Abstract
Objective
Understanding tumor microenvironment and its impact on prognosis of HIV-related lymphomas may provide insight into novel therapeutic strategies.
Design
We characterized the relationship between infiltrating immune cells with tumor characteristics, HIV disease history and survival in 80 HIV-related diffuse large B-cell lymphoma (DLBCL) patients diagnosed in the era of combined antiretroviral therapy (1996–2007) at Kaiser Permanente (KP) California. Eighty HIV-unrelated DLBCL patients were included for comparison.
Methods
Data on patients’ clinical history were obtained from KP’s electronic health records. The density of stromal CD4+, CD8+ and FOXP3+ T cells and CD68+ macrophages, as well as tumor molecular characteristics were examined using immunohistochemistry. The associations between stromal immune infiltration and patient’s clinical history or tumor characteristics were examined using Kruskal Wallis tests or Peasrons’ correlation coefficient. The effect of stromal immune infiltration on two-year mortality was evaluated in multivariable logistic regression.
Results
Compared to HIV-unrelated DLBCL, patients with HIV-related DLBCL had significantly reduced stromal CD4+ and FOXP3+ T cells, but increased density of macrophages. Increased density of stromal macrophages was correlated with lower circulating CD4 cell count at DLBCL diagnosis. Tumor molecular characteristics, including BCL6, p53 and cMYC expression, but not EBV infection status, were significantly correlated with stromal immune infiltration, particularly FOXP3+ T cells. A higher density of infiltrating CD8+ T cell was significantly associated with reduced mortality in HIV-related DLBCL patients [odds ratio=0.30 (0.09–0.97) for ≥25% vs. <10%].
Conclusion
These data provide evidence for the prognostic significance of cytotoxic T cells in determining outcomes of HIV-related lymphoma.
Keywords: HIV, Diffuse large B-cell lymphoma, Tumor Microenvironment, Stromal, Prognosis
Introduction
The tumor microenvironment plays a vital role in cancer development[1, 2]. The interaction between tumor cells and stromal cells, such as infiltrating immune cells, fibroblasts and endothelial cells may create a local environment that supports or inhibits tumor growth. Anti-tumor immunity mediated by infiltrating T cells, macrophages and dendritic cells play an important role in determining disease progression and patient outcome in a variety of cancers, including diffuse large B-cell lymphomas[3–5]. To this end, several studies have demonstrated the prognostic significance of the stromal immune cells through gene expression profiling[6–8], immunohistochemistry[9–11] or functional/animal studies[12, 13], lending support to therapeutic strategies that aim to restore immune surveillance in the tumor microenvironment.
Despite knowledge advancements about anti-tumor immunity and its treatment implications in lymphomas in the general population, there is a lack of studies examining the microenvironment for HIV-related lymphomas. As such, little is known about the microenvironment and the role of anti-tumor immunity among immunodeficient populations, such as HIV-infected patients. Liapis and colleagues have for the first time attempted to characterize the microenvironment of HIV-related DLBCL[14]. They showed increased vessel density and altered infiltrating T cell populations compared to patients with sporadic DLBCL. These findings highlighted the need to better understand tumor microenvironment unique to HIV-related lymphomas and its therapeutic implications.
In this study we examined the immune component of the microenvironment, including stromal T-cell subpopulations and tumor-associated macrophages in HIV-related DLBCL. We sought to understand (1) if the composition of infiltrating immune cells differs by clinical and molecular characteristics of the tumor, (2) if the composition of infiltrating immune cells may be affected by HIV disease history such as severe immunosuppression, and (3) if the density of specific infiltrating immune cells can predict patient survival in HIV-related DLBCL. To warrant generalizability to current clinical management of DLBCL in HIV-infected patients, the present study is entirely based on contemporary patients diagnosed in the post-combination antiretroviral therapy (ART) era.
Methods
Study Population
All adult HIV-infected patients (≥18 years) diagnosed with incident DLBCL between 1996 and 2007 were identified from the Kaiser Permanente (KP) Southern and Northern California Health Plans, two large integrated health care delivery systems. DLBCL diagnoses were ascertained from KP’s Surveillance, Epidemiology, and End Results (SEER)-affiliated cancer registries. HIV infection status was identified through record linkage with the health plans’ HIV registries.
A group of HIV-unrelated DLBCL patients were also selected for comparison. Tumor biology can differ by age and DLBCL tend to be diagnosed at younger age in HIV-infected persons. Furthermore, the HIV-infected cohort in California was 95% male and the majority was of non-Hispanic white race. To ensure comparability of HIV-unrelated DLBCL patients to the HIV-related DLBCL patients, we matched subjects 1:1 by age groups (i.e., <30, 30–50 and >50 years), gender and race (white vs. non-white). Appropriate tumor specimens of matched HIV-unrelated DLBCL patients for 4 HIV-related DLBCL patients were unavailable. The comparisons for those 4 HIV-infected patients were thus replaced by additional matched HIV-unrelated DLBCL patients for other HIV-infected patients.
Pathology Review and Tissue Microarray Construction
Archived tumor specimens were retrieved and hematoxylin and eosin stained (H&E) slides were reviewed to confirm the DLBCL diagnosis as well as to identify representative tumor blocks for tissue microarray (TMA) construction (at the UCLA Core Microarray Facility). Whenever possible three 0.6-mm cores from different areas of the donor block were obtained from each case and inserted in a grid pattern into a recipient paraffin block using a tissue arrayer (Beecher Instruments, Silver Spring, MD).
Immunohistochemistry Staining
Immunohistochemistry staining was performed on TMA cores to analyze the stromal expression of CD4 (helper T-cells), CD8 (cytotoxic T-cells), FOXP3 (Treg cells), and CD68 (macrophage). In addition, the expression of the following markers was assessed in the tumor cells: BCL2, BCL6, p53, Ki-67, cMYC. Expression of CD10, MUM1 and BCL6 were used to determine the germinal center (GC) phenotype using the Hans’ algorithm[15]. The percent of stromal cells that expressed CD4, CD8, FOXP3 and CD68, and the percent of tumor cells that expressed Ki-67 were digitally scored on a computerized automated platform. The percent of tumor cells that expressed BCL2, BCL6, p53 and cMYC was scored manually by one study pathologist and confirmed by another. Cases with discrepant scores (about 10%) were resolved by re-review with double headed microscope. Tumor EBV infection was determined by in situ hybridization of EBV encoded RNA and was considered positive if ≥75% of the DLBCL cells had detectable EBV. Among EBV-positive tumors, LMP1 expression was determined based on immunohistochemistry staining.
Normal tonsillar lymphoid tissue was included as a positive control. Negative controls for each case consisted of substituting the primary antibody with isotype specific non-cross reacting antibody matching the primary antibody. The detailed information on TMA construction, antibody, incubation method and signal detection for each marker were described elsewhere[16].
DLBCL Morphologic Variants
DLBCL morphologic variant subtyping was performed by the two study pathologists (Said J and Zha H), who independently reviewed pathology reports, H&E slides and stained tumor marker expression data. Minor classification discrepancies on two patients were resolved by the pathologists after applying the World Health Organization’s 2008 classification system of tumors of the hematopoietic and lymphoid tissues.
Ascertainment of Patient Survival
Two-year mortality was chosen as the outcome because most deaths in HIV-infected patients (85% in our study) occurred within two years after DLBCL diagnosis. Overall mortality ascertainment was complete for all subjects (even if the person terminated KP membership) through record linkage with KP’s membership and utilization files, California’s state death file, and Social Security death records. As such, there was no loss-to-follow up.
Covariates
The International Prognostic Index (IPI) was calculated based on age, clinical stage, extranodal involvement, serum lactose dehydrogenase (LDH), and performance status[17, 18]. Age at DLBCL diagnosis, stage at diagnosis, extranodal involvement, and initial chemotherapy were collected from KP’s cancer registries. Serum LDH level and circulating CD4 cell counts at DLBCL diagnosis (and their nadir) were obtained from the laboratory databases. Performance status and chemotherapy were ascertained from standardized medical record review[19]. We collected HIV disease factors from HIV registries, including prior AIDS diagnosis, use of ART, and duration of known HIV infection.
Statistical Analysis
The density of the five stromal immune cells were calculated and compared by HIV status using the t-test statistic. Next, among HIV-infected patients, we compared the density of the stromal immune cells by stage at diagnosis, DLBCL variant (centroblastic, immunoblastic and plasmablastic), GC phenotype, prior AIDS diagnosis, ART use prior to DLBCL diagnosis, and tumor EBV infection and LMP1 status, using the Kruskal Wallis tests. The association between circulating CD4 cell count (both at DLBCL diagnosis and nadir) and infiltrating immune cells was examined using Pearson’s correlation coefficient. Similarly, the association between infiltrating immune cells and tumor molecular characteristics, including the expression of BCL2, BCL6, p53, Ki-67 and cMYC, was examined using Pearson’s correlation coefficient.
Kaplan-Meier survival curves were generated for CD8+ and FOXP3+ T cells and CD68+ macrophage for the following density categories: <10%, 10–24%, 25–49%, 50–74%, and ≥75%. However, because less than 5 subjects had stromal immune cell density at 50% or greater, the following combined categories were presented: <10%, 10–24%, and ≥25%. Because most HIV-related DLBCL patients had minimal CD4+ T cell infiltration, we dichotomized CD4+ T cells into two categories: <1% and ≥1%. The association between infiltrating immune cells and 2-year overall mortality was examined using bivariate and multivariable logistic regression adjusting for IPI, GC phenotype and DLBCL variant. Multivariable models restricted to those who received chemotherapy treatment were also performed. Missing data were handled using the multiple imputation method described by Rubin[20]. All analyses were performed with SAS Version 9.2; Cary, North Carolina, USA.
Results
The demographic and clinical characteristics of the 80 HIV-related and the 80 matched HIV-unrelated DLBCL patients are presented in Table 1. The mean age at DLBCL diagnosis was similar by HIV status (50 years old) due to matching. Compared with HIV-uninfected patients, HIV-infected patients were more likely to be diagnosed at advanced stage (48% and 29%; p=0.01), with extranodal involvement (43% vs. 11%, p<0.01), GC phenotype (39% vs. 26%; p=0.01), and immunoblastic (23% vs. 6%) or plasmablatic subtypes (8% vs. 1%, p<0.01). HIV-infected patients in this study had a mean circulating CD4 cell count at DLBCL diagnosis of 206 cells/mm3, and a mean 5-years duration of known HIV infection prior to DLBCL diagnosis. In addition, 43% of the HIV-infected DLBCL patients had a prior AIDS diagnosis, and 65% patients had a history of ART use at time of DLBCL diagnosis (Table 1).
Table 1.
Diffuse large B-cell lymphoma patient characteristics and two-year mortality by HIV infection status.
| HIV-positive (N=80) | HIV-negative (N=80) | P value | |
|---|---|---|---|
| Age, year, mean (SD) | 47.9 (9.2) | 50.6 (15.9) | 0.97 |
| Male gendera | 74 (92.5%) | 70 (87.5%) | 0.29 |
| Race/Ethnicity | |||
| White | 47 (58.8%) | 47 (58.8%) | 1.00 |
| Non-White | 33 (41.2%) | 33 (41.2%) | |
| Known duration of HIV infection, year, mean (SD) | 5.2 (5.80) | – | – |
| Prior AIDS diagnosis | 34 (42.5%) | – | – |
| Prior use of ART | 52 (65.0%) | – | – |
| CD4 cell count at DLBCL diagnosis, cells/mm3, mean (SD) | 206.2 (166.9) | – | – |
| Lowest CD4 cell count recorded in KP prior to DLBCL diagnosis, cells/mm3, mean (SD) | 71.2 (66.7) | – | – |
| SEER summary stage | |||
| I(Localized) | 20 (25%) | 38 (47.5%) | 0.01 |
| II(Regional) | 14 (17.5%) | 15 (18.8%) | |
| III(Distant) | 38 (47.5%) | 23 (28.8%) | |
| Unknown | 8 (10%) | 4 (5%) | |
| Extranodal involvement | |||
| Yes | 33 (41.3%) | 9 (11.3%) | <0.01 |
| Unknown | 0 (0%) | 19 (23.8%) | |
| Germinal Center (GC) Phenotype | |||
| GC | 31 (38.8%) | 21 (26.3%) | 0.01 |
| Non GC | 41 (51.3%) | 58 (72.5%) | |
| Unknown | 8 (10.0%) | 1 (1.3%) | |
| DLBCL subtype | |||
| Centroblastic | 56 (70.0%) | 72 (90%) | <0.01 |
| Immunoblastic | 18 (22.5%) | 5 (6.3%) | |
| Plasmablastic | 6 (7.5%) | 1 (1.3%) | |
| Unknown | 0 (0%) | 2 (2.5%) | |
| Received chemotherapy | 60 (75.0%) | 67 (83.8%) | 0.17 |
| Two-year mortality | 37 (46.3%) | 13 (16.3%) | <0.01 |
HIV infection status and stromal immune cells
Density of infiltrating immune cells by HIV infection status is shown in Table 2. The majority of HIV-related DLBCL patients had minimal CD4 T cell infiltration (i.e., 75% of the patients had <1% of stromal CD4+ T cells, data not shown). Compared to HIV-unrelated DLBCL, HIV-related DLBCL showed significantly reduced density of infiltrating CD4 T cells (mean = 1.8% vs. 14.8% for HIV-related vs. HIV-unrelated DLBCL, respectively. P<0.001) and FOXP3+ Treg cells (7.3% vs. 22.5%, p<0.001), but significantly increased density of infiltrating CD8+ T cells (19.9% vs. 14.6%, p=0.017) and macrophages (15.0% vs. 8.7%, p=0.006).
Table 2.
Density of infiltrating immune cells in diffuse large B-cell lymphoma by HIV infection status.
| Stromal marker | HIV-infected N=80 |
HIV-uninfected N=80 |
P-value | |
|---|---|---|---|---|
|
|
||||
| % among all stromal cells | ||||
| CD4+ | Mean (SD) | 1.8% (5.3%) | 14.8% (21.7%) | <0.001 |
| Range | (0.0%–41.6%) | (0.0%–94.6%) | ||
| CD8+ | Mean (SD) | 19.9% (16.1%) | 14.6% (15.6%) | 0.017 |
| Range | (0.4%–67.5%) | (0.0%–73.8%) | ||
| FOXP3+ | Mean (SD) | 7.3% (9.5%) | 22.5% (20.3%) | <0.001 |
| Range | (0.2%–62.5%) | (0.5%–82.8%) | ||
| CD68+ | Mean (SD) | 15.0% (15.4%) | 8.7% (9.7%) | 0.006 |
| Range | (0.6%–68.0%) | (0.1%–49.6%) | ||
Stromal immune cells and HIV disease history
There was a greater degree of stromal macrophage infiltration in those with lower CD4 cell count at DLBCL diagnosis (Pearson’s correlation coefficient = −0.25, p-value= 0.05). A similar but weaker association with stromal macrophages and the nadir circulating CD4 cell count was also observed (Pearson’s correlation coefficient = −0.18, p-value= 0.14). No association was found between circulating CD4 T cells and stromal density of any of the T cells examined (data not shown). However, having an AIDS diagnosis prior to the DLBCL diagnosis was associated with a substantially reduced infiltrating CD4 T cells (mean (SD) =0.6% (6.91%) and 2.7% (0.9%), p-value=0.09). Although all our HIV-related DLBCL patients were diagnosed in the ART era, not all patients had initiated ART at the time of DLBCL diagnosis. To this end, we did not find an association between ART use prior to DLBCL diagnosis and any of the stromal immune cells examined (data not shown).
EBV infection status and stromal immune cells in HIV-related DLBCL
Seventy HIV-related DLBCL patients who had a valid EBV results were included in this analysis. One-third (22 of 70) of them were positive for tumor EBV infection. When we examined the stromal immune cells by EBV status, a greater density of CD4+ T cells was found in the EBV-negative compared to the EBV-positive DLBCLs (mean: 2.2% vs. 0.5%, respectively), although this difference was not statistically significant (p=0.19). EBV-negative HIV-related DLBCL also demonstrated slightly lower density of FOXP3+ Treg cells compared to EBV-positive DLBCL (mean: 6.2% vs. 9.6%, respectively, p=0.07). The level of stromal CD8+ T cell (20.6% vs. 19.8%) and CD68+ macrophages (13.4% vs. 15.9%) appeared to be similar by tumor EBV status. When we explored the link between LMP1 expression and these stromal immune cells among EBV-positive HIV-related DLBCLs, no association was found between LMP1 expression and CD4+ or FOXP3+ T cells (data not shown).
Stromal immune cells and DLBCL characteristics
When we examined the stromal immune cells by DLBCL variant in HIV-related DLBCL, we observed a significantly elevated density of stromal FOXP3+ Treg cells in plasmablastic subtype, compared to centroblastic or immunoblastic subtypes (mean (SD)=13.4% (9.1%), 6.5% (9.2%), and 7.7% (10.6%), respectively. P=0.04). Stromal macrophage density appeared to be elevated in immunoblastic subtype compared to the centroblastic subtype despite lack of statistical significance (mean (SD) = 24.2% (21.3%) and 11.8% (12.0%), respectively). No clear association was found between the stromal immune cells examined and clinical stage or cell-of-origin (data not shown).
When we examined the relationship between stromal immune cells and tumor molecular characteristics, stromal FOXP3+ Treg cells were found to be positively associated with tumor expression of p53 and cMYC [Pearson’s correlation coefficient =0.37 (p<0.01) and 0.32 (p<0.1), respectively], and inversely associated with BCL6 [Pearson’s correlation coefficient = −0.31 (p=0.01)]Table 3).
Table 3.
Correlation between selected tumor marker expression and infiltrating immune cells in HIV-related DLBCL.
| Stromal immune cells | CD4 | CD8 | FOXP3 | CD68 |
|---|---|---|---|---|
|
|
||||
| Selected tumor markers | Pearson’s Correlation Coefficienta (P-value) |
|||
| BCL2 | −0.01 (p=0.93) | 0.08 (p=0.52) | −0.08 (p=0.53) | −0.07 (p=0.57) |
| BCL6 | −0.17 (p=0.17) | −0.00 (p=0.98) | −0.31 (p=0.01) | −0.14 (p=0.26) |
| P53 | 0.25 (p=0.04) | −0.1 (p=0.40) | 0.37 (p<0.01) | 0.13 (p=0.30) |
| cMYC | 0.16 (p=0.18) | −0.01 (p=0.95) | 0.32 (p<0.01) | 0.01 (p=0.91) |
| Ki-67 | 0.04 (p=0.73) | 0.03 (p=0.83) | 0.09 (p=0.50) | −0.22 (p=0.07) |
Numbers in bold are correlations that are statistically significant at P-value <0.10 level.
Pearson’s correlation coefficient ranges from 0 to 1, with 0 being independent and 1 being perfect correlation. A negative value indicates an inverse association.
Stromal immune cells and patient survival
Figure 1 shows the Kaplan-Meier survival curve in HIV-related DLBCL by stromal immune cell density. In multivariable logistic regression adjusting for IPI, germinal center phenotype and DLBCL variant, a higher density of stromal CD8+ T cells was significantly associated with reduced mortality [odds ratio (OR) for ≥25% vs. < 10% = 0.30, 95% confidence interval 0.09–0.97]. Reduced mortality was also suggested for those with higher density of stromal CD4+ T cells [OR for >1% vs. ≤1% = 0.43 (0.14–1.31)], and those with lower density of stromal macrophages [OR for >25% vs. <10% = 2.14 (0.48–9.43)], despite lack of statistical significance (Table 4). Similar OR estimates were obtained when we restricted the analyses to only those who received chemotherapy (data not shown).
Figure 1. Kaplan-Meier survival curve for HIV-related DLBCL by density of infiltrating immune cells.
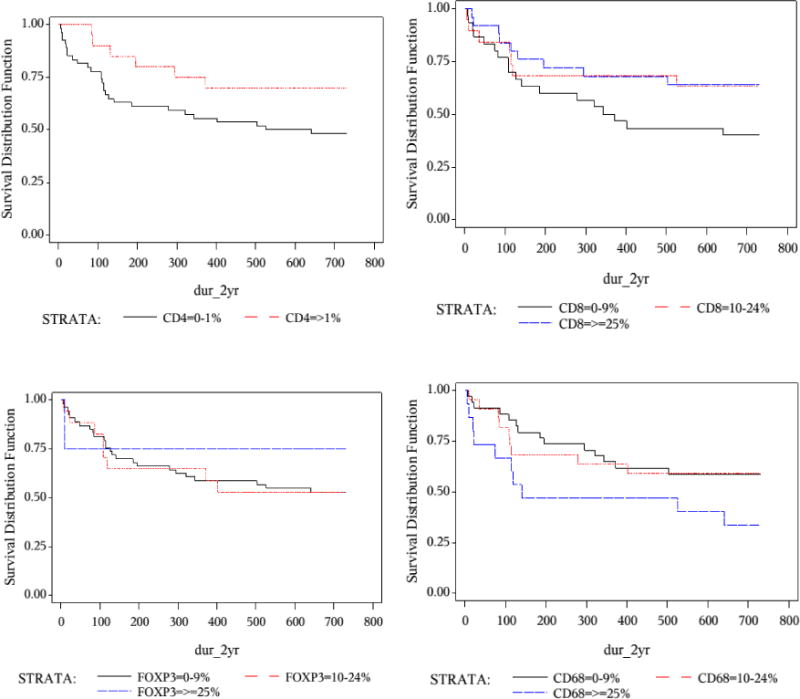
Log-rank test P-value:
p=0.08 for CD4+ T cells; p= 0.17 for CD8+ T cells;
p=0.81 for FOXP3+ T cells; p=0.14 for CD68+ macrophage.
Table 4.
Association between infiltrating immune cells and two-year overall mortality in HIV-related DLBCL patients, crude and adjusted for International Prognostic Index (IPI).
| HIV-infected patients (n=80) Stromal immune cells |
Crude | Adjusted for IPI, germinal center phenotype and DLBCL variant | |
|---|---|---|---|
|
| |||
| Odds ratio (95% CI) | Odds ratio (95% CI) | P-value | |
| CD4 (reference: <1%) | |||
| ≥1% | 0.49 (0.18–1.31) | 0.43 (0.14–1.31) | 0.14 |
| CD8 (reference: <10%) | |||
| 10–24% | 0.41 (0.12–1.37) | 0.37 (0.10–1.31) | 0.12 |
| ≥25% | 0.37 (0.13–1.06) | 0.30 (0.09–0.97) | 0.05 |
| ≥10% vs. <10% | 0.38 (0.15–0.99) | 0.33 (0.12–0.92) | 0.04 |
| FOXP3 (reference: <10%) | |||
| 10–24% | 0.97 (0.32–2.95) | 0.73 (0.21–2.51) | 0.62 |
| ≥ 25% | 0.36 (0.04–3.33) | 0.14 (0.01–1.68) | 0.12 |
| CD68 (reference: <10%) | |||
| 10–24% | 0.86 (0.30–2.48) | 1.04 (0.33–3.21) | 0.95 |
| ≥ 25% | 2.49 (0.74–8.34) | 2.14 (0.48–9.43) | 0.32 |
| ≥ 25% vs. <25% | 2.65 (0.86–8.20) | 2.11 (0.54–8.14) | 0.28 |
Discussion
Little is known about the tumor microenvironment in the setting of HIV-related lymphomas. In this study, we found significant differences in the density of important stromal immune cells between HIV-related and HIV-unrelated DLBCL, confirming findings previously reported by Liapis and colleagues based on a smaller sample[14]. In addition, we observed an association between stromal immune cells and HIV disease history, a novel finding that may have implication for HIV disease management. Most importantly, we are among the first to link the density of stromal immune cells to patient survival outcomes in HIV-related DLBCL and reported the prognostic significance of CD8+ T cells on survival. These findings suggest that novel therapeutic approaches targeting the microenvironment, e.g., by restoring T-cell mediated tumor surveillance may have particular benefits among HIV-infected patients who do not respond well to standard chemotherapy.
The reduced density of Treg cells and elevated infiltration of macrophages seen in HIV-related DLBCL is consistent with a pattern generally associated with poorer prognosis. Intratumoral T cells are thought to have an important impact on antitumor immunity[21]. Treg cells affects immune responses of a variety of immune cells, including T cells, B cells and natural killer cells[22]. Although Treg cells are thought to induce immunosuppressive phenotype, several studies of DLBCL and follicular lymphoma have linked high number of Treg cells to superior survival[23–25]. Tumor-associated macrophages, on the other hand, have generally been shown to correlate with disease progression in a variety of cancers, including DLBCL and follicular lymphomas[26, 27]. As shown in in vitro and in vivo studies, macrophages may promote angiogenesis by secreting cytokines and other angiogenic factors such as the VEGFA and MMP9, which also support the growth of lymphomas[28]. We also observed very limited infiltrating CD4+ T cell population in HIV-related DLBCL. The prognostic significance of infiltrating CD4+ T cells is less clear and may depend on the distribution of subpopulations of CD4+ T cells. However, some studies suggest favorable survival profiles for patients with higher number of overall stromal CD4+ T cells[29, 30].
Interestingly, HIV-related DLBCL patients did not have reduced level of stromal CD8+ T cells in our study. Studies suggest that CD8+ T cells may directly target lymphoma cells[31]. Higher stromal density of CD8+ T cells significantly predicted improved patient survival in our cohort of HIV-related DLBCL, independent of the IPI score. Our finding is consistent with those in lymphomas in the general population[32–34]. The relationship between stromal CD8+ T cells and patient survival outcomes may reflect compromised major histocompatibility complex (MHC) restricted immune function, resulting in a loss of effective tumor immunosurveillance[8]. Loss of cell adhesion molecules in the microenvironment has also been linked to loss of stromal CD8+T cell function, when loss of stromal CD8+ T cell function has been linked to poor patient outcomes[35]. Our findings suggest that CD8+ T cells may be an important component of lymphoma-specific immune response in HIV-related lymphomas. These stromal immune cells may also serve as useful markers for patient risk stratification beyond the traditional clinical IPI algorithm.
An additional novel contribution of this study is the preliminary evidence that host immune history may play a role in shaping the tumor microenvironment. We showed that patients with prior AIDS-defining conditions, primarily opportunistic infections, and low circulating CD4 cell count at DLBCL diagnosis tended to have reduced density of stromal CD4+ T cells and increased macrophage infiltration, respectively. Several previous studies, including our own work, have shown that circulating CD4 cell count and/or prior AIDS are independent prognostic predictors in patients with HIV-related lymphoma[36, 37]. Our data suggest that one of the potential mechanisms of the prognostic impact of these HIV disease factors may be though modifying the tumor microenvironment. If this is confirmed, then the importance of maintaining circulating CD4 cell count would not only be relevant to preventing the development of HIV-related lymphomas, but also would promote a better prognosis in those who develop such malignancies.
EBV infection of tumor cells is seen in about one-third of the contemporary HIV-related DLBCL cases. Recent data suggest that EBV-mediated pathogenesis involved interaction with the tumor microenvironment[38]. Thus, we hypothesized that EBV-positive DLBCLs recruited a particular subset of immune cells. However, contrary to our hypothesis, no clear pattern of stromal immune cells was seen in DLBCLs positive for EBV infection. Although a statistically significant difference was found for Treg cells, the degree of the difference appears unlikely to be clinically meaningful. LMP1 expression was not associated with stromal CD4+ T cells or Treg cells. However, only 22 EBV-positive DLBCL patients were included in this comparison; thus the LMP1 analysis should be considered exploratory. A previous study that examined the presence of EBV infection in pediatric DLBCL patients also found no alteration of the T-cell subsets in EBV-positive tumors[39]. However, these results do not exclude the possibility that the presence of EBV may adversely affect the function of these T cells. In fact, studies suggest that EBV may impair the function of cytotoxic T cells and contribute to decreased immune surveillance[40].
Our results provide some evidence for the bi-directional interactions between the tumor and the microenvironment, as we observed significant correlations between certain molecular tumor characteristics and stromal immune cells. We examined the expression of five oncogenic proteins that are known to play a central role in lymphoma development and progression; three of these are correlated with the stromal density of Treg cells. We also observed a positive association between p53 expression and infiltrating CD4+ T cells population. It has been proposed that functional p53 protein is involved in the induction of anti-tumor CD4+ T-cell response via expression of major-histocompatibility-complex (MHC) class-II antigens, and the absence of p53 may reduce the induction of CD4+ T cell activity[41]. Another potential explanation to the association we observed might be that the p53 peptides presented to immune cells may serve as a potential mechanism for triggering anti-tumor immune response[42]. It is also possible that some of the correlations we observed simply reflected certain microenvironment characteristics that simultaneously promote/inhibit biologic processes in both the tumor and the stromal. For example, pro-inflammatory cytokines such as IL6 and TNF-α have been shown to trigger the expression of BCL6 in multiple myeloma cells[43]. IL-6 is known to be a negative regulator for Treg cells[44]. It is thus possible that the inverse association observed between tumor BCL6 expression and infiltrating Treg cells may be due to a pro-inflammatory cytokine environment. Our study, however, was not designed to address if it is the specific tumor characteristics that recruit (or through de novo generation) the Treg cells, or whether the specific stromal immunity that induces or promotes the expression of certain oncogenic proteins, or both. To date, knowledge on the biologic processes that underlie the interplay between tumor and its microenvironment remains limited. Additional laboratory and clinical studies are needed to further elucidate the interaction between tumor, tissue and system level factors.
A potential limitation of this study was the limited sample size for certain subgroup comparisons. This limitation in sample size may also explain the lack of statistical significance between the density of stromal Treg cells and macrophages with survival outcomes. However, our study is based on a well-defined cohort of HIV-related DLBCL patients from the ART era that is among the largest reported in the literature. Another limitation is that we did not distinguish the sub-populations of stromal CD4+ T cells and tumor associated macrophages, and we did not measure cytokine production or other factors that may affect immune cell functions.
In conclusion, we found that the composition of stromal immune cells in HIV-related DLBCL was significantly different from that found in HIV-unrelated DLBCL, and were consistent with a pattern associated with poorer prognosis. We also determined the prognostic significance of stromal CD8+ T cells in HIV-related lymphomas in a clinical population. We found that host HIV disease history, including low circulating CD4 cell count and prior AIDS diagnosis were associated with the composition of tumor microenvironment. As such, early initiation of ART and maintenance of immune function may have an impact not only on the incidence of lymphomas but also on disease prognosis. These data suggest that antitumor immunity plays an important role in disease progression and treatment outcomes in HIV-infected patients, but it can be compromised. Patient management strategies directed at restoring immunosurveillance at the tumor microenvironment level may thus hold promise for treating HIV-related lymphomas.
Acknowledgments
The authors would like to thank Ms. Wendy Leyden for programming support and Ms. Courtney Ellis and Ms. Michelle McGuire for project management support.
Source of Funding:
This work is supported by National Cancer Institute grant R01CA134234-01 Prognostic Markers for HIV-Positive Diffuse Large B-Cell Lymphoma.
Footnotes
Authorship Contribution:
CC conceptualized the study. CC, MJS and JS led the study design. CC and MJS led the collection of clinical data. JS and HDZ performed pathology review and diagnosis confirmation. JS and BC performed laboratory assays and tumor marker data collection. LX and LC performed data cleaning, editing and statistical analyses. OM, DIA and RH assisted with the study design, data collection and result interpretation.
Final approval of manuscript:
All authors have critically reviewed and edited the manuscript.
Duplicate Publication:
This manuscript has not been previously published and is not under consideration elsewhere. The contents of this manuscript will not be copyrighted, submitted, or published elsewhere while acceptance by your journal is under consideration.
Conflicts of Interest:
The authors have no financial conflict of interest to disclose.
References
- 1.Sonnenschein C, Soto AM. Theories of carcinogenesis: an emerging perspective. Semin Cancer Biol. 2008;18:372–377. doi: 10.1016/j.semcancer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bizzarri M, Cucina A. Tumor and the microenvironment: a chance to reframe the paradigm of carcinogenesis? Biomed Res Int. 2014;2014:934038. doi: 10.1155/2014/934038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coupland SE. The challenge of the microenvironment in B-cell lymphomas. Histopathology. 2011;58:69–80. doi: 10.1111/j.1365-2559.2010.03706.x. [DOI] [PubMed] [Google Scholar]
- 4.Galand C, Donnou S, Molina TJ, Fridman WH, Fisson S, Sautes-Fridman C. Influence of Tumor Location on the Composition of Immune Infiltrate and Its Impact on Patient Survival. Lessons from DCBCL and Animal Models. Front Immunol. 2012;3:98. doi: 10.3389/fimmu.2012.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott DW, Steidl C. The classical Hodgkin lymphoma tumor microenvironment: macrophages and gene expression-based modeling. Hematology Am Soc Hematol Educ Program. 2014;2014:144–150. doi: 10.1182/asheducation-2014.1.144. [DOI] [PubMed] [Google Scholar]
- 6.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 8.Rimsza LM, Roberts RA, Miller TP, Unger JM, LeBlanc M, Braziel RM, et al. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: a follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood. 2004;103:4251–4258. doi: 10.1182/blood-2003-07-2365. [DOI] [PubMed] [Google Scholar]
- 9.Chang KC, Huang GC, Jones D, Lin YH. Distribution patterns of dendritic cells and T cells in diffuse large B-cell lymphomas correlate with prognoses. Clin Cancer Res. 2007;13:6666–6672. doi: 10.1158/1078-0432.CCR-07-0504. [DOI] [PubMed] [Google Scholar]
- 10.Hasselblom S, Sigurdadottir M, Hansson U, Nilsson-Ehle H, Ridell B, Andersson PO. The number of tumour-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br J Haematol. 2007;137:364–373. doi: 10.1111/j.1365-2141.2007.06593.x. [DOI] [PubMed] [Google Scholar]
- 11.Lippman SM, Spier CM, Miller TP, Slymen DJ, Rybski JA, Grogan TM. Tumor-infiltrating T-lymphocytes in B-cell diffuse large cell lymphoma related to disease course. Mod Pathol. 1990;3:361–367. [PubMed] [Google Scholar]
- 12.Wang W, Kardosh A, Su YS, Schonthal AH, Chen TC. Efficacy of celecoxib in the treatment of CNS lymphomas: an in vivo model. Neurosurg Focus. 2006;21:E14. doi: 10.3171/foc.2006.21.5.15. [DOI] [PubMed] [Google Scholar]
- 13.Mineo JF, Scheffer A, Karkoutly C, Nouvel L, Kerdraon O, Trauet J, et al. Using human CD20-transfected murine lymphomatous B cells to evaluate the efficacy of intravitreal and intracerebral rituximab injections in mice. Invest Ophthalmol Vis Sci. 2008;49:4738–4745. doi: 10.1167/iovs.07-1494. [DOI] [PubMed] [Google Scholar]
- 14.Liapis K, Clear A, Owen A, Coutinho R, Greaves P, Lee AM, et al. The microenvironment of AIDS-related diffuse large B-cell lymphoma provides insight into the pathophysiology and indicates possible therapeutic strategies. Blood. 2013;122:424–433. doi: 10.1182/blood-2013-03-488171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 16.Chao C, Silverberg MJ, Martinez-Maza O, Chi M, Abrams DI, Haque R, et al. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clin Cancer Res. 2012;18:4702–4712. doi: 10.1158/1078-0432.CCR-11-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi G, Donisi A, Casari S, Re A, Cadeo G, Carosi G. The International Prognostic Index can be used as a guide to treatment decisions regarding patients with human immunodeficiency virus-related systemic non-Hodgkin lymphoma. Cancer. 1999;86:2391–2397. [PubMed] [Google Scholar]
- 18.Mounier N, Spina M, Gabarre J, Raphael M, Rizzardini G, Golfier JB, et al. AIDS-related non-Hodgkin lymphoma: final analysis of 485 patients treated with risk-adapted intensive chemotherapy. Blood. 2006;107:3832–3840. doi: 10.1182/blood-2005-09-3600. [DOI] [PubMed] [Google Scholar]
- 19.Salloum RG, Smith TJ, Jensen GA, Lafata JE. Using claims-based measures to predict performance status score in patients with lung cancer. Cancer. 2011;117:1038–1048. doi: 10.1002/cncr.25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 21.Afshar-Sterle S, Zotos D, Bernard NJ, Scherger AK, Rodling L, Alsop AE, et al. Fas ligand-mediated immune surveillance by T cells is essential for the control of spontaneous B cell lymphomas. Nat Med. 2014;20:283–290. doi: 10.1038/nm.3442. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta A, Saxena R. Regulatory T cells: a review. Natl Med J India. 2012;25:341–351. [PubMed] [Google Scholar]
- 23.Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW, et al. An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma. 2014 doi: 10.3109/10428194.2013.879713. [DOI] [PubMed] [Google Scholar]
- 24.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 25.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 26.Marchesi F, Cirillo M, Bianchi A, Gately M, Olimpieri OM, Cerchiara E, et al. High density of CD68+/CD163+ tumour-associated macrophages (M2-TAM) at diagnosis is significantly correlated to unfavorable prognostic factors and to poor clinical outcomes in patients with diffuse large B-cell lymphoma. Hematol Oncol. 2014 doi: 10.1002/hon.2142. [DOI] [PubMed] [Google Scholar]
- 27.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 28.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keane C, Gill D, Vari F, Cross D, Griffiths L, Gandhi M. CD4(+) tumor infiltrating lymphocytes are prognostic and independent of R-IPI in patients with DLBCL receiving R-CHOP chemo-immunotherapy. Am J Hematol. 2013;88:273–276. doi: 10.1002/ajh.23398. [DOI] [PubMed] [Google Scholar]
- 30.Wahlin BE, Sundstrom C, Holte H, Hagberg H, Erlanson M, Nilsson-Ehle H, et al. T cells in tumors and blood predict outcome in follicular lymphoma treated with rituximab. Clin Cancer Res. 2011;17:4136–4144. doi: 10.1158/1078-0432.CCR-11-0264. [DOI] [PubMed] [Google Scholar]
- 31.Grube M, Rezvani K, Wiestner A, Fujiwara H, Sconocchia G, Melenhorst JJ, et al. Autoreactive, cytotoxic T lymphocytes specific for peptides derived from normal B-cell differentiation antigens in healthy individuals and patients with B-cell malignancies. Clin Cancer Res. 2004;10:1047–1056. doi: 10.1158/1078-0432.ccr-03-0075. [DOI] [PubMed] [Google Scholar]
- 32.Rajnai H, Heyning FH, Koens L, Sebestyen A, Andrikovics H, Hogendoorn PC, et al. The density of CD8+ T-cell infiltration and expression of BCL2 predicts outcome of primary diffuse large B-cell lymphoma of bone. Virchows Arch. 2014;464:229–239. doi: 10.1007/s00428-013-1519-9. [DOI] [PubMed] [Google Scholar]
- 33.Laurent C, Muller S, Do C, Al-Saati T, Allart S, Larocca LM, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118:5371–5379. doi: 10.1182/blood-2011-04-345777. [DOI] [PubMed] [Google Scholar]
- 34.Alvaro T, Lejeune M, Salvado MT, Lopez C, Jaen J, Bosch R, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24:5350–5357. doi: 10.1200/JCO.2006.06.4766. [DOI] [PubMed] [Google Scholar]
- 35.Stopeck AT, Gessner A, Miller TP, Hersh EM, Johnson CS, Cui H, et al. Loss of B7.2 (CD86) and intracellular adhesion molecule 1 (CD54) expression is associated with decreased tumor-infiltrating T lymphocytes in diffuse B-cell large-cell lymphoma. Clin Cancer Res. 2000;6:3904–3909. [PubMed] [Google Scholar]
- 36.Bower M, Gazzard B, Mandalia S, Newsom-Davis T, Thirlwell C, Dhillon T, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med. 2005;143:265–273. doi: 10.7326/0003-4819-143-4-200508160-00007. [DOI] [PubMed] [Google Scholar]
- 37.Robotin MC, Law MG, Milliken S, Goldstein D, Garsia RJ, Dolan GM, et al. Clinical features and predictors of survival of AIDS-related non-Hodgkin’s lymphoma in a population-based case series in Sydney, Australia. HIV Med. 2004;5:377–384. doi: 10.1111/j.1468-1293.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 38.Cader FZ, Vockerodt M, Bose S, Nagy E, Brundler MA, Kearns P, et al. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood. 2013;122:4237–4245. doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- 39.Cohen M, De Matteo E, Narbaitz M, Carreno FA, Preciado MV, Chabay PA. Epstein-Barr virus presence in pediatric diffuse large B-cell lymphoma reveals a particular association and latency patterns: analysis of viral role in tumor microenvironment. Int J Cancer. 2013;132:1572–1580. doi: 10.1002/ijc.27845. [DOI] [PubMed] [Google Scholar]
- 40.Liu WL, Lin YH, Xiao H, Xing S, Chen H, Chi PD, et al. Epstein-Barr Virus Infection Induces Indoleamine 2,3-Dioxygenase Expression in Human Monocyte-Derived Macrophages through p38/Mitogen-Activated Protein Kinase and NF-kappaB Pathways: Impairment in T Cell Functions. J Virol. 2014;88:6660–6671. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeki K, Tanaka Y, Morimoto I, Nishimura Y, Kimura A, Yamashita U, et al. Induction of expression of MHC-class-II antigen on human thyroid carcinoma by wild-type p53. Int J Cancer. 1998;75:391–395. doi: 10.1002/(sici)1097-0215(19980130)75:3<391::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol Immunother. 2005;54:1072–1081. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hideshima T, Mitsiades C, Ikeda H, Chauhan D, Raje N, Gorgun G, et al. A proto-oncogene BCL6 is up-regulated in the bone marrow microenvironment in multiple myeloma cells. Blood. 2010;115:3772–3775. doi: 10.1182/blood-2010-02-270082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]


