Abstract
Recent models predict contrasting impacts of climate change on tropical and temperate species, but these models ignore how environmental stress and organismal tolerance change during the life cycle. For example, geographical ranges and extinction risks have been inferred from thermal constraints on activity during the adult stage. Yet, most animals pass through a sessile embryonic stage before reaching adulthood, making them more susceptible to warming climates than current models would suggest. By projecting microclimates at high spatio-temporal resolution and measuring thermal tolerances of embryos, we developed a life cycle model of population dynamics for North American lizards. Our analyses show that previous models dramatically underestimate the demographic impacts of climate change. A predicted loss of fitness in 2% of the USA by 2100 became 35% when considering embryonic performance in response to hourly fluctuations in soil temperature. Most lethal events would have been overlooked if we had ignored thermal stress during embryonic development or had averaged temperatures over time. Therefore, accurate forecasts require detailed knowledge of environmental conditions and thermal tolerances throughout the life cycle.
Keywords: life cycle, embryo, climate change, extreme events, acute stress, lizard
1. Introduction
In the coming years, global warming will redistribute and potentially eliminate species [1,2]. With each passing decade, extreme heat and drought have become more frequent and more intense [3,4]. These climatic events directly impact the performances of species [5] and alter the interactions among them [6,7]. The outlook for biodiversity depends on the frequency and magnitude of extreme events relative to organismal tolerances and their capacity to evolve. Even a small degree of warming will greatly impact species if the resulting temperatures exceed lethal limits.
To identify those species facing the greatest risks, biologists have built a mechanistic theory of population dynamics and range shifts during climate change [8–10]. Unfortunately, these models quantify thermal stress at far coarser resolutions than those experienced by organisms [11]. For example, rates of survival and fecundity have been gleaned from experiments involving chronic heat stress [12]. Yet, organisms often thrive during intermittent exposure to temperatures that would prove lethal during chronic exposure [13,14]. By using responses to artificially chronic stresses to forecast responses to naturally intermittent stresses, biologists have overestimated the impacts of warming. To complicate matters, an organism's exposure to and tolerance of heat depend on its stage in the life cycle [15,16]. When organisms pass through vulnerable and sensitive stages, brief exposures to extreme temperatures can catastrophically impact populations [10]. Therefore, mechanistic models should incorporate the appropriate frequency, duration and magnitude of heat stress for each stage. By quantifying operative temperatures and organismal performances at the appropriate scales for embryos and adults, we show that such details qualitatively alter the predicted impacts of climate change.
Our analyses focus on North American lizards, which have been a model system for understanding range shifts and extinction risks during global warming [9,17–21]. Lizards face different thermal stresses during the life cycle, because embryos develop in a confined space while adults enjoy freedom and mobility. Consequently, embryo temperatures conform to changes in soil temperature [22], while adults thermoregulate with great precision [23]. Mothers deposit their embryos in shallow nests, where they experience fluctuating and unpredictable temperatures for more than two months [24]. Thermal stress can occur daily during a brief period in the afternoon when soil temperatures peak. Although embryos cannot survive prolonged exposure to such extremes [25,26], they grow and develop rapidly during brief daily exposures [14,24]. Still, even a brief exposure to a temperature of sufficient magnitude causes death by cardiac arrest [27]. Consequently, we must know the frequency and magnitude of thermal changes in soil and the chance that embryos will survive these stresses if we want to infer demographic impacts.
Our study integrated computational and experimental approaches. First, we downscaled climatic data to derive hourly microclimates available to lizards during embryonic and adult stages. Then, we quantified the lethal limits of lizard embryos from four regions during realistic rates of warming and cooling. Finally, we modelled the impacts of climatic extremes on local extinctions and range shifts, with and without considering the embryonic stage. For this modelling, we assumed that adults behaviourally thermoregulate and lay eggs at certain depths and exposures. Ignoring thermal stress on embryos dramatically underestimated the negative impacts of climate change. This bias became worse when considering responses to future climates, which will impose a greater frequency and magnitude of thermal stress.
2. Material and methods
(a). Scaling from global climates to operative temperatures
To see how lizards experience extreme climatic events at each stage of the life cycle, we calculated operative temperatures using hourly microclimates for the past and the future. We started by downscaling bias-corrected data from the Community Earth System Model (dataset ds316.1; [28]) with the Weather Research & Forecasting Model (v. 3.4; [29]). The resulting regional climates were used to compute microclimates for 1980–1999 and 2080–2099 with the Noah Microphysics Model [30]. Microclimatic data—temperatures of air and soil, wind speed, relative humidity and radiation—spanned vertical positions from 198 cm above-ground to 198 cm below-ground, at levels of shade ranging from 0 to 100%. Models were written and compiled in Fortran and were simulated on the Stampede Supercomputer at the Texas Advanced Computing Center. The electronic supplementary material describes how we calculated and validated the microclimatic variables.
(b). Survival of embryos during thermal stress
To understand how thermal stress impacts the survivorship of embryos, we conducted experiments with lizards from the Sceloporus undulatus complex. This North American group comprises several evolutionary species connected by various degrees of gene flow [31,32]. Although lizards throughout the range of this group reproduce at different frequencies, lizards in all populations reproduce in May and June; therefore, we collected gravid females from four populations in these months during 2012 and 2013. Although experiments were divided between years, gravid females from each population were included each year. The populations were chosen to yield genotypes from northern and southern regions of two phylogenetic clades: Atlantic County, NJ (S. undulates; 39.8° N, 74.6° W); Edgefield County, SC (S. undulates; 33.7° N, 82.0° W); Costilla County, CO (Sceloporus tristichus; 37.10° N, 105.74° W) and Gila County, AZ (S. tristichus, 33.3° N, 111.0° W). Methods used to house females and to collect and incubate eggs were described by Angilletta et al. [27].
In our first experiment, we measured the effect of realistic heat stress on the survival of embryos. Embryos in natural nests heat gradually throughout the day, reaching a maximal temperature in the afternoon. To quantify the risk of mortality during warming, we exposed embryos to daily cycles of temperature with different amplitudes. The embryos represented a range of developmental stages between 50 and 95% of the incubation period, approximated from day of incubation and a mean incubation period of 70 days [14]. Prior to entering these treatments, embryos had developed at the same thermal cycle, which peaked at 35°C each afternoon and fell to 20°C each night (figure 1a); this thermal cycle mimics those of natural nests [24]. At the beginning of the experiment, eggs were assigned to remain at the same temperatures or to shift to one of three warmer cycles (peaking at either 38°, 40° or 42°C; figure 1a). A single egg per clutch was assigned to each treatment. Every morning, the survival of each embryo was inferred by detecting heart rate with a commercially available system of infrared sensors (Buddy Egg Monitor, Avitronics, Cornwall, UK). Embryos without a pulse for three continuous days were scored as dead on the first day that a pulse went undetected. None of these eggs hatched, suggesting that we accurately assessed mortality. We used a Cox proportional hazards model to estimate the effects of population and temperature on the survival of embryos. Because the responses of embryos from the same mother were probably correlated, we also included a robust sandwich estimator of the variance attributable to maternal identity. Statistical models were fit using the survival package of the R statistical software [33].
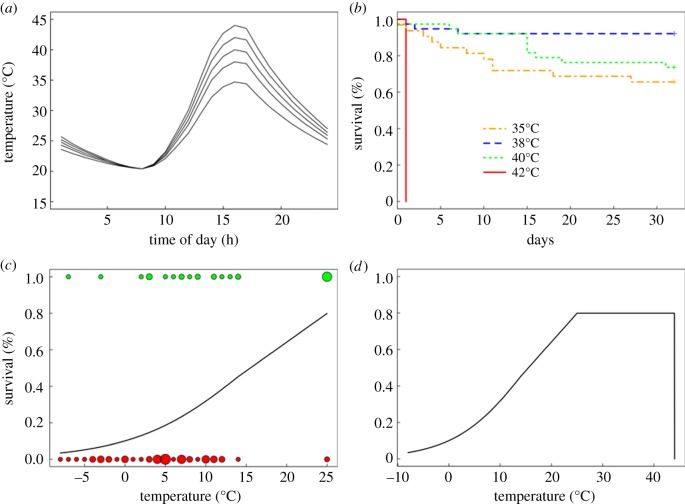
Figure 1.
Realistic rates of warming and cooling were used to estimate the temperatures that reduce the survival of lizard embryos. (a) Four thermal cycles used to estimate the effect of acute warming. (b) The proportion of embryos that survived daily cycles of temperature depended on the maximal temperature of the cycle (35–42°C). (c) The survival of embryos after cooling depended on the minimal temperature (top circles, survived to hatching; bottom circles, died before hatching; size of circles represents the number of embryos). (d) Empirical data were used to construct a theoretical relationship between body temperature and the rate of embryonic survival. (Online version in colour.)
In a second experiment, we exposed embryos to cooling and measured survival after rewarming. We already knew that Sceloporus embryos survive prolonged exposures to temperatures as low as 15°C [34] and cooled to temperatures as low as −10°C before freezing in our preliminary observations. Therefore, we quantified survivorship following brief exposure to temperatures between these extremes. Eggs were placed individually in sealed glass vials, which were then submerged in a bath of water and ethylene glycol. A thermocouple passed through a hole in the cap of the vial and came into contact with the shell of the egg (OctTemp2000, MadgeTech, Warner, NH, USA). A refrigerated circulator (Proline 855C, LAUDA-Brinkmann, LP., Delran, NJ, USA) lowered the temperature of the bath continuously at a rate of 3°C per hour, which resembles rates of cooling in soils. Each egg was cooled from 15°C to a randomly selected temperature between 15° and –10°C, at intervals of 1°C. Upon reaching the target, the egg was warmed to air temperature and returned to a daily thermal cycle from 20° to 35°C. Survival following cooling was inferred from daily measures of heart rate, as described above. We tested the relationship between embryonic survival and the minimal temperature using a Bernoulli mixed effect regression with the logit function (lmer function, [35]).
Having quantified survival during warming and cooling, we asked whether prior exposure to extreme yet nonlethal warming helps an embryo tolerate future warming. An embryo from each mother was randomly assigned to one of two treatments: a diel cycle that peaked at either 35°C (control) or 40°C (stressed). Embryos experienced these treatments for 2 days before being exposed to a cycle peaking at 42°C followed by a cycle peaking at 44°C. After each cycle, we monitored cardiac activity to determine whether each embryo had survived. Cox proportional hazards models were used to quantify effects of population and treatment (control versus heated) on the survival of embryos during each cycle (42°C and 44°C).
(c). Modelling the performance of embryos
We tracked survival and development according to hourly soil temperatures derived from dynamical downscaling and our microclimate model. On each day of the year, we allocated a nest to microhabitats with each combination of shade (0, 25, 50, 75 or 100%) and depth (3, 6, 9 or 12 cm). These combinations captured the range of microhabitats for natural nests [24]. For each nest, we calculated: (i) minimal and maximal temperatures, (ii) the mean survival rate of embryos, (iii) the mean incubation period, and (iv) the number of days between hatching and winter dormancy (i.e. when climate precludes activity for at least 7 days). Nests were added to the model on days when: (i) climate enabled activity by adults, and (ii) at least 30 days of activity had passed for a female to produce a clutch of eggs.
We modelled the survivorship of embryos based on our experimental results (figure 1). Since we observed a threshold for mortality during warming, we assumed that embryos died if soil temperature exceeded 44°C. Since extreme cooling had a graded effect on survival, we calculated survivorship from the empirical relationship between the minimal temperature during cooling and the probability of survival. Eggs that neither warmed to 44°C nor cooled below 25°C were assigned a survivorship to hatching of 80%, based on the mean survivorship in our incubation treatments and similar treatments in other experiments [14]. In our model, the minimal temperature of an embryo equalled the minimal soil temperature during incubation. Surviving embryos developed at an hourly rate (D, dec %) described as
where Tsoil equals the temperature of soil (°C) at the shade and depth of the nest. We parametrized this function with rates of development recorded at constant temperatures [25], because the variance of temperature has little or no direct effect on developmental rates of Sceloporus embryos [26].
(d). Modelling rates of population growth
Rates of population growth (r0, lizards d−1) were computed according to Buckley [17]:
where enet equals the net energy gain by an adult, μ equals the daily rate of mortality (197.26 × 10−5 lizards d−1; [17]), and m equals the number of eggs produced per Joule (3.2 × 10−4 eggs J−1; [17]) multiplied by the probability of surviving to adulthood. For each location on the map, we calculated the survival to adulthood component of m as the product of the survivorship of embryos and the survivorship of juveniles. In contrast to previous analyses [17–19,21], we allowed the survivorship of embryos to vary according to soil temperatures (figure 1d) and compared our results to those obtained assuming a constant survivorship (m = 2.78 × 10−5 eggs J−1; [17]). In all cases, the survivorship of juveniles to adulthood was 7.98 × 10−5 (lizards J−1), which we obtained by averaging values from demographic studies [36].
The net energy gain of an adult was calculated as
where tf and tr equal durations of foraging and resting, respectively (s d−1), and ef and er equal energy gained while foraging and energy lost while resting, respectively (J s−1). These variables were estimated by simulating feeding and digestion at predicted body temperatures (Tb). Body temperatures were predicted from operative temperatures derived from air temperatures, radiative loads and wind speeds in each microhabitat. We simulated behavioural thermoregulation by assuming that lizards could select between exposed or shaded microhabitats, assuming either a laying or standing posture. If body temperature resided in the range for activity (between 29.4°C and 36.3°C, central 80% of field body temperature, [23]), a lizard actively maintained its preferred body temperature (33.1°C; [23]). Otherwise, we assigned the lizard the available temperature closest to its preferred temperature. During the night, thermoregulation was impossible, and lizards were assigned a temperature by assuming no solar radiation. See the electronic supplementary material for additional information about the calculation of energy gain and body temperature.
To see how predictions depended on the resolution of climate, we simulated population growth using either hourly projections of microclimatic variables (hourly data) or monthly means of daily minima and maxima for the same variables (monthly data).
3. Results
(a). Survival of embryos during thermal stress
We observed a threshold effect on the survivorship of embryos during warming. All embryos died when briefly exposed to 42°C, but the majority of embryos survived daily exposures to lower temperatures (figure 1b). Based on the most likely statistical model (electronic supplementary material, table S3), embryos survived best when warming to 38°C each day. Survivorship with warming to 35° or 40°C each day was high but significantly lower than survivorship at 38°C. The effect of population was omitted from the most likely model and was non-significant in the maximal model (p-value ranged from 0.31 to 0.79).
Embryos failed to improve their heat tolerance when exposed to 41°C before experiencing higher temperatures. All embryos, regardless of acclimation treatment or source population, survived to 42°C but died when exposed to 44°C (β = 2.46, z = 6.63, p < 0.001). The effects of acclimation and population were omitted from the most likely model and were estimated as non-significant in the maximal model (acclimation: p = 0.96; population: p-value ranged from 0.76 to 0.84).
In contrast to warming, cooling to temperatures as low as –8°C caused a graded increase in mortality. This continuous relationship was described by a linear model: logit(survival) = −2.19 + 0.14 · Tsoil,lowest (p = 0.001; figure 1c). As with heat tolerance, cold tolerance of embryos did not differ among populations (p-value ranged from 0.34 to 0.87).
(b). Thermal variation and embryonic survival
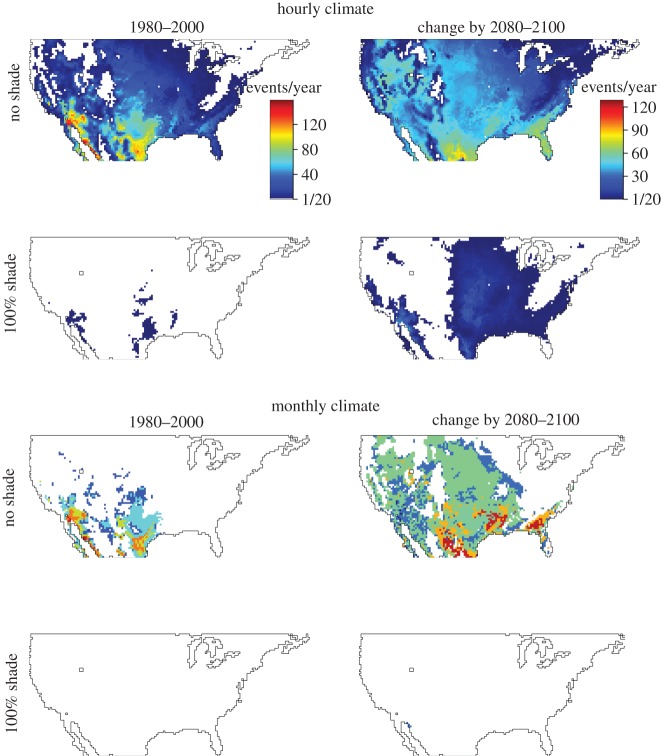
In our climate simulation, maximal soil temperatures exceeded the lethal limit for embryos more frequently and at more locations by 2100 (figure 2). Assuming that females lay eggs at a depth of 6 cm and no shade, lethal events spread from 77 to 96% of the USA between 2000 and 2100 and increased in frequency from 24 to 50 days per year. Under full shade, lethal events spread from only 3 to 48% of the USA and increased in frequency from 0.2 to 4 days per year.
Figure 2.
Frequencies of lethal events under monthly and hourly data during past (1980–2000) climate and the predicted change in the future (2080–2100). Data are presented for nests at a depth of 6 cm under either 0 or 100% shade. White areas represent locations for which no heat events were predicted. For frequencies at other depths, see the electronic supplementary material, figures S1–S4. (Online version in colour.)
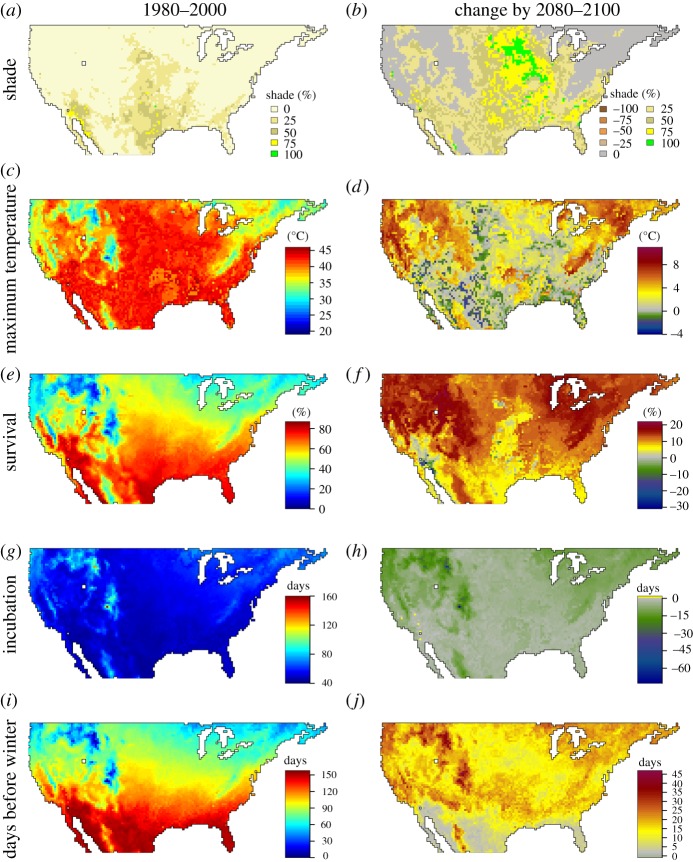
Since geographical patterns of embryonic survival depended on the timing and location of nests (figure 3 and electronic supplementary material, figures S5–S32), mothers could enhance reproductive success through nesting behaviour. Embryos survival was greatest in the deepest nests, which experience dampened thermal cycles. However, the amount of shade that maximized survival varied spatially, with warmer regions at lower altitudes favouring more shade (figure 3 and electronic supplementary material, figures S33–S60). Assuming that females lay eggs during June at a depth of 6 cm under 50% shade, embryonic survival will decrease from 38 to 16% by 2100 in the majority of the USA (electronic supplementary material, figure S14).
Figure 3.
The shade conditions that maximize embryonic survival vary across space and time. Here, we show the optimal levels of shade for eggs laid at a maximal depth of 12 cm during the month of June in the period of 1980–2000 (a) and the optimal change in shade by 2080–2100 (b). The remaining plots depict the maximal soil temperatures (c,d), maximal survival rates (e,f), incubation period (g,h) and time between hatching and the first day of winter (i,j), assuming that mothers construct nests in the optimal level of shade. See the electronic supplementary material, figures S33–S60 for plots based on maximal depths of 3, 6 or 9 cm of eggs laid through April to October. (Online version in colour.)
Dynamical downscaling of climates to an hourly resolution yielded a more realistic view of embryonic survival. Specifically, monthly data severely underestimated the geographical distribution of lethal events while overestimating the frequency of lethal events at each location. For unshaded nests, the distribution of lethal events increased spread from 14 to 54% of the USA between 2000 and 2100 (versus 77 to 96% for hourly data). At locations where lethal events are predicted in the monthly data, the mean frequency of lethal events for an unshaded nest increased from 64 to 77 days per year (versus 24–50 days per year for hourly data). Moreover, monthly data overestimated thermal extremes during summer and underestimated thermal extremes during winter. Assuming that lizards deposit their eggs at a depth of 6 cm under 50% shade [24], using monthly rather than hourly climates underestimated the frequency of lethal events in 33 and 68% of the USA for past and future climates, respectively (figure 2). Results were qualitatively similar for other levels of depth and shade (electronic supplementary material, figures S1–S4).
(c). Rates of population growth
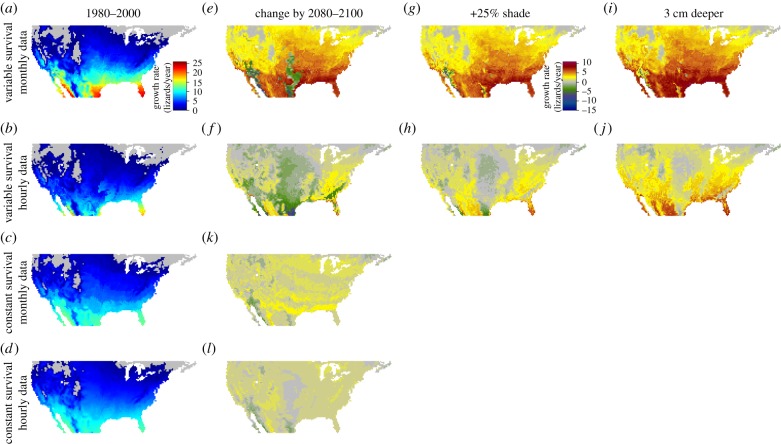
The potential range of lizards and its shift during global warming depended strongly on our assumptions about embryonic performance. When embryos survived at a constant rate, a strong latitudinal pattern of fitness emerged (figure 4d). The projected change in climate by 2100 would create more opportunities for lizards to forage in 98% of the USA, leading to greater predicted fitness. Nevertheless, when embryonic performance depended on soil temperatures, the thermal threshold for survival disrupted the smooth latitudinal gradient in predicted fitness (figure 4b). Considering embryonic performance also shaped our view of the impacts of climate change. When embryos survived at a constant rate, climate change was predicted to decrease fitness in only 4% of the USA (based on hourly climate data); yet, when survival depended on temperature, we predicted a decrease in fitness in 35% of the USA. This negative impact can be avoided in many locations if females lay eggs in shadier (figure 4h) or deeper locations (figure 4j). Nesting in shadier locations would compensate for warming in all but 5% of the USA, and nesting in deeper soil would compensate for warming in all but 0.1% of the USA.
Figure 4.
Population growth rates for the period 1980–2000 and the predicted spatial distribution of changes by 2080–2100. The survival of embryos is assumed to be either temperature-dependent or -independent. Body temperatures of lizards were calculated from either monthly averages of climate or hourly projections of climate. If embryonic survival depends on temperature, changes in population growth rates are shown at three scenarios of nesting behaviour: mothers either fail to alter the depth and shade of their nests, or construct nests under more shade or in deeper soil. These results were based on simulations in which the depth of nests was 6 cm and the level of shade was 50%. See the electronic supplementary material, figures S61–S72 for results of simulations under other depths and shade conditions. (Online version in colour.)
4. Discussion
Global extinctions of lizards have been connected to thermal constraints on the activity of adults [21]. For example, tropical and subtropical lizards were predicted to face extinction because warming will restrict activity, reducing opportunities to growth and reproduction [21]. By contrast, temperate lizards might gain opportunities to grow and reproduce if their environments warm [17]. Although models based on a single life stage enable researchers to predict outcomes for many species [9,21], such models omit critical processes by which climate determines the fitness an organism. Our model, which considers heat stress at multiple life stages, predicts population dynamics that differ qualitatively from a model that ignores heat stress during the embryonic stage (figure 4 and electronic supplementary material, figures S61–S72). In previous studies, models based solely on the adult stage predicted greater fitness in warmer environments, resulting from more time for activity [17,18,37]. However, fitness decreased in our model, because embryos cannot escape lethal temperatures by moving [22]. Thus, a loss of performance at one stage can cause rapid extinction [16] or limit species abundance [38] even when performance increases at other stages. Demographic models should benefit from understanding how each life stage avoids or tolerates thermal stress.
For embryos, the consequences of global warming depend on where mothers will lay eggs in future environments [39]. In past climates, even mothers in the warmest regions could nest in sunnier patches, where embryos would survive better, hatch earlier, and have more time to grow before winter. With the projected change in climate, females should construct shadier nests at all but a few locations, where temperatures will not increase sufficiently to threaten embryos or where mothers should already nest in full shade. Although mothers can lay eggs in shadier places, nests might still exceed the lethal limits of embryos if heatwaves become more frequent. Mothers that construct deeper nests can compensate for losses of fitness (figure 4 and electronic supplementary material, figures S33–S60), assuming that their offspring can still reach the surface after hatching [40]. Even if we consider the potential for adaptive nesting behaviour, repeated exposure to temperatures below the lethal limit could still impact the morphology, physiology and behaviour of offspring [41]. Therefore, our analyses provide conservative forecasts of the impact of climate warming on population dynamics.
The resolution of climatic variables determined whether we detected lethal events and hence drew accurate conclusions about the risk of local extinction. Simulations based on monthly data overestimated rates of population growth, regardless of whether we considered embryonic performance. However, overestimation was far more severe when the lethal temperature of embryos was included in the model. Because monthly data were created by averaging extreme temperatures among days, they failed to capture rare lethal events. Since biologists have relied on climate data aggregated to a monthly resolution [42,43], their analyses might have missed significant ecological impacts. For example, Deutsch et al. [12] used monthly data to conclude that temperate species would benefit from climate warming; however, a re-analysis based on hourly data showed that temperate species might also be at risk [44,45]. As in our model, this discrepancy resulted from the increasing variance of temperature in the future, which was dampened by averaging daily extremes within months.
By measuring survivorship under realistic durations of thermal stress, we learned that intermittent warming can even enhance the fitness of a species (figure 1). Although lizard embryos were able to withstand acute exposures to temperatures as high as 42°C, they did not survive a constant temperature above 34°C in a previous experiment [25]. Based on our climate data, nests would temporarily exceed 34°C in more than 90% of the current range of the S. undulatus complex (figure 5). Thus, lethal limits based on chronic exposure would lead one to ridiculously underestimate the potential range. In fact, natural nests often exceed the temperatures that kill embryos during chronic exposure [41]. Yet influential analyses of the biological impacts of climate change have relied on thermal limits of animals during chronic exposure (e.g. [12,45,46]). In contrast to these efforts, our analysis accounts for a thermal threshold of survival during acute exposure, which combines with climatic fluctuations to produce nonlinear effects on demography (figure 4). Similar thresholds exist in other species; for example, the survivorship of two-spotted spider mites (Tetranychus urticae Koch) drops from 70 to 0% between brief exposures to 45.5°C and 46°C [47]. By combining tolerances of acute thermal stress with highly resolved climate data, biologists will gain a more accurate perception of the consequences for population growth and geographical ranges.
Figure 5.
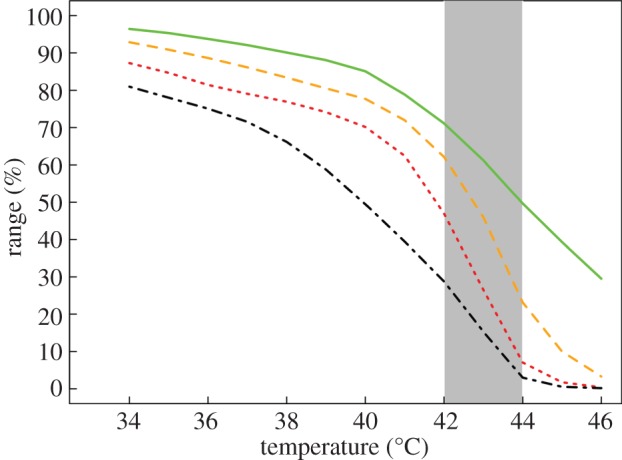
The percentage of the geographical range in which nests at various depths below the ground (3, solid line; 6, dashed line; 9, dotted line; 12, dotted-dashed line in centimetres) had experienced warm temperatures from 34°C to 46°C under shade conditions that maximized survival. The grey area represents empirically based lethal temperatures for embryos. (Online version in colour.)
By explicitly modelling the embryonic stage, we also explained the seasonal timing of reproduction by lizards. In the coldest portion of the S. undulatus complex range, adults are active from April to October but produce only two clutches of eggs during this period [48]. This contracted period of reproduction reflects the thermal tolerance of embryos rather than that of adults. Specifically, eggs laid before May or after July would probably die from thermal stress or would hatch with little time to acquire energy for winter (electronic supplementary material, figures S33–S60). If minimal and maximal soil temperatures increase by 2100, as predicted by our simulations (see the electronic supplementary material, figures S73–S100 and figures S101–S128, respectively), lizards in the coldest portion of the range environments will enjoy a longer period of reproduction each year.
Although environments vary dramatically throughout the range, embryos sampled from four populations shared the same lethal temperature. This conservation of the thermal niche makes sense if similar extreme temperatures occur periodically in all of these populations [27]. To evaluate this idea, we mapped locations at which soil temperatures reached extreme temperatures at least once during 1980–1999 (figure 5). Despite clear differences in mean temperatures among sites, soils in 60% of the range reached 41°C at least once, regardless of their depth (0–12 cm) or shade (0–100%). Such a pervasive frequency of thermal stress explains why embryos from different locations tolerate temperatures up to 42°C. Moreover, the phylogeography of the S. undulatus complex indicates repeated northward expansion of range, which might have transported alleles from warm-adapted populations in the south [32]. Therefore, either periodic strong selection for thermal tolerance throughout the range or gene flow from populations in the southern part of the range could explain the remarkable niche conservatism we observed in this species.
For animals that literally place all of their eggs in one basket, lethal temperatures will impede recruitment and depress the mean fitness of the population. Moreover, repeated exposure to sublethal stresses can cause cumulative damage that reduces the quality of offspring or their survival after hatching [41]. These issues became evident only after considering the interactions between embryonic physiology and adult behaviour in the context of hourly fluctuations in climate. In the future, biologists should carefully dissect the temporal structure of environmental stress during life cycles when assessing the ecological impacts of climate change.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Rory Telemeco for helpful comments.
Ethics
All procedures were approved by the Animal Care and Use Committee of Arizona State University (11–1161, 14–1338). The collections of lizards were approved by Arizona Game and Fish Department (SP628482), Colorado Parks and Wildlife (12HP968), New Jersey Department of Environmental Protection (SC20113051), and South Carolina Department of Natural Resources (26–2012).
Data accessibility
Embryonic tolerance data have been uploaded as part of the electronic supplementary material. Microclimate data will be available as a data repository through the Knowledge Network for Biocomplexity.
Authors' contributions
O.L., L.B.B., T.H.K., C.S. and M.J.A. conceived and designed the experiments; O.L., C.S., K.B. and D.K. performed the experiments; O.L. and M.J.A. analysed the data; L.B.B., T.H.K. and M.J.A. contributed materials/analysis tools; O.L., L.B.B., T.H.K. and M.J.A. co-wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by grants from the National Science Foundation to M.J.A. (EF-1065638), L.B.B. (EF-1065638) and T.H.K. (EF-1064901). O.L. was also supported by a Rothschild Post-Doctoral fellowship.
References
- 1.Javeline D, Hellmann JJ, Cornejo RC, Shufeldt G. 2013. Expert opinion on climate change and threats to biodiversity. Bioscience 63, 666–673. ( 10.1525/bio.2013.63.8.9) [DOI] [Google Scholar]
- 2.Moritz C, Agudo R. 2013. The future of species under climate change: resilience or decline? Science 341, 504–508. ( 10.1126/science.1237190) [DOI] [PubMed] [Google Scholar]
- 3.Hansen J, Sato M, Ruedy R. 2012. Perception of climate change. Proc. Natl Acad. Sci. USA 109, E2415–E2423. ( 10.1073/pnas.1205276109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocker TF, et al. 2013. Technical Summary. In Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM), pp. 33–115. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heatwaves. Biol. Lett. 6, 253–256. ( 10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blois JL, Zarnetske PL, Fitzpatrick MC, Finnegan S. 2013. Climate change and the past, present, and future of biotic interactions. Science 341, 499–504. ( 10.1126/science.1237184) [DOI] [PubMed] [Google Scholar]
- 7.Valiente-Banuet A, et al. 2015. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. ( 10.1111/1365-2435.12356) [DOI] [Google Scholar]
- 8.Heffernan JB, et al. 2014. Macrosystems ecology: understanding ecological patterns and processes at continental scales. Front. Ecol. Environ. 12, 5–14. ( 10.1890/130017) [DOI] [Google Scholar]
- 9.Buckley LB. 2010. The range implications of lizard traits in changing environments. Glob. Ecol. Biogeogr. 19, 452–464. ( 10.1111/j.1466-8238.2010.00538.x) [DOI] [Google Scholar]
- 10.Thomson JA, Burkholder DA, Heithaus MR, Fourqurean JW, Fraser MW, Statton J, Kendrick GA. 2015. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Glob. Change Biol. 21, 1463–1474. ( 10.1111/gcb.12694) [DOI] [PubMed] [Google Scholar]
- 11.Potter KA, Arthur Woods H, Pincebourde S. 2013. Microclimatic challenges in global change biology. Glob. Change Biol. 19, 2932–2939. ( 10.1111/gcb.12257) [DOI] [PubMed] [Google Scholar]
- 12.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6669–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niehaus AC, Angilletta MJ, Sears MW, Franklin CE, Wilson RS. 2012. Predicting the physiological performance of ectotherms in fluctuating thermal environments. J. Exp. Biol. 215, 694–701. ( 10.1242/jeb.058032) [DOI] [PubMed] [Google Scholar]
- 14.Oufiero CE, Angilletta MJ. 2006. Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60, 1066–1075. ( 10.1111/j.0014-3820.2006.tb01183.x) [DOI] [PubMed] [Google Scholar]
- 15.Kingsolver JG, Woods HA, Buckley LB, Potter KA, MacLean HJ, Higgins JK. 2011. Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 51, 719–732. ( 10.1093/icb/icr015) [DOI] [PubMed] [Google Scholar]
- 16.Radchuk V, Turlure C, Schtickzelle N. 2013. Each life stage matters: the importance of assessing the response to climate change over the complete life cycle in butterflies. J. Anim. Ecol. 82, 275–285. ( 10.1111/j.1365-2656.2012.02029.x) [DOI] [PubMed] [Google Scholar]
- 17.Buckley LB. 2008. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19. ( 10.1086/523949) [DOI] [PubMed] [Google Scholar]
- 18.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. 2010. Can mechanism inform species’ distribution models? Ecol. Lett. 13, 1041–1054. ( 10.1111/j.1461-0248.2010.01506.x) [DOI] [PubMed] [Google Scholar]
- 19.Kearney M. 2013. Activity restriction and the mechanistic basis for extinctions under climate warming. Ecol. Lett. 16, 1470–1479. ( 10.1111/ele.12192) [DOI] [PubMed] [Google Scholar]
- 20.Kearney M, Porter WP. 2004. Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology 85, 3119–3131. ( 10.1890/03-0820) [DOI] [Google Scholar]
- 21.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–849. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 22.Du W-G, Shine R. 2015. The behavioural and physiological strategies of bird and reptile embryos in response to unpredictable variation in nest temperature. Biol. Rev. 90, 19–30. ( 10.1111/brv.12089) [DOI] [PubMed] [Google Scholar]
- 23.Angilletta MJ. 2001. Thermal and physiological constraints on energy assimilation in a widespread lizard (Sceloporus undulatus). Ecology 82, 3044–3056. ( 10.1890/0012-9658%282001%29082%5B3044%3ATAPCOE%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 24.Angilletta MJ, Sears MW, Pringle RM. 2009. Spatial dynamics of nesting behavior: lizards shift microhabitats to construct nests with beneficial thermal properties. Ecology 90, 2933–2939. ( 10.1890/08-2224.1) [DOI] [PubMed] [Google Scholar]
- 25.Angilletta MJ, Winters RS, Dunham AE. 2000. Thermal effects on the energetics of lizard embryos: implications for hatchling phenotypes. Ecology 81, 2957–2968. ( 10.1890/0012-9658%282000%29081%5B2957%3ATEOTEO%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 26.Andrews RM, Mathies T, Warner DA. 2000. Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol. Monogr. 14, 420–431. ( 10.2307/1467055) [DOI] [Google Scholar]
- 27.Angilletta MJ, Zelic MH, Adrian GJ, Hurliman AM, Smith CD. 2013. Heat tolerance during embryonic development has not diverged among populations of a widespread species (Sceloporus undulatus). Conserv. Physiol. 1, cot018 ( 10.1093/conphys/cot018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaghan AJ, Steinhoff DF, Bruyere CL. 2014. NCAR Community Earth System Model, bias corrected model output. Research data archive at the National Center for Atmospheric Research, Computational and Information Systems Laboratory. See http://rda.ucar.edu/datasets/ds316.1/. (accessed 22 November 2014).
- 29.Skamarock WC, Klemp JB, Dudhia J, Gill DO, Barker DM, Wang W, Powers JG. 2008. A Description of the Advanced Research WRF Version 3, 113 Boulder, CO: National Center for Atmospheric Research. [Google Scholar]
- 30.Niu GY, et al. 2011. The community Noah land surface model with multiparameterization options (Noah-MP): 1. Model description and evaluation with local-scale measurements. J. Geophys. Res.-Ocean Atmos. 116, D12109 ( 10.1029/2010jd015139) [DOI] [Google Scholar]
- 31.Leache AD. 2009. Species tree discordance traces to phylogeographic clade boundaries in North American fence lizards (Sceloporus). Syst. Biol. 58, 547–559. ( 10.1093/sysbio/syp057) [DOI] [PubMed] [Google Scholar]
- 32.Leache AD, Reeder TW. 2002. Molecular systematics of the eastern fence lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Syst. Biol. 51, 44–68. ( 10.1080/106351502753475871) [DOI] [PubMed] [Google Scholar]
- 33.Therneau T, Lumley T. 2009. Survival: survival analysis, including penalised likelihood. R package version 235–8. See http://CRANR-projectorg/package=survival.
- 34.Christian KA, Tracy CR, Porter WP. 1986. The effect of cold exposure during incubation of Sceloporus undulatus eggs. Copeia 1986, 1012–1014. ( 10.2307/1445303) [DOI] [Google Scholar]
- 35.Bates D, Maechler M, Bolker B. 2012. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999–0. See http://CRAN.R-project.org/package=lme4.
- 36.Tinkle DW, Ballinger RE. 1972. Sceloporus undulatus: a study of the intraspecific comparative demography of a lizard. Ecology 53, 570–584. ( 10.2307/1934772) [DOI] [Google Scholar]
- 37.Kearney M. 2012. Metabolic theory, life history and the distribution of a terrestrial ectotherm. Funct. Ecol. 26, 167–179. ( 10.1111/j.1365-2435.2011.01917.x) [DOI] [Google Scholar]
- 38.Woods HA. 2013. Ontogenetic changes in the body temperature of an insect herbivore. Funct. Ecol. 27, 1322–1331. ( 10.1111/1365-2435.12124) [DOI] [Google Scholar]
- 39.Telemeco RS, Elphick MJ, Shine R. 2009. Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90, 17–22. ( 10.1890/08-1452.1) [DOI] [PubMed] [Google Scholar]
- 40.Kolbe JJ, Janzen FJ. 2002. Impact of nest-site selection on nest success and nest temperature in natural and disturbed habitats. Ecology 83, 269–281. ( 10.1890/0012-9658%282002%29083%5B0269%3AIONSSO%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 41.Bowden RM, Carter AW, Paitz RT. 2014. Constancy in an inconstant world: moving beyond constant temperatures in the study of reptilian incubation. Integr. Comp. Biol. 54, 830–840. ( 10.1093/icb/icu016) [DOI] [PubMed] [Google Scholar]
- 42.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 43.Kearney MR, Isaac AP, Porter WP. 2014. microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci. Data 1, 140006 ( 10.1038/sdata.2014.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingsolver JG, Diamond SE, Buckley LB. 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct. Ecol. 27, 1415–1423. ( 10.1111/1365-2435.12145) [DOI] [Google Scholar]
- 45.Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley CDG, McCann KS, Savage V, Tunney TD, O'Connor MI. 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc. R. Soc. B 281, 20132612 ( 10.1098/rspb.2013.2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavallo C, Dempster T, Kearney MR, Kelly E, Booth D, Hadden KM, Jessop TS. 2015. Predicting climate warming effects on green turtle hatchling viability and dispersal performance. Funct. Ecol. 29, 768–778. ( 10.1111/1365-2435.12389) [DOI] [Google Scholar]
- 47.Caillon R, Suppo C, Casas J, Arthur Woods H, Pincebourde S. 2014. Warming decreases thermal heterogeneity of leaf surfaces: implications for behavioural thermoregulation by arthropods. Funct. Ecol. 28, 1449–1458. ( 10.1111/1365-2435.12288) [DOI] [Google Scholar]
- 48.Niewiarowski PH, Angilletta MJ, Leache AD. 2004. Phylogenetic comparative analysis of life-history variation among populations of the lizard Sceloporus undulatus: an example and prognosis. Evolution 58, 619–633. ( 10.1554/02-415) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Embryonic tolerance data have been uploaded as part of the electronic supplementary material. Microclimate data will be available as a data repository through the Knowledge Network for Biocomplexity.