Abstract
Thyroid hormones (TH) are bound to three major serum transport proteins, thyroxin-binding globulin (TBG), transthyretin (TTR) and human serum albumin (HSA). TBG has the strongest affinity for TH, whereas HSA is the most abundant protein in plasma. Individuals harboring genetic variations in TH transport proteins present with altered thyroid function tests, but are clinically euthyroid and do not require treatment. Clinical awareness and early recognition of these conditions are important to prevent unnecessary therapy with possible untoward effects. This review summarizes the gene, molecular structure and properties of these TH transport proteins and provides an overview of their inherited abnormalities, clinical presentation, genetic background and pathophysiologic mechanisms.
Keywords: Thyroid hormone transport proteins, thyroxine-binding globulin, transthyretin, human serum albumin, TBG deficiency, familial dysalbuminemic hyperthyroxinemia, mutations
INTRODUCTION
Thyroxine (T4) has a long half-life and a high serum concentration. These features are attributed to binding of the majority of T4 and the principal iodothyronines [triiodothyronine (T3) and reverse T3 (rT3)] to three serum thyroid hormone (TH)-binding proteins, thyroxin-binding globulin (TBG), transthyretin (TTR) and human serum albumin (HSA) (1). Binding of TH by other serum proteins (high density lipoproteins) is considered negligible without biological relevance. Although HSA is the most abundant TH-binding protein, its affinity for TH is significantly lower compared to that of TBG. The affinity of TBG to T4 is 50- and 7000-fold higher than that of TTR and HSA respectively (Table 1).
Table 1.
Some Properties and Metabolic Parameters of the Principal TH-Binding Proteins in Serum
| TBG | TTR | HSA | |
|---|---|---|---|
| Molecular weight (K daltons) | 54* | 55 | 66.5 |
| Structure | Monomer | Tetramer | Monomer |
| Carbohydrate content (%) | 20 | 0 | 0 |
| Number of binding sites for T4 and T3 | 1 | 2 | 4 |
| Association constant, Ka (M−1) | |||
| For T4 | 1 × 1010 | 2 × 108** | 1.5 × 106** |
| For T3 | 1 × 109 | 1 × 106 | 2 × 105 |
| Concentration in serum | |||
| (mean normal, mg/liter) | 16 | 250 | 40,000 |
| Total T4 binding capacity (mg T4 / liter) | 0.2 | 3 | >1000 |
| Relative distribution of T4 and T3 in serum (%) | |||
| For T4 | 75 | 20 | 5 |
| For T3 | 75 | <5 | 20 |
| Half-life (days) | 5*** | 2 | 15 |
| Degradation rate (mg/day) | 15 | 650 | 17,000 |
Apparent molecular weight on acrylamide gel electrophoresis 60 K daltons.
Value given is for the high affinity binding site only.
Longer under the influence of estrogen.
These proteins function mainly as a buffer system to maintain a large extrathyroidal TH pool and stable free T4 concentration. Only 0.03% of total serum T4 and 0.3% of total serum T3 are in an unbound form. In the absence of TH-binding proteins- with TBG being the major TH carrier- any abrupt decrease in TH secretion would result in a rapid depletion of the extrathyroidal T4 pool. Additionally, TH-binding proteins may serve as a protective mechanism against urinary iodine loss. A third proposed function involves the uniform distribution of TH across cells, which enhances tissue sensitivity to circulating TH levels (2). Lastly, TH-binding proteins may be subject to conformational changes in pathological states and thus regulate targeted TH delivery to tissues. Indeed, TBG is cleaved by leukocyte elastase at sites of inflammation, which reduces its affinity for TH (3).
Abnormalities in TH-binding proteins do not result in thyroid dysfunction but rather to altered serum TH concentrations, which may be misinterpreted and lead to unneeded treatment and side effects. When the affinity for TH is impaired, assays used in clinical practice to estimate free TH levels commonly yield spurious results. In such circumstances measurement of free TH concentration by equilibrium dialysis or ultrafiltration is recommended.
Serum TH-binding protein abnormalities, both acquired and inherited, are characterized by hyper- or hypo-iodothyroninemia, while subjects are clinically euthyroid (4). However, it is possible that other thyroid disease, such as thyrotoxicosis or hypothyroidism, may be concurrently present and further perplex both diagnosis and management (5). The aim of this review is to provide an update on the heritable defects of serum TH-binding proteins, their structure, genetic background, laboratory tests and consequences.
THYROXIN-BINDING GLOBULIN (TBG)
THE MOLECULE, GENE AND PROPERTIES
TBG is a 54KDa single polypeptide chain synthesized by the liver. The pre-protein is an acidic glycoprotein of 415 amino acids (aa). The mature molecule (395 aa, minus the signal peptide) has four N-linked oligosaccharides. The latter are important for the correct post-translational folding and secretion of TBG and are responsible for the microheterogeneity of TBG when subjected to isoelectric focusing (IEF). Improper folding of the molecule or changes in the polypeptide core alter TBG secretion, TH binding capacity and immunoreactivity. For details regarding TBG properties see Table 1,
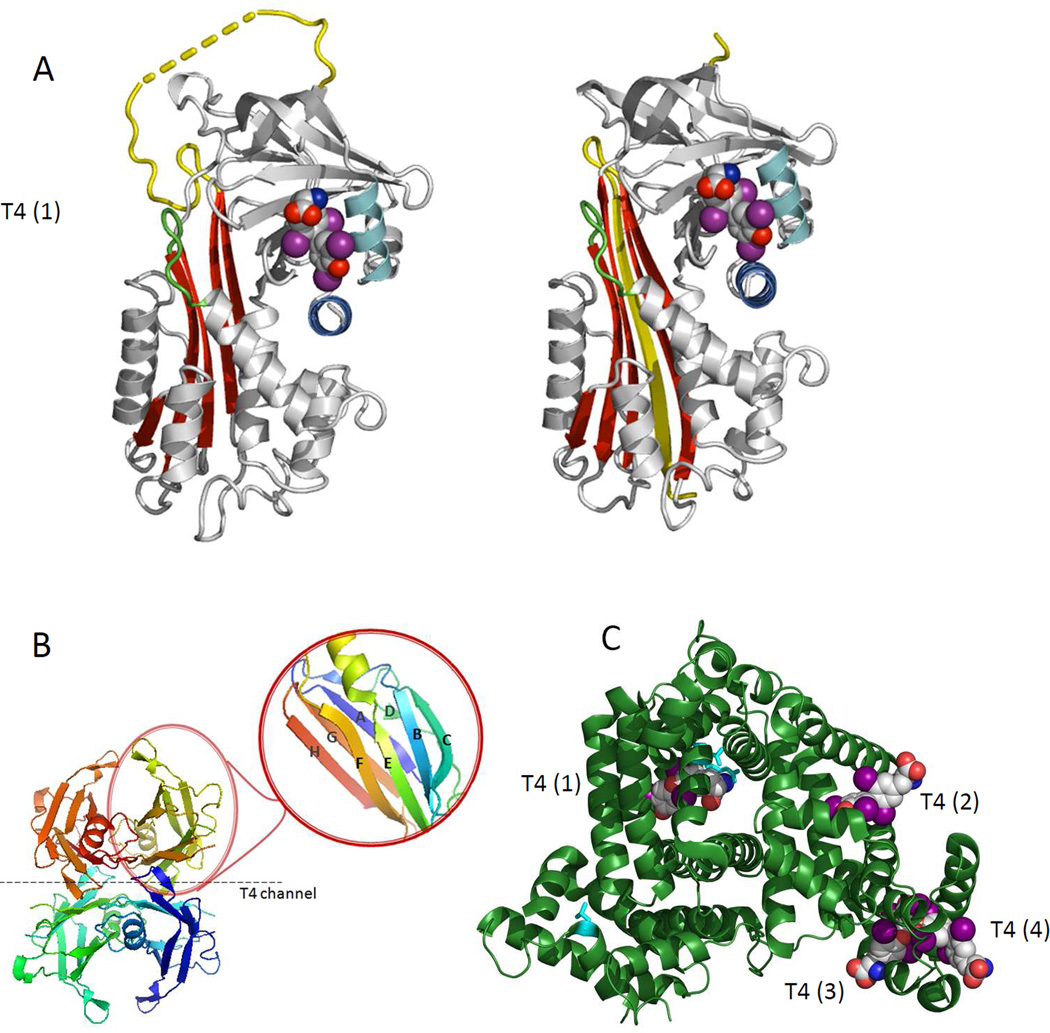
TBG is a member of the serine protease inhibitor (serpin) superfamily, also known as SERPINA7. TBG differs from other serpins in having the upper half of its main beta-sheet fully opened, so that its reactive center peptide loop can readily move in and out of the sheet to facilitate equilibrated binding and release of TH (Fig. 1). The coordinated movements of the reactive loop and the hormone-binding site allow the allosteric regulation of hormone release (6).
Figure 1.
Crystal structures of serum TH-binding proteins. A. Structure of the TBG molecule: intact S-form (left) and cleaved R-form (right). Insertion of the reactive loop (in yellow) occurs following its cleavage by proteases to give an extra strand in the main sheet of the molecule but the T4-binding site can still retain its active conformation. The S-to-R change in TBG results in a 6 -fold decrease but not a total loss of affinity. B. The homotetrameric structure of TTR composed of four monomers of 127 amino acids. TTR contains eight stands (A-H) and a small α-helix. The contacts between the dimers form two hydrophobic pockets where T4 binds (T4 channel). As shown in the magnified insert, each monomer contains one small α-helix and eight β-strands (CBEF and DAGH). C. The structures of HSA in the presence of T4 as modeled on the structures 1BM0, 1HK1, 1HK3 in the Protein Data Bank (http://www.rcsb.org/pdb/home/home.do). The entire WT HSA molecule (in green) with its four T4 binding sites [T4 (1) to T4(4)].
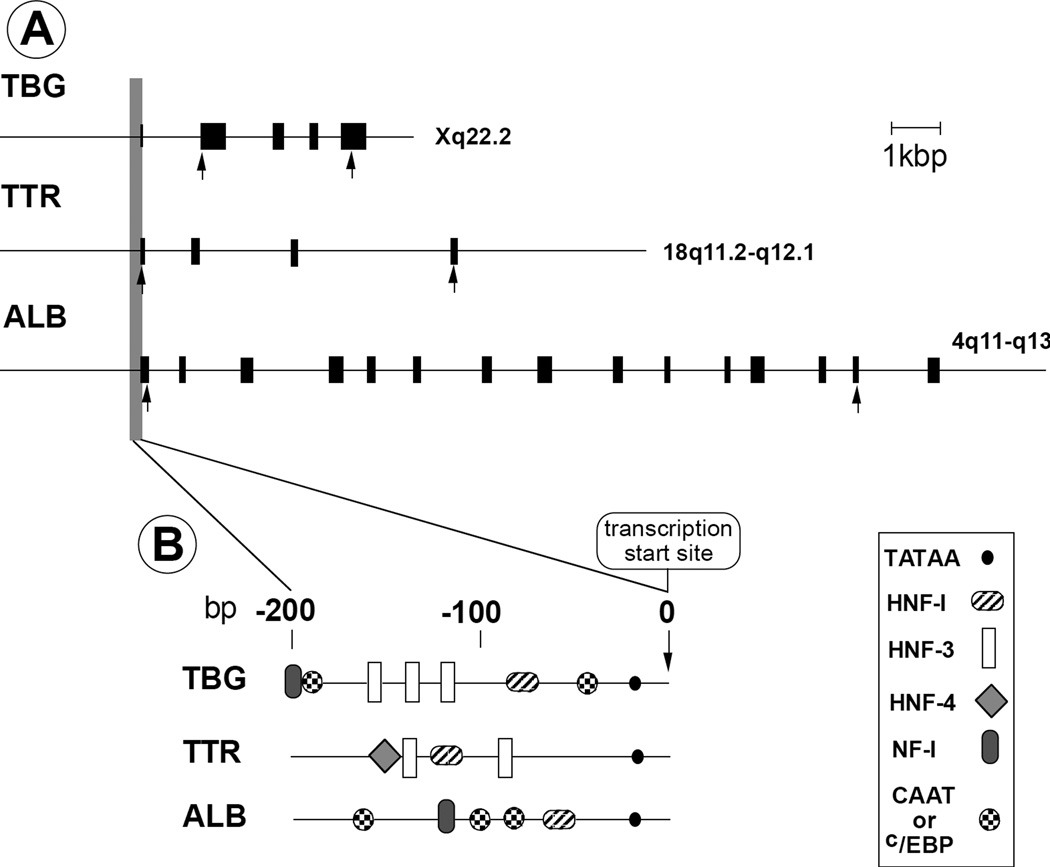
TBG is encoded by a single gene copy located on the long arm of the X-chromosome (Xq21–22). The gene consists of 5 exons, of which 4 are coding. The TBG gene promoter contains several transcription factor binding sites (hepatic nuclear factors (HNF)1α, HNF3α and HNF3β) imparting strong liver-specific transcriptional regulation (Fig. 2) (7). An enhancer region, 20 kbp downstream of the TBG gene, was recently shown to be involved in the regulation of the gene expression (8).
Figure 2.
A. Genomic organization and chromosomal localization of TH-binding proteins. Filled boxes represent exons. Arrows indicate location of initiation codons and termination codons. B. Structure of promoter regions with the location of cis-acting transcriptional regulatory elements. Reproduced with permission.
INHERITED TBG ABNORMALITIES
Beierwaltes et al (9) report of TBG excess was the first demonstration of an inherited disorder of a TH transport protein, followed, 30 years later, by a report of the first mutation in the TBG gene, causing X-linked TBG deficiency (10). TBG abnormalities are fully expressed in hemizygous males. Due to dosage compensation, through the random inactivation of one of the two X chromosomes, heterozygous females usually show TBG levels intermediate between affected and unaffected males (7). Occasionally, allele selective inactivation may result in a phenotype indistinguishable from that of affected males (11). TBG defects are classified according to their serum levels in hemizygotes expressing only the mutant allele: complete and partial TBG deficiency (TBG-CD and TBG-PD respectively) and TBG excess (TBG-E) (5). In TBG-CD, affected males have undetectable TBG and carrier females have on average half the normal TBG concentration. In TBG-PD, the mean TBG concentration in heterozygous females is usually above half the normal. Serum TBG concentration in males with TBG-E is 2- to 4- fold the normal mean and that in the corresponding carrier females is slightly higher than half that of the affected males.
Usually, TBG deficiency or excess are diagnosed by the incidental finding of abnormal serum T4 and normal free T4 levels. The absence of factors causing acquired TBG abnormalities should raise the suspicion of an inherited TBG defect that can be easily confirmed by the presence of similar TBG abnormalities in family members. However, confirmation of inherited TBG defects requires genetic analysis (12). Fifty-seven TBG variants have been so far identified and in 49 the precise defect has been genetically determined (Table 2).
Table 2.
TBG Variants and Gene Mutations (for references see http://www.thyroidmanager.org)
| TBG NAME | Abbreviated name |
Intron Exon |
CODON1 | AMINO ACID | NUCLEOTIDE | ||
|---|---|---|---|---|---|---|---|
| WT | Variant | WT | Variant | ||||
| Complete Deficiency (CD) | |||||||
| Milano (fam A) | CDMi2 | IVS 1 | fs | 5' DSS | unknown | gtaagt | gttaagt |
| Andrews3 | CDAN | IVS 1 | fs | 5' DSS | unknown | gtaagt | gcaagt |
| Portuguese 1 (pt A) | CDP1 | 1 | 23 | S (Ser) | X (OCH) | TCA | TAA |
| Yonago | CDY | 1 | 28–29fs-51 | D F | X (OPA) | GA(CT)TT | GAATT |
| Negev (Bedouin | CDN | 1 | 38fs-51 | T (Thr) | X (OPA) | ACT | T del |
| Nikita (fam B) | CDNi | 1 | 50fs-51 | P (Pro) | X (OPA) | CCT | T del |
| Taiwanese 1 | CDT12 | 1 | 52 | S (Ser) | N (Asn) | AGC | AAC |
| Parana | CDPa2 | 1 | 61 | S (Ser) | C (Cys) | TCC | TGC |
| No name | CD6 | 1 | 165fs-168 | V (Val) | X (OCH) | GTT | T del |
| Kankakee | CDK | IVS 2 | 188fs-195 | 3' ASS | X (OPA) | agCC | ggCC |
| Poland | CDPL | 2 | 201fs-206 | D (Asp) | X (OCH) | GAC | G del |
| Portuguese 2 (pt B) | CDP2 | 2 | 223 | Q (Gln) | X (OCH) | CAA | TAA |
| No name | CD52 | 2 | 227 | L (Leu) | P (Pro) | CTA | CCA |
| Portuguese 34 | CDP3 | 2 | 233 | N (Asn) | I (Ile) | ACC | ATC |
| Berlin3 | CDBn | IVS 3 | fs | −28 bp | ? | ? | ? |
| Houston | CDH | IVS 3 | 279fs-374 | 3’ ASS | X (OPA) | agAT | aaAT |
| Buffalo | CDB | 3 | 280 | W (Trp) | X (AMB) | TGG | TAG |
| Taiwanese 2 | CDT2 | 3 | 280 | W (Trp) | X (OPA) | TGG | TGA |
| Lisle | CDL | IVS 4 | 280fs-325 | 5' DSS | X (OPA) | gtaaa | ggaaa |
| Jackson (fam K) | CDJa | IVS 4 | 280fs-325 | 5' DSS | X (OPA) | gtaaa | gtaag |
| No name | CD7 | 3 | 283fs-301 | L (Leu) | X (OPA) | TGT | G del |
| No name | CD82 | 4 | 329fs-374 | A (Ala) | X (OPA) | GCT | G del |
| Japan | CDJ | 4 | 352fs-374 | L (Leu) | X (OPA) | CTT | C del |
| Penapolis | CDPe | 4 | 332fs-374 | K (Lys) | X (OPA) | AAG | A del |
| Kyoto4 | CDKo | 4 | 370 | S (Ser) | F (Phe) | TCT | TTT |
| Harwichport | CDH | 4 | 381fs-396 | Y (Tyr) | X (OPA) | AGG | 19nt del |
| NeuIsenburg | CDNl | 4 | 384fs-402 | L (Leu) | 7 aa add | CTC | TC del |
| Partial Deficiency (PD) | |||||||
| Allentown | PDAT | 1 | −2 | H (His) | Y (Tyr) | CAC | TAC |
| San Diego | PDSD2 | 1 | 23 | S (Ser) | T (Thr) | TCA | ACA |
| Brasilia | PDB | 1 | 35 | R (Arg) | W (Trp) | CGG | TGG |
| Wanne-Eickel5 | PDWE | 1 | 35 | R (Arg) | E (Glu) | CGG | CAG |
| Mainz 1′ | PDMZ1 | 1 | 52 | S (Ser) | R (Arg) | AGT | AGA |
| Mainz 2′ | PDMZ2 | 1 | 64 | A (Ala) | D (Asp) | GCC | GAC |
| Korea6 | PDKa | 1 | 74 | E (Glu) | K (Lys) | GAG | AAG |
| Gary | PDG | 1 | 96 | I (Ile) | N (Asn) | ATC | AAC |
| Mainz 3′ | PDMZ3 | 1 | 112 | N (Asn) | L (Lys) | AAT | AAG |
| Montréal | PDM | 1 | 113 | A (Ala) | P (Pro) | GCC | CCC |
| Aborigine | PDA2 | 2 | 191 | A (Ala) | T (Thr) | GCA | ACA |
| Glencoe | PDGe | 2 | 215 | V (Val) | G (Gly) | GTG | GGG |
| Quebec | PDQ2 | 4 | 331 | H (His) | Y (Tyr) | CAT | TAT |
| Japan (Kumamoto) | PDJ | 4 | 363 | P (Pro) | L (Leu) | CCT | CTT |
| Heidelberg | PDHg | 4 | 368 | D (Asp) | G (Gly) | GAT | GGT |
| Mainz 4′ | PDMZ4 | 4 | 381 | R (Arg) | G (Gly) | AGG | GGG |
| Mainz 5′ | PDMZ5 | 4 | 382 | S (Ser) | R (Arg) | AGT | CGT |
| No name | enhancer | - | - | - | G | A | |
| Other Variants | |||||||
| Slow | S | 1 | 171 | D (Asp) | N (Asn) | GAC | AAC |
| Polymorphism | Poly | 3 | 283 | L (Leu) | F (Phe) | TTG | TTT |
| Chicago | CH or Cgo | 3 | 309 | Y (Tyr) | F (Phe) | TAT | TTT |
Codon numbering from fist amino acid of the mature protein. The 20 amino acids of the signal peptide are numbered −1 to −20, from N- to C-terminus. The codon at the site of mutation is followed by the codon at the site of termination of translation.
coexistence of TBG Poly
Moeller LC, Appiagyei-Dankah Y, Köhler B, Biebermann H, Janssen OE, Führer D. Two novel mutations in the Serpina7 gene are associated with complete deficiency of thyroxine-binding globulin. Eur Thyroid J (in press)
complete deficiency is uncertain as the TBG assay used was unable to detect values <10% the mean normal
Moeller LC, Vinzelberg P, Jaeger A, Appiyagyei-Dankah Y, Fingerhut A, Mann K, Janssen OE. Two novel mutations leading to partial and complete thyroxine-binding globulin deficiency. 51. Symposium of the German Society for Endocrinology, March 7–11 2007, Salzburg, Austria
Also a silent mutation at codon 55: GCA -> GCG
Personal communication Joachim Pohlenz, Universitätsmedizin Mainz, Germany
del, delete; add, addition; aa, amino acid; fs, frame shift
Pt, patent; fam, family
IVS, intervening sequence or intron; 3, acceptor splice site; DSS, donor splice site
Complete Deficiency of TBG (TBG-CD)
TBG-CD was first identified in a female with Turner syndrome with complete absence of TBG binding, similar to affected male family members. Her very low serum T4 concentration and short stature led to treatment with increasing doses of TH resulting in restlessness, perturbed sleep and deterioration of school performance (13). By definition, TBG-CD requires TBG serum concentration in affected hemizygous males below 5 mg/L (0.9 nmol/L) or 0.003% the average normal (5). The prevalence of TBG-CD is approximately 1:15,000 newborn males. So far, 27 TBG gene mutations causing TBG-CD have been identified (Table 2). Eighteen have truncated molecules produced by single nucleotide substitution or frame shifts due to nucleotide deletion(s). In 7 variants, mutations occurred in introns close to splice sites causing frame shift (TBG-CDK) or complete exon splicing (TBG-CDL and TBG-CDJa) (Table 2). TBG-CDBn, lacking 79- nucleotides, (28 in intron 3 and the first 51 of exon 3) has the longest deletion in the TBG gene. TBG-CDMI was recently found in association with the P453A mutation in the thyroid hormone receptor beta (THRB) gene (14). While single aa substitutions usually produce TBG-PD, in 6 instances they resulted in TBG-CD.
Partial Deficiency of TBG (TBG-PD)
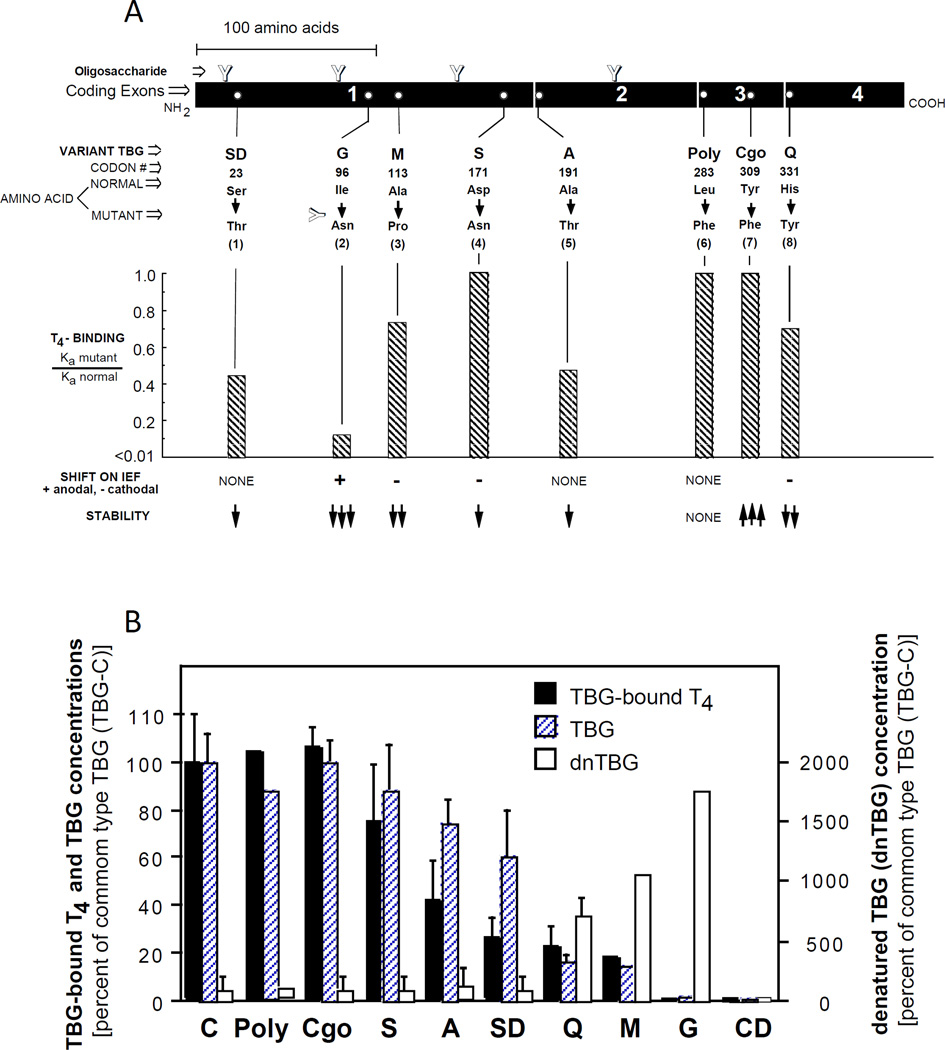
TBG-PD is the most common form of inherited TBG deficiency (prevalence of1:4,000 newborns). Serum TBG levels in heterozygous females often overlap the normal range. Contrary to TBG-CD, all TBG-PDs are caused by missense mutations. TBG-CDs with missense mutations may have been misclassified due to the low sensitivity of routine assays measuring TBG. Eighteen families with variable reduction of TBG concentration have TBG gene mutations (Table 2). Few variants are unstable, have lower binding affinity for TH or abnormal migration patterns on IEF (Fig. 3A). Mutations with decreased affinity for T4 have a disproportionate reduction in hormone concentration relative to the corresponding serum TBG level (Fig. 3B).
Figure 3.
A Properties of some TBG variants causing partial TBG deficiency (TBG-PD). [Modified from Refetoff et al (51)]. B Serum T4-bound to TBG and the concentration of TBG and denatured TBG (dnTBG) in hemizygous subjects expressing the different TBG variants. Results, graphed as mean ± SD, were normalized by expressing them as % of those for the common type TBG (TBG-C). For abbreviations used in the nomenclature of the TBG variants, see legend to figure 1. [Adapted from Janssen et al (52)].
A Japanese family with autosomal dominant TBG-PD showed half normal TBG concentration in affected males and females (Table 2). The molecule had normal affinity for T4, IEF migration and heat liability. No sequence changes were found in the coding and promoter regions of the TBG gene, suggesting an abnormality in a factor regulating TBG gene transcription.
TBG A64D was found in association with TTR A109T, known to have increased affinity for T4 (see below). All affected males carrying both TH-binding protein variants had low serum TT4 level indicating that the defect in TBG has a stronger effect compared to the TTR variant (15).
In 5% (4 of 74) of families with TBG deficiency, studied in the author’s laboratory, no mutations were identified in the entire TBG gene and surrounding sequences. Next-generation sequencing identified, in all four families (three of which had Arab ethnicity), a novel single nucleotide substitution within a liver-specific enhancer, 20 kb downstream of the TBG gene. In vitro studies confirmed that the mutation reduced the activity of the enhancer. Affected subjects shared a haplotype of 8 Mb surrounding the mutation indicating a common ancestor 20 generations ago (8).
TBG Excess (TBG-E)
Estrogen excess is the most common cause of increased serum TBG concentration, mediated by prolonged TBG half-life. In contrast, the estimated prevalence of TBG-E is around 1:25,000 (5). In 1995, it was demonstrated that gene duplication and triplication was the cause of TBG-E (16). Hemizygous males have approximately 2- and 3-fold the average normal serum TBG concentration, respectively.
TBG Variants with no Alteration in Serum TBG Concentration
Five TBG variants have normal or clinically insignificant alterations in their serum concentration. Four occur with high frequency in some population groups. TBG-Poly (Fig. 2), with normal physical and biological properties, has been detected in 16% and 20% of the French Canadian and Japanese populations, respectively (Table 2). TBG-S (D171N) with cathodal iEF shift due to loss of a negative charge has an allele frequency of 5–16% in African Blacks and 2–10% in Pacific Islanders (Figs. 3A) and TBG-F a frequency of 3.2% in Eskimos residing on the Kodiac and St. Lawrence islands. TBG-C1 with allele frequency of 5.1% was found in inhabitants of two Mali villages, whereas TBG-Cgo (Y309F), with normal affinity for TH, is resistant to high temperatures due to more loop inserted conformation.
Consequences of Structural Changes Caused by Mutations in the TBG Gene
The pathophysiologic mechanisms explaining the phenotype of the various TBG variants have been investigated. Contrary to previous speculations, it is not the increased extracellular degradation but rather the intracellular retention and degradation of the defective TBG molecules that are responsible for the low serum TBG concentrations (17). Few of the underlying mechanisms include alterations in cotranslational TBG processing and secretion, retention of the variant TBG within the endoplasmic reticulum, instability of the binding pocket or increase of denatured TBG that reduce T4-binding (18).
TRANSTHYRETIN (TTR)
THE MOLECULE, GENE AND PROPERTIES
TTR, previously known as thyroxine-binding prealbumin (TBPA), transports 15–20% of circulating T4 and also binds vitamin A-binding protein. It is a highly acidic, non-glycosylated, 55 kDa protein with four identical 127 aa subunits. Each subunit has a small α-helix and 8 β-strands (19). The four subunits form a symmtetrical barrel structure with a double hydrophobic channel running through the center of the molecule to create two iodothyronine binding sites (Figure 1B).
A single copy of the 6.8kbp TTR gene is located on the long arm of chromosome 18 18q11.2–12.1) (Figure 2). It has a TATAA box and binding sites for HNF-1, 3 and -4. TTR is expressed in liver, the retinal pigment epithelia of the eye and the choroid plexus, and is the principal T4-binding protein of the cerebrospinal fluid.
The first T4 molecule binds to TTR with a Ka intermediate between TBG and HSA (Table 1). The binding affinity for the second T4 is significantly reduced through negative cooperativeness at the binding site (20). TTR contribution to TH transport is small and its complete absence has no untoward effects. For details regarding TTR properties see Table 1,
INHERITED TTR DEFECTS
TTR gene mutations are subdivided into amyloidogenic and non-amyloidogenic. The majority occurs in CpG hot spots and inheritance is dominant. The former give rise to familial amyloidotic polyneuropathy (FAP), first described by Andrade et al (21), characterized by neuropathy and cardiomyopathy due to amyloid deposition of the variant TTR, ultimately causing multiple organ failure and death at early childhood. The affinity to bind T4 is not related with the ability of the variant TTR to form amyloid fibrils.
Because of the low amount of T4 bound to TTR, alterations of TTR levels only minimally affect TH concentration. Out of over 70 TTR gene mutations, heterozygotes for the V30M, S77Y and the I84S mutations and homozygotes for the V122I mutation show decreased binding affinity for T4 (Table 3) (22). Only in variants with significant increase in binding affinity for TH are serum total T4 and rT3 substantially elevated. Of all subjects with euthyroid hyperthyroxinemia, approximately 2% harbors TTR gene defects which are not pathogenic.
Table 3.
TTR variants with altered affinity for T4 and potentially an effect on serum thyroid function tests (for references see http://www.thyroidmanager.org),
| AFFINITY FOR T4 Mutant / Normal |
TTR CONCENTRATION |
CODON Number |
AMINO ACID (Normal -Variant) |
|
|---|---|---|---|---|
| HOMO* | HETERO* | |||
| DECREASED | ||||
| <0.1 | 0.17 – 0.41 | N | 30 | Val - Met |
| 0.54 | 58 | Leu - His | ||
| 0.45 | 77 | Ser - Tyr | ||
| 0.19 – 0.46 | N | 84 | Ile - Ser | |
| ~1.0 | 0.44 | 122 | Val - Ile | |
| INCREASED | ||||
| 0.35† | N | 6 | Gly - Ser | |
| 8.3–9.8 | 3.2 – 4.1‡ | N | 109 | Ala - Thr |
| N | 109 | Ala - Val | ||
| Inc or N | 119 | Thr - Met | ||
HOMO, homozygous; HETERO, heterozygous.
Probably overestimated since the subjects harboring this TTR variant have normal serum TT4 concentrations.
Affinity of recombinant TTR Thr109 is 9-fold that of the normal TTR.
Variant TTR tested and shown not to have altered affinity to T4 are: Ala60, (hetero) (22).
N, normal; Inc, increased.
The first reported family with a TTR-dependent hyperthyroxinemia had the A109T variant with increased binding affinity for T4 10-fold), rT3 and tetraiodothyroacetic acid and less for T3 and trioiodothyroacetic acid (23, 24). TTR A109V has a similar thyroid phenotype and binding affinity for T4 (25). Subjects with TTR T119M (26) have normal or slightly elevated serum T4 and rT3 concentrations. The mechanism underlying the slight increase in serumT4 has been a subject of debate. Alves et al (27) failed to show a higher Ka for T4 but rather found an increase in TTR concentration, probably because of altered stability of the molecule. On the contrary, Almeida et al (28) showed increased T4-binding affinity. Interestingly, the presence of hyperthyroxinemia in these cases is transient and brought about by non-thyroidal illness, either by increasing the proportion of heterotetramers or through an unexpected effect of non-thyroidal illness on T4 binding to the mutant TTR (26). Finally, TTR G6S, with a frequency of 12% (29), identified in a family with euthyroid hyperthyroxinemia and a 4-fold higher T4-binding affinity than normal TTR (30), was shown to have normal T4-binding in another study.
HUMAN SERUM ALBUMIN (HSA)
THE MOLECULE, GENE AND PROPERTIES
HSA, the most abundant circulating protein, is a single negatively charged polypeptide chain synthetized in the liver. It consists of 585 aa and has a molecular mass of 66.5 kDa (31). Its major function is to maintain stable colloid osmotic pressure. Furthermore, it plays an important role in binding and transporting a large number of endogenous (including TH) and exogenous compounds, thus forming a circulating depot for ligands and prolonging their half-life. It is also an important circulating antioxidant. Despite a high binding capacity, HSA affinity for T4 and T3 is almost 10,000-fold lower than that of TBG (Table 1). From a physiology standpoint, even extreme alterations of HSA concentration, such as analbuminemia, do not result in a significantly change of TH levels (32).
HSA has a single-gene copy close to the centromere on the long arm of chromosome 4 (4q11–13). The gene has 15 exons, 14 of which are coding (Figure 2). Its mRNA encodes a pre-pro-molecule of 609 aa. The mature HSA is released after cleavage of the signal peptide (18 aa) and the propeptide (6 aa). The promoter has a TATA motif and six binding sites for nuclear proteins, such as HNF1 and the CCAAT/enhancer-binding protein (C/EBP), which regulate the HSA gene expression.
INHERITED HSA DEFECTS
Familial dysalbuminemic hyperthyroxinemia (FDH) is an autosomal dominant form of euthyroid hyperiodothyroninemia caused by gain-of-function mutations in the HSA gene. First described in 1979 (33), its prevalence varies from 0.01 to 1.8% depending on the ethnic origin, being highest in Hispanics (34).
The characteristic thyroid biochemical profile in FDH is elevated serum iodothyronine levels, the relative magnitude of T4, T3 and rT3 being dependent on the type of HSA mutation (Table 4). The most common variant HSA R218H manifests with increased serum TT4 concentration without proportional increase of TT3 and unsuppressed TSH. TrT3 in most cases is also elevated (35). Free T4 concentration is within the reference interval when measured by equilibrium dialysis or by ultrafiltration. Furthermore, T4 production rate and the response of TSH to TRH stimulation are normal, as are peripheral markers of thyroid hormone action and cellular T4 uptake, all indicators of euthyroidism (33), (36). The latter is often questioned when fT4 levels are measured by indirect/analog methods which yield falsely elevated results due to the interference of variant albumin. The mechanisms responsible for this interference are different depending on the assay and include interaction of the T4 analogue and the assay buffer used, since chloride inhibits binding to HSA (37). Misdiagnosis has led to inappropriate ablative or medical treatment. The presence of FDH should be confirmed by demonstration of increased T4-binding to HSA, using IEF or immunoprecipitation, confirmed by HSA gene sequencing.
Table 4.
Albumin variants with increased affinity for iodothyronines, their effect on serum concentration of and affinity to these hormones.
| VARIANT | SERUM CONCENTRATION | BINDING AFFINITY (Ka) of the variant albumins |
Reference | ||||
|---|---|---|---|---|---|---|---|
| T4 µg/dl |
T3 ng/dl |
rT3 ng/dl |
N | T4 | T3 | ||
| (fold the normal mean) | (fold the normal mean) | ||||||
| WT | 8.0 ± 0.2 | 125 ± 4 | 22.5 ± 0.9 | 83 | 1 | 1 | Personal observation |
| R218H | 16.0 ± 0.5 (2.0) |
154 ± 3 (1.2) |
33.1 ± 1.1 (1.5) |
83 | (10 – 15) | (4) | (38) |
| R218P | 135 ± 17 (16.8) |
241 ± 25 (1.9) |
136 ± 13 (6.1) |
8 | (11–13*) | (1.1*) | (41,42) |
| R218S | 70 (8.8) |
159 (1.3) |
55 7 (2.6) |
1 | NM | NM | (43) |
| R222I | 21±1.4 (2.6) |
135±18 (1.2) |
1417±107 (86) |
8 | NM | NM | (44) |
| L66P | 8.7 (1.1) |
320 (3.3) |
22 3 (1) |
6 | (1.5) | (40) | (4) |
Values reported are mean ± standard error, and the number of subjects per genotype is indicated under “N”.
Determined at saturation. Affinities are higher at the concentrations of T4 and T3 found in serum.
NM, not measured.
All data were generated in the Chicago laboratory except for 4 of the 8 individuals with ALB R218P and those with ALB R222I, provided by Nadia Schoenmakers, University of Cambridge, UK.
FDH was first linked to the HSA gene in a large Amish kindred by haplotyping, using markers 1 cM from the HSA gene (LOD score 5.63) (35). This same family and 5 others were found to harbor a missense mutation producing HSA R218H (38). This mutation was associated with an intragenic polymorphism creating a SacI+ restriction site, strongly suggesting a founder effect in agreement with the ethnic predilection of FDH R218H. By virtue of its frequency, this type of FDH has been found in families with TTR (39) and TBG (40) gene mutations.
Two other mutations in the same codon of the HSA have been identified, R218P and R218S. HSA R218P was found in three Japanese and one Swiss family. Heterozygote individuals harboring this mutation have extremely high serum total T4 concentration, 17- fold higher than the mean normal level, a TrT3 6-fold higher and a TT3 only twice the mean normal (Table 4) (41), (42). Although the Kα value obtained by Scatchard analysis at T4 saturation was similar to that of HSA R218H, measurement of the dialysable T4 concentration was 11-fold lower in serum from HSA R218P than that in serum from HSA R218H (42). HSA R218S was recently identified in a Canadian family of Bangladeshi origin (43). TT4 concentration was intermediate between HSA R218P and R218H (Table 4).
HSA R222I was identified in four unrelated families (three from Somalia and one from Croatia). This mutant HSA has a remarkably high TrT3 level but relatively modest increase in TT4, similar to that in HSA R218H (44) (Table 4). HSA L66P has only high serum TT3. Found in a Thai family it is unique, as both TT4 and TrT3 concentrations are normal, thus termed FDH-T3 (4) (Table 4). When T3 was measured using a T3 analog rather than a radioisotope, TT3 concentration was falsely low or undetectable, leading to unnecessary treatment with TH. Despite lacking genetic information, FDH-T3 could have been the cause for increased T3-binding activity of HSA in a Japanese patient with Graves’ disease (45) and another patient with the FDH-T3 phenotype (46).
Molecular mechanism of increased TH-binding to mutant HSAs
In-vitro assays and in-silico modeling were used to elucidate the molecular mechanisms underlying the HSA gene defects in FDH. Codons 218 and 222 are located in subdomain 2A of HSA, where the high-affinity T4-binding pocket is localized (47). Petersen et al (48) demonstrated that in the wild type (WT) HSA, the large guanidino group of arginine results in an unfavorable interaction with the amino group of T4, whereas replacement by a smaller side-chain histidine or alanine enhances binding to T4. Similarly, in-silico analysis predicts that the side chains of arginines 218 and 222 must undergo a marked displacement to accommodate T4. Their substitution with histidine or proline at position 218 and isoleucine at position 222 results in a conformational adjustment forming a more favorable binding pocket for T4 (44).
Bisalbuminemia and analbuminemia
Besides the HSA variants resulting in altered TH binding, there are variant proalbumin or albumin molecules (alloalbuminemia or bisalbuminemia), with altered electrophoretic mobility (showing up as a double band in heterozygotes) but no clear association with disease (49). Although T4 was found to preferentially bind to the variant rather than the normal albumin in few reports (50), bisalbuminemia does not result in significant changes in TH levels.
Analbuminemia is the complete absence or very low HSA. It is an extremely rare condition caused by nonsense mutations resulting in premature termination of translation or splicing defects and only mild symptoms (edema, fatigability). The defect has no clear effect on iodothyronine concentration, other than a slight TH elevation caused by the increased TBG and TTR levels (32).
SUMMARY
Inherited defects of TH transport proteins result in altered thyroid function tests, while affected individuals remain clinically euthyroid. With a single exception, TBG defects are X-chromosome linked, and therefore, fully expressed in males. Partial TBG deficiency is more common than complete deficiency and is caused by mutations in the coding as well as enhancer region of the TBG gene. High frequency of TBG variants has been identified in some ethnic groups. TBG-excess is caused by gene duplication or triplication. The majority of TTR gene mutations cause familial amyloidotic polyneuropathy, whereas mutations causing euthyroid hyperthyroxinemia are not pathogenic. Some variants produce transient hyperthyroxinemia during non-thyroidal illness. Familial dysalbuminemic hyperthyroxinemia is caused by gain-of-function mutations in the HSA gene, the R218H being the most common with prevalence in Hispanics. fT4 levels are within normal range when measured with equilibrium dialysis, whereas indirect/ analog methods may yield spurious results. A variant with increased affinity for T3 has been identified in an extended Thai family. Study of the molecular properties of variant TH transport proteins has helped understand the structural requirements for iodothyronine binding and their clinical significance.
Practice points.
-
⊙
Inherited defects of circulating TH-binding proteins result in alterations of thyroid function tests, which may be misinterpreted and lead to incorrect diagnosis. Affected individuals are clinically euthyroid and require no treatment.
-
⊙
Equilibrium dialysis is the recommended method of estimating fT4 levels in FDH, whereas analog/indirect methods may yield falsely elevated results.
-
⊙
TBG has the strongest affinity for T4; TTR and HSA bind a small proportion of T4 despite large capacity. Gain-of-function mutations of TTR and HSA but not altered concentrations alter serum TT4 concentration.
Research agenda.
-
⊙
Further work on the pathophysiologic mechanisms underlying the mutation in the enhancer of the TBG gene causing partial TBG deficiency will fully characterize its properties.
-
⊙
Advances of in-silico tools, such as protein structure modeling, will help update our knowledge on the conformational changes occurring in variant TH-binding proteins and correlate them with the thyroid phenotype.
Acknowledgments
This work was supported in part by Grants R37DK15070 from the National Institutes of Health USA and the Seymour J. Abrams fund for thyroid research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Theodora Pappa, The University of Chicago, 5841 South Maryland Avenue, Chicago, Illinois 60637, USA, <tpappa@medicine.bsd.uchicago.edu>
Alfonso Massimiliano Ferrara, Istituto Oncologico Veneto, Padova 35128, Italy, <massi.ferrara@gmail.com>
Samuel Refetoff, The University of Chicago, 5841 South Maryland Avenue, Chicago, Illinois 60637, USA, <refetoff@uchicago.edu>, tel: 1 773 702-6939 fax: 1 773 702-6940
REFERENCES
- 1.Oppenheimer JH. Role of plasma proteins in the binding, distribution, and metabolism of the thyroid hormones. New England Journal of Medicine. 1968;278:1153–1162. doi: 10.1056/NEJM196805232782107. [DOI] [PubMed] [Google Scholar]
- 2.Mendel CM, Weisinger RA, Jones AL, Cavalieri RR. Thyroid hormone-binding proteins in plasma facilitate uniform distribution of thyroxine within tissues: A perfused rat liver study. Endocrinology. 1987;120:1742–1749. doi: 10.1210/endo-120-5-1742. [DOI] [PubMed] [Google Scholar]
- 3.Janssen OE, Golcher HMB, Grasberger H, et al. Characterization of thyroxine-binding globulin cleaved by human leukocyte elastase. Journal of Clinical Endocrinology and Metabolism. 2002;87:1217–1222. doi: 10.1210/jcem.87.3.8332. [DOI] [PubMed] [Google Scholar]
- 4.Sunthornthepvarakul T, Likitmaskul S, Ngowngarmratana S, et al. Familial dysalbuminemic hypertriiodothyroninemia: a new dominantly inherited albumin defect. Journal of Clinical Endocrinology and Metabolism. 1998;83:1448–1454. doi: 10.1210/jcem.83.5.4815. [DOI] [PubMed] [Google Scholar]
- 5.Refetoff S. Inherited thyroxine-binding globulin (TBG) abnormalities in man. Endocrine Reviews. 1989;10:275–293. doi: 10.1210/edrv-10-3-275. [DOI] [PubMed] [Google Scholar]
- 6.Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proceedings of the National Academy of Science. 2006;103:13321–13326. doi: 10.1073/pnas.0604080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi Y, Mori Y, Janssen OE, et al. Human thyroxine-binding globulin gene: Complete sequence and transcriptional regulation. Molecular Endocrinology. 1993;7:1049–1060. doi: 10.1210/mend.7.8.8232304. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara AM, Pappa T, Fu J, et al. A novel mechanism of inherited TBG deficiency: mutation in a liver-specific enhancer. Journal of Clinical Endocrinololgy and Metabolism. 2015;100:E173–E181. doi: 10.1210/jc.2014-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beierwaltes WH, Robbins J. Familial increase in the thyroxine-binding sites in serum alpha globulin. Journal of Clinical Investigation. 1959;38:1683–1688. doi: 10.1172/JCI103946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori Y, Seino S, Takeda K, et al. A mutation causing reduced biological activity and stability of thyroxine-binding globulin probably as a result of abnormal glycosylation of the molecule. Molecular Endocrinology. 1989;3:575–579. doi: 10.1210/mend-3-3-575. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto H, Mori Y, Tani Y, et al. Molecular analysis of females manifesting thyroxine-binding globulin (TBG) deficiency: selective X-chromosome inactivation responsible for the difference between phenotype and genotype in TBG-deficient females. Journal of Clinical Endocrinololgy and Metabolism. 1996;81:2204–2208. doi: 10.1210/jcem.81.6.8964852. [DOI] [PubMed] [Google Scholar]
- 12.Refetoff S, Murata Y, Mori Y, et al. Thyroxine-binding globulin: organization of the gene and variants. Hormone Research. 1996;45:128–138. doi: 10.1159/000184775. [DOI] [PubMed] [Google Scholar]
- 13.Refetoff S, Selenkow HA. Familial thyroxine-binding globulin deficiency in a patient with Turner's syndrome (X0): Genetic study of a kindred. New England Journal of Medicine. 1968;278:1081–1087. doi: 10.1056/NEJM196805162782002. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara AM, Cakir M, Henry PH, Refetoff S. Coexistence of THRB and TBG gene mutations in a Turkish family. Journal of Clinical Endocrinology and Metabolism. 2013;98:E1148–E1151. doi: 10.1210/jc.2013-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sklate RT, Olcese MC, Maccallini GC, et al. Novel mutation p.A64D in the Serpina7 gene as a cause of partial thyroxine-binding globulin deficiency associated with increases affinity in transthyretin by a known p.A109T mutation in the TTR gene. Hormone and Metabolic Reserach. 2014;46:100–108. doi: 10.1055/s-0033-1358741. [DOI] [PubMed] [Google Scholar]
- 16.Mori Y, Miura Y, Takeuchi H, et al. Gene amplification as a cause for inherited thyroxine-binding globulin excess in two Japanese families. Journal of Clinical Endocrinololgy and Metabolism. 1995;80:3758–3762. doi: 10.1210/jcem.80.12.8530630. [DOI] [PubMed] [Google Scholar]
- 17.Janssen EO, Chen B, Büttner C, et al. Molecular and structural characterization of the heat-resistant thyroxine-binding globulin-Chicago. The Journal of Biological Chemistry. 1995;270:28234–28238. doi: 10.1074/jbc.270.47.28234. [DOI] [PubMed] [Google Scholar]
- 18. thyroidmanager.org. [Google Scholar]
- 19.Blake CC, Geisow MJ, Oatley SJ, et al. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. Journal of Molecular Biology. 1978;121:339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 20.Irace G, Edelhoch H. Thyroxine induced conformational changes in prealbumin. Biochemistry (Moscow) 1978;17:5729–5733. doi: 10.1021/bi00619a020. [DOI] [PubMed] [Google Scholar]
- 21.Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- 22.Refetoff S, Dwulet FE, Benson MD. Reduced affinity for thyroxine in two of three structural thyroxine-binding prealbumin variants associated with familial amyloidotic polyneuropathy. Journal of Clinical Endocrinololgy and Metabolism. 1986;63:1432–1437. doi: 10.1210/jcem-63-6-1432. [DOI] [PubMed] [Google Scholar]
- 23.Moses AC, Lawlor J, Haddow J, Jackson IMD. Familial euthyroid hyperthyroxinemia resulting from increased thyroxine binding to thyroxine-binding prealbumin. New England Journal of Medicine. 1982;306:966–969. doi: 10.1056/NEJM198204223061605. [DOI] [PubMed] [Google Scholar]
- 24.Moses C, Rosen HN, Moller DE, et al. A point mutation in transthyretin increases affinity for thyroxine and produces euthyroid hyperthyroxinemia. Journal of Clinical Investigation. 1990;86:2025–2033. doi: 10.1172/JCI114938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Refetoff S, Marinov VSZ, Tunca H, et al. A new family with hyperthyroxinemia due to transthyretin Val109 misdiagnosed as thyrotoxicosis and resistance to thyroid hormone. Journal of Clinical Endocrinololgy and Metabolism. 1996;81:3335–3340. doi: 10.1210/jcem.81.9.8784093. [DOI] [PubMed] [Google Scholar]
- 26.Scrimshaw BJ, Fellowes AP, Palmer BN, et al. A novel variant of transthyretin (prealbumin), Thr119 to Met, associated with increased thyroxine binding. Thyroid. 1992;2:21–26. doi: 10.1089/thy.1992.2.21. [DOI] [PubMed] [Google Scholar]
- 27.Alves IL, Divino CM, Schussler GC, et al. Thyroxine binding in a TTR met 119 kindred. Journal of Clinical Endocrinololgy and Metabolism. 1993;76:484–488. doi: 10.1210/jcem.77.2.8102146. [DOI] [PubMed] [Google Scholar]
- 28.Almeida Mo, Dumas AM, Lans MC, et al. Thyroxine binding to tranthyretin met 119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine. 1997;6:309–315. doi: 10.1007/BF02820508. [DOI] [PubMed] [Google Scholar]
- 29.Murrell JR, Schoner RG, Liepnieks JJ, et al. Production and functional analysis of normal and variant recombinant human transthyretin proteins. Journal of Biological Chemistry. 1992;267:16595–16600. [PubMed] [Google Scholar]
- 30.Fitch NJS, Akbary MT, Ramsden DB. An inherited non-amyloidogenic transthyretin variant, [Ser6]-TTR, with increased thyroxine-binding affinity, characterized by DNA sequencing. Journal of Endocrinology. 1991;129:309–313. doi: 10.1677/joe.0.1290309. [DOI] [PubMed] [Google Scholar]
- 31.Peters T., Jr Serum albumin. Advances in Protein Chemistry. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- 32.Hollander CS, Bernstein G, Oppenheimer JH. Abnormalities of thyroxine binding in analbuminemia. Journal of Clinical Endocrinology and Metabolism. 1968;28:1064–1066. doi: 10.1210/jcem-28-7-1064. [DOI] [PubMed] [Google Scholar]
- 33.Henneman G, Krenning EP, Otten M, et al. Raised total thyroxine and free thyroxine index but normal free thyroxine. A serum abnormality due to inherited increased affinity of iodothyronines for serum binding protein. Lancet. 1979;1:639–642. doi: 10.1016/s0140-6736(79)91080-8. [DOI] [PubMed] [Google Scholar]
- 34.DeCosimo DR, Fang SL, Braverman LE. Prevalence of familial dysalbuminemic hyperthyroxinemia in Hispanics. Annals of Internal Medicine. 1987;107:780–781. doi: 10.7326/0003-4819-107-5-780_2. [DOI] [PubMed] [Google Scholar]
- 35.Weiss RE, Angkeow P, Sunthornthepvarakul T, et al. Linkage of familial dysalbuminemic hyperthyroxinemia to the albumin gene in a large Amish family. Journal of Clinical Endocrinology and Metabolism. 1995;80:116–121. doi: 10.1210/jcem.80.1.7829599. [DOI] [PubMed] [Google Scholar]
- 36.Sarne DH, Refetoff S. Normal cellular uptake of thyroxine from serum of patients with familial dysalbuminemic hyperthyroxinemia or elevated thyroxine-binding globulin. Journal of Clinical Endocrinology and Metabolism. 1988;67:1166–1170. doi: 10.1210/jcem-67-6-1166. [DOI] [PubMed] [Google Scholar]
- 37.Ross HA, de Rijke YB, Sweep FC. Spuriously high free thyroxine values in familial dysalbuminemic hyperthyroxinemia. Clinical Chemistry. 2011;57:524–525. doi: 10.1373/clinchem.2010.158170. [DOI] [PubMed] [Google Scholar]
- 38.Sunthornthepvarakul T, Angkeow P, Weiss RE, et al. An identical missense mutation in the albumin gene produces familial disalbuminemic hyperthyroxinemia in 8 unrelated families. Biochemical and Biophysical Research Communications. 1994;202:781–787. doi: 10.1006/bbrc.1994.1998. [DOI] [PubMed] [Google Scholar]
- 39.Lalloz MR, Byfield PG, Goel KM, et al. Hyperthyroxinemia due to the coexistence of two raised affinity thyroxine-binding proteins (albumin and prealbumin) in one family. Journal of Clinical Endocrinology and Metabolism. 1987;64:346–352. doi: 10.1210/jcem-64-2-346. [DOI] [PubMed] [Google Scholar]
- 40.Langsteger W, Stockigt JR, Docter R, et al. Familial disalbuminaemic hyperthyroxinaemia and inherited partial TBG deficiency: fist report. Clinical Endocrinology (Oxford) 1994;40:751–758. doi: 10.1111/j.1365-2265.1994.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 41.Wada NHC, Shimizu C, Kijima H, et al. A novel misssense mutation in codon 218 of the albumin gene in a distinct phenotype of familial dysalbuminemic hyperthyroxinemia in a Japanese kindred. Journal of Clinical Endocrinology and Metabolism. 1997;82:3246–3250. doi: 10.1210/jcem.82.10.4276. [DOI] [PubMed] [Google Scholar]
- 42.Pannain S, Feldman M, Eiholzer U, et al. Familial dysalbuminemic hyperthyroxinemia in a Swiss family caused by a mutant albumin (R218P) shows an apparent discrepancy between serum concentration and affinity for thyroxine. Journal of Clinical Endocrinology and Metabolism. 2000;85:2786–2792. doi: 10.1210/jcem.85.8.6746. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg SM, Ferrara AM, Nicholas ES, et al. A Novel Mutation in the Albumin Gene (R218S) Causing Familial Dysalbuminemic Hyperthyroxinemia in a Family of Bangladeshi Extraction. Thyroid. 2014;24:945–950. doi: 10.1089/thy.2013.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenmakers N, Moran C, Campi I, et al. A novel albumin gene mutation (R222I) in familial dysalbuminemic hyperthyroxinemia. Journal of Clinical Endocrinology and Metabolism. 2014;99:E1381–E1386. doi: 10.1210/jc.2013-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yabu Y, Miyai K, Kobayashi A, et al. A new type of albumin with predominantly increased binding affinity for 3,3',5-triiodothyronine in a patient with Graves' disease. Journal of Endocrinological Investigation. 1987;10:163–169. doi: 10.1007/BF03347183. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura S, Kajita Y, Ochi Y. Familial dysalbuminemic hypertriiodothyroninemia in a Japanese kindred. Internal Medicine. 2000;39:50–54. doi: 10.2169/internalmedicine.39.50. [DOI] [PubMed] [Google Scholar]
- 47.Petitpas I, Petersen CE, Ha CE, et al. Structural basis of albumin-thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. Proceedings of the National Academy of Science. 2003;100:6440–6445. doi: 10.1073/pnas.1137188100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen CE, Ha C-E, Harohalli K, et al. Mutagenesis studies of thyroxine binding to human serum albumin define an important structural characteristic of subdomain 2A. Biochemistry (Moscow) 1997;36:7012–7017. doi: 10.1021/bi970225v. [DOI] [PubMed] [Google Scholar]
- 49.Kragh-Hansen U, Minchiotti L, Galliano M, Peters T., Jr Human serum albumin isoforms: genetic and molecular aspects and functional consequences. Biochimica et Biophysica Acta. 2013;1830:5405–5417. doi: 10.1016/j.bbagen.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 50.Sarcione EJ, Aungst CW. Bisalbuminemia associated with albumin thyroxine binding defect. Clinica Chimica Acta. 1962;7:297–298. doi: 10.1016/0009-8981(62)90024-4. [DOI] [PubMed] [Google Scholar]
- 51.Refetoff S, Dumont JE, Vassart G. Thyroid disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogeldstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. 8th edn. Vol. 3. New York: MacGraw-Hill; 2000. pp. 4029–4075. [Google Scholar]
- 52.Janssen OE, Bertenshaw R, Takeda K, et al. Molecular basis of inherited thyroxine-binding globulin defects. Trends in Endocrinololgy and Metabolism. 1992;3:49–53. doi: 10.1016/1043-2760(92)90043-z. [DOI] [PubMed] [Google Scholar]