Abstract
Two recent studies provide provocative experimental findings about the potential influence of kin recognition and cooperation on the level of sexual conflict in Drosophila melanogaster. In both studies, male fruit flies apparently curbed their mate-harming behaviours in the presence of a few familiar or related males, suggesting some form of cooperation mediated by kin selection. In one study, the reduction in agonistic behaviour by brothers apparently rendered them vulnerable to dramatic loss of paternity share when competing with an unrelated male. If these results are robust and generalizable, fruit flies could be a major new focus for the experimental study of kin selection and social evolution. In our opinion, however, the restrictive conditions required for male cooperation to be adaptive in this species make it unlikely to evolve. We investigated these phenomena in two different populations of D. melanogaster using protocols very similar to those in the two previous studies. Our experiments show no evidence for a reduction in mate harm based upon either relatedness or familiarity between males, and no reduction in male reproductive success when two brothers are in the presence of an unfamiliar, unrelated, ‘foreign’ male. Thus, the reduction of sexual conflict owing to male cooperation does not appear to be a general feature of the species, at least under domestication, and these contrasting results call for further investigation: in new populations, in the field and in the laboratory populations in which these phenomena have been reported.
Keywords: Drosophila, sexual conflict, cooperation, mate harm, kin selection, familiarity
1. Introduction
Because males depend upon females to transmit the genetic material contained in their spermatozoa, male mate harm would not appear to be adaptive. Yet harm can evolve when the injurious behaviour performed by males either discourages the female from remating with rival males or increases the immediate fecundity of the female, as when injured females allocate more resources to current reproduction as part of a ‘terminal investment strategy’ [1,2]. More commonly, mate harm represents collateral damage experienced by females resulting from male–male competition. Whether harm is adaptive or incidental, theory predicts that kin selection can moderate sexual conflict when there are inclusive fitness benefits.
Thus, the adaptive value of competitive behaviours that inflict harm on the female should be diminished when competitor males are related, in part because when a male curbs the behaviour that causes harm to a mate the female will be in better reproductive condition. Such benefits to reducing harm would be magnified if the females were also relatives, as may be the case in viscous, patch-structured populations where individuals remain near to where they grew up [3,4]. Moreover, by reducing harming behaviours, a male may save energy or resources (seminal fluids, etc.), thereby increasing his odds of surviving to find another mate and being in good condition once he does. Several conditions are necessary for this kind of kin selection to occur: (i) males must somehow harm the female, reducing her lifetime reproductive success, (ii) there must be signals of kinship, and (iii) populations need to be sufficiently structured that groups of closely related males stably interact with females over sufficient periods of time for benefits to accrue. A number of highly social animals show evidence for cooperation among related males when reproducing, with examples including cooperative lekking and mate-sharing in some birds and mammals (reviewed in [5]). These studies may provide proof of principle, but so far there has been little investigation as to how kinship might influence mate harm by males that are closely related to each other.
A recent experimental study by Carazo et al. [6] using Drosophila melanogaster—a species in which males are well-known to harm the female before, during and after copulation (e.g. [2,7,8])—provided evidence that, when housed together with a female, fruit fly brothers reduced aggressive behaviours towards one another as well as courting the female less vigorously. Females that were maintained with trios of sibling males (their ‘AAA’ treatment) produced more offspring over a longer period of time than females housed with males from different families (their ‘ABC’ treatment). The authors interpreted these findings as indicating that the brothers were cooperating to reduce injury to the female. These results are novel and exciting but surprising for several reasons. First, a number of studies of insects have shown scant evidence for relatedness influencing cooperative behaviour (see [9] for review). Moreover, the population sizes and dispersal capacity of this little insect are generally believed to be large [10,11], although dispersal is likely to be facultative, sexually dimorphic and related to the stability of available resources, among other things [12–14]. Drosophila melanogaster females typically mate with more than one male in aggregations around food resources that are also their oviposition sites, and these are likely to attract other, unrelated, individuals. For example, Imhof et al. [15] estimated that a typical wild-caught female from a Viennese population carried sperm from four to six different males, such that the resulting broods would not comprise full siblings.
Carazo et al. [6] also report that when trios were composed of two brothers and an unrelated male (‘AAB treatment’ in their experiment 4), the (unrelated) B male sired about 50% of the female's offspring. Thus, this ‘foreign’ male's paternity share was much greater than the one-third predicted from random mating, and about double the average paternity share of each ‘local’ brother. These results suggest that kin alliances may be vulnerable to usurpation of paternity by unrelated males. Clearly, if Drosophila males do cooperate with siblings (or other kin), then there are important implications for the population structure and mating behaviour of this species in the wild.
But were the effects reported by Carazo et al. [6] due to male–male relatedness? In social insects, cues for cooperative behaviour often come from the rearing environment rather than genetic relatedness per se [9]. For example, the mix of hydrocarbons on the insect epicuticle can be altered by microenvironmental factors such as diet and gut microbe composition, in turn influencing mate choice [16]. Carazo et al. [6] overlooked this potential factor by conflating relatedness with the common rearing environment of sibling males. Indeed, Hollis et al. [17] studied a different population of D. melanogaster and found similar benefits to females from being held with trios of brothers that had grown up together (as in Carazo et al., experiment 1) but not when brothers were reared in different vials, and thus were familial but not familiar. Of course, recognition of familiarity may be a proxy for relatedness in patch-structured populations, such as colonies of social insects, so these two studies could be in agreement, although different in the proximate mechanism underlying to the apparent cooperative behaviour. It is difficult to imagine what possible benefit could result from cooperation based upon familiarity alone.
Whatever the natural biology of D. melanogaster be, cooperative reduction in mate harm by males would almost certainly be maladaptive in the laboratory environment where the typical population is panmictic, crowded and unlikely to be genetically structured. For a male fruit fly under common laboratory-rearing protocols, self-inhibition of competitive behaviours based upon environmental cues would have no kin selection benefits and clear deleterious effects. Both Carazo et al. [6] and Hollis et al. [17] used populations that had decades (40–45 years) of laboratory adaptation, raising the question of why such plastic responses would persist in the face of selection. One also wonders how much heterogeneity there actually could be among rearing vessels in the laboratory. Most laboratory stocks are reared on medium prepared according to a strict formula, sterilized and mass dispensed into vials or bottles with a preservative to inhibit microbial growth. Are there sufficient microenvironmental differences between two vials for recognition of vial-mates to occur? The effect observed by Hollis et al. [17] suggests that there may be such cues, at least under some conditions, but this result calls for corroboration in other laboratories and other conditions. Unfortunately, Hollis et al. [17] did not conduct a fully crossed experiment, as they left out the unrelated + familiar treatment that is needed to fully support the assertion that being raised together is sufficient to reduce mate-harming behaviour.
Together, the studies of Carazo et al. [6] and Hollis et al. [17] present evidence that the ubiquitous fruit fly may be an exciting new system in which to investigate kin selection and social evolution experimentally. But their results are sufficiently unexpected that further investigation would be wise before heavily investing in this line of research. To replicate their studies, we examined female age-specific fertility in response to the same two treatments employed by Carazo et al. [6]. We also looked at the impact of both relatedness and familiarity between two males on the paternity share of a third unrelated male, using a fully crossed design. We used two distinct but related laboratory populations, both descended from the same base population used by Hollis et al. [17]. Our results do not support the previous findings with respect to the effects of either kin or familiarity on male mate harm to female fitness, suggesting that, at the very least, such responses are not general features of laboratory populations of D. melanogaster.
2. Material and methods
(a). Drosophila stocks
Our base population (IV) is nominally and ancestrally the same as that used by Hollis et al. [17], though separated by decades of maintenance in different laboratories. The IV laboratory population was founded from wild stock collected in Massachusetts in 1975 and subsequently passed on to various laboratories worldwide, especially to the academic offspring of Brian and Deborah Charlesworth. Our IV population [18] was split from that used by Hollis et al. [17] sometime before 1984 and has a history of 14-day, discrete generation culture [19] in 25 × 95 mm vials at a density of 80–120 flies per vial, with a total population size of 1.5–2.5 × 103 adults per generation that are mixed prior to female oviposition. We acquired the stock from Dr M. R. Rose in 2002 and have continued with the same maintenance protocol except switching to a 12 L : 12 D light cycle (from 24 : 0). A visibly marked derivative, the IVbw population, was established through 10 rounds of backcrossing, with episodic refreshers of several generations to maintain genetic similarity between the two populations. The recessive bw1 marker has a mildly deleterious effect in this genetic background, causing a fitness loss of 10% or less in males [20].
Our first experiment on mate harm employed a laboratory population designated CO1. This population is descended from IV and part of a selection treatment initiated in 1989 [21] that has been maintained on a four week, discrete generation cycle, with the first two weeks in vials and the last two weeks in population cages. We chose this population because of its history of extended adult life in population cages, which might allow greater population structure and more extended interactions to occur; Dahomey, the population studied by Carazo et al. [6] is also a cage-maintained stock.
(b). Mate harm experiment
To generate triplets of males that were siblings (AAA) or non-siblings (ABC), virgin males and females from the CO1 population were haphazardly paired in vials and held for several days prior to transfer to fresh medium for egg collection over the next 24 h. These pairs were maintained for three weeks, with egg collections at the same time each week, so that younger males could replace ageing male flies in the treatment vials, thereby reducing the effect of co-ageing of males and females (see below).
For the experimental treatments, virgin males and females were collected using light CO2 anaesthesia as they emerged from pupae, 9–10 days after egg laying; handling thereafter was via mouth-aspiration without anaesthesia. Males were held for 1–2 days before being assorted into AAA or ABC experimental treatments. Parents that produced sons assigned to the AAA treatment did not contribute sons to the ABC treatment or vice versa. As all three males came from the same parents and the same vial, AAA trios were both related and familiar, whereas ABC trios were unrelated and unfamiliar, as in Carazo et al. [6]. Males were assorted into social groups 48 h before an unrelated virgin female was aspirated into each vial containing the three males; all flies were 3–4 days in adult age when females were introduced. These groups of three males and a female were held in vials for 24 h before transfer to a new vial with live yeast to stimulate egg production. The flies remained in the second vial for 3 days, then transferred to another yeasted vial. Thus, each week the flies spent 1 day on unsupplemented medium followed by two consecutive 3-day periods on medium with yeast. The experiment began with 75 AAA and 74 ABC vials.
To maintain a relatively constant environment for the female with respect to male age and vigour, males were replaced at the beginning of each week with brothers that were one week younger. These new males were handled exactly the same way as the first males put into each vial but came from eggs produced by the parental females that were either one week (week 2 of the assay) or two weeks older (week 3 of the assay). All vials of flies were maintained until the death, loss, or sustained lack of viable offspring produced by the female. Vials were incubated until the offspring eclosed and could be counted to determine the female's lifetime reproductive success and any changes in fecundity as she aged. Counts were performed blind to the treatment group in all experiments reported on herein.
Males also died during this experiment, though at a much lower rate than females. When males died, the level of intrasexual competition and female mate harm probably declined, so we removed these vials from our analyses. Sometimes we were also unable to replace males from the same parents with their younger siblings as some parents failed to produce enough male offspring as they aged, so those samples were also not analysed. In all, we analysed data from 43 AAA vials and 52 ABC vials.
(c). Foreign male experiment
In this experiment, we examined the combined reproductive success of two brothers housed with both an unrelated male and an unrelated female. This AAB treatment here comprised all four combinations of related × familiar factors for the two A males. In the unrelated and unfamiliar treatment, these two males were more similar to one another than to the B male only in eye colour, and this treatment serves as a check on the expectation of one-third paternity share by each male if each male obtained an equal share, on average. This experiment was conducted with flies from the IV population, using two wild-type ‘A’ males and a brown-eyed ‘B’ male—as well as the reverse—to test for any effects of the eye-colour mutation on male performance.
To generate brothers in this experiment, 100 virgin male–female pairs were allowed to mate for 2 days in vials with supplementary yeast to increase female fecundity. These parents were then transferred to new vials and allowed to oviposit overnight for 10 h. Small brushes were used to count the eggs into smaller (13 mm diameter) test tubes with ad libitum medium at 12 eggs per tube. The smaller surface area of the tubes created larval densities and a social environment comparable with the normal maintenance regime for this population in the laboratory. Two tubes were collected from each of 100 pairs, half wild-type and half brown-eyed. Once the offspring began to eclose, related and familiar A male dyads were drawn from the same test tube, while related but unfamiliar A males were drawn from different tubes.
Unrelated ‘A’ and ‘B’ males were produced in test tubes handled as above, except that the eggs were laid onto food plates in two population cages containing approximately 500 flies each. Eggs were sampled from different regions of the plates and from different plates to minimize the chance of relatedness in rearing tubes. As with the related males, unrelated but familiar ‘A’ male pairs were drawn from the same test tube, while unrelated and unfamiliar ‘A’ males were drawn from different tubes.
Experimental male AAB trios were put together less than 15 h after eclosion, on day 11 after their egg was laid, and allowed to interact for 48 h prior to the introduction of a virgin female. All females were from the IVbw population, so that paternity could be scored by offspring eye colour. Females were reared at densities of 100 per vial and were put into treatment vials 5 days after eclosion. Every other day at the same time for 8 days, all flies were transferred to fresh vials and the old vials incubated until all adults emerged. We thus had five different samples from each ‘vial’ over the course of 10 days, with separate samples for each 2-day period. Progeny were counted once all of the adults had eclosed, 12+ days later.
We began the experiment with a total of 50 AAB social groups (‘vials’) in each of the four experimental treatments, with the B male as wild-type in 25 of these and brown-eyed in the other 25 per treatment. Declining sample sizes as the experiment progressed largely reflect female mortality (see also [22]). There were also a few losses of flies during handling and some obvious data recording errors; those vials were also deleted from the dataset analysed here. We have no reason to expect any bias due to the samples deleted.
(d). Statistical analyses
All statistical analyses were performed using R v. 3.2.0 [23], and we provide all of the data, as well as R code and output from our analyses which are archived on the Dryad data repository. Following Carazo et al. [6], we report the results of t-tests for two-sample comparisons, and generalized linear models (GLM) and generalized linear mixed models (GLMM) to compare treatments when controlling for other variables. For GLMs and GLMMs, we report likelihood ratio chi-square tests (LR χ2) comparing full models with a model without the factor of interest. To compare the risks of mortality (survival during the experiment) and reproductive failure (cessation of progeny production) among treatments, we fit the data to Cox Proportional Hazards models. Descriptive statistics are presented as mean ± 95% confidence Limit (CL).
3. Results
(a). Mate harm experiment
Mate harm levels were apparently high and similar in both treatments, with females surviving an average of 11.4 [9.97, 12.72] (n = 52) days after introduction to vials containing three unrelated males (ABC), and 10.7 [9.14, 12.17] (n = 43) days in vials where the three males were related (full siblings) and reared together (AAA). There was no significant difference between treatments in mean female longevity (figure 1a; t-test, t = 0.68, p = 0.50).
Figure 1.
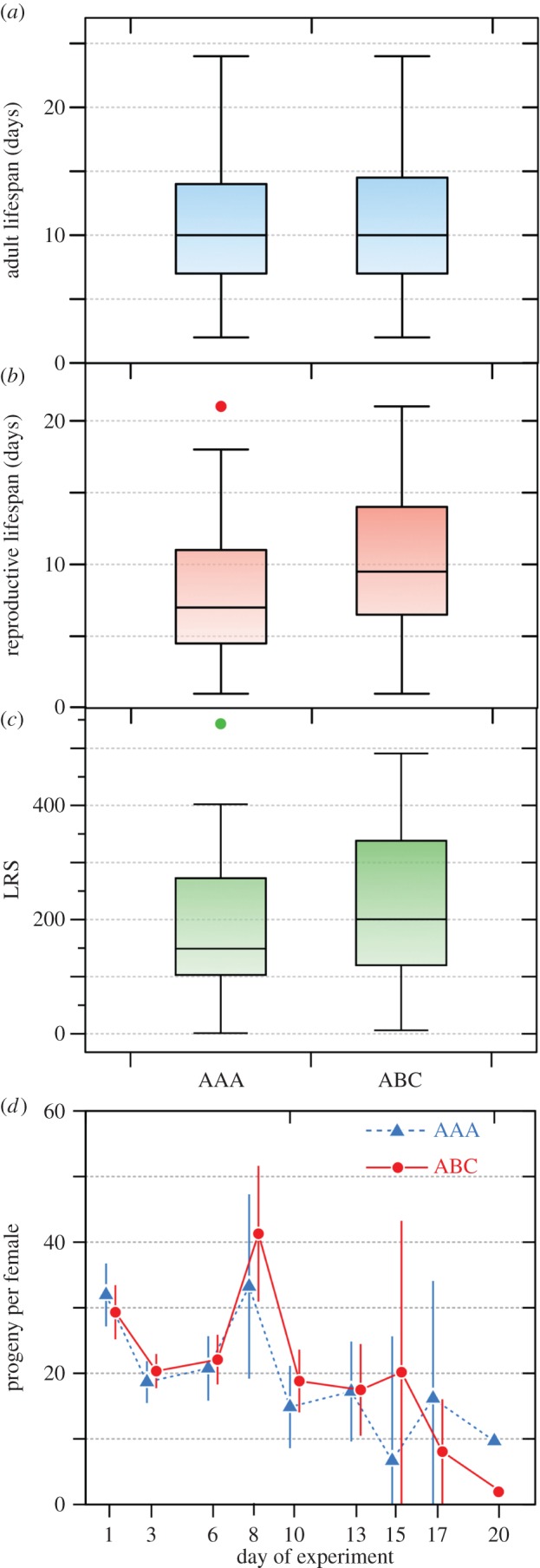
Mate harm experiment. Tukey box plots compare treatments designed to evaluate the effects of three brothers (AAA; n = 43 vials) versus three unrelated males (ABC; n = 52) on a female's (a) longevity, (b) period of offspring production and (c) lifetime reproductive success (LRS; total number of progeny). Graph (d) shows the average number of progeny (±95% CL) produced per day by females in the presence of AAA and ABC male threesomes; symbols for each day (shown on the x-axis) are jittered for clarity. (Online version in colour.)
Reproductive lifespan was estimated as the period from females being introduced to the males until they no longer produced viable eggs. Females housed with brothers (AAA; n = 43) produced offspring for an average of 8.6 [7.18, 10.03] days while those in the unrelated male treatment (ABC; n = 52) had a reproductive lifespan of 9.7 [8.4, 11.0] days, and the small difference was not statistically significant (t = 1.12, p = 0.27; figure 1b). The approximately 2 day difference between total lifespan and reproductive lifespan indicates a short period of female survival after they stopped producing viable eggs.
Nor was there a significant difference in the total number of offspring produced (lifetime reproductive success, LRS) by females housed with AAA (183.7 [143.7, 223.6] n = 43) or ABC (218.3 [182.3, 254.2] n = 52) triplets of males (t = 1.30, p = 0.20; figure 1c). Lifetime reproductive output is likely to be positively associated with longevity, and indeed the correlation was strong and positive for both AAA (r = 0.77, p < 0.0001) and ABC (r = 0.75, p < 0.0001) treatments. Controlling for female longevity, there was still no significant difference in the reproductive output of females in these two treatments (negative binomial GLM, effect of treatment, LR χ2 = 1.06, p = 0.30).
There was also no significant difference in female survival between the two treatments (mortality risk, LR χ2 = 0.45, p = 0.50, n = 95), nor between the lengths of the egg laying periods (risk of reproductive failure, LR χ2 = 0.72, p = 0.40), or the time courses of viable egg production (t-tests comparing treatments at each time period, all p > 0.20; figure 1d).
(b). Foreign male experiment
There was no significant effect of male eye colour (LR 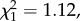 p = 0.29) or treatment (LR χ2 = 3.24, p = 0.36) on the proportion of the female's progeny (LRS) sired by the ‘foreign’ male in this experiment (figure 2). For each treatment, the 95% CL for the mean siring success of the foreign (B) males included 0.33 (figure 2) as expected if each male achieved equal reproductive success. Although the foreign males obtained the highest proportion of reproductive success when they were with two brothers raised in the same environment, the difference is slight and non-significant (p > 0.55 in each case, GLM controlling for foreign male eye colour).
p = 0.29) or treatment (LR χ2 = 3.24, p = 0.36) on the proportion of the female's progeny (LRS) sired by the ‘foreign’ male in this experiment (figure 2). For each treatment, the 95% CL for the mean siring success of the foreign (B) males included 0.33 (figure 2) as expected if each male achieved equal reproductive success. Although the foreign males obtained the highest proportion of reproductive success when they were with two brothers raised in the same environment, the difference is slight and non-significant (p > 0.55 in each case, GLM controlling for foreign male eye colour).
Figure 2.
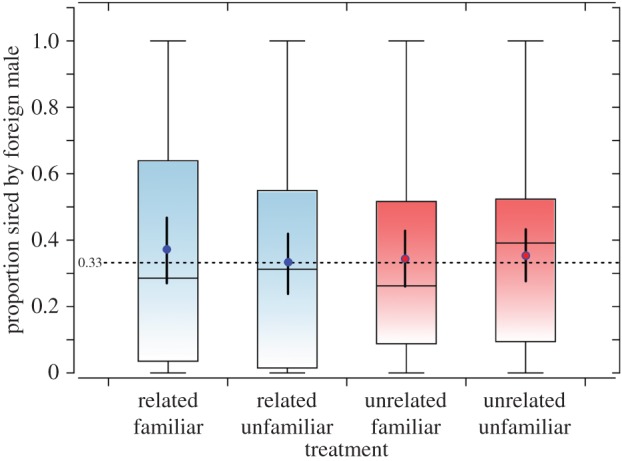
Foreign male experiment. Tukey box plots compare the proportion of progeny sired by the ‘foreign’ male in treatments where the female was also housed with two brothers raised together (n = 43) two brothers raised apart (n = 39), two unrelated males raised together (n = 47), or two unrelated males raised apart (n = 47). The dot and solid line inside the box are the mean ± 95% CL of the proportions for each treatment, and the horizontal dashed line is at 0.33, the expected value if the males achieve equal reproductive success. (Online version in colour.)
Comparing treatments in this experiment, there were also no significant differences in either LRS per female (LR 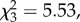 p = 0.14, controlling for the significant effects of female longevity and foreign male eye colour) or female survival (mortality risk, LR
p = 0.14, controlling for the significant effects of female longevity and foreign male eye colour) or female survival (mortality risk, LR 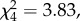 p = 0.43, GLM controlling for the non-significant effect of the foreign male's eye colour).
p = 0.43, GLM controlling for the non-significant effect of the foreign male's eye colour).
4. Discussion
Compelled by the remarkable findings of both Carazo et al. [6] and Hollis et al. [17], we undertook experiments varying social conditions for males by changing both genetic relatedness (brothers versus unrelated males) and familiarity (co-reared versus reared in isolation). We replicated the experiments of Carazo et al. [6] as closely as possible based upon published protocols and used comparable or larger sample sizes, extending their experimental design to include controls and the effect of familiarity. However, we found no evidence that either relatedness or familiarity influenced mate harm in our laboratory populations, with no treatment effects on the female's day-to-day or total offspring production, reproductive lifespan or longevity. There was also no suggestion in our experiments that two males that were brothers, or raised in the same environment, or both, were vulnerable to losing paternity share to a foreign male. Below, we consider whether differences in population origin and subsequent maintenance, or small differences in experimental protocols, can explain the disparate outcomes from these similar studies. As we outlined in the Introduction, we feel that the adaptive reduction of mate harm in D. melanogaster is unlikely to evolve based upon the species' ecology in the field or the laboratory. We call upon researchers to corroborate our findings with the same and different populations of this species.
The 3 : 1 (male : female) sex ratio used in the mate harm experiment was clearly stressful to females. Females from the CO1 population used in this experiment had been selected for total reproductive output at about 18 days of adult age (i.e. 28 days from egg being laid) for more than 300 generations. Typical mean longevity of these females under 1 : 1 sex ratios is more than 5 weeks (e.g. 41 days in [24]), yet the females in our experiments most often lived less than two weeks, surviving an average of 11 days (11.3 days for ABC and 10.7 days for AAA treatments) after the introduction of males and supplemental yeast. Female longevity and reproductive lifespan were remarkably similar to those reported by Carazo et al. [6], suggesting that the absence of measurable differences between treatments in our assays is not due to a low intensity of male harassment and other mate-harming behaviours. Furthermore, differences in harm as measured by female longevity and total reproductive success, though not statistically significant, were in the opposite direction to those reported by Carazo et al. [6] and Hollis et al. [17] with respect to experimental treatment.
Once supplied with ad libitum dietary yeast at a young age, D. melanogaster females typically rapidly increase their rate of egg production, peaking at 60 or more eggs per day after 3 or 4 days and then declining (e.g. [25]). In our experiments, females given unlimited yeast after 1 day in the presence of three males had a median offspring production of only 38 per day and this peaked 7 days after yeast was added (figure 1d). We suspect that this somewhat muted and delayed spike in female reproductive output reflects male interference with female feeding or oviposition, compounded by elevated female activity caused by harassment. Gains in daily fertility through the first week of this experiment may be a reflection of reduced intensity of harassment as males aged or became disinterested in courtship. Once those males were replaced by younger males at the beginning of the second week, the reproductive output of females declined and mortality rates soared.
Our offspring production data differ somewhat from the patterns reported by Carazo et al. [6] and Hollis et al. [17]. In both of those studies, females began the experiment with the maximum reproductive rate observed (approx. 50 offspring d−1 and 60–70 d−1, respectively) and declined rapidly soon after the addition of males. It seems likely that females in those experiments had richer base medium or received dietary yeast supplementation as virgins prior to the introduction of males, whereas our females received extra yeast the day after being introduced to males. Irrespective of these details, the pattern of fertility in females in all three studies is telling, in that the male-biased sex ratio appears to cause an immediate reduction in reproductive rate. This suggests that direct interference, such as exclusion from access to the food, is an important component of harm in addition to the chronic detrimental effects on females of male harassment, copulation and seminal fluids [7,8,26]. Many females in our experiment also died at or immediately after their peak reproductive output, suggesting catastrophic mortality rather than extended wear and tear under these conditions.
Because different populations were used by the three research groups, it is possible that plasticity for cooperative behaviours has been lost in some lineages but not in others. This might occur via vestigialization and selection against those behaviours in the laboratory (as we argued above) operating upon different pools of genetic variation in different stocks, or due to differences in protocols. While all four populations have been cultured for similar numbers of generations in the laboratory (approx. four decades), our IV population, collected in Massachusetts, has a very different history from the Dahomey population used by Carazo et al. [6], collected in Benin. Differences in behaviour have been reported between Zimbabwean D. melanogaster and North American (cosmopolitan) flies [27], so the presence/absence of cooperation may reflect fundamental differences between these source populations. Moreover, Dahomey has been maintained with overlapping generations, allowing both young and old individuals to contribute to the population [22], whereas our IV population and its derivatives undergo a discrete generation culture schedule, with selection for a specific age of reproduction. Interestingly, although separated by decades of maintenance in different laboratories, our populations share a common laboratory stock ancestor (IV) with those of Hollis et al. [17]. It is not clear how differences in laboratory culture would affect the maintenance of the plastic behaviours concerned, as confined space and admixture are common to most laboratory stocks, reducing the potential for local population structure.
Carazo et al. [6] generated a scenario (their experiment 4) where two males that were related and familiar (AA males were brothers from the same source vial) competed with a foreign (B) male for fertilizations. Cooperating males apparently had a sizeable proportion of their reproductive success usurped by foreign (unrelated and unfamiliar) males. Three features of their experiment reduce our confidence in the robustness of this result. First, two visible genetic markers, spa and se, in addition to the dominant wild-type were used to detect paternity, and the fitness asymmetries among these genotypes appear to account for part of the extraordinary magnitude of the effect reported. Carazo et al. [6] conducted all six combinations of A and B males, and in each case the foreign (B) male obtained significantly more than 33% paternity share. But when wild-type males played the role of the foreign (B) male, they gained nearly 70% of the paternity, compared with 15% for the local (A) males; when mutants were the foreign male, their paternity share was 43% (analysis from archived Dryad files associated with the paper). Second, that experiment was performed on a small scale, with each male type represented as the local (A) males 18 times, presumably with half challenged by each of the other two genotypes as the B male (i.e. nine per treatment; total n = 54). Third, they did not run any control treatments—for example, one in which the local (A) males were not related, to verify that their null expectation of 0.33 paternity share was appropriate. Using a larger sample size with an orthogonal design, and no measurable asymmetries in wild-type and marker stock fitness, we were unable to corroborate the findings of the Carazo et al. [6] experiment—neither relatedness nor familiarity affected male paternity share.
If a reduction in competitiveness owing to familiarity is indeed present in some populations of D. melanogaster, it is important to carefully consider the female perspective and her potential role. For example, female signals of receptivity may modulate the intensity of courtship and aggression. The importance of females to group behaviour in D. melanogaster has been shown by Krupp et al. [28] and Billeter et al. [29] who found a higher mating rate when females were housed with males from a mixture of strains or genotypes, compared with females with a single strain or genotype. Female choice could explain the result obtained by Carazo et al. [6] if females remate to maximize offspring genetic diversity—encountering two co-reared sibling males and a third ‘foreign’ male, the female could increase the genetic diversity of her offspring by mating disproportionately with the foreigner. Thus, their results may have been owing to polyandry benefits rather than related male cooperation and vulnerability to usurpation. In our view, female-driven effects are a viable alternative to the hypotheses forwarded by Carazo et al. [6].
Carazo et al. [6, p. 674] wrote that ‘… the benefits of relaxed competition among relatives may be dynamic, diminishing rapidly as populations become less viscous…’. We questioned whether or not Drosophila populations satisfy the restrictive conditions required for kin selection often enough for plasticity in competitiveness to evolve, leading to the research we report here. Research in behavioural and evolutionary ecology is rarely replicated, even in model systems where the cost of such replication would be relatively low, the protocols precisely described and repeatable, and even the original study populations or their descendants readily available. The recent ‘Many Labs’ Replication Project [30] attempting to repeat 13 classic studies in psychology across several research groups worldwide has revealed the ample benefits of such an endeavour. In a few cases the original conclusions did not hold up, whereas in others the original effect sizes turned out to be seriously overestimated, though underestimated in a few others [30]. The message, however, is clear—replication is important both in kind (same population, same protocols) and in spirit (different populations or species, similar protocols that should not unduly influence results). Only with such careful replication can we ensure that a field of study moves forward productively.
The results reported by Carazo et al. [6] and Hollis et al. [17] are exciting and could potentially provide a foundation for experimental work on the evolution of kin selection and social behaviour in the Drosophila model system. Taylor [4] argues from theory that, in patch-structured populations, benefits to altruism are likely to be seen when altruistic behaviour immediately precedes dispersal. Perhaps reduced mate harm is a facet of Drosophila breeding biology in early adult life, and thus experiments with extended co-association of individuals are artificial and as a result may yield inconsistent results. We remain sceptical of the results and interpretations of Carazo et al. [6] and Hollis et al. [17] based upon our understanding of the biology of this species and our own attempts at replication reported here, but further work is warranted. We call upon researchers interested in this issue to conduct the relatively simple experiments (especially the related-and-familiar versus unrelated-and-unfamiliar control) required to determine the extent of male harm in this species and its association with social group composition.
Acknowledgements
We are grateful to Ellen Avery, Julia Duszczyszyn, Alexa Kuczynski, Michelle Lemieux and Jacqueline Tattle for assistance with fly ranching and wrangling. Two anonymous reviewers provided helpful input on the manuscript.
Data accessibility
All of the raw data analysed in this paper are publicly available from the Dryad digital repository at http://datadryad.org/resource/doi:10.5061/dryad.q6ds5; all analyses and R code are provided in the electronic supplementary material.
Authors' contributions
A.C. and R.M. conceived and designed the study; A.C., M.B. and J.A. collected the data; R.M. analysed the data; A.C. and R.M. drafted the manuscript; and all authors edited and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by discovery grants from the Natural Sciences and Engineering Research Council of Canada to A.C. and R.M., and a grant from Queen's University to R.M.
References
- 1.Lessells CM. 1999. Sexual conflict in animals. In Levels of selection in evolution (ed. Keller L.), pp. 75–99. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Morrow EH, Arnqvist G, Pitnick S. 2003. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 14, 802–806. (doi:10.1093/beheco/arg073) [Google Scholar]
- 3.Hamilton WD. 1964. The genetical evolution of social behaviour, I and II. J. Theor. Biol. 7, 1–52. (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 4.Taylor PD. 1992. Altruism in viscous populations: an inclusive fitness model. Evol. Ecol. 6, 352–356. (doi:10.1007/BF02270971) [Google Scholar]
- 5.Pizzari T, Biernaskie JM, Carazo P. 2014. Inclusive fitness and sexual conflict: how population structure can modulate the battle of the sexes. BioEssays 37, 155–166. (doi:10.1002/bies.201400130) [DOI] [PubMed] [Google Scholar]
- 6.Carazo P, Tan CKW, Allen F, Wigby S, Pizzari T. 2014. Within-group male relatedness reduces harm to females in Drosophila. Nature 505, 672–675. (doi:10.1038/nature12949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18, 41–47. (doi:10.1016/S0169-5437(02)00004-6) [Google Scholar]
- 8.Rice WR, Stewart AD, Morrow EH, Linder JE, Ortieza N, Byrne PG. 2006. Assessing sexual conflict in the Drosophila melanogaster laboratory model system. Phil. Trans. R. Soc. B 361, 287–299. (doi: 10.1098/rstb.2005.1787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breed MD. 2014. Kin and nestmate recognition: the influence of W.D. Hamilton on 50 years of research. Anim. Behav. 92, 271–279. (doi:10.1016/j.anbehav.2014.02.030) [Google Scholar]
- 10.Coyne JA, Boussy IA, Prout T, Bryant SH, Jones JS, Moore JA. 1982. Long-distance migration of Drosophila. Am. Nat. 119, 589–595. (doi:10.1086/283936) [Google Scholar]
- 11.Keller A. 2007. Drosophila melanogaster’s history as a human commensal. Curr. Biol. 17, R77–R81. (doi:10.1016/j.cub.2006.12.031) [DOI] [PubMed] [Google Scholar]
- 12.Schlötterer C, Neumeier H, Sousa C, Nolte V. 2006. Highly structured Asian Drosophila melanogaster populations: a new tool for hitchhiking mapping? Genetics 172, 287–292. (doi:10.1534/genetics.105.045831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon JC, Dickson WB, Dickinson MH. 2011. Prior mating experience modulates the dispersal of Drosophila in males more than in females. Behav. Genet. 41, 754–767. (doi:10.10007/s10519-011-9470-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S-P, Guo W-Y, Muhammad SA, Chen RR, Mu L-L, Li G-Q. 2014. Mating experience and food deprivation modulate odor preference and dispersal in Drosophila melanogaster males. J. Insect Sci. 14, 131 (doi:10.1093/jis/14.1.131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imhof M, Harr B, Gottfried B, Schlötterer C. 1998. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 7, 915–917. (doi:10.1046/j.1365-294x.1998.00382.x) [DOI] [PubMed] [Google Scholar]
- 16.Lizé A, McKay R, Lewis Z. 2013. Gut microbiota and kin recognition. Trends Ecol. Evol. 28, 325–326. (doi:10.1016/j.tree.2012.10.013) [DOI] [PubMed] [Google Scholar]
- 17.Hollis B, Kawecki TJ, Keller L. 2015. No evidence that within-group relatedness reduces harm to females in Drosophila. Ecol. Evol. 5, 979–983. (doi:10.1002/ece3.1417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose MR, Charlesworth B. 1981. Genetics of life history in Drosophila melanogaster. I. Sib analysis of adult females. Genetics 97, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose MR. 1984. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38, 1004–1010. (doi:10.2307/2408434) [DOI] [PubMed] [Google Scholar]
- 20.Mallet MA, Chippindale AK. 2011. Inbreeding reveals stronger net selection on Drosophila melanogaster males: implications for mutation load and the fitness of sexual females. Heredity 106, 994–1002. (doi:10.1038/hdy.2010.148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rose MR, Vu LN, Park SU, Graves JL. 1992. Selection on stress resistance increases longevity in Drosophila melanogaster. Exp. Gerontol. 27, 241–250. (doi:10.1016/0531-5565(92)90048-5) [DOI] [PubMed] [Google Scholar]
- 22.Wigby S, Chapman T. 2004. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution 58, 1028–1037. (doi:10.1111/j.0014-3820.2004.tb00436.x) [DOI] [PubMed] [Google Scholar]
- 23.R Core Team 2015. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 24.Phelan JP, Archer MA, Beckman KA, Chippindale AK, Nusbaum TJ, Rose MR. 2003. Breakdown in correlations during laboratory evolution. I. Comparative analyses of Drosophila populations. Evolution 57, 527–535. (doi:10.1111/j.0014-3820.2003.tb01544.x) [DOI] [PubMed] [Google Scholar]
- 25.Chippindale AK, Leroi AM, Kim SB, Rose MR. 1993. Phenotypic plasticity and selection in Drosophila life history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biol. 6, 171–193. (doi:10.1046/j.1420-9101.1993.6020171.x) [Google Scholar]
- 26.Partridge L, Green A, Fowler K. 1987. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 33, 745–749. (doi:10.1016/0022-1910(87)90060-6) [Google Scholar]
- 27.Wu CI, Hollocher H, Begun DJ, Aquadro CF, Wu MH. 1995. Sexual isolation in Drosophila melanogaster: a possible case of incipient speciation. Proc. Natl Acad. Sci. USA 92, 2519–2523. (doi:10.1073/pnas.92.7.2519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupp JJ, et al. 2008. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383. (doi:10.1016/j.cub.2008.07.089) [DOI] [PubMed] [Google Scholar]
- 29.Billeter J-C, Jagadeesh S, Stepek N, Azanchi R, Levine JD. 2012. Drosophila melanogaster females change behaviour and offspring production based on social context. Proc. R. Soc. B 279, 2417–2425. (doi:10.1098/rspb.2011.2676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein RA, et al. 2014. Investigating variation in replicability: a ‘many labs’ replication project. Social Psychol. 45, 142–152. (doi:10.1027/1864-9335/a000178) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the raw data analysed in this paper are publicly available from the Dryad digital repository at http://datadryad.org/resource/doi:10.5061/dryad.q6ds5; all analyses and R code are provided in the electronic supplementary material.


