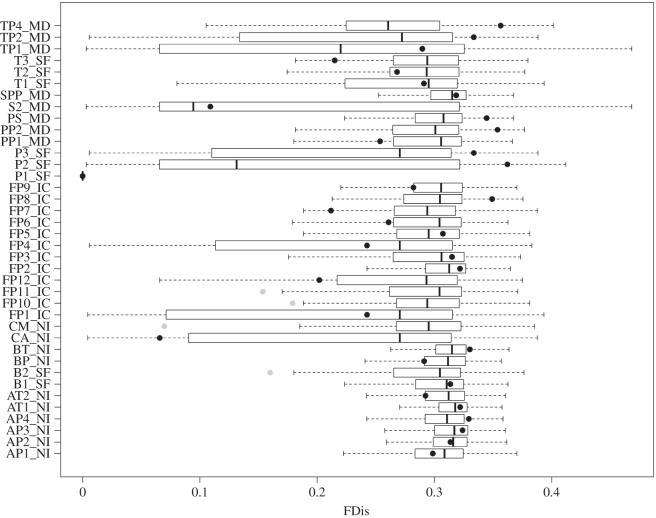
We detected that the model with a bell-curved association between FDis values and depth gradient of ponds (p<0.001, figure 1) was the most parsimonious (table 2). Some FDis values (10%) were lower than expected by the null expectation (figure 2), indicating that some species do not occur in ponds with extreme gradients (e.g. shallow and deep depth) probably because their traits are poorly adapted to these scenarios.
Figure 1.
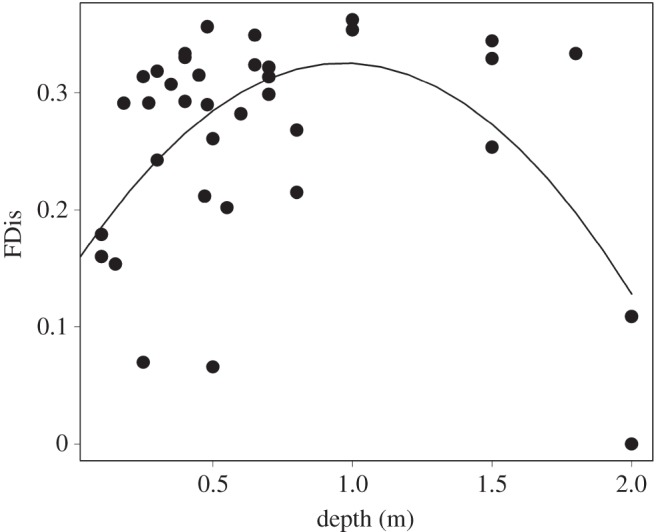
Relationship between FDis values and the depths of 38 ponds in an agricultural area in southeastern Brazil.
Table 2.
Generalized least-squares models predicting the relationship between FDis values and the environmental variables of ponds. DEPTH, maximum depth of ponds; NVI, number of vegetation types in the interior of ponds; AICc, Akaike information criterion, corrected for the small sample size; Δ AICc, difference in Akaike's information criterion; ω, Akaike weights to evaluate model selection uncertainty; NULL, model without predictor variable (considering only intercept). Significant results (p≤0.05) are italicized.
| AICc | ΔAICc | ωAICc | p | |
|---|---|---|---|---|
| DEPTH (quadratic) | −63.93 | 0 | 0.49 | <0.001 |
| NULL | −63.92 | 0.1 | 0.48 | — |
| DEPTH (linear) | −56.61 | 7.3 | 0.01 | >0.05 |
| NVI (linear) | −55.47 | 8.5 | 0.007 | >0.05 |
| NVI (quadratic) | −49.44 | 14.5 | <0.001 | >0.05 |
| DEPTH+NVI | −47.93 | 16.0 | <0.001 | >0.05 |
Figure 2.
Boxplot showing 999 randomized FDis values for each pond. Circles are observed FDis values. Grey circles represent p<0.05, whereas black circles represent p>0.05. Nomenclature of the ponds is the same as used in the original articles.
4. Discussion
Our results showed that ponds with intermediate depth harboured higher functional trait diversity (FDis) than ponds with extreme depths (e.g. shallow or deep depth). Thus, extreme gradients not only may alter total species richness [32] but also can cause a shift in functional space occupation by filtering species with traits that are poorly adapted to these scenarios [33]. We observed that ponds with shallow and deep depths each harboured a set of species with similar traits, whereas ponds with intermediate depths harboured species with distinct traits. Wellborn et al. [34] highlighted that tadpoles of some species are not found in either short-hydroperiod ponds (i.e. shallow depth) because of their high risk of desiccation, or permanent-water ponds (i.e. deep depth) because of their elevated number of predators. We observed that the low functional trait diversity in shallow and deep ponds is caused by the low occurrence of treefrog tadpoles (i.e. species from the genera Dendropsophus, Scinax and Trachycephalus) with traits associated with midwater dwelling. These treefrog tadpoles occurred predominantly in ponds having intermediate depths. Therefore, tadpoles with triangular bodies, high dorsal and ventral fins, and the presence of flagella may have their performance enhanced in intermediate ponds. For example, kinematic studies of both fish and frog tadpoles suggest that traits associated with fins and tails improve swimming [19,35–37]. Furthermore, these nektonic tadpoles are susceptible to fish predation [38]. Thus, shallow and deep depths may act as filters for some frog tadpole traits, contributing to this discrepancy in functional diversity along a depth gradient.
Currently, ecologists are increasingly emphasizing the need to predict how community and ecosystem function will respond to rapid environmental change [39,40]. Although it is widely recognized that small reproductive habitats are important for the maintenance of aquatic and semi-aquatic organismal biodiversity, they remain ignored, no matter what the outcome [41]. Our results show that the depth gradient of ponds inserted in a pasture matrix has a bell-curve-shaped association with frog tadpole functional diversity. However, we still do not know how the expansion of agriculture, currently represented mostly by sugarcane, and the homogenization of reproductive habitats affect the diversity of functional traits in the long term. Because we are witnessing increasing human pressure on land use, simple acts (e.g. maintaining reproductive habitats with medium depth) can be the first step in guiding us to protect the diversity of Neotropical frog tadpole traits in agricultural landscapes. This recommendation becomes even more important because regulations to protect small reproductive habitats are absent and overlooked in Brazilian legislation.
Supplementary Material
Data accessibility
The datasets supporting this article can be accessed at the Dryad Repository (http://dx.doi.org/10.5061/dryad.16q12).
Author' contributions
D.C.R.F. and C.S.Q. developed the initial concepts; F.R.S. conceived the present study version; C.S.Q. compiled data; F.R.S. performed analyses and prepared figures, tables and appendices; F.R.S. wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
F.R.S. was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP 2013/50714-0). D.C.R.F. was supported by the SISBIOTA programme (CNPq 563075/2010-4 and FAPESP 2010/52321-7) and a fellowship from CNPq (303522/2013-5). C.S.Q. was supported by a CAPES/SISBIOTA fellowship.
References
- 1.Carstensen DW, Lessard JP, Holt BG, Krabbe Borregaard M, Rahbek C. 2013. Introducing the biogeographic species pool. Ecography 36, 1310–1318. (doi:10.1111/j.1600-0587.2013.00329.x) [Google Scholar]
- 2.Southwood TRE. 1977. Habitat, the templet for ecological strategies. J. Anim. Ecol. 46, 337–365. (doi:10.2307/3817) [Google Scholar]
- 3.Towsend CR, Scarsbrook MR, Dolédec S. 1997. Quantifying disturbance in streams: alternative measures of disturbance in relation to macroinvertebrate species traits and species richness. J. N. Am. Benthol. Soc. 16, 531–544. (doi:10.2307/1468142) [Google Scholar]
- 4.Ricklefs RE. 1987. Community diversity: relative roles of local and regional processes. Science 235, 167–171. (doi:10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- 5.Webb CT, Hoeting JA, Ames GM, Pyne MI, Poff NL. 2010. A structured and dynamic framework to advance traits-based theory and prediction in ecology. Ecol. Lett. 13, 267–283. (doi:10.1111/j.1461-0248.2010.01444.x) [DOI] [PubMed] [Google Scholar]
- 6.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. (doi:10.1111/j.2007.0030-1299.15559.x) [Google Scholar]
- 7.McGill BJ, Enquist BJ, Weiher E, Westboy M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. (doi:10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 8.Lavorel S, et al. 2007. Plant functional types: are we getting any closer to the Holy Grail? In Terrestrial ecosystems in a changing world (eds J Canadell, LF Pitelka, D Pataki), pp. 149–164. The IGBP series. New York, NY: Springer.
- 9.Santos TG, Rossa-Feres DC, Casatti EL. 2007. Diversidade e distribuição espaço temporal de anuros em região com pronunciada estação seca no sudeste do Brasil. Iheringia 97, 37–49. (doi:10.1590/S0073-47212007000100007) [Google Scholar]
- 10.da Silva FR, Gibbs JP, Rossa-Feres DC. 2011. Breeding habitat and landscape correlates of frog diversity and abundance in tropical agricultural landscape of southeastern Brazil. Wetlands 31, 1079–1087. (doi:10.1007/s13157-011-0217-0) [Google Scholar]
- 11.Díaz-Paniagua C. 1987. Tadpole distribution in relation to vegetal heterogeneity in temporary ponds. Herpetol. J. 1, 167–169. [Google Scholar]
- 12.Kopp K, Wachlevski M, Eterovick PC. 2006. Environmental complexity reduces tadpole predation by water bugs. Can. J. Zool. 84, 136–140. (doi:10.1139/z05-186) [Google Scholar]
- 13.Rodrigues RR, et al. 2008. Diretrizes para conservação e restauração da biodiversidade no estado de São Paulo. São Paulo, Brazil: FAPESP. [Google Scholar]
- 14.da Silva FR, Candeira CP, Rossa-Feres DC. 2011. Dependence of anuran diversity on environmental descriptors in farmland ponds. Biodiv. Conserv. 21, 1411–1424. (doi:10.1007/s10531-012-0252-z) [Google Scholar]
- 15.Vasconcelos TS. 2009. Diversidade, padrões espaciais e temporais de anfíbios anuros em uma floresta estacional semidecidual atlântica, Parque Estadual do Morro do Diabo (PEMD). PhD thesis, Universidade Estadual Paulista, campus Rio Claro, São Paulo, Brazil. See http://acervodigital.unesp.br/handle/unesp/160704?locale=pt_BR (accessed 17 March 2014).
- 16.Vasconcelos TS, Rossa-Feres DC. 2005. Diversidade, distribuição espacial e temporal de anfíbios anuros (Amphibia, Anura) na região noroeste do estado de São Paulo, Brasil. Biota Neotrop. 5, 1–14. (doi:10.1590/S1676-06032005000300010) [Google Scholar]
- 17.Altig R, Johnston GF. 1989. Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetol. Monogr. 3, 81–90. (doi:10.2307/1466987) [Google Scholar]
- 18.Van Buskirk J, McCollum SA. 2000. Influence of tail shape on tadpole swimming performance. J. Exp. Biol. 203, 2149–2158. [DOI] [PubMed] [Google Scholar]
- 19.Van Buskirk J, McCollum SA. 2001. Functional mechanisms of an inducible defense in tadpoles: morphology and behaviour influence mortality risk from predation. J. Evol. Biol. 13, 336–347. (doi:10.1046/j.1420-9101.2000.00173.x) [Google Scholar]
- 20.Rossa-Feres DC, Jim J, Fonseca MG. 2004. Diets of tadpoles from a temporary pond in southeastern Brazil (Amphibia, Anura). Rev. Bras. Zool. 21, 745–754. (doi:10.1590/S0101-81752004000400003) [Google Scholar]
- 21.Strauß A, Reeve E, Randrianiaina RD, Vences M, Glos J. 2010. The world's richest tadpole communities show functional redundancy and low functional diversity: ecological data on Madagascar's stream-dwelling amphibian larvae. BMC Ecol. 10, 1–10. (doi:10.1186/1472-6785-10-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laliberté E, Legendre P. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. (doi:10.1890/08-2244.1) [DOI] [PubMed] [Google Scholar]
- 23.Podani J, Schmera D. 2006. On dendrogram-based measures of functional diversity. Oikos 115, 179–185. (doi:10.1111/j.2006.0030-1299.15048.x) [Google Scholar]
- 24.Ding Z, Feeley KJ, Wang Y, Pakeman RJ, Ding P. 2013. Patterns of bird functional diversity on land-bridge island fragments. J. Ecol. 82, 781–790. (doi:10.1111/1365-2656.12046) [DOI] [PubMed] [Google Scholar]
- 25.Dormann FC, et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628. (doi:10.1111/j.2007.0906-7590.05171.x) [Google Scholar]
- 26.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information- theoretic approach. New York, NY: Springer. [Google Scholar]
- 27.Swenson NG. 2014. Functional and phylogenetic ecology in R. Springer UseR! Series New York, NY: Springer. [Google Scholar]
- 28.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 29.Laliberté E, Shipley B. 2011. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package v. 1.0-11; See http://cran.r-project.org/web/packages/FD. [DOI] [PubMed] [Google Scholar]
- 30.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. (doi:10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 31.Bolker B, R Development Core Team. 2014. bbmle tools for general maximum likelihood estimation. R package v. 1.0.17. See http://CRAN.R-project.org/ackage=bbmle.
- 32.Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310. (doi:10.1126/science.199.4335.1302) [DOI] [PubMed] [Google Scholar]
- 33.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. 2013. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. (doi:10.1016/j.tree.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 34.Wellborn GA, Skelly DK, Werner EE. 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Syst. 27, 337–363. (doi:10.1146/annurev.ecolsys.27.1.337) [Google Scholar]
- 35.Wassersug RJ, Hoff KVS. 1985. Kinematics of swimming in anuran larvae. J. Exp. Biol. 119, 1–30. [Google Scholar]
- 36.Wassersug RJ. 1989. Locomotion in amphibian larvae (or ‘Why aren't tadpoles built like fishes?’). Am. Zool. 29, 65–84. (doi:10.1093/icb/29.1.65) [Google Scholar]
- 37.Liu H, Wassersug RJ, Kawachi K. 1996. A computational fluid dynamics study of tadpole swimming. J. Exp. Biol. 199, 1245–1260. [DOI] [PubMed] [Google Scholar]
- 38.Nomura F, Prado VHM, da SILVA FR, Borges RE, Dias NYN, Rossa-Feres DC. 2011. Are you experienced? Predator type and predator experience trade-offs in relation to tadpole mortality rates. J. Zool. 284, 144–150. (doi:10.1111/j.1469-7998.2011.00791.x) [Google Scholar]
- 39.Naeem S, Thompson LJ, Lawler SP, Lawton JH. 1994. Declining biodiversity can alter the performance of ecosystems. Nature 368, 734–737. (doi:10.1038/368734a0) [Google Scholar]
- 40.Tilman D, Hill J, Lehman C. 2006. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314, 1598–1600. (doi:10.1126/science.1133306) [DOI] [PubMed] [Google Scholar]
- 41.Semlitsch RD, Bodie JR. 1998. Are small, isolated wetlands expendable? Conserv. Biol. 12, 1129–1133. (doi:10.1046/j.1523-1739.1998.98166.x) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article can be accessed at the Dryad Repository (http://dx.doi.org/10.5061/dryad.16q12).