Abstract
Evidence that the social environment at critical stages of life-history shapes individual trajectories is accumulating. Previous studies have identified either current or delayed effects of social environments on fitness components, but no study has yet analysed fitness consequences of social environments at different life stages simultaneously. To fill the gap, we use an extensive dataset collected during a 24-year intensive monitoring of a population of Alpine marmots (Marmota marmota), a long-lived social rodent. We test whether the number of helpers in early life and over the dominance tenure length has an impact on litter size at weaning, juvenile survival, longevity and lifetime reproductive success (LRS) of dominant females. Dominant females, who were born into a group containing many helpers and experiencing a high number of accumulated helpers over dominance tenure length showed an increased LRS through an increased longevity. We provide evidence that in a wild vertebrate, both early and adult social environments influence individual fitness, acting additionally and independently. These findings demonstrate that helpers have both short- and long-term effects on dominant female Alpine marmots and that the social environment at the time of birth can play a key role in shaping individual fitness in social vertebrates.
Keywords: cooperative breeding, subordinate, sociality, Marmota marmota, lifetime reproductive success, longevity
1. Introduction
Sociality is expected to have both positive and negative effects on fitness. However, sociality can only evolve when its benefits exceed its costs. Helpers might be beneficial to dominant individuals in cooperative breeders because they should reduce costs associated with parental care, thereby increasing survival, reproductive lifespan and ultimately lifetime reproductive success (LRS) [1]. Previous studies have focused on the role of the social environment experienced during adulthood on fitness, or have reported that more helpers during the whole reproductive life lead to increased adult survival [2], annual fecundity [3], reproductive success [4] or LRS [5]. However, other studies have failed to report any effect (e.g. [6]), and all studies performed so far have only focused on a unique and fixed measure of individual social environment.
Recently, several studies have emphasized the importance of the quality of the early-life environment in shaping adult performance [7,8] and researchers increasingly recognize that the early social environment might be a key factor shaping fitness [9]. A favourable social environment during early life increases an individual's reproductive performance. In mammals, having more helpers during early life leads to increased growth [10] and survival of juveniles [11], and to an earlier age at first reproduction [12] (but see [13]). However, although the influence of the social environment at early-life stages on fitness has been well investigated [13,14], our current knowledge of the fitness consequences of the interplay between early and adult social environments is still limited. Such a gap probably results from the requirement of detailed information on both early social environment and life-history traits over an entire lifetime to conduct such studies. Recently, an exciting study on red wolves (Canis rufus) revealed that the presence of helpers at birth leads females to postpone their last reproduction by 2 years without any delay in the age at first reproduction, which consequently leads to an increased reproductive lifespan [15]. However, the possible contribution of the adult social environment was not accounted for, so we still do not know how early adult social environments interplay to determine individual fitness. Considering the number of helpers present at both early and adult stages should allow a better understanding of the mechanisms underpinning the relationship between sociality and individual fitness.
Taking advantage of an exceptional individual-based longitudinal monitoring, we analyse simultaneously whether the social environments experienced by dominant females, both when juvenile and during adult life, influence individual fitness and its components in Alpine marmots (Marmota marmota). To do this, we first analysed the LRS as an integrative measure of fitness and then we analysed three fitness components (longevity, litter size and juvenile survival). The Alpine marmot is a ground-dwelling social rodent. Based on previous studies highlighting the beneficial effects of the social environments provided by helpers on the fitness of female marmots [11,15], we expect both early and adult social environments of high quality to cause increased individual fitness.
2. Material and methods
(a). Study species
The Alpine marmot is a socially monogamous species living in families of two to 16 individuals composed of a dominant pair, sexually mature (i.e. 2 years and older) or immature (i.e. 1 year old) subordinates of both sexes, and juveniles. All members of a family share a common territory, mostly defended by the dominant pair [15]. Sun exposure and home-range size are major components of the quality of a territory [16]. Territory quality varies principally with the aspect of the territory. Territories with a southern or eastern aspect (where snow melts relatively early) and low grassy slopes are of higher quality than the North aspect territories [17].
Only 15% of females born (N = 549) in the study site have obtained a dominant position. On average, females become dominant between 2 and 6 years of age (with a median of 4 and a mean of 3.76) and among females obtaining a breeding status, 77% first reproduce when they are either 3 or 4 years of age. Dominant pairs monopolize reproduction and remain established for several years until eviction or death. Mating occurs during the 15 days following emergence from hibernation (from early to late April) and dominant females give birth to the sole litter of the year after 30 days of gestation. The altricial juveniles remain in the natal burrow for 40 days and emerge above ground once weaned (between mid-June and mid-July). We do not know what factors determine whether an individual becomes dominant. Early-life conditions (litter size) did not influence the chance to become dominant [18]. However, high adult survival suggests that the eviction of a dominant from a family group is the main reason why it loses its status. At sexual maturity (2 years of age), 27% of females and 12% of males stay in their natal group either as subordinates or as dominants if they inherit dominance, but most disperse to gain dominance in another group. Dispersing individuals never join another family group as subordinates. They gain a dominant position at immigration (35% and 37% of the dominant females and males, respectively, are born in a neighbouring territory in the studied population). Dispersing individuals that do not manage to become dominant are at a very high risk of dying [19]. Indeed, secondary dispersal is an extremely rare phenomenon (only one case for 25 years).
Subordinates are either sexually immature (yearling) or sexually mature (2-year-olds and older) individuals of both sexes that delay dispersal for one or several years. Reproduction of subordinates is physiologically suppressed in both sexes [20] and sexually immature and mature subordinate males are considered to be helpers [21,22] because they actively contribute to heat production in the family hibernacula (social thermoregulation sensu [22]). Indeed, hibernation is characterized by a cyclic process of alternating phases of hypothermia and euthermia. In each cycle, subordinate males wake up earlier and have longer euthermic periods than other family members, thus warming the burrow [22]. Consequently, subordinate males present in a family group increase the probability of juveniles to survive their first hibernation [11] but endure costs in terms of body mass loss [21]. On the contrary, female subordinates decreased juvenile survival during hibernation and therefore are costly for dominants most probably because subordinate females compete with juveniles for heat [11].
(b). Data collection
We collected all data from a wild population of Alpine marmots located in La Grande Sassière nature reserve (2340 m.a.s.l., French Alps, 45°29′ N, 6°59′ E). From 1990 to 2014, every year from mid-April to mid-July, we monitored 24 different family groups using both capture–mark–recapture and observation.
We caught marmots using two-door live traps baited with dandelion (Taraxacum densleonis). We placed traps in front of the entrance of the main burrow of each territory so that the assignment of each trapped individual to its family group was possible. We caught juveniles by hand from their first day of emergence. Once captured, we tranquillized animals with Zolétil 100 (0.1 ml kg−1). Animals were individually marked with a transponder (Trovan™, Munich, Germany) and a numbered ear tag placed either on the right ear (females) or on the left ear (males). An additional coloured plastic ear tag was placed on the opposite ear of dominant individuals. We assessed the social status of adults by considering scrotum development for males and the development of teats for females. We further confirmed social status by daily observations of scent marking and aggressive behaviour [23].
We determined group composition by combining daily observations during the activity periods of the marmots and capture–recapture data. For each family group, we assessed the number of subordinates of each sex and age class (yearling versus adult). Intensive observations of burrows enabled us to know the date of juvenile emergence and the exact number of weaned juveniles. We thus assessed the age of individuals in years from birth; the age of 0 was assigned to juveniles.
(c). Measures of reproductive performance
For all dominant females that produced at least one juvenile in a given reproductive event, we recorded litter size at weaning and survival of their juveniles from weaning to their first hibernation. We also recorded longevity and LRS of dominant females.
We measured litter size as the number of juveniles at weaning (range = 1–7, mean = 3.60, median = 4), juvenile survival as the probability for a juvenile to survive its first hibernation (mean = 0.63) and recorded individual longevity (range = 3–16, mean = 7.70, median = 7).
LRS is a reliable integrative measure of fitness [24] and is defined as the number of juveniles produced throughout a female's lifetime that survive a critical stage in life history [25]. In Alpine marmots 60% of offspring die during the first hibernation [26] and annual survival afterwards is high and quite constant, contributing little to among-female variation in fitness. Therefore, in Alpine marmots, the total number of juveniles produced by a female throughout her lifespan that survived their first period of hibernation (range = 0–20, mean = 6.18, median = 5) provides the most accurate and reliable measure of LRS.
(d). Assessing the social context
For each dominant female, we recorded the number of natal helpers as the number of subordinate males present at birth (i.e. early social environment) in its natal family group, and the number of helpers over the female dominance tenure length (i.e. adult social environment) as the number of helpers present during the entire dominance tenure divided by the number of years as dominant.
The number of helpers a female had during each year of her dominance tenure varied slightly in response to offspring survival and dispersal (see electronic supplementary material, section A, repeatability was equal to 0.45). Hence a female's average number of helpers throughout her dominance tenure is a relatively good measure of the social environment she experienced during adulthood.
(e). Sample sizes
Among the 72 known-aged dominant females, we assessed both early and adult social environments for 42. We recorded litter size for 153 litters produced by these 42 females and 496 juvenile survival events from 137 litters produced by 40 females. Longevity and LRS were available for 29 females because 13 dominant females were still alive at the end of the study period.
(f). Direct effects of early and adult social environments on female fitness and its components
Given that early and adult social environments could be correlated, two scenarios emerge. First, early and adult social environments can directly influence fitness and its components (scenario 1). Second, early social environment can indirectly influence fitness and its components through its effect on adult social environment (scenario 2). In scenario 2, even if early social environment acts indirectly on fitness and its components, a direct effect of adult social environment on fitness and its components can occur.
Following the recommendations of Shipley [27], we built a first path analysis for LRS. We implemented LRS as a dependent variable with a log-link function and a negative binomial error distribution. Then, we constructed separate path analyses for each fitness component (i.e. longevity, litter size and juvenile survival). Longevity was entered as a dependent variable with a normal error distribution. We entered litter size at weaning as a dependent variable with a log-link function and a Poisson error distribution. We included as fixed factors year and the aspect of the territory (south, north or valley), the latter encompassing the location of a marmot home range in a particular direction and associated features, such as exposition, slope and vegetation cover. Litter size of marmots in this population remains constant until females reach 10 years of age, and declines thereafter [28]. We thus included a threshold effect of age in the litter size model. We included mother identity as a random effect. Juvenile survival (included as the number of juveniles in a given litter that survived until the following year as the response variable, and litter size as the binomial denominator) was entered as a dependent variable with a logit-link function and a binomial distribution. We included year, the aspect of the territory and the change of dominant male as fixed factors. Litter size does not influence offspring survival in the studied population [18]. From full models of litter size and juvenile survival, we selected a baseline model for these two reproductive traits by retaining only the confounding variables with statistically significant effects on a given reproductive trait (see electronic supplementary material, section B). To test the fit of the data to the indirect scenario (scenario 2), we compared the C value associated with scenario 2 to a χ2-distribution with 2k degrees of freedom (where k is the number of pairs of variables in the graph that are not associated in the focal model) [27]. We rejected scenario 2 when the p-value was below 0.05 [27]. In addition, we used hierarchical partitioning [29] to evaluate the independent influence of social environments on fitness components. We reported the proportion of independent explained variance (γ) by social environments when both have statistically significant effects on fitness components. The proportion of explained variance cannot be compared among two path analyses.
Models were fitted with generalized linear models for LRS, linear models for longevity and generalized linear mixed models for juvenile survival and litter size to account for repeated measures on the same individuals. We estimated parameters (±1 s.e.) of LRS and litter size on a log-scale and juvenile survival on a logit-scale. We performed all analyses in R [30] using the function glm.nb in the ‘MASS’ library [31], the function lm in the ‘stats’ library [32] and the function glmmPQL in the ‘nlme’ library [33]. For hierarchical partitioning, we used the function hier.part in the ‘hier.part’ library [34].
3. Results
Early and adult social environments are independent (N = 29, β = −0.09 ± 0.13, p = 0.53, R = −0.13). Early (N = 29, β = 0.17 ± 0.09, p = 0.04) and adult (N = 29, β = 0.69 ± 0.12, p < 0.01) social environments increase LRS only directly (figures 1 and 2), leading scenario 2 to be rejected (C = 8.40, p = 0.04). Adult social environment explains a greater part of the explained variance in LRS (85.13%) than early social environment (14.87%).
Figure 1.
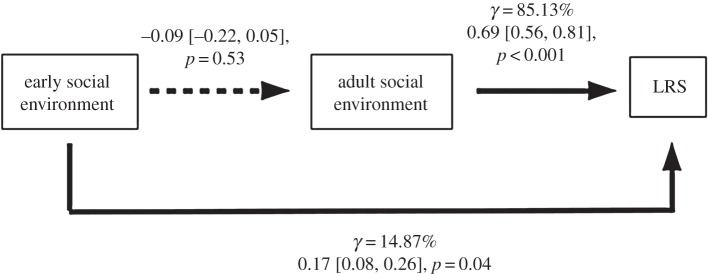
Path diagrams showing direct and indirect influences of early social environment on LRS in dominant female Alpine marmots (Marmota marmota). Path coefficients are given with their associated standard errors in brackets. Thick lines indicate statistically supported effects. Percentages of explained independent variance (γ) associated with the early and adult social environments were indicated.
Figure 2.
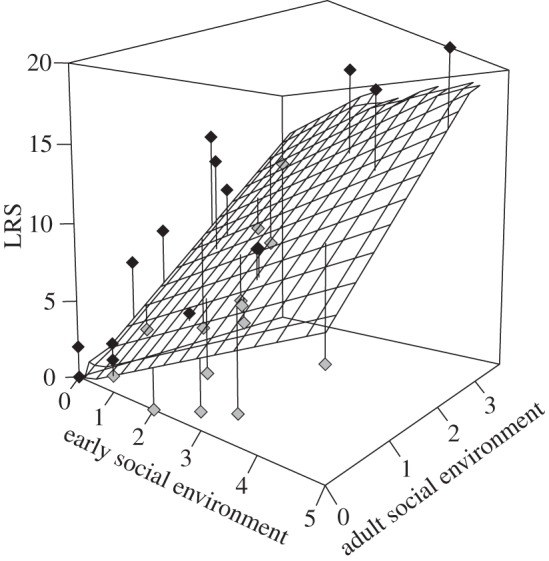
Lifetime reproductive success of dominant female Alpine marmots as a function of the number of helpers at birth (i.e. early social environment) and the total number of helpers over a female reproductive lifetime (i.e. adult social environment). Black squares represent observed data greater than the predicted values. Grey squares represent observed data lower than the predicted values. The surface corresponds to the fitted model.
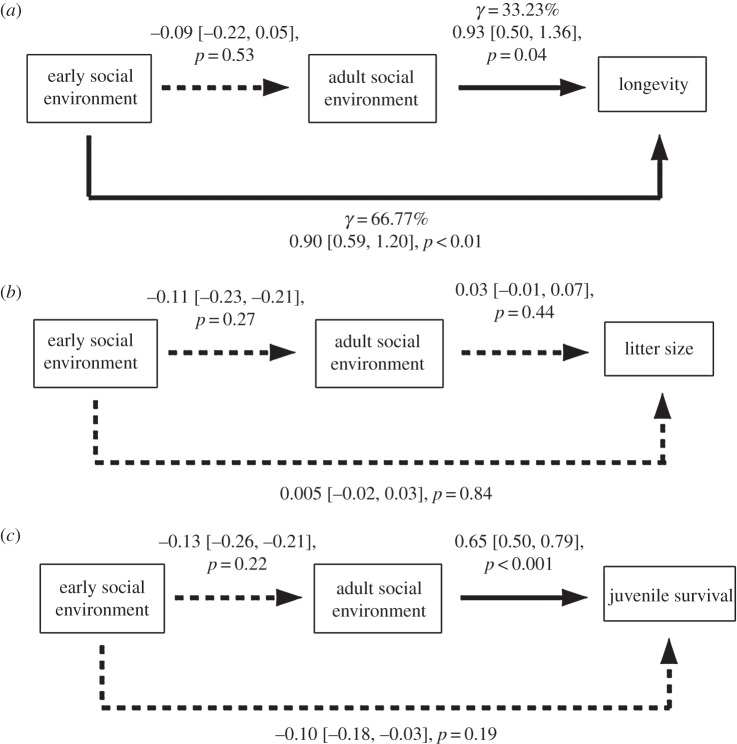
Early (N = 29, β = 0.90 ± 0.31, p < 0.01) and adult (N = 29, β = 0.93 ± 0.43, p = 0.04) social environments directly increase longevity (figure 3a), leading to rejection of scenario 2 (C = 9.92, p < 0.01). Contrary to LRS, the early social environment explains a greater part of the explained variance in longevity (66.77%) than the adult social environment (33.23%). Social environments do not influence litter size (early: N = 153, β = 0.005 ± 0.03, p = 0.84; adult: N = 153, β = 0.03 ± 0.04, p = 0.44; figure 3b). Moreover, the early social environment does not influence juvenile survival (N = 137, β = −0.10 ± 0.08, p = 0.19), whereas the adult social environment does (N = 137, β = 0.65 ± 0.14, p < 0.01; figure 3c).
Figure 3.
Path diagrams showing direct and indirect influences of early social environment on (a) longevity, (b) litter size at weaning and (c) juvenile survival in dominant female Alpine marmots (Marmota marmota). Path coefficients are given with their associated standard errors in brackets. Thick lines indicate statistically supported effects. Percentages of explained independent variance (γ) associated with the early and adult social environments were indicated for longevity.
4. Discussion
Our results provide evidence that both early and adult social environments have independent additive effects on LRS in a social species. The LRS of breeding female Alpine marmots markedly increases with both early and adult social environments through a positive effect on longevity (electronic supplementary material, section C). The positive effect of the early social environment is the main driver of longevity while the positive effect of the adult social environment is the main driver of LRS. In addition, favourable adult social environments increase juvenile survival, and thereby LRS, but neither early nor adult social environment influence litter size.
Although compelling evidence shows that good early-life environmental conditions positively influence fitness [9,35], evolutionary mechanisms underlying the positive delayed effect of helping on individual fitness in social species have not been yet investigated. The silver spoon hypothesis [36] predicts that individuals facing good environmental conditions during early life might enjoy relatively good body condition when reaching adulthood and have therefore greater fitness than individuals suffering adverse early environmental conditions. Our results on the influence of social environments are thus consistent with this hypothesis because female marmots born with many helpers outperform in terms of LRS females born with few or no helpers.
Life-history theory predicts that individuals have to allocate resources either to growth, reproduction or survival [37]. As the early-life environment influences the whole individual trajectory mostly through changes in allocation to growth [9], the relative allocation (sensu [38]) to survival is likely to be modified. In Alpine marmots, a favourable social environment is especially beneficial during hibernation. Indeed, juveniles have low fat reserves and suffer from huge fat losses during winter [22], therefore, reducing the costs associated with heat production is crucial for them. The presence of adult subordinates is particularly important because these latter arouse before juveniles and increase the heat in the hibernaculum. This social thermoregulation (sensu [22]) leads to a decrease in the costs of hibernation and thereby to increase juvenile survival [11]. We hypothesize that a good early social environment should decrease energy expenditure in juveniles associated with the succession of euthermic and hypothermic phases during their first hibernation. Therefore, a good early social environment could permit juveniles to reduce body reserve expenses during the first hibernation. The saved energy could be allocated to other functions, potentially allowing females to reproduce earlier or to live longer, as expected from the principle of allocation [39].
The positive effect of a good adult social environment on juvenile survival, longevity and LRS is consistent with the literature [2,5,11]. Our findings further reveal that the social environment does not influence litter size. Until now, studies that have investigated the influence of social environment on litter size have shown contrasting results. In agreement with our findings, increasing the number of helpers did not increase litter size at emergence in Mednyi Island arctic foxes (Alopex lagopus semenovi), in which most helpers were adult females [40]. On the other hand, a high number of male and female helpers increased litter size at weaning in meerkats [3] and African wild dogs (Lycaon pictus) [41] but decreased litter size at weaning in the European badger (Meles meles) [6]. In Alpine marmots, the benefit of having helpers occurs after weaning and particularly during hibernation [11]. Indeed, from birth to weaning, lactation falls entirely to mothers and there is no evidence that helpers care for juveniles between birth and weaning. However, helpers participate in raising juveniles after weaning. Specifically during hibernation, helpers wake up before the other members of the family, allowing mothers to decrease body warmth costs. These results are in line with the load-lightening hypothesis [1], which predicts that dominant individuals should reduce parental allocation as the number of helpers increases, which will then enhance their survival, reproductive lifespan and LRS. For example, in long-tailed tits (Aegithalos caudatus), males with large clutch size decrease their work-rate in presence of helpers, leading their survival to increase [42]. Therefore, in Alpine marmots a trade-off between parental care and survival might explain the increased longevity of females benefiting from favourable social environment throughout their life.
Our study provides evidence that individual fitness can be shaped by additive and independent effects of social environments experienced during early life and adulthood. In Alpine marmots, a high number of helpers at early and adult stages independently leads to increase longevity and LRS. While longevity is mostly influenced by the early social environment, LRS is mostly influenced by the adult social environment because of the effect of the adult environment on juvenile survival. These independent effects of social environments on longevity could possibly be an adaptive process. Indeed, when a female marmot becomes dominant, she will keep this social status until death, as indicated by the strong correlation between longevity and dominance tenure (N = 29, R = 0.95). Therefore, if both early and adult social environments positively influence longevity, they increase the length of the dominant tenure. Given that only dominant females produce offspring and that female marmots only reproduce once a year, a strong selection could occur for increasing the number of reproductive attempts [25], and thereby the length of a female's dominant tenure.
As path analysis remains a correlative approach, we cannot exclude that spatio-temporal variation in environmental factors could shape annual variation in the number of helpers and then influence fitness and its components [43]. Indeed, a high-quality territory could allow both a larger number of helpers and higher fitness. However, we can exclude a temporal variation in the adult social environment because of the high repeatability of the number of helpers over the dominant's lifetime. Additionally, the phenotypic and genotypic quality of dominant females could also influence their fitness and its components. Indeed, as individuals in better than average quality are expected to live longer, the high LRS and longevity can also reflect a viability selection favouring high-quality females. Moreover, our study focussed on the 15% of females that became dominant. Social factors acting on non-breeding females can markedly differ from social factors acting on breeding females.
Overall, our findings reveal the importance of considering the whole social environment experienced by dominant females from birth to death to understand fitness variation among females. The evidence that early and adult social environments have strong and independent effects on fitness allows a better understanding of the role of the social environment in shaping fitness in cooperative breeders.
Supplementary Material
Acknowledgements
We warmly thank all the students and Earthwatch volunteers involved in marmot trapping, Kevin Chow, Johanna Chen, Paula Fan, Kathryn A. Angell and Herman B. Leonard for editing the language. Thanks are also extended to the authorities of the Vanoise National Park for granting us permission to work in La Grande Sassière Nature Reserve. We are also grateful to Anne Charmantier and two anonymous reviewers for insightful comments on previous versions of this paper.
Ethics
The fieldwork conducted on the Alpine marmots was undertaken after acceptance of the project by the Vanoise National Park, and after the deliverance of the permit number AP n°2010/21 by the Préfecture de la Savoie. V.B. and A.C. are authorized for experimentation with animals, issued by the French Ministry of Agriculture and Fisheries. The protocol was approved by the ethical committee of the University Claude Bernard Lyon 1 (no. BH2012-92 V1).
Data accessibility
Data from this study have been deposited in Dryad (http://dx.doi.org/10.5061/dryad.8974j)
Authors' contributions
A.C. and D.A. conceived the study. V.B. performed the analysis. V.B., J.F.L., D.A., J.M.G. and A.C. wrote the manuscript. A.C., D.A. and V.B. contributed to the data collection. All authors provided editorial advice and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the ‘Agence Nationale de la Recherche’ (ANR, project ANR-13-JSV7-0005) and Earthwatch. V.B. is supported by a French Ministry of Education Research grant.
References
- 1.Crick HQP. 1992. Load-lightening in cooperatively breeding birds and the cost of reproduction. Ibis 134, 56–61. ( 10.1111/j.1474-919X.1992.tb07230.x) [DOI] [Google Scholar]
- 2.Khan MZ, Walters JR. 2002. Effects of helpers on breeder survival in the red-cockaded woodpecker (Picoides borealis). Behav. Ecol. Sociobiol. 51, 336–344. ( 10.1007/s00265-001-0441-3) [DOI] [Google Scholar]
- 3.Russell AF, Brotherton PNM, McIlrath GM, Sharpe LL, Clutton-Brock TH. 2003. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav. Ecol. 14, 486–492. ( 10.1093/beheco/arg022) [DOI] [Google Scholar]
- 4.Shreeves G, Field J. 2002. Group size and direct fitness in social queues. Am. Nat. 159, 81–95. ( 10.1086/324125) [DOI] [PubMed] [Google Scholar]
- 5.Lardy S, Allainé D, Bonenfant C, Cohas A. In press Sex-specific determinants of fitness in a social mammal. Ecology. [DOI] [PubMed] [Google Scholar]
- 6.Woodroffe R, Macdonald DW. 2000. Helpers provide no detectable benefits in the European badger (Meles meles). J. Zool. 250, 113–119. ( 10.1111/j.1469-7998.2000.tb00582.x) [DOI] [Google Scholar]
- 7.Douhard M, Gaillard JM, Delorme D, Capron G, Duncan P, Klein F, Bonenfant C. 2013. Variation in adult body mass of roe deer: early environmental conditions influence early and late body growth of females. Ecology 94, 1805–1814. ( 10.1890/13-0034.1) [DOI] [PubMed] [Google Scholar]
- 8.Garratt M, Lemaître JF, Douhard M, Bonenfant C, Capron G, Warnant C, Klein F, Brooks RC, Gaillard JM. 2015. High juvenile mortality is associated with sex-specific adult survival and lifespan in wild roe deer. Curr. Biol. 25, 759–763. ( 10.1016/j.cub.2014.11.071) [DOI] [PubMed] [Google Scholar]
- 9.Lummaa V, Clutton-Brock TH. 2002. Early development, survival and reproduction in humans. Trends Ecol. Evol. 17, 141–147. ( 10.1016/S0169-5347(01)02414-4) [DOI] [Google Scholar]
- 10.Clutton-Brock TH, Russell AF, Sharpe LL, Brotherton PNM, McIlrath GM, White S, Cameron EZ. 2001. Effects of helpers on juvenile development and survival in meerkats. Science 293, 2446–2449. ( 10.1126/science.1061274) [DOI] [PubMed] [Google Scholar]
- 11.Allainé D, Theuriau F. 2004. Is there an optimal number of helpers in Alpine marmot family groups? Behav. Ecol. 15, 916–924. ( 10.1093/beheco/arh096) [DOI] [Google Scholar]
- 12.Russell AF, Young AJ, Spong G, Jordan NR, Clutton-Brock TH. 2007. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B 274, 513–520. ( 10.1098/rspb.2006.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparkman AM, Adams J, Beyer A, Steury TD, Waits L, Murray DL. 2011. Helper effects on pup lifetime fitness in the cooperatively breeding red wolf (Canis rufus). Proc. R. Soc. B 278, 1381–1389. ( 10.1098/rspb.2010.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höner OP, Wachter B, Hofer H, Wilhelm K, Thierer D, Trillmich F, Burke T, East ML. 2010. The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nat. Commun. 60, 1–7. ( 10.1038/ncomms1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allainé D. 2000. Sociality mating system and reproductive skew in marmots: evidence and hypotheses. Behav. Process. 51, 21–34. ( 10.1016/S0376-6357(00)00116-9) [DOI] [PubMed] [Google Scholar]
- 16.Allainé D, Rodrigue I, Le Berre M, Ramousse R. 1994. Habitat preferences of alpine marmots, Marmota marmota. Can. J. Zool. 72, 2193–2198. ( 10.1139/z94-293) [DOI] [Google Scholar]
- 17.Allainé D, Graziani L, Coulon J. 1998. Postweaning mass gain in juvenile alpine marmots Marmota marmota. Oecologia, 113, 370–376. ( 10.1007/s004420050388) [DOI] [PubMed] [Google Scholar]
- 18.Dupont P, Pradel R, Lardy S, Allainé D, Cohas A. 2015. Litter sex composition influences dominance status of Alpine marmots (Marmota marmota). Oecologia. ( 10.1007/s00442-015-3375-6) [DOI] [PubMed] [Google Scholar]
- 19.Stephens PA, Frey-Roos F, Arnold W, Sutherland WJ. 2002. Model complexity and population predictions. The alpine marmot as a case study. J. Anim. Ecol. 71, 343–361. ( 10.1046/j.1365-2656.2002.00605.x) [DOI] [Google Scholar]
- 20.Hackländer K, Mostl E, Arnold W. 2003. Reproductive suppression in female Alpine marmots (Marmota marmota). Anim. Behav. 65, 1133–1140. ( 10.1006/anbe.2003.2159) [DOI] [Google Scholar]
- 21.Arnold W. 1990. The evolution of marmot sociality: II. Costs and benefits of joint hibernation. Behav. Ecol. Sociobiol. 27, 239–246. [Google Scholar]
- 22.Arnold W. 1993. Social evolution in marmots and the adaptive value of joint hibernation. Verh. Dtsch. Zool. Ges. 86, 79–93. [Google Scholar]
- 23.Bel M, Porteret C, Coulon J. 1995. Scent deposition by cheek rubbing in the Alpine marmot (Marmota marmota) in the French Alps. Can. J. Zool. 73, 2065–2071. ( 10.1139/z95-243) [DOI] [Google Scholar]
- 24.Brommer JE, Gustafsson L, Pietiainen H, Merila J. 2004. Single-generation estimates of individual fitness as proxis for long-term genetic contribution. Am. Nat. 163, 505–517. ( 10.1086/382547) [DOI] [PubMed] [Google Scholar]
- 25.Clutton-Brock TH. 1988. Reproductive success (ed. Clutton-Brock TH.). Chicago, IL: University of Chicago Press. [Google Scholar]
- 26.Cohas A, Bonenfant C, Gaillard JM, Allainé D. 2007. Are extra-pair young better than within-pair young? A comparison of survival and dominance in alpine marmot. J. Anim. Ecol. 76, 771–781. ( 10.1111/j.1365-2656.2007.01253.x) [DOI] [PubMed] [Google Scholar]
- 27.Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363–368. ( 10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 28.Berger V, Lemaître JF, Gaillard JM, Cohas A. 2015. How do animals optimize the size-number trade-off when aging? Insights from reproductive senescence patterns in marmots. Ecology 96, 46–53. ( 10.1890/14-0774.1) [DOI] [PubMed] [Google Scholar]
- 29.Chevan A, Sutherland M. 1991. Hierarchical partitioning. Am. Stat. 45, 90–96. [Google Scholar]
- 30.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Venables WN, Ripley BD. 2002. Modern applied statistic with S. Berlin, Germany: Springer. [Google Scholar]
- 32.Wilkinson GN, Rogers CE. 1973. Symbolic description of factorial models for analysis of variance. Appl. Stat. 22, 392–399. ( 10.2307/2346786) [DOI] [Google Scholar]
- 33.Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-PLUS. Berlin, Germany: Springer. [Google Scholar]
- 34.Walsh C, MacNally R. 2004. hier.part: hierarchical partitioning. R package version 1.0. http://cran.r-project.org.
- 35.Douhard M, Plard F, Gaillard JM, Capron G, Delorme D, Klein F, Duncan P, Loe LE, Bonenfant C. 2014. Fitness consequences of environmental conditions at different life stages in a long-lived vertebrate. Proc. R. Soc. B 281, 20140276 ( 10.1098/rspb.2014.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grafen A. 1988. On the uses of data on lifetime reproductive success. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), pp. 454–471. Chicago, IL: University of Chicago Press. [Google Scholar]
- 37.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Cody ML. 1966. A general theory of clutch size. Evolution 20, 174–184. ( 10.2307/2406571) [DOI] [PubMed] [Google Scholar]
- 39.Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM. 2015. Early-late life trade-offs and the evolution of ageing in the wild. Proc. R. Soc. B 282, 20150209 ( 10.1098/rspb.2015.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruchenkova EP, Goltsman M, Sergeev S, Macdonald DW. 2009. Is alloparenting helpful for Mednyi Island arctic foxes, Alopex lagopus semenovi? Naturwissenschaften 96, 457–466. ( 10.1007/s00114-008-0494-5) [DOI] [PubMed] [Google Scholar]
- 41.McNutt JW, Silk JB. 2008. Pup production, sex ratios, and survivorship in African wild dogs, Lycaon pictus. Behav. Ecol. Sociobiol. 62, 1061–1067. ( 10.1007/s00265-007-0533-9) [DOI] [Google Scholar]
- 42.Meade J, Nam K-B, Beckerman AP, Hatchwell BJ. 2010. Consequences of ‘load-lightening’ for future indirect fitness gains by helpers in a cooperatively breeding bird. J. Anim. Ecol. 79, 529–537. ( 10.1111/j.1365-2656.2009.01656.x) [DOI] [PubMed] [Google Scholar]
- 43.Cockburn A, Sims RA, Osmond HL, Green DJ, Double MC, Mulder RA. 2008. Can we measure the benefits of help in cooperatively breeding birds: the case of superb fairy-wrens Malurus cyaneus? J. Anim. Ecol. 77, 430–438. ( 10.1111/j.1365-2656.2007.01351.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study have been deposited in Dryad (http://dx.doi.org/10.5061/dryad.8974j)