Abstract
The dispersal of parasites is critical for epidemiology, and the interspecific vectoring of parasites when species share resources may play an underappreciated role in parasite dispersal. One of the best examples of such a situation is the shared use of flowers by pollinators, but the importance of flowers and interspecific vectoring in the dispersal of pollinator parasites is poorly understood and frequently overlooked. Here, we use an experimental approach to show that during even short foraging periods of 3 h, three bumblebee parasites and two honeybee parasites were dispersed effectively onto flowers by their hosts, and then vectored readily between flowers by non-host pollinator species. The results suggest that flowers are likely to be hotspots for the transmission of pollinator parasites and that considering potential vector, as well as host, species will be of general importance for understanding the distribution and transmission of parasites in the environment and between pollinators.
Keywords: host–parasite interaction, pollinators, disease vector, parasite transmission, honeybee, bumblebee
1. Introduction
Parasites are of major ecological and evolutionary importance [1,2], and understanding the mechanisms of parasite dispersal is key to the epidemiology of parasite dynamics [3]. The shared use of resources, such as water sources and transport hubs, can play a pivotal role in the dynamics of disease spread in humans and other animals by acting as sites of parasite dispersal [4,5]. When parasites are transmitted between transient hosts, they can be widely dispersed to novel areas with the travelling host [1,6]. Consequently, sites that facilitate parasite dispersal are frequently restricted or monitored during times of pandemic threat or conservation concern [7,8].
Our understanding of host–parasite epidemiology comes primarily from studies of single host–parasite systems. However, all parasites exist in an environment in which they will, in addition to their hosts, encounter very many other species, creating significant potential for non-host species to be important in the dispersal of the parasite [9,10]. There are many classic cases of organisms vectoring parasites by acting as an intermediate host in which the parasite completes part of its life cycle [11,12]. However, the incidental dispersal of parasites on the body surface of a vector, or following passage through the gut without infection taking place, may also be of great importance, particularly for parasites that transmit faecal-orally or via contact.
The potential for vectoring of parasites will be especially great when multiple closely related host species share the same resource. An extreme example of such shared resources is exhibited by plant–pollinator mutualisms. While some plant–pollinator systems are specific, in the vast majority of cases flowers are visited by multiple pollinator species in a complex web of interactions [13,14]. It then follows that vectoring of parasites by non-host species during shared flower use may be of great importance in pollinator–parasite interactions [15,16]. There is currently great interest in the stress factors affecting pollinators, many of which are showing substantial population declines with knock-on effects on the plants that rely on them for pollination [17–19]. Parasites are well established as being an important factor in at least some of these declines, with several bumblebee species showing population declines that correlate with pathogen spillover from commercially produced bumblebees [20–28], and honeybee colony losses in many countries being associated with emerging parasites such as the Varroa mite and the microsporidian Nosema ceranae [29–34]. Importantly, there is increasing evidence of parasite transmission between pollinator taxa being more significant than has generally been appreciated. Several honeybee viruses and the N. ceranae microsporidian parasite of honeybees have been detected infecting bumblebees [22,35–42], and the Apicystis bombi neogregarine parasite of bumblebees has been shown experimentally to infect honeybees [22,43].
Remarkably, however, the epidemiology and transmission of pollinator parasites is still very poorly understood and the potentially profound role of shared flower use, in particular, little investigated [15,16]. Several studies have detected the presence of parasites in bee collected pollen [22,42,44], but it is unclear if these parasites were on the flowers and collected along with the pollen or if they originate from the foraging bee [45]. The Israeli acute paralysis virus has been shown experimentally to transmit between honeybee and bumblebee colonies, and vice versa, in a greenhouse, but whether transmission was via shared flowers, interspecific drifting or robbing, or some other mechanism was not determined [42]. Bumblebees have been shown to avoid flowers contaminated with high doses of parasite [46], implying that the threat is present and sufficient for them to have evolved this capability. However, the only direct experimental evidence of the transmission of pollinator parasites via flowers comes from a single study, in which Crithidia bombi, a trypanosome parasite of bumblebees, was shown to infect foraging bumblebees after it was applied to flowers [47]. Here, we investigate experimentally the potential for flowers to act as dispersal platforms for pollinator parasites, and for non-host species to vector them, using bumblebees and honeybees as both hosts and vectors.
2. Material and methods
(a). Dispersal
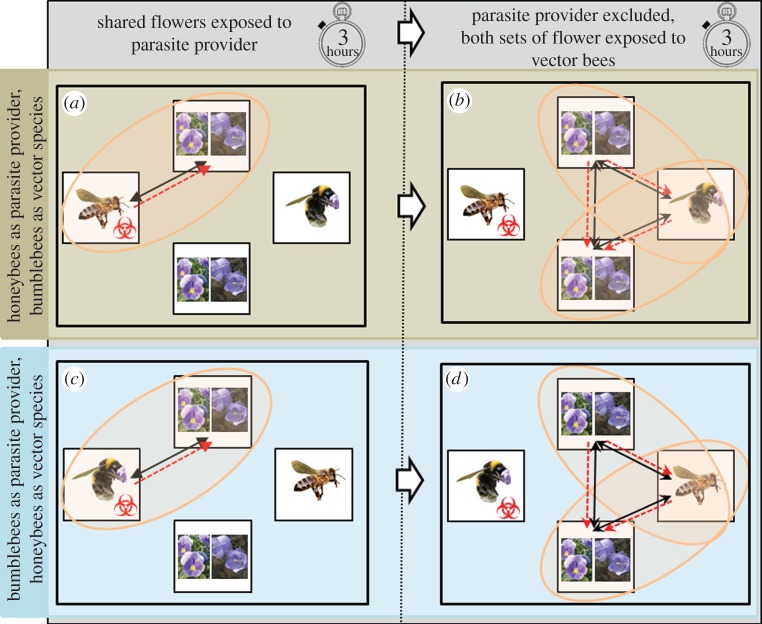
The experiment used mixed groups of 80 flowers, with each group consisting of 50 purple Campanula cochleariifolia (fairies’ thimbles) which have bell-shaped flowers, and 30 purple Viola tricolor (pansy) which have flat, platform-like flowers. All plants were kept in a newly built flight cage for 24 h prior to their flowers opening in order to prevent visitation by any non-experimental bees. The bees used in the experiment were colonies of Apis mellifera carnica honeybees and Bombus terrestris audax bumblebees. The honeybee colonies each consisted of three frames of bees, brood and food, in a mini-nucleus box. The bumblebee colonies were obtained from a commercial producer and contained approximately 60–80 workers at the time of the experiment. All colonies had two-way and one-way entrance/exit doors fitted to allow the exit and entry of bees to be easily controlled. Three honeybee colonies and three bumblebee colonies were used as source of ‘parasite provider’ bees for the experiment. These honeybee colonies had been determined by PCR screening (see below) to be infected by the Nosema apis and N. ceranae parasites, while the bumblebee colonies had been determined by PCR to be infected by A. bombi, C. bombi and Nosema bombi; the colonies of neither bee species were infected by the parasites of the other bee species. Nosema apis is apparently unable to infect bumblebees, and C. bombi and N. bombi are unable to infect honeybees, whereas N. ceranae and A. bombi are capable of infecting both hosts [15,22,40]. Three additional honeybee colonies and three additional bumblebee colonies, which had been confirmed by PCR to be free of any of these parasite infections, were selected to provide the ‘vector bees' for the experiment. The experiment was run for 6 h in total using three infected honeybee colonies as the parasite provider species and three uninfected bumblebee colonies as the vector species. The experiment was then repeated for another 6 h using three infected bumblebee colonies as the parasite provider species and three uninfected honeybee colonies as the vector species. Each experimental combination was carried out once. In each case, three colonies of the species providing the parasites were placed in a flight cage (6 × 4 × 1.5 m; L × W × H), and left for a day to acclimatize. A first group of mixed flowers (50 C. cochleariifolia and 30 V. tricolor) was then placed in the flight cage, and the bees allowed to forage on them for 3 h. After this period, the colonies of the parasite provider species were excluded from the foraging area, into which a second group of mixed flowers (50 C. cochleariifolia and 30 V. tricolor) was then placed, and three colonies of the vector species were allowed to forage for 3 h on both groups of flowers: the group of flowers which had been foraged on by the parasite provider species (shared flowers) and the group of flowers which were only available to the vector species (vector-only flowers; figure 1). The size of colonies and flight cage used meant that both honeybees and bumblebees foraged actively on the flowers during the experiment, and did not exhibit unnatural behaviour such as aggregating in the corners of the flight cage. Immediately prior to the experiment, 30 flowers of each species and 10 bees from the entrance of each colony (both parasite provider and vector species) were collected (n = 30). At the end of the experiment, 50 C. cochleariifolia and 30 V. tricolor flowers from each flower patch (n = 80 per patch) and a further 10 bees from the entrance of each colony were collected (n = 30). All bees and flowers were screened for parasites.
Figure 1.
Experimental set-up. The movement of bees (black, solid arrows) and potential movement of parasites (red, dashed arrows) during experiments in which either honeybees provided parasites and bumblebees were the vectors (a,b), or vice versa (c,d). Initially, the bees providing parasites were allowed to forage on a set of flowers (a,c). The parasite provider bees were then excluded, and the vector bees allowed to forage on both sets of flowers (b,d). Flowers consisted of a mix of the flat-formed V. tricolor flowers and bell-shaped C. cochleariifolia flowers. (Online version in colour.)
(b). Parasite screening
Parasite screening was done using sensitive PCR-based methodology that can reliably detect even low intensity or latent infections. Bees were first washed and surface sterilized with UV, and the malpighian tubules, fatbody and entire gut (including crop) were then dissected from each bee taking great care to ensure the sample was not contaminated by the insect integument. The tissues were homogenized with a micropestle. Flowers were removed from their stem and vortexed in 1 ml of 100% ethanol for 2 min. A 20 µl subsample of the solution was taken for spore (choanomastigotes in the case of Crithidia) detection by microscopy following a 1 : 3 dilution in ethanol. The characteristic size and morphology of Nosema and Apicystis spores, and choanomastigotes of Crithidia, makes them easily distinguishable from each other by eye, and the accuracy of this was confirmed by the PCR results. The remaining wash of 980 µl ethanol and particles from the flower (including any parasites present) was centrifuged at 14 000g for 5 min, before the upper 800 µl of solution was discarded and the remaining 180 µl homogenized with a micropestle. The homogenized sample was then washed by adding 800 µl of Tris-EDTA buffer, vortexed for 30 s and centrifuged at 14 000g for 5 min, after which 800 µl of the supernatant was discarded. This wash procedure was repeated two further times, with 950 µl of supernatant being removed on the final occasion to leave 30 µl of sample. The DNA from each sample was then extracted using 5% Chelex solution and screened for the honeybee parasites N. ceranae and N. apis, and for the bumblebee parasites C. bombi, N. bombi and A. bombi, by conventional PCR with parasite-specific primers (electronic supplementary material, table S1). Presence of a parasite was identified by the presence of a band of the correct size after gel electrophoresis. Positive and negative controls were included in all assays.
(c). Statistical analysis
The frequency of samples (bees or flowers) in which each parasite was detected were compared between before and after the experiments using generalized linear models with binomial distribution, logit link function and the likelihood ratio χ2 statistic. All models were checked for overdispersion and a scale parameter included in the models where necessary to control for this. In both the experiment with honeybees as the parasite providers and the experiment with bumblebees as the parasite providers, comparisons were made between before and after the experiment in the prevalence of parasites in: (i) the parasite provider bees, (ii) the flowers shared between parasite provider and vector bees, (iii) the flowers visited only by the vector bees, and (iv) the vector bees themselves. Comparisons were made separately for each parasite in all cases. Flower type and colony of origin were included as factors, and non-significant interaction terms were removed stepwise in all cases to obtain the minimum adequate models. All analyses were carried out in PASW Statistics 18 (IBM, Armonk, NY, USA).
3. Results
No honeybee or bumblebee parasites were found to be present on the flowers sampled immediately prior to the experiment, which had been exposed in the flight cage with no bees present for 24 h. No honeybee parasites were detected in the bumblebee vectors sampled prior to exposure in the experiment, and no bumblebee parasites were detected in the honeybee vectors sampled prior to their exposure in the experiment. All vector bees sampled prior to the experiment were therefore negative for the parasites that were present in the parasite provider bees. No parasites of the (uninfected) vector species were detected on the flowers or in the parasite provider bees in either case. During the experiment, both honeybees and bumblebees were observed to actively forage on the flowers provided to them in the experiment. Honeybees (16–20%), which acted as parasite providers, had N. apis and 46–53% had N. ceranae, while 33–36% of the bumblebees which acted as parasite providers had A. bombi, 70–73% had C. bombi, and 7% had N. bombi. Parasite provider bees never tested positive for a vector species parasite following the experiment, and the prevalence of parasites in the parasite provider bees did not differ between before and after the foraging period either when honeybees were the parasite providers or when bumblebees were the parasite providers (p > 0.05 in all cases).
(a). Honeybees as parasite providers
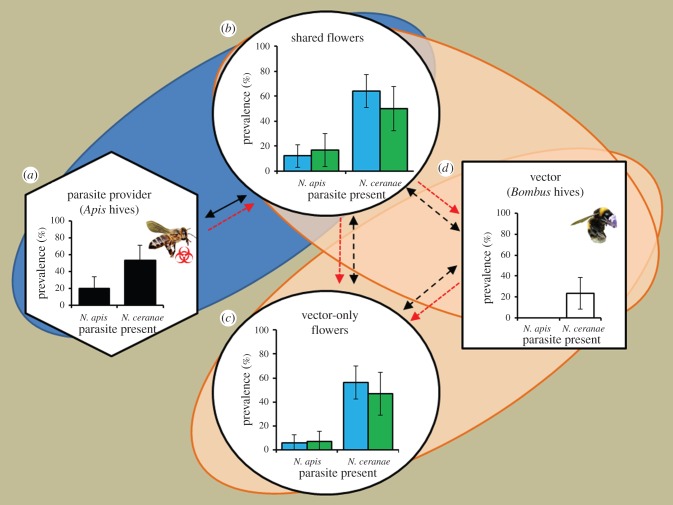
The prevalence of shared flowers that were contaminated with N. apis and N. ceranae increased significantly from 0% before the experiment to 14% and 59%, respectively, after the experiment (χ2 = 13.3, p < 0.001, and χ2 = 68.2, p < 0.001, respectively; figure 2). The prevalence of N. apis and N. ceranae on flowers visited only by the bumblebee vectors increased during the experiment from 0% to 6% and 52%, respectively (χ2 = 5.73, p = 0.017, and χ2 = 58.7, p < 0.001, respectively; figure 2). Dispersal of N. apis and N. ceranae onto flowers was equally likely regardless of the flower species (χ2 = 0.30, p = 0.586, and χ2 = 2.07, p = 0.15, respectively). When subsamples of flower washes were examined with microscopy, Nosema spores were observed in subsamples from 9% of flowers shared by the honeybee parasite providers and bumblebee vectors (with an estimated average of 1.2 × 104 spores per flower), and on 10% of flowers visited only by bumblebee vectors (with an average of 7.2 × 103 spores per flower). At the end of the experiment, the prevalence of N. ceranae in the vector bumblebee colonies had increased from 0% to 23% (χ2 = 10.7, p = 0.001), while N. apis remained undetected (figure 2).
Figure 2.
Bumblebees vector honeybee parasites. The prevalence of the honeybee parasites N. apis and N. ceranae within the honeybee colonies acting as the parasite providers (a), or after the experiment on the bell-shaped C. cochleariifolia and flat-formed V. tricolor flowers (b,c, blue (left) and green (right) columns, respectively), or within bumblebee colonies that acted as vectors (d). All flowers and bumblebees were free of the two parasites prior to the experiment. One set of flowers (b) was initially exposed to honeybees for 3 h, while the bumblebees were excluded from the foraging arena. The honeybees were then excluded from the arena, and the bumblebees allowed to forage freely on the same set of shared flowers, and also on a new set of clean, vector-only flowers (c). Solid black arrows represent movement of the parasite provider, dashed black arrows represent movements of vector species between flowers and hive, and red arrows indicate possible dispersal routes of the parasites. Error bars represent 95% CIs. (Online version in colour.)
(b). Bumblebees as parasite providers
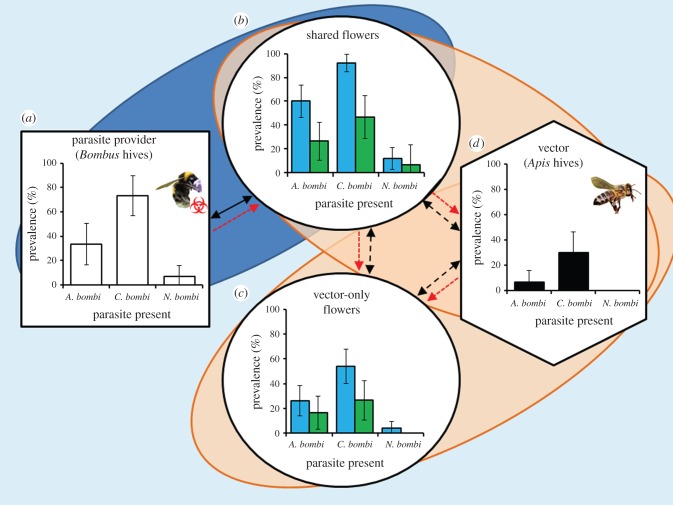
The prevalence of A. bombi, C. bombi and N. bombi on shared flowers increased significantly during the experiment from 0% to 48%, 75% and 10%, respectively (χ2 = 50.9, p < 0.001, χ2 = 105.0, p < 0.001, and χ2 = 8.77, p = 0.003, respectively; figure 3). The prevalence of A. bombi and C. bombi on honeybee exclusive flowers also increased significantly during the experiment from 0% to 22% and 43%, respectively (χ2 = 21.1, p < 0.001, and χ2 = 45.63, p < 0.001, respectively), while the increase in the prevalence of N. bombi from 0% to 3% of flowers was non-significant (χ2 = 4.17, p = 0.124; figure 3). Dispersal of the A. bombi and C. bombi parasites was more likely to occur on the bell-shaped C. cochleariifolia flowers (χ2 = 11.75, p = 0.001 and χ2 = 7.5, p = 0.006, respectively), but was not affected by flower species for N. bombi (χ2 = 1.56, p = 0.212; figure 3). When the subsamples of flower washes were examined by microscopy, spores/choanomastigotes of A. bombi, C. bombi and N. bombi were observed on 6%, 13% and 1% of the flowers shared by bumblebees and honeybees (with on average 3.1 × 102, 1.3 × 104 and 7.8 × 102 spores/choanomastigotes per flower, respectively), and on 5%, 14% and 3% of the flowers visited only by honeybee vectors (with an average of 5.6 × 102, 1.3 × 104 and 1.7 × 103 spores/choanomastigotes per flower, respectively). The prevalence of A. bombi and C. bombi in colonies of the honeybee vectors increased during the experiment from 0% to 7% and 30%, respectively (χ2 = 2.9, p = 0.09, and χ2 = 14.2, p < 0.001, respectively), while N. bombi remained undetected (figure 3).
Figure 3.
Honeybees vector bumblebee parasites. The prevalence of the bumblebee parasites A. bombi, C. bombi and N. bombi within the bumblebee colonies acting as the parasite providers (a), or after the experiment on bell-shaped C. cochleariifolia and flat-formed V. tricolor flowers (b,c, blue (left) and green (right) columns, respectively), or within honeybee colonies that acted as vectors (d). All flowers and bumblebees were free of the three parasites prior to the experiment. One set of flowers (b) was initially exposed to bumblebees for 3 h, while the honeybees were excluded from the foraging arena. The bumblebees were then excluded from the arena, and the honeybees allowed to forage freely on the same set of shared flowers, and also on a new set of clean, vector-only flowers (c). Solid black arrows represent movement of the parasite provider, dashed black arrows represent movements of vector species between flowers and hive, and red arrows indicate possible dispersal routes of the parasites. Error bars represent 95% CIs. (Online version in colour.)
4. Discussion
The results show that flowers can act as dispersal platforms for a variety of pollinator parasites. Parasites were dispersed onto flowers by their host pollinators, and then vectored on to further flowers and back to colonies by non-host pollinators, with this being the case for both honeybee and bumblebee parasites. Flower species affected the dispersal of some parasites, but the results suggest that once contaminated, flowers can apparently become hotspots for disease dispersal via vectoring bees. The lack of any parasites on flowers collected immediately prior to the experiment but after 24 h in the flight cage without bees, the lack of any parasites of the (uninfected) vector species on flowers after the experiments, and the lack of any contamination of parasite provider bees with parasites of the (uninfected) vector species, given the sensitive PCR-based methodology used, along with the increase following the vectoring stage of several parasites which are not able to infect the vector species; all confirms that the parasite contamination detected had originated from the parasite provider bees and not from outside the experiment or from latent infections in the vector bees.
The bumblebee parasites A. bombi, C. bombi and N. bombi, plus the honeybee parasites N. apis and N. ceranae, were all rapidly dispersed from infected individuals to flowers within a 3 h foraging period. Although the two flower species require different methods of flower handling by the bees [48], the three Nosema species showed no evidence of a relationship between flower species and dispersal. Apicystis bombi and C. bombi, however, dispersed onto the bell-shaped C. cochleariifolia flowers more frequently than the flat-formed V. tricolour (with 21% and 36% greater dispersal, respectively). This may potentially be owing to increased physical contact and/or handling time with the bell-shaped C. cochleariifolia flowers during foraging or owing to foraging preference between the two flower species. This demonstrates not only that shared flowers are sites for the dispersal of all five of the pollinator parasites investigated, but also suggests that some flowers may provide a more effective transmission platform for parasites than others. The link with dispersal and physical contact between bee and flower also suggests that some parasite dispersal may be from spore adhesion to the bee cuticle and subsequently rubbing off onto surfaces. Further work to examine the effect of flower species and form on parasite transmission will be worthwhile. Whether parasite dispersal in nature is higher or lower than found in the experiment here will depend upon the density and species composition of the flower, and pollinator, communities. However, the very high level of parasite dispersal within just the short 3 h time span of the experiment makes it probable that parasite dispersal in the natural environment is widespread. It may also be expected that infected bees could have increased flower handling times and foraging demands owing to the cognitive and energetic costs of infection which would drive parasite dispersal rate even higher [49–53]. The propensity of a parasite to transfer between pollinator and flower is remarkably high, with clear vectoring between foraging sites taking place very rapidly.
After honeybees had foraged on flowers that had been visited by bumblebees, the bumblebee parasites A. bombi and C. bombi were detected in 6% and 30%, respectively, of honeybees collected from the entrances of their hives. As these bees were screened using only internal tissues, and great care was taken to avoid any contamination of the samples by the insect integument, this suggests that the honeybees had ingested the parasites during either the collection of nectar and pollen from the contaminated flowers, or the subsequent grooming of contaminated body surfaces by the bees. The internal tissue sample screened for each bee included the crop, which is most likely where the parasites were contained given the short 3 h duration of the experiment. Apicystis bombi has been detected in honeybees previously, though its virulence in this host is unknown [43]. In bumblebees, it reduces the fatbody and survival of workers and over-wintering queens [54,55]. Crithidia bombi does not appear able to infect honeybees, but the parasite is able to retain viability after passage through the honeybee gut if ingested [56]. This suggests that honeybees could act as reservoir hosts for the A. bombi bumblebee parasite, as well as vectoring C. bombi via their guts in addition to on their bodies. After bumblebees had foraged on flowers that had been visited by honeybees, 23% of bumblebees collected from the entrances of their hives had N. ceranae within them. This again means that the bumblebees had ingested the parasite during either foraging, food processing or grooming. Nosema ceranae is traditionally thought of as being a honeybee parasite and has been implicated in colony losses in some areas [31–33]. However, N. ceranae has more recently been identified as an emerging pathogen in several bumblebee species, causing both lethal and sublethal effects [22,37,39,40]. The results here highlight the potential role of shared flower use as a mode of transmission, which will facilitate the spillover of harmful parasites between different pollinators and populations.
These results provide strong evidence that many parasites may benefit from the shared use of flowers by multiple pollinator species, with non-host as well as host pollinators dispersing the parasites around the environment. The frequent, polylectic contact that bees and pollinators in general have with flowers provides the ideal transmission platform for parasites to spread between host species and landscapes [16]. The potential role that risk of infection may play in shaping foraging choices by pollinators may be an important area for future research. Our findings suggest the need to widen parasite screening regimes for imported/exported bees and flower products to include parasites that may be vectored by the bees or flowers, and which may pose a potentially devastating threat to naive pollinator communities. The results highlight that ecological communities may often include multiple potential mechanisms and agents of parasite dispersal, in addition to the simple bipartite host–parasite interactions that are typically considered. Many host–parasite interactions take place in communities in which there are shared use of resources, and parasite vectoring by multiple host and non-host species may be far more significant for parasite ecology than generally realized.
Acknowledgements
We thank Eamonn Mallon, Alison Dunn, members of the Hughes Lab, and two anonymous reviewers for their constructive comments on the manuscript, Katherine Roberts, Sophie Evison, Crystal Frost, Brian and Sandra Graystock, for technical assistance.
Data accessibility
Data are available through the Dryad Digital Repository.
Authors' contributions
P.G., D.G. and W.O.H.H. conceived and designed the work; P.G. carried out the experiments and analysed the data; P.G., D.G. and W.O.H.H. wrote the paper. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Financial support was provided by the Natural Environment Research Council (NE/G012113/1 to D.G. and W.O.H.H.), and the Bumblebee Conservation Trust.
References
- 1.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 2.Hatcher MJ, Dunn AM. 2011. Parasites in ecological communities. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Kamo M, Boots M. 2006. The evolution of parasite dispersal, transmission, and virulence in spatial host populations. Evol. Ecol. Res. 8, 1333–1347. [Google Scholar]
- 4.Pruss A, Kay D, Fewtrell L, Bartram J. 2002. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ. Health Perspect. 110, 537–542. ( 10.1289/ehp.02110537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatem AJ, Rogers DJ, Hay SI. 2006. Global transport networks and infectious disease spread. Adv. Parasitol. 62, 293–343. ( 10.1016/S0065-308X(05)62009-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatem AJ, Hay SI, Rogers DJ. 2006. Global traffic and disease vector dispersal. Proc. Natl Acad. Sci. USA 103, 6242–6247. ( 10.1073/pnas.0508391103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malone JD, Brigantic R, Muller GA, Gadgil A, Delp W, McMahon BH, Lee R, Kulesz J, Mihelic FM. 2009. US airport entry screening in response to pandemic influenza: modeling and analysis. Travel Med. Infect. Dis. 7, 181–191. ( 10.1016/j.tmaid.2009.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pysek P, Richardson DM. 2010. Invasive species, environmental change and management, and health. Ann. Rev. Environ. Resour. 35, 25–55. ( 10.1146/annurev-environ-033009-095548) [DOI] [Google Scholar]
- 9.Rigaud T, Perrot-Minnot M-J, Brown MJF. 2010. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B 277, 3693–3702. ( 10.1098/rspb.2010.1163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn AM, Perkins SE. 2012. Invasions and infections. Funct. Ecol. 26, 1234–1237. ( 10.1111/1365-2435.12022) [DOI] [Google Scholar]
- 11.Cox FEG. 2010. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 3, 5 ( 10.1186/1756-3305-3-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coura JR, Vinas PA. 2010. Chagas disease: a new worldwide challenge. Nature 465, S6–S7. ( 10.1038/nature09221) [DOI] [PubMed] [Google Scholar]
- 13.Fontaine C, Dajoz I, Meriguet J, Loreau M. 2005. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 4, e1 ( 10.1371/journal.pbio.0040001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulson D, Darvill B. 2004. Niche overlap and diet breadth in bumblebees; are rare species more specialized in their choice of flowers? Apidologie 35, 55–63. ( 10.1051/apido:2003062) [DOI] [Google Scholar]
- 15.Ruiz-González MX, Bryden J, Moret Y, Reber-Funk C, Schmid-Hempel P, Brown MJF. 2012. Dynamic transmission, host quality, and population structure in a multi-host parasite of bumblebees. Evolution 66, 3053–3066. ( 10.1111/j.1558-5646.2012.01655.x) [DOI] [PubMed] [Google Scholar]
- 16.McArt SH, Koch H, Irwin RE, Adler LS. 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624–636. ( 10.1111/ele.12257) [DOI] [PubMed] [Google Scholar]
- 17.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 18.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 19.Burkle LA, Marlin JC, Knight TM. 2013. Plant-pollinator interactions over 120 years: loss of species, co-ocurrence, and function. Science 339, 1611–1615. ( 10.1126/science.1232728) [DOI] [PubMed] [Google Scholar]
- 20.Meeus I, Brown MJF, De Graaf DC, Smagghe GUY. 2011. Effects of invasive parasites on bumble bee declines. Conserv. Biol. 25, 662–671. ( 10.1111/j.1523-1739.2011.01707.x) [DOI] [PubMed] [Google Scholar]
- 21.Szabo ND, Colla SR, Wagner DL, Gall LF, Kerr JT. 2012. Do pathogen spillover, pesticide use, or habitat loss explain recent North American bumblebee declines? Conserv. Lett. 5, 232–239. ( 10.1111/j.1755-263X.2012.00234.x) [DOI] [Google Scholar]
- 22.Graystock P, Yates K, Evison SEF, Darvill B, Goulson D, Hughes WOH. 2013. The Trojan hives: pollinator pathogens, imported and distributed in bumblebee colonies. J. Appl. Ecol. 50, 1207–1215. ( 10.1111/1365-2664.12134) [DOI] [Google Scholar]
- 23.Schmid-Hempel R, et al. 2014. The invasion of southern South America by imported bumblebees and associated parasites. J. Anim. Ecol. 83, 823–837. ( 10.1111/1365-2656.12185) [DOI] [PubMed] [Google Scholar]
- 24.Graystock P, Goulson D, Hughes WO. 2014. The relationship between managed bees and the prevalence of parasites in bumblebees. PeerJ 2, e522 ( 10.7717/peerj.522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colla SR, Otterstatter MC, Gegear RJ, Thomson JD. 2006. Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biol. Conserv. 129, 461–467. ( 10.1016/j.biocon.2005.11.013) [DOI] [Google Scholar]
- 26.Arbetman M, Meeus I, Morales C, Aizen M, Smagghe G. 2012. Alien parasite hitchhikes to Patagonia on invasive bumblebee. Biol. Invasions 15, 489–494. ( 10.1007/s10530-012-0311-0) [DOI] [Google Scholar]
- 27.Otterstatter MC, Thomson JD. 2008. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS ONE 3, e2771 ( 10.1371/journal.pone.0002771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goka K, Okabe K, Yoneda M. 2006. Worldwide migration of parasitic mites as a result of bumblebee commercialization. Popul. Ecol. 48, 285–291. ( 10.1007/s10144-006-0010-8) [DOI] [PubMed] [Google Scholar]
- 29.Kraus B, Page RE. 1995. Effect of Varroa jacobsoni (Mesostigmata: Varroidae) on feral Apis mellifera (Hymenoptera: Apidae) in California. Environ. Entomol. 24, 1473–1480. ( 10.1093/ee/24.6.1473) [DOI] [Google Scholar]
- 30.Genersch E, Aubert M. 2010. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 41, 54 ( 10.1051/vetres/2010027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higes M, Martin-Hernandez R, Meana A. 2010. Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41, 375–392. ( 10.1051/apido/2010019) [DOI] [Google Scholar]
- 32.Higes M, et al. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 10, 2659–2669. ( 10.1111/j.1462-2920.2008.01687.x) [DOI] [PubMed] [Google Scholar]
- 33.Klee J, et al. 2007. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invert. Pathol. 96, 1–10. ( 10.1016/j.jip.2007.02.014) [DOI] [PubMed] [Google Scholar]
- 34.McMahon DP, Fürst MA, Caspar J, Theodorou P, Brown MJF, Paxton RJ. 2015. A sting in the spit: widespread cross-infection of multiple RNA viruses across wild and managed bees. J. Anim. Ecol. 84, 615–624. ( 10.1111/1365-2656.12345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genersch E, Yue C, Fries I, de Miranda JR. 2006. Detection of deformed wing virus, a honey bee viral pathogen, in bumble bees (Bombus terrestris and Bombus pascuorum) with wing deformities. J. Invert. Pathol. 91, 61–63. ( 10.1016/j.jip.2005.10.002) [DOI] [PubMed] [Google Scholar]
- 36.Evison SEF, Roberts KE, Laurenson L, Pietravalle S, Hui J, Biesmeijer JC, Smith JE, Budge G, Hughes WOH. 2012. Pervasiveness of parasites in pollinators. PLoS ONE 7, e30641 ( 10.1371/journal.pone.0030641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey L, Gibbs AJ. 1964. Acute infection of bees with paralysis virus. J. Ins. Pathol. 6, 395–407. [Google Scholar]
- 39.Plischuk S, Martín-Hernández R, Prieto P, Lucía M, Botías C, Meana A, Abrahamovich AH, Lange C, Higes M. 2009. South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environ. Microbiol. Rep. 1, 131–135. ( 10.1111/j.1758-2229.2009.00018.x) [DOI] [PubMed] [Google Scholar]
- 40.Graystock P, Yates K, Darvill B, Goulson D, Hughes WOH. 2013. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. J. Invert. Pathol. 114, 114–119. ( 10.1016/j.jip.2013.06.005) [DOI] [PubMed] [Google Scholar]
- 41.Li JL, Chen WF, Wu J, Peng WJ, An JD, Schmid-Hempel P, Schmid-Hempel R. 2012. Diversity of Nosema associated with bumblebees (Bombus spp.) from China. Int. J. Parasitol. 42, 49–61. ( 10.1016/j.ijpara.2011.10.005) [DOI] [PubMed] [Google Scholar]
- 42.Singh R, et al. 2010. RNA viruses in hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS ONE 5, e14357 ( 10.1371/journal.pone.0014357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipa JJ, Triggiani O. 1996. Apicystis gen nov and Apicystis bombi (Liu, Macfarlane & Pengelly) comb nov (Protozoa: Neogregarinida), a cosmopolitan parasite of Bombus and Apis (Hymenoptera: Apidae). Apidologie 27, 29–34. ( 10.1051/apido:19960104) [DOI] [Google Scholar]
- 44.Flores JM, Gutierrez I, Espejo R. 2005. The role of pollen in chalkbrood disease in Apis mellifera: transmission and predisposing conditions. Mycologia 97, 1171–1176. ( 10.3852/mycologia.97.6.1171) [DOI] [PubMed] [Google Scholar]
- 45.Copley TR, Jabaji SH. 2012. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 112, 15–24. ( 10.1111/j.1365-2672.2011.05192.x) [DOI] [PubMed] [Google Scholar]
- 46.Fouks B, Lattorff HMG. 2011. Recognition and avoidance of contaminated flowers by foraging bumblebees (Bombus terrestris). PLoS ONE 6, e26328 ( 10.1371/journal.pone.0026328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durrer S, Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302. ( 10.1098/rspb.1994.0176) [DOI] [Google Scholar]
- 48.Dafni A. 1992. Pollination ecology: a practical approach. Oxford, UK: Oxford University Press. [Google Scholar]
- 49.Gegear RJ, Otterstatter MC, Thomson JD. 2005. Does parasitic infection impair the ability of bumblebees to learn flower-handling techniques? Anim. Behav. 70, 209–215. ( 10.1016/j.anbehav.2004.09.025) [DOI] [Google Scholar]
- 50.Gegear RJ, Otterstatter MC, Thomson JD. 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B 273, 1073–1078. ( 10.1098/rspb.2005.3423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otterstatter MC, Gegear RJ, Colla SR, Thomson JD. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 58, 383–389. ( 10.1007/s00265-005-0945-3) [DOI] [Google Scholar]
- 52.Alghamdi A, Dalton L, Phillis A, Rosato E, Mallon EB. 2008. Immune response impairs learning in free-flying bumble-bees. Biol. Lett. 4, 479–481. ( 10.1098/rsbl.2008.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyler ER, Adams S, Mallon EB. 2006. An immune response in the bumblebee, Bombus terrestris leads to increased food consumption. BMC Physiol. 6, 6 ( 10.1186/1472-6793-6-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu HJ, Macfarlane RP, Pengelly DH. 1974. Mattesia bombi n. sp. (Neogregarinida: Ophrocystidae), a parasite of Bombus (Hymenoptera: Apidae). J. Invert. Pathol. 23, 225–231. ( 10.1016/0022-2011(74)90188-8) [DOI] [PubMed] [Google Scholar]
- 55.Macfarlane RP, Lipa JJ, Liu HJ. 1995. Bumble bee pathogens and internal enemies. Bee World 76, 130–148. ( 10.1080/0005772X.1995.11099259) [DOI] [Google Scholar]
- 56.Ruiz-González MX, Brown MJF. 2006. Honey bee and bumblebee trypanosomatids: specificity and potential for transmission. Ecol. Entomol. 31, 616–622. ( 10.1111/j.1365-2311.2006.00823.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available through the Dryad Digital Repository.