Abstract
Ecologically important traits do not evolve without limits. Instead, evolution is constrained by the set of available and viable phenotypes. In particular, natural selection may only favour a narrow range of adaptive optima constrained within selective regimes. Here, I integrate data with theory to test whether selection explains phenotypic constraint. A global database of 599 plant species from 94 families shows that stomatal ratio, a trait affecting photosynthesis and defence against pathogens, is highly constrained. Most plants have their stomata on the lower leaf surface (hypostomy), but species with half their stomata on each surface (amphistomy) form a distinct mode in the trait distribution. A model based on a trade-off between maximizing photosynthesis and a fitness cost of upper stomata predicts a limited number of adaptive solutions, leading to a multimodal trait distribution. Phylogenetic comparisons show that amphistomy is the most common among fast-growing species, supporting the view that CO2 diffusion is under strong selection. These results indicate that selective optima stay within a relatively stable set of selective regimes over macroevolutionary time.
Keywords: selective regime, adaptive landscape, phenotypic constraint, stomata, amphistomy, phylogenetic comparative methods
1. Introduction
What determines the major features of phenotypic evolution [1,2]? When selection predominates, the topography of the macroevolutionary adaptive landscape—a ‘map’ of phenotype to fitness—determines the broad patterns of life's diversity [3]. In particular, a limited number of adaptive solutions corresponding to distinct selective regimes should result in convergent evolution of similar phenotypes across independent evolutionary lineages. In such cases, surveys across species should reveal a multimodal trait distribution in which the modes point to the underlying selective regimes. Multimodality has been observed frequently among plants and animals, including traits such as self-incompatibility [4], the precocial–altricial spectrum [5], pollination syndromes [6], ecomorphology in Anolis [7] and plant height [8]. That such disparate classes of traits show broadly similar patterns suggests that partially discrete selective regimes may be a general feature of macroevolution. However, we rarely know whether multimodality reflects constraints imposed by selection and not some other constraint on phenotypic evolution.
In particular, certain phenotypes may be common not because they are more fit, but rather because they are genetically, developmentally or functionally accessible. Conversely, rare phenotypes might be inaccessible. Since the term ‘constraint’ is not used consistently in biology, I specifically follow the definitions given by Arnold [9]: genetic constraints are limitations set by the ‘pattern of genetic variation and covariation for a set of traits' [p. S86]; developmental constraints are limitations on ‘possible developmental states' [p. S95] and functional constraints are imposed by ‘time, energy or the laws of physics' [p. S97]. Arnold contrasts these with selective constraints determined by the adaptive landscape. There are examples of genetic [10], developmental [11] and functional [12] constraints on phenotypic evolution acting in nature, meaning that we cannot assume selection alone shapes trait evolution. Compelling evidence from cross-species comparisons that selection constrains phenotypic evolution requires showing that phenotypic evolution is constrained, that selection is sufficient to explain the inferred constraint and that non-selective constraints are inconsistent with these observations.
Here, I evaluate evidence for selective constraints on a functionally important plant trait, stomatal ratio (SR), using comparative methods and theory. SR, defined as the ratio of upper to lower stomatal density, impacts how plants assimilate CO2 from the atmosphere via photosynthesis. Physiological experiments and biophysical theory demonstrate that amphistomatous leaves, those that have equal stomatal densities on both upper and lower surfaces, maximize photosynthetic rate by minimizing the distance between substomatal cavities and chloroplasts, facilitating rapid CO2 diffusion [13–16]. Hence, nearly all plants should be amphistomatous to maximize photosynthesis, yet paradoxically up to 90% of plant species in some communities are hypostomatous [17–20], meaning that most stomata are on the lower surface. In rare cases, most stomata are on the upper surface (hyperstomy). I use upper and lower rather than abaxial and adaxial, because position relative to the ground, rather than to the stem, best explains patterns across both normal and ‘upside-down’ (i.e. resupinate) leaves. SR is a quantitative metric that describes continuous variation between hypo- and hyperstomy.
Multiple lines of evidence indicate selection on SR, but there is little consensus on the adaptive significance. SR varies widely, but non-randomly [13,17,19,21–23] and evolves rapidly in some taxa, possibly owing to selection [24,25]. Several environmental and anatomical factors have been hypothesized to favour amphistomy (table 1). The mechanistic details and literature underlying these hypotheses and predictions are described in the electronic supplementary material, appendix S1. The preponderance of hypostomy almost certainly reflects a cost of upper stomata. For example, hypostomy has evolved anew in resupinate leaves [31]. Upper stomata might be costly because they increase susceptibility to foliar pathogens (e.g. rust fungi) that infect through stomata [15], suggesting that SR mediates a trade-off between photosynthetic rate and defence [24], but other costs have been proposed (electronic supplementary material, appendix S1). Identifying the selective forces (i.e. fitness benefits and costs) shaping SR have been hampered by four methodological limitations. Namely, previous studies were typically qualitative rather than quantitative, confined to specific geographical regions or clades, did not account for phylogenetic non-independence or did not take into account multiple confounding factors. To overcome these limitations, I assembled a quantitative, global and phylogenetically extensive database that disentangles correlated predictor variables (e.g. light level and leaf thickness).
Table 1.
Adaptive hypotheses and predictions for SR. (The first and second columns indicate the hypothesized ecological factors and the predicted direction of association with amphistomy, respectively. References to key studies are provided, but see the electronic supplementary material, appendix S1 for additional detail.)
This new dataset revealed that SR is a multimodal trait (figure 1), strongly suggesting some form of constraint. In other words, phenotypic values between modes are rare either because they are selected against (selective constraint) or rarely arise (other constraints). To test whether the observed pattern is consistent with constraint, I modified existing evolutionary process models to accommodate proportion traits like SR. Fitting this model to the data demonstrates that SR is highly constrained by multiple selective regimes, where selective regimes are defined as ‘the aggregate of all such environmental and organismic factors that combine to determine how natural selection will act upon character variation’ ([32, p. 4], see also [7,33]). Next, I leveraged knowledge on the relationship between SR, photosynthesis and a cost of upper stomata (most probably decreased resistance to foliar pathogens) to construct a simple cost–benefit model consistent with the underlying physics and a minimum of additional assumptions. This model indicates that distinct selective regimes can result from a simple trade-off between photosynthesis and defence, even when the underlying ecological gradients are smooth. By contrast, a review of the literature does not support a primary role for genetic, developmental and functional constraints. Finally, phylogenetic multiple regression identifies life-history evolution as the primary selective agent underlying shifts in selective regime, but anatomical and climatic factors are also important. By merging theory and data, this study adduces new evidence that selection is the primary constraint on phenotypic evolution and offers a simple mechanistic explanation for a seemingly complex macroevolutionary pattern.
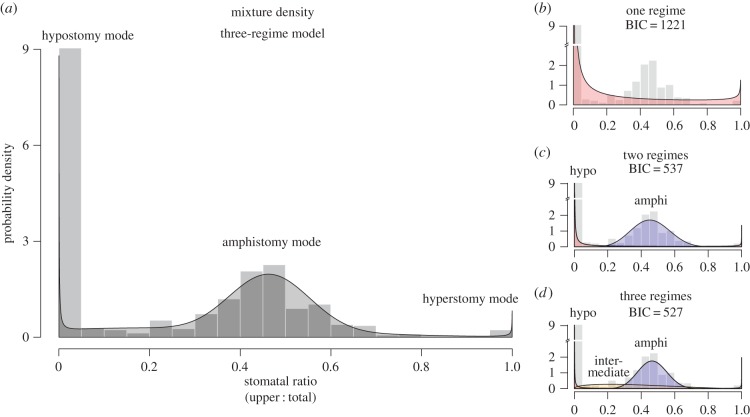
Figure 1.
SR is a multimodal trait. (a) A density histogram of SR across 599 species reveals three noticeable modes. The plurality of species are completely hypostomatous (all stomata on the lower surface). There is a smaller, broader mode of amphistomatous species (approximately equal density of stomata on upper and lower surfaces). Finally, there are a small number of hyperstomatous species (all stomata on the upper surface). A mixture of selective regimes (shaded grey polygon) manifests these three modes, indicating phenotypic constraint. The grey polygon is the mixture density of the maximum-likelihood three-regime model. Panel (d) breaks down the mixture density into three regimes depicted as red, blue and yellow polygons. The three-regime model fitted better (lower Bayesian information criterion (BIC)) than one- and two-regime models (panels (b) and (c), respectively).
2. Material and methods
(a). Assembling a comparative dataset
To test the hypotheses in table 1, I surveyed the literature for trait data on SR and leaf thickness. I compiled a new, global dataset from 25 previously published studies (electronic supplementary material, appendix S2) containing trait data (SR and leaf thickness) on 599 species across 94 plant families. I obtained environmental data (elevation, habitat openness and precipitation) from GIS layers and growth form from online databases or primary literature. The dataset with trait and climate data comprised a 552 species subset covering 90 families. Complete details on how the dataset was assembled are available in the electronic supplementary material, appendix S2.
(b). Evolutionary process model: connecting multimodality to phenotypic constraint
I inferred constraint by comparing the fit of evolutionary process models with and without constraint. The phenotypic optimum under an unconstrained model evolves stochastically without limits other than the fact that proportions are bounded between 0 and 1. By contrast, the optimum under the constrained model varies within a limited range of values around a long-run average or ‘optimum’. I developed a new evolutionary process model that extends the Ornstein–Uhlenbeck (OU) model for proportion traits like SR that are bounded by 0 and 1. In general, OU models infer phenotypic constraint around a long-run average [7,33–35], but the standard OU model assumes traits are normally distributed. The new method, based on an underlying process model derived in the electronic supplementary material, appendix S3, assumes that proportion traits are beta-distributed at stationarity (see Results). While the beta distribution fits proportion data better than a normal distribution, it is not yet possible to account for phylogenetic non-independence with this method, unlike standard OU methods. Therefore, I tested whether species rapidly approach stationarity, as assumed in non-phylogenetic approaches, using standard OU methods to estimate phylogenetic signal relative to the transition rate between regimes (the next paragraph describes how regimes were inferred). I used Simmap [36] in the R package phytools version 0.4-56 [37] to estimate transition rates between regimes and fit standard OU models to the data using the R package OUwie version 1.45 [38] (see the electronic supplementary material, appendix S4 for complete detail). Hereafter I refer to methods using the new OU model for proportional traits as ‘OUbeta’ and methods using the standard OU model as ‘OUnorm’. The limitations of each approach complement each other: OUbeta uses a good distribution for proportion traits, but is non-phylogenetic; OUnorm uses a normal distribution, but accounts for phylogeny.
Multimodality suggests the presence of multiple regimes, selective or non-selective, associated with different modes. I used finite mixture models to infer the number of regimes constraining SR evolution under the OUbeta model (see [8] for a similar approach). That is, I assume the distribution of trait values across species can be represented as a mixture of multiple regimes at stationarity, each of which is modelled as a beta-distributed variable. To fit models, I used an expectation–maximization algorithm to find the maximum-likelihood mixture model (electronic supplementary material, appendix S5). I selected the best model using the conservative Bayesian information criterion (BIC). I accepted models with an additional regime if they decreased BIC by two or more. I also tested for multiple regimes within families where there were sufficient data (n ≥ 15). Ten families met this criterion. For each family, I compared the fit of mixtures with k = 1,2 or 3 regimes using BIC. Further, I rejected additional regimes supported by BIC if one of those regimes contained fewer than three species (this affected Poaceae and Salicaceae). If multiple similar regimes are found repeatedly in disparate families, this provides compelling evidence for convergent evolution because of phenotypic constraints imposed by a shared set of regimes.
(c). Cost–benefit model: can a photosynthesis–defence trade-off account for constraint?
I used theory to ask whether multiple selective regimes could be an emergent property of more fundamental fitness costs and benefits associated with SR. Full details of the model are given in the electronic supplementary material, appendix S6. The model assumes a fitness benefit (denoted Bmax) and cost (denoted Cmax) of upper stomata. Based on biophysical theory, having stomata on both surfaces (holding total stomatal density constant) increases photosynthetic rate. The model does not specify a particular fitness cost of upper stomata—in fact, there could be multiple costs—but recent evidence suggests that infection by foliar pathogens may be the main cost (electronic supplementary material, appendix S1 discusses the fitness benefits and costs associated with SR). For a given benefit : cost ratio and shape of the benefit function (denoted σ2), I solved for the SR that optimizes fitness, which I denote as Sfit. The model therefore generates fitness landscapes that relate some environmental gradient, through variation in fitness benefits and costs, to the fitness of different SR phenotypes. Next, I examined whether such landscapes generate a trait distribution that qualitatively resembles the data, even when the underlying environmental gradients are smooth. I specifically focused on the pattern observed within families, where there was generally one mode of amphistomatous species and another mode of hypostomatous species (hyperstomatous in the case of Poaceae). I generated hypothetical trait distributions by assuming that the benefit : cost ratio varies smoothly and using different shapes of the benefit function (see the electronic supplementary material, appendix S6).
(d). Testing adaptive hypotheses for stomatal ratio using phylogenetic regression
I tested whether SR varies as a function of leaf thickness, mean annual precipitation, elevation, leaf area index (a proxy for habitat openness) and growth form using type 2 phylogenetic ANOVA with both categorical (growth form) and continuous (e.g. leaf thickness) predictor variables. I accounted for phylogeny using a Phylomatic [39] megatree for this relatively large and phylogenetically extensive dataset. Complete details on phylogenetic regression are in the electronic supplementary material, appendix S2.
3. Results
(a). Stomatal ratio evolution is constrained by multiple selective regimes
The most striking feature of the data is that SR is highly multimodal (figure 1), with apparent modes at 0 (hypostomatous), ≈0.5 (amphistomatous) and 1 (hyperstomatous). The distribution of SR values across species is consistent with an evolutionary process model that includes constraints imposed by multiple selective regimes. To infer regimes, I augmented the standard OU model for proportion traits (see the electronic supplementary material, appendix S3), which shows that the stationary distribution of SR (or any proportion trait) r follows a beta distribution:
| 3.1 |
where B(·) refers to the beta function. Under the OUbeta model, a selective regime at stationarity is characterized by two parameters, a long-run average in the adaptive landscape, θ, and a precision, ϕ, around the average.
If there are a limited number of partially distinct adaptive solutions in the world, then a model with multiple selective regimes should fit the data better than a model with a single regime [7,33]. Using finite mixture model analysis (electronic supplementary material, appendix S5), I inferred three selective regimes (electronic supplementary material, table S1), but note that the mapping between modes and regimes is not always one-to-one. In particular, one regime produces modes at both 0 and 1 (figure 1d). Nevertheless, the data clearly supports the large number of hypostomatous (SR = 0) species as a distinct mode. There was also strong support for an amphistomatous regime (compare figure 1b to 1c). Finally, the best-supported model also included a small mode for hyperstomatous species and a separate, smaller regime for species intermediate between hypo- and amphistomy (figure 1d).
The OUbeta method assumes that the trait distribution rapidly approaches its stationary distribution. OUnorm methods, which assume normally distributed traits but account for phylogeny, suggest that hypo- and amphistomatous regimes are close to stationarity (electronic supplementary material, appendix S4). Most species have spent much longer in their present-day regime (median of 45–225 Myr depending on regime) than the phylogenetic half-life (22 Myr), suggesting that the trait distribution should be close to stationarity. However, I caution that transition rates are rough estimates because of sparse taxonomic sampling and failure to account for different diversification rates associated with each regime [40].
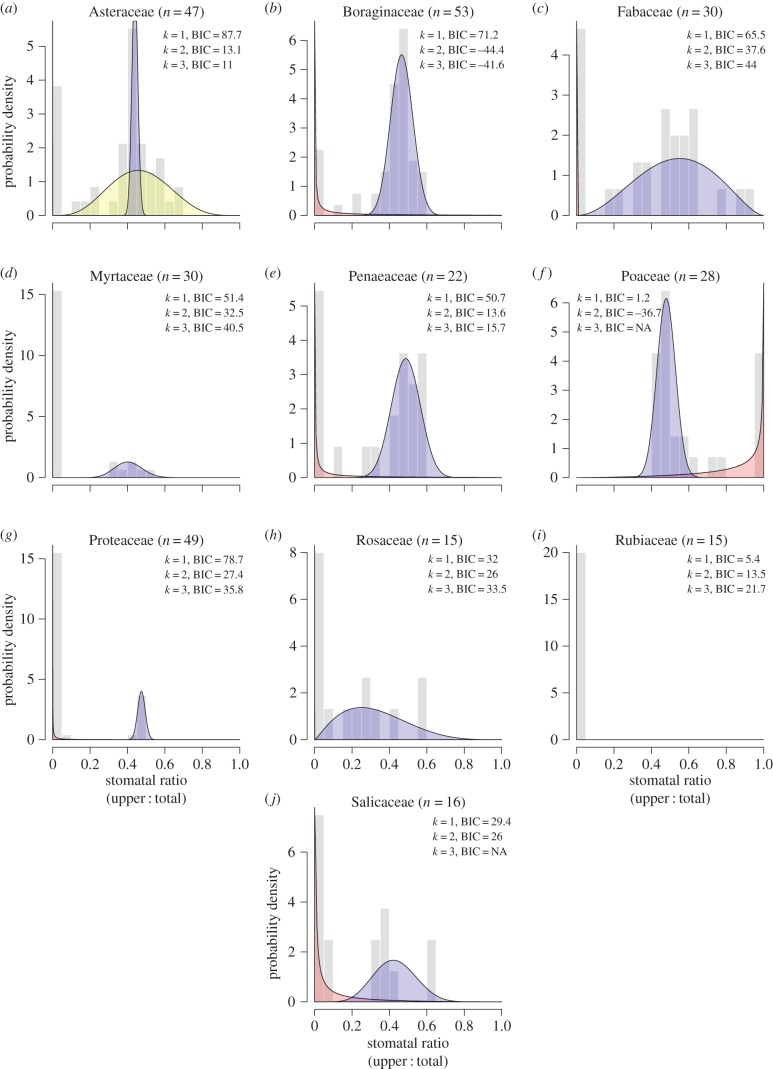
The same general pattern seen at the global scale—multiple selective regimes leading to distinct modes—is recapitulated within nine of 10 families best-represented in the global dataset (figure 2). Additional detail is given in the electronic supplementary material, appendix S8. Species from the one exception to this pattern (Rubiaceae) were mostly woody (i.e. more likely to be hypostomatous, see below). Among herbaceous Rubiaceae, there are some amphistomatous species [41], suggesting a broader sample may evince multiple modes. In summary, the apparent pattern of constraint on SR is strikingly similar across multiple disparate families and at a global scale, suggesting convergent evolution because of shared phenotypic constraint.
Figure 2.
Repeated evolution of multimodality suggests that selective regimes are conserved across land plants. Shaded polygons of inferred regimes are plotted atop a histogram of SR from a given plant family (grey bars). Note that some distributions are very narrow spikes near the origin. n is the sample size for each family. (a) Three regimes were inferred for Asteraceae; (b–j) two regimes were inferred for other families except the Rubiaceae. The number of regimes was inferred from information theoretic (BIC) comparisons of finite mixture models with beta-distributed components (k is the number of components). Lower BIC indicates better fit. In Poaceae and Salicaeae, I rejected models with k = 3 because some components had very low membership.
(b). Selection is sufficient to accommodate constraint
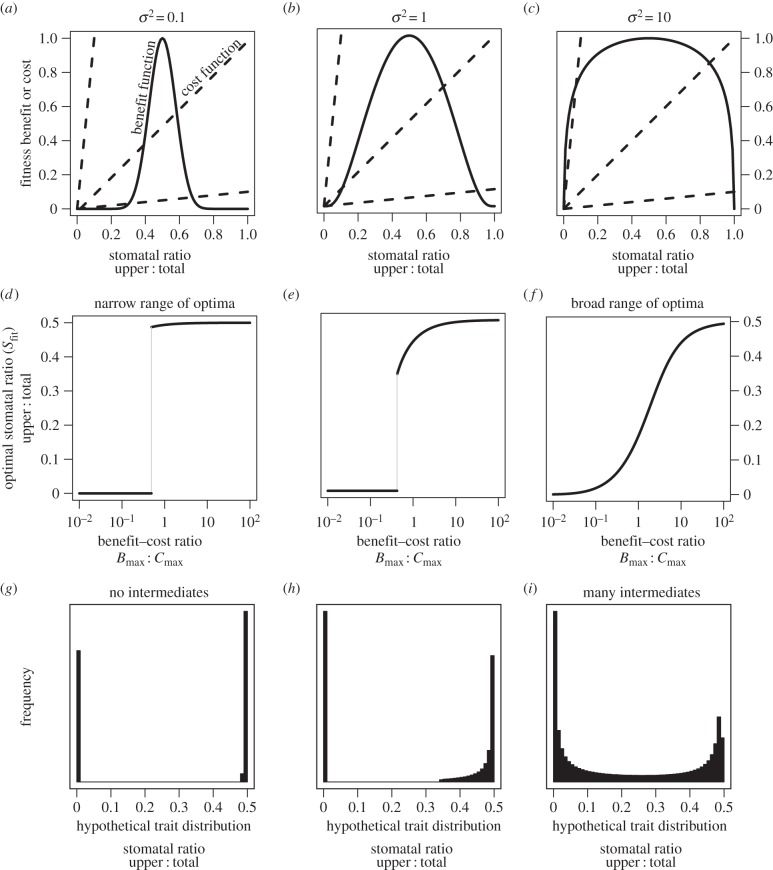
I analysed a cost–benefit model of SR to ask whether selection is sufficient to account for apparent phenotypic constraint. Not surprisingly, selection favours greater SR (Sfit) as the fitness benefit of greater photosynthesis increases relative to the cost of upper stomata (figure 3a–c), but the shape of the function is highly sensitive to one parameter in the model, the shape of the benefit function σ2. In particular, there are a broad array of adaptive optima when σ2 is high, but a much narrower range when σ2 is low (figure 3d–f). When the range of optima is broad, intermediate phenotypes between complete hypostomy and amphistomy are best when the benefit : cost ratio itself is intermediate. By contrast, when the range of adaptive optima is narrow, intermediates are universally less fit than either of the boundary phenotypes. As the benefit : cost ratio decreases, there is a sudden shift from amphistomy being favoured to hypostomy being favoured. The dearth of species with intermediate SR in nature, especially within families, therefore suggests either a narrow range of adaptive optima or that there are a broad range of adaptive optima, but that intermediate environments are rare. Numerical simulations based on smooth variation in the benefit : cost ratio indicate that the simple, yet realistic assumptions of this model are sufficient to account for qualitatively different selective regimes and generate patterns of multimodality similar to those seen in nature (figure 3g–h).
Figure 3.
Selection is sufficient to explain why intermediate phenotypes are rare. (a–c) In each panel, a benefit function (solid line, see the electronic supplementary material, equation S33) is shown with three different cost functions (dashed line, see electronic supplementary material, equation S35). In all panels, Bmax is fixed at 1 and three slopes of the cost function, Cmax are illustrated: 0.1, 1 and 10. The fitness benefit is always maximized when SR is 0.5 (amphistomy). The shape factor σ2 changes the benefit function from bell-shaped in a to an inverted-U shape in (c). Panels (d–f) show that the shape of the benefit function affects the range of optima permitted by the adaptive landscapes. Solid lines are the SR that optimizes fitness (Sfit) as a function of the benefit : cost ratio (Bmax : Cmax). The transition between amphistomy being favoured over hypostomy can be abrupt (d) or gradual (f), leading to a narrow or broad range of adaptive optima. The light grey line indicates the range of universally unfit phenotypes. Panels (g–i) show hypothetical trait distributions assuming that the benefit : cost ratio varies uniformly from 10−2 to 102. Histograms were generated by solving for Sfit with 104 evenly spaced values of Bmax : Cmax. Note that the trait values range from hypostomatous to amphistomatous, but a mirror image distribution with hyperstomatous species would be seen if fitness costs accrued to lower stomata.
(c). Growth form, leaf thickness and precipitation shape stomatal ratio evolution
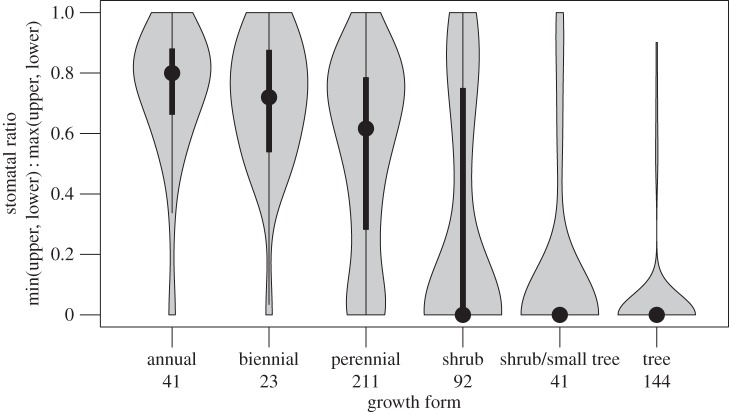
If SR is strongly associated with other traits or climatic factors, especially if there are compelling a priori hypotheses (table 1) supporting such associations, then it suggests that trait variation is shaped by selection. Phylogenetic multiple regression identified growth form and, to a lesser extent, leaf thickness and precipitation as the best predictors of SR (electronic supplementary material, table S2). Amphistomy is strongly associated with fast growth forms (herbaceous plants), whereas hypostomy is most common in slower growing shrubs and trees (figure 4). As predicted by biophysical theory [13,15], thicker leaves also tend to be amphistomatous, although the correlation is weak (electronic supplementary material, figure S1a). Finally, amphistomy is more common in dry environments, whereas hypo/hyperstomy is associated with higher precipitation (electronic supplementary material, figure S1b). Elevation and leaf area index, a proxy for open habitat of leaves below the canopy, were not significantly associated with SR in this dataset (electronic supplementary material, table S2). In single regressions, amphistomy is more common in open environments, as in previous studies [14,20,21,23], but this correlation is not significant after precipitation is factored into multiple regression (precipitation and leaf area index are positively correlated).
Figure 4.
Association between amphistomy and fast growth forms points to selection on life-history shaping SR evolution. The violin plot shows SR as a function of growth form. The width of the grey polygons indicates the density of data. Length of grey polygon indicates the range of the data; the point indicates the median; the thick lines indicate the 0.25 and 0.75 quantiles. Sample sizes per growth form in the dataset are given below the label.
4. Discussion
This study posits that multimodal traits reveal the presence of distinct selective regimes. Hence, the prevalence of certain phenotypes and the dearth of others directly reflects selective constraints on phenotypic evolution. Evidence from a new, global dataset clearly shows that SR is a multimodal trait (figure 1) and that multimodality has evolved repeatedly in plants (figure 2). These patterns are difficult to reconcile with models omitting constraint, but are consistent with multiple selective regimes (electronic supplementary material, table S1). This means that although the selective optimum experienced by a given species varies over macroevolutionary time, the range of optima is highly constrained by ecological factors. In particular, the cost–benefit model shows that a trade-off between photosynthesis and defence against pathogens is sufficient to generate multiple selective regimes. Shifts from one regime to another (i.e. hypo- to amphistomy or vice versa) appears to be primarily driven by growth form, suggesting that the fitness benefit of amphistomy—faster diffusion of CO2 to chloroplasts—is greatest in species with ‘fast’ life histories.
(a). Multimodality implies constraint on the macroevolutionary adaptive landscape
Multimodality (figure 1) cannot be explained by an evolutionary process model neglecting constraint. However, apparent clustering could occur by systematic underrepresentation of intermediate trait values [42] or non-random taxon sampling. It is highly improbable that the data are biased against intermediate phenotypes because the studies used in the dataset generally have no a priori hypothesis about SR in their focal species. Any bias is likely in the opposite direction because I omitted many studies that report only qualitative data, which is most probable when species are completely hypo- or amphistomatous. Non-random taxon sampling could also give the appearance of multimodality. To give an extreme example, if there had been a single transition from hypo- to amphistomy followed by stasis, then sampling the tips of the phylogeny would produce a multimodal pattern with apparently strong statistical support, even though it only represents a single evolutionary event. The fact that multimodality reappears in multiple distantly related families (figure 2) makes non-random taxon sampling alone an unlikely explanation, though it might accentuate the pattern. Future work is needed to extend regime-inference methods [33,43,44] to non-Gaussian traits, as this study begins to do with a new evolutionary process model for proportion traits.
(b). Selection is the most probable explanation for phenotypic constraint
In principle, constraint could reflect a mix of selective, genetic, developmental and functional factors [9]. However, there is little evidence for non-selective constraints on SR, whereas selection explains the precise position of modes in the trait distribution and the association with growth form. Although the molecular mechanisms have not been identified, plants exhibit the ability to fine-tune SR through independent control of upper and lower stomatal densities: loci capable of generating intermediate SR phenotypes occur spontaneously during mutagenesis [45], segregate among natural populations [24,46,47] and are fixed between closely related species [25,48]. That such intermediate phenotypes are rare in nature implicates selective constraint. However, I cannot rule out that a tight link between epidermal cell fate and ab-adaxial polarity established early in leaf development could reinforce the preponderance of hypostomy. Even if hypostomy was initially favoured by selection, epidermes with (without) stomata could become tightly linked to abaxial (adaxial) identity over time. If this developmental constraint were important, then transitions from hypo- to amphistomy should be rare. Future studies with dense taxonomic sampling and well-resolved phylogenies should be able to test this prediction.
In contrast to non-selective forces, the cost–benefit model shows that with a small number of realistic, evidence-based assumptions, selection is sufficient to accommodate the data. First, selection predicts not only multimodality, but the location of the dominant modes near 0 and 0.5, a pattern that is hard to reconcile with non-selective constraints. Stomata are often distributed equally on both surfaces (amphistomy), because this arrangement maximizes photosynthetic rate even in leaves with a highly differentiated palisade and spongy mesophyll [13,15,49]. More often, all stomata are on the lower surface because the costs of upper stomata outweigh the benefits. A dearth of intermediates between hypo- and amphistomy occurs when these phenotypes often fall in a fitness valley or intermediate environments are rare. However, the best mixture model includes a small peak of intermediates (electronic supplementary material, table S1; figure 1d), suggesting that some environments support a broader array of adaptive optima. However, the fact that most species, especially within families (figure 2), cluster around particular modes suggests constraint around a narrow range of optima.
(c). Life history, more than anatomy and climate, determines stomatal ratio
Selection also best explains non-random association between SR, growth form and climate. In particular, growth form is strongly associated with SR (figure 4), as suggested by earlier ecological surveys [17,30]. Two hypotheses that might explain this relationship are: (i) herbaceous plants have shorter leaf lifespans [50], requiring higher photosynthetic rates to pay their construction costs in a shorter time [51]; and (ii) herbaceous plants have faster life histories, leading to stronger selection on high growth rates, mediated in part by higher leaf-level photosynthetic rate [52]. That the relationship between SR and whole-plant lifespan holds within herbaceous (annuals versus perennials) species, supports the second hypothesis (selection on faster life history favours amphistomy). If correct, this hypothesis implies remarkably strong selection on leaf-level photosynthesis, especially the rate of leaf CO2 diffusion, in herbs. Stomatal conductance, which is determined by stomatal aperture and density, is one major component of total leaf CO2 conductance, which also includes internal factors affected by leaf anatomy [53]. For hypostomatous leaves, increasing SR can increase total leaf diffusive conductance more than adding stomata to the lower surface, as long as there is some internal resistance to CO2 [16]. Hence, selection for increased photosynthesis in herbs should select for some combination of increased stomatal size/density and ratio.
Surprisingly, there was little evidence supporting the most common adaptive explanation for amphistomy that thicker leaves select for stomata on both sides to facilitate CO2 diffusion [13]. In actuality, support for this hypothesis is mixed (electronic supplementary material, appendix S1), because the trend is weak (electronic supplementary material, figure S1a). Less powerful studies could easily have failed to detect a significant relationship. I also found that amphistomy, perhaps counterintuitively, was more common in plants from low precipitation environments (electronic supplementary material, figure S1b), as predicted (electronic supplementary material, appendix S1). Although low precipitation was correlated with leaf area index (a proxy for habitat openness), multiple phylogenetic regression indicated that precipitation was causal, in contrast to previous studies [20,23]. This is especially surprising given association between SR and fast growth form. CO2 limits photosynthesis most under the high light of an open canopy, implying that fast growth, high light and amphistomy should co-occur, but there was no evidence for this association. The discrepancy between studies could reflect different methods—plant-level descriptions of light environment (previous studies) versus coarser, remotely sensed measurements of canopy cover (this study)—or that previous patterns were restricted to particular families. I was unable to test the effects of leaf orientation and stomatal packing on SR, although these are likely to be important factors [22,54]. The evidence from this and previous studies shows that SR is an ecologically relevant functional trait that could be valuable in physiological ecological and evolution [55].
That many ecologically important traits, like SR, cluster around particular values but not others suggests pervasive constraint on phenotypic evolution. The emerging evidence from this and other recent studies on SR (see especially [24]) is that peaks of high fitness are constrained by a trade-off between photosynthetic rate and defence against foliar pathogens that preferentially infect through upper stomata. In particular, the cost–benefit model analysed here predicts that even a small change in the fitness costs or benefits are sufficient to shift trait evolution into qualitatively different selective regimes. If it is generally true that multimodal traits are associated with rapid regime shifts, then one way forward is to look for signatures of such shifts in closely related species that sit astride different regimes. For example, Muir et al. [25] recently identified two large effect loci that together account for the phenotypic difference between hypostomatous and amphistomatous species, perhaps suggesting that these loci enabled a regime shift. Integrating comparative biology, mechanistic studies of organismal function and the genetics of adaptation points to a general approach for evaluating the common features of macroevolutionary adaptive landscapes and, hence, the role of selection in constraining phenotypic evolution.
Supplementary Material
Acknowledgements
Ranessa Cooper, Jenny Read, Gregory Jordan and Tim Brodribb generously made data available. I thank two anonymous reviewers, Tim Brodribb, Lawren Sack, William Smith, Sally Otto and members of the Angert, Otto, Schluter and Whitlock laboratories for their feedback on earlier versions of this manuscript.
Data accessibility
All data are accessible from the Dryad digital repository, http://dx.doi.org/10.5061/dryad.rj7g1.
Competing interests
I have no competing interests.
Funding
I am supported by a Biodiversity Postdoctoral Fellowship funded by NSERC-CREATE.
References
- 1.Uyeda JC, Hansen TF, Arnold SJ, Pienaar J. 2011. The million-year wait for macroevolutionary bursts. Proc. Natl Acad. Sci. USA 108, 15 908–15 913. ( 10.1073/pnas.1014503108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold SJ. 2014. Phenotypic evolution: the ongoing synthesis. Am. Nat. 183, 729–746. ( 10.1086/675304) [DOI] [PubMed] [Google Scholar]
- 3.Simpson GG. 1944. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 4.Raduski AR, Haney EB, Igić B. 2012. The expression of self-incompatibility in angiosperms is bimodal. Evolution 66, 1275–1283. ( 10.1111/j.1558-5646.2011.01505.x) [DOI] [PubMed] [Google Scholar]
- 5.Martin R, MacLarnon A. 1985. Gestation period, neonatal size and maternal investment in placental mammals. Nature 313, 220–223. ( 10.1038/313220a0) [DOI] [Google Scholar]
- 6.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403. ( 10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 7.Mahler DL, Ingram T, Revell LJ, Losos JB. 2013. Exceptional convergence on the macroevolutionary landscape in island lizard radiations. Science 341, 292–295. ( 10.1126/science.1232392) [DOI] [PubMed] [Google Scholar]
- 8.Scheffer M, Vergnon R, Cornelissen JHC, Hantson S, Holmgren M, van Nes EH, Xu C. 2014. Why trees and shrubs but rarely trubs? Trends Ecol. Evol. 29, 433–434. ( 10.1016/j.tree.2014.06.001) [DOI] [PubMed] [Google Scholar]
- 9.Arnold SJ. 1992. Constraints on phenotypic evolution. Am. Nat. 140, S85–S107. ( 10.1086/285398) [DOI] [PubMed] [Google Scholar]
- 10.Barton N, Partridge L. 2000. Limits to natural selection. BioEssays 22, 1075–1084. () [DOI] [PubMed] [Google Scholar]
- 11.Gould SJ. 2002. The structure of evolutionary theory. Cambridge, MA: Harvard University Press. [Google Scholar]
- 12.Kimball S, Gremer JR, Huxman TE, Venable DL, Angert AL. 2013. Phenotypic selection favors missing trait combinations in coexisting annual plants. Am. Nat. 182, 191–207. ( 10.1086/671058) [DOI] [PubMed] [Google Scholar]
- 13.Parkhurst DF. 1978. The adaptive significance of stomatal occurrence on one or both surfaces of leaves. J. Ecol. 66, 367–383. ( 10.2307/2259142) [DOI] [Google Scholar]
- 14.Mott KA, Gibson AC, O'Leary JW. 1984. The adaptive significance of amphistomatic leaves. Plant Cell Environ. 5, 455–460. ( 10.1111/1365-3040.ep11611750) [DOI] [Google Scholar]
- 15.Gutschick VP. 1984. Photosynthesis model for C3 leaves incorporating CO2 transport, propagation of radiation, and biochemistry 2. Ecological and agricultural utility. Photosynthetica 18, 569–595. [Google Scholar]
- 16.Parkhurst DF, Mott KA. 1990. Intercellular diffusion limits to CO2 uptake in leaves studied in air and helox. Plant Physiol. 94, 1024–1032. ( 10.1104/pp.94.3.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salisbury E. 1927. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Phil. Trans. R. Soc. Lond. B 216, 1–65. ( 10.1098/rstb.1928.0001) [DOI] [Google Scholar]
- 18.Leick E. 1927. Untersuchungen über den Einfluß des Lichtes auf die öffnungsweite unterseitiger und oberseitiger Stomata desselben Blattes. Jahrbücher für Wissenschaftliche Botanik 67, 771–848. [Google Scholar]
- 19.Wood JG. 1934. The physiology of xerophytism in Australian plants: the stomatal frequencies, transpiration and osmotic pressures of sclerophyll and tomentose-succulent leaved plants. J. Ecol. 22, 69–87. ( 10.2307/2256096) [DOI] [Google Scholar]
- 20.Fitter A, Peat H. 1994. The ecological flora database. J. Ecol. 82, 415–425. ( 10.2307/2261309) [DOI] [Google Scholar]
- 21.Gibson AC. 1996. Structure-function relations of warm desert plants. Berlin, Germany: Springer. [Google Scholar]
- 22.Smith WK, Bell DT, Shepherd KA. 1998. Associations between leaf structure, orientation, and sunlight exposure in five Western Australian communities. Am. J. Bot. 85, 51–63. [PubMed] [Google Scholar]
- 23.Jordan GJ, Carpenter RJ, Brodribb TJ. 2014. Using fossil leaves as evidence for open vegetation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 395, 168–175. ( 10.1016/j.palaeo.2013.12.035) [DOI] [Google Scholar]
- 24.McKown AD, et al. 2014. Association genetics, geography and ecophysiology link stomatal patterning in Populus trichocarpa with carbon gain and disease resistance trade-offs. Mol. Ecol. 23, 5771–5790. ( 10.1111/mec.12969) [DOI] [PubMed] [Google Scholar]
- 25.Muir CD, Pease JB, Moyle LC. 2014. Quantitative genetic analysis indicates natural selection on leaf phenotypes across wild tomato species (Solanum sect. Lycopersicon; Solanaceae). Genetics 198, 1629–1643. ( 10.1534/genetics.114.169276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohr PL. 1919. Untersuchungen über die Blattantomie von Alphen- und Ebenenpflanzen. PhD thesis, Universität Basel, Basel, Switzerland. [Google Scholar]
- 27.Spinner H. 1936. Stomates et altitude. Berichte der Schweizerischen Botanischen Gesellschaft 46, 12–27. [Google Scholar]
- 28.Woodward FI. 1986. Ecophysiological studies on the shrub Vaccinium myrtillus l. taken from a wide altitudinal range. Oecologia 70, 580–586. ( 10.1007/BF00379908) [DOI] [PubMed] [Google Scholar]
- 29.Körner C, Neumayer M, Menendez-Riedl SP, Smeets-Scheel A. 1989. Functional morphology of mountain plants. Flora 182, 353–383. [Google Scholar]
- 30.Peat H, Fitter A. 1994. Comparative analyses of ecological characteristics of British angiosperms. Biol. Rev. 69, 95–115. ( 10.1111/j.1469-185X.1994.tb01487.x) [DOI] [Google Scholar]
- 31.Lyshede OB. 2002. Comparative and functional leaf anatomy of selected Alstroemeriaceae of mainly Chilean origin. Bot. J. Linnean Soc. 140, 261–272. ( 10.1046/j.1095-8339.2002.00091.x) [DOI] [Google Scholar]
- 32.Baum DA, Larson A. 1991. Adaptation reviewed: a phylogenetic methodology for studying character macroevolution. Syst. Biol. 40, 1–18. ( 10.1093/sysbio/40.1.1) [DOI] [Google Scholar]
- 33.Butler M, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 164, 683–695. ( 10.1086/426002) [DOI] [PubMed] [Google Scholar]
- 34.Hansen TF. 1997. Stabilizing selection and the comparative analysis of adaptation. Evolution 51, 1341–1351. ( 10.2307/2411186) [DOI] [PubMed] [Google Scholar]
- 35.Muir CD, Thomas-Huebner M. In press Constraint around quarter-power allometric scaling in wild tomatoes (Solanum sect. Lycopersicon; Solanaceae). Am. Nat. 186 ( 10.1086/682409) [DOI] [PubMed] [Google Scholar]
- 36.Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Syst. Biol. 52, 131–158. ( 10.1080/10635150390192780) [DOI] [PubMed] [Google Scholar]
- 37.Revell LJ. 2012. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 38.Beaulieu JM, Jhwueng D-C, Boettiger C, O'Meara BC. 2012. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383. ( 10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 39.Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183. ( 10.1111/j.1471-8286.2004.00829.x) [DOI] [Google Scholar]
- 40.Maddison WP. 2006. Confounding asymmetries in evolutionary diversification and character change. Evolution 60, 1743–1746. ( 10.1111/j.0014-3820.2006.tb00517.x) [DOI] [PubMed] [Google Scholar]
- 41.Holm T. 1907. Rubiaceae: Anatomical studies of North American representatives of Cephalanthus, Oldenlandia, Houstonia, Mitchella, Diodia, and Galium. Bot. Gazette 43, 153–186. ( 10.1086/329140) [DOI] [Google Scholar]
- 42.McGlone MS, Richardson SJ, Jordan GJ, Perry GL. 2015. Is there a ‘suboptimal’ woody species height? A response to Scheffer et al. Trends Ecol. Evol. 30, 4–5. ( 10.1016/j.tree.2014.09.007) [DOI] [PubMed] [Google Scholar]
- 43.Ingram T, Mahler DL. 2013. SURFACE: detecting convergent evolution from comparative data by fitting Ornstein–Uhlenbeck models with stepwise Akaike information criterion. Methods Ecol. Evol. 4, 416–425. ( 10.1111/2041-210X.12034) [DOI] [Google Scholar]
- 44.Uyeda JC, Harmon LJ. 2014. A novel Bayesian method for inferring and interpreting the dynamics of adaptive landscapes from phylogenetic comparative data. Syst. Biol. 64, 902–918. ( 10.1093/sysbio/syu057) [DOI] [PubMed] [Google Scholar]
- 45.Dow GJ, Berry JA, Bergmann DC. 2014. The physiological importance of developmental mechanisms that enforce proper stomatal spacing in Arabidopsis thaliana. New Phytol. 201, 1205–1217. ( 10.1111/nph.12586) [DOI] [PubMed] [Google Scholar]
- 46.Hall N, Griffiths H, Corlett J, Jones H, Lynn J, King G. 2005. Relationships between water-use traits and photosynthesis in Brassica oleracea resolved by quantitative genetic analysis. Plant Breeding 124, 557–564. ( 10.1111/j.1439-0523.2005.01164.x) [DOI] [Google Scholar]
- 47.Rae AM, Ferris R, Tallis MJ, Taylor G. 2006. Elucidating genomic regions determining enhanced leaf growth and delayed senescence in elevated CO2. Plant Cell Environ. 29, 1730–1741. ( 10.1111/j.1365-3040.2006.01545.x) [DOI] [PubMed] [Google Scholar]
- 48.Chitwood DH, et al. 2013. A quantitative genetic basis for leaf morphology in a set of precisely defined tomato introgression lines. Plant Cell 25, 2465–2481. ( 10.1105/tpc.113.112391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith WK, Vogelmann TC, DeLucia EH, Bell DT, Shepherd KA. 1997. Leaf form and photosynthesis. BioScience 11, 785–793. ( 10.2307/1313100) [DOI] [Google Scholar]
- 50.Wright IJ, et al. 2005. Assessing the generality of global leaf trait relationships. New Phytol. 166, 485–496. ( 10.1111/j.1469-8137.2005.01349.x) [DOI] [PubMed] [Google Scholar]
- 51.Reich PB, Wright IJ, Cavender-Bares J, Craine J, Oleksyn J, Westoby M, Walter M. 2003. The evolution of plant functional variation: traits, spectra, and strategies. Int. J. Plant Sci. 164, S143–S164. ( 10.1086/374368) [DOI] [Google Scholar]
- 52.Field C, Mooney HA. 1986. The photosynthesis–nitrogen relationship in wild plants. In On the economy of plant form and function (ed. Givnish TJ.), pp. 25–56. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 53.Parkhurst DF. 1994. Diffusion of CO2 and other gases inside leaves. New Phytol. 126, 449–479. ( 10.1111/j.1469-8137.1994.tb04244.x) [DOI] [PubMed] [Google Scholar]
- 54.Brodribb TJ, Jordan GJ, Carpenter RJ. 2013. Unified changes in cell size permit coordinated leaf evolution. New Phytol. 199, 559–570. ( 10.1111/nph.12300) [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Harguindeguy N, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. ( 10.1071/BT12225) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are accessible from the Dryad digital repository, http://dx.doi.org/10.5061/dryad.rj7g1.