Abstract
High and low nuclear levels of the conserved transcriptional regulator β-catenin distinguish multiple sister cell fates to specify endoderm and mesoderm during early embryogenesis in a chordate embryo.
β-catenin, a protein that functions both as a cell adhesion molecule and as a transcriptional regulator, has essential and diverse roles in animal embryogenesis [1,2]. One very early role in many animal phyla is the specification of three distinct germ layers — ectoderm, mesoderm and endoderm — along the animal-vegetal axis. Cells at the animal pole have low levels of nuclear β-catenin and adopt the ectoderm fate, producing skin, nervous system, and head structures. Higher nuclear β-catenin levels instruct cells at the opposite, vegetal pole to adopt endomesodermal fates that later segregate into endoderm (gut) and mesoderm (muscle and blood). Most animal embryos examined use nuclear β-catenin to specify endomesoderm, suggesting an ancient origin for this early role. Remarkably, in two animal species, the nematode Caenorhabditis elegans and the annelid Platynereis dumerilii, β-catenin further acts to distinguish sister cell fates after nearly all embryonic cell divisions [3]. In these two species, the distinct fates of sister cells depend on high and low levels of nuclear β-catenin. This highly reiterative cell-fate specification mechanism had so far been found only in two protostome invertebrates. Now, in a recent Current Biology paper Hudson et al. [4] show that sequential differences in β-catenin levels specify endomesoderm very early in a chordate embryo, that of the ascidian sea squirt Ciona intestinalis. Finding such β-catenin driven binary cell fate decisions in a chordate embryo suggests that these β-catenin functions might share an ancient and common evolutionary origin.
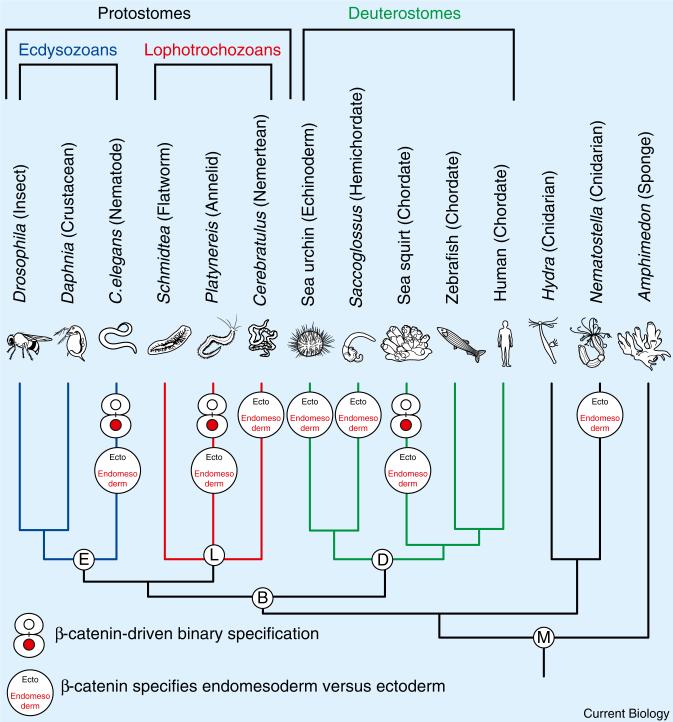
How do different β-catenin levels specify different cell fates? In brief, β-catenin is a potent regulator that, typically in response to Wnt pathway signaling, accumulates to high levels and converts a transcriptional repressor into an activator. High levels of nuclear β-catenin thereby promote transcription of target genes that specify cell fate, while low β-catenin levels repress target genes, and a different fate ensues. Thus, high and low levels of β-catenin provide a simple switch for specifying distinct cell fates [1]. β-catenin specifies endomesodermal (vegetal pole) versus ectodermal (animal pole) cell fates in embryos of animals from all over the animal tree of life (Figure 1) [5–7]. Species in which such a role has been found include all major subdivisions of the tree: deuterostomes (sea urchins, hemichordates, ascidians), lophotrochozoans (nemerteans, annelids), and ecdysozoans (nematodes) [8–14]. Thus, the β-catenin-dependent specification of endomesoderm versus ectoderm might constitute an evolutionarily ancient mechanism of early animal development.
Figure 1. β-catenin-driven cell fate specification processes in metazoan species.
Endomesoderm and binary cell fate specification functions for β-catenin in different species, and how these species are related to each other within the metazoan tree of life, are shown. Higher bilaterian animals are subdivided into three major subdivisions: the deuterostomes (e.g., sea urchins (echinoderms), sea squirts and vertebrates (chordates)), and two protostome divisions, the ecdysozoans (e.g. C. elegans (nematodes) and Drosophila (arthropods)) and the lophotrochozoans (e.g. Platynereis (annelids) and Cerebratulus (nemerteans)).
β-catenin also controls cell fate in many other developmental contexts. For instance, in C. elegans [15], β-catenin operates a cell fate switch that controls anterior versus posterior cell fate choices throughout embryogenesis, a process termed ‘binary cell fate specification’ [16,17]. β-catenin levels are low in anterior and high in posterior sister cells. Furthermore, experimental increase of β-catenin levels in anteriorly born cells causes them to adopt posterior fates, while reducing β-catenin function causes posteriorly born cells to adopt anterior fates. This mechanism reiteratively specifies anterior versus posterior cell fates for nearly every sister cell pair born throughout C. elegans embryogenesis [18]. More recently, a β-catenin-mediated binary specification mechanism was discovered in an animal only distantly related to nematodes, the annelid Platynereis dumerilii, where high β-catenin levels specify vegetal fates and low levels animal fates [13].
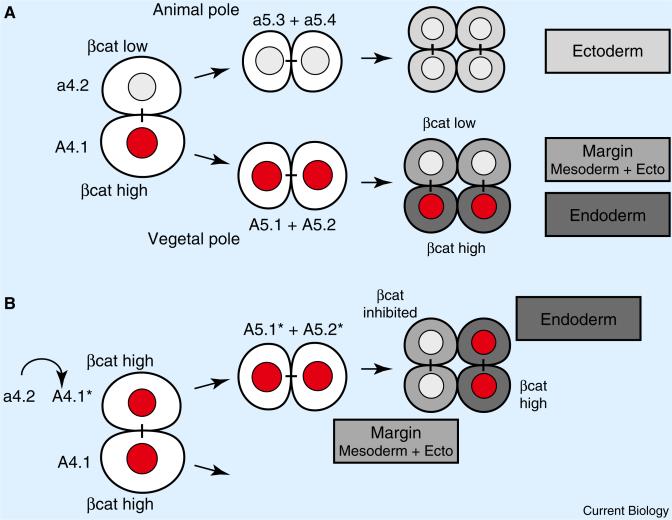
While β-catenin-dependent endomesoderm specification has been well established in deuterostomes (Figure 1), the use of reiterative β-catenin switches to specify lineages of sister cell fates had not been seen outside of nematode and polychaete worms [3,6]. Now Hudson et al. [4] elegantly document in chordate embryos two binary β-catenin switches that distinguish animal and vegetal sister cell fates to specify endomesodermal germ layers. The early embryos of the ascidian Ciona intestinalis that Hudson et al. [4] manipulated exhibit a stereotyped and bilaterally symmetric cleavage pattern. The authors examined β-catenin levels in early embryos and found that high levels of β-catenin, in the vegetal pole daughter cells produced by two consecutive rounds of animal-vegetal oriented cell divisions, correlate with the specification first of endomesoderm and then endoderm (Figure 2A).
Figure 2. β-catenin-driven binary cell fate switches in the ascidian embryo.
(A) Two β-catenin switches in wild-type embryos after animal-vegetal oriented cell divisions. The scheme depicts one of the two sister cell pairs a4.2 and A4.1 from an 8-cell stage ascidian embryo, and its progeny (anterior views, animal pole to the top, vegetal pole to the bottom). The animal-pole sister a4.2 and its descendants exhibit only low levels of nuclear β-catenin protein, and will adopt ectodermal cell fates. The vegetal-pole sister A4.1, and its descendants A5.1 and A5.2 exhibit high levels of nuclear β-catenin adopting endomesodermal cell fate. Both, A5.1 and A5.2 divide to form animal-pole sisters with low β-catenin levels adopting margin cell fate (mesoderm and ectoderm), and vegetal-pole sisters with high β-catenin levels adopting endodermal cell fates. (B) Ectopic β-catenin switches specify endoderm versus margin cell fate in the ectodermal cell lineage a4.2. Ectopically elevating β-catenin levels in the animal pole sister a4.2 causes a4.2 to adopt the endomesodermal cell fate of its vegetal pole sister A4.1, and the subsequent formation of an ectopic A5.1* and A5.2* daughter cell pair. Ectopic inhibition of β-catenin in A5.1* causes its sister cell pair to adopt margin cell fate, whereas high levels of β-catenin in A5.2* promote endodermal cell fate. High and low nuclear levels of β-catenin protein are indicated (red nucleus: βcat high; white nucleus: βcat low). Sister cells are connected by black bars. Adopted cell fates are shown in boxes, and grey scale-coded accordingly in sister cells.
To test whether the high and low levels of β-catenin are important for distinguishing sister cell fates, Hudson et al. [4] manipulated β-catenin levels in subsets of early embryonic cells [4]. Raising β-catenin levels in animal pole cells caused them to adopt the fates of their vegetal sister cells. Conversely, blocking β-catenin's transcriptional function in vegetal pole cells caused them to adopt the fates of their animal sister cells. Most impressively, Hudson et al. [4] generated ectopic and sequential β-catenin switches entirely within an ectodermal lineage that normally never shows high levels of nuclear β-catenin (Figure 2B). To do this, they first raised β-catenin levels in animal pole cells, causing them to adopt the endomesodermal fate of their vegetal pole sisters. The transformed animal pole sister cells divide horizontally to form two daughter cells. Then, the authors blocked β-catenin function in one transformed daughter cell, causing its descendants to adopt mesodermal fates. More precisely, these descendants adopted margin cell fates, producing both mesoderm and some ectoderm. At the same time, the authors maintained high β-catenin levels in the other transformed daughter cell, causing its descendants to adopt endodermal cell fates. Thus, Hudson et al. [4] ectopically recreated the β-catenin switch sequence that specifies endoderm and mesoderm/margin, and thereby convincingly demonstrate that this β-catenin switch is sufficient for the binary control of vegetal versus animal sister cell fates in early ascidian embryos. In contrast to C. elegans and Platynereis, there is, however, no evidence that this switch acts beyond these early stages of ascidian embryogenesis.
As β-catenin-mediated binary specification has now been found in protostome and deuterostome species: a nematode, a lophotrochozoan [13,16,17], and now a chordate [4], its presence in all major higher metazoan animal lineages is consistent with an ancient evolutionary origin for this developmental mechanism (Figure 1). But thus far global binary β-catenin-mediated specification has been found only in embryos that develop rapidly, with stereotyped and nearly invariant cell lineages, where it may be particularly useful.
What, then, is the evolutionary relationship of binary specification and endomesoderm specification? In C. elegans, Platynereis and Ciona, β-catenin-mediated binary specification segregates endomesoderm from ectoderm, suggesting an ancient and common evolutionary origin for these β-catenin functions [4,12,13]. Whether and how global β-catenin switches evolved from the more general and widely conserved role of β-catenin in specifying endomesoderm, or vice versa, are intriguing questions. Finally, it will be interesting to see if animal embryos have applied binary β-catenin switches to other developmental contexts, including vertebrate stem cell maintenance and differentiation [19,20].
References
- 1.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munro E, Bowerman B. Cellular symmetry breaking during Caenorhabditis elegans development. Cold Spring Harb. Perspect. Biol. 2009;1:a003400. doi: 10.1101/cshperspect.a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson C, Kawai N, Negishi T, Yasuo H. β-catenin-driven binary fate specification segregates germ layers in ascidian embryos. Curr. Biol. 2013;23:491–495. doi: 10.1016/j.cub.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 6.Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu R, Martindale MQ. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- 8.Darras S, Gerhart J, Terasaki M, Kirschner M, Lowe CJ. beta-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development. 2011;138:959–970. doi: 10.1242/dev.059493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry JQ, Perry KJ, Wever J, Seaver E, Martindale MQ. Beta-catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Dev. Biol. 2008;317:368–379. doi: 10.1016/j.ydbio.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Takada N, Satoh N, Satou Y. (beta)-catenin mediates the specification of endoderm cells in ascidian embryos. Development. 2000;127:3009–3020. doi: 10.1242/dev.127.14.3009. [DOI] [PubMed] [Google Scholar]
- 11.Logan CY, Miller JR, Ferkowicz MJ, McClay DR. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development. 1999;126:345–357. doi: 10.1242/dev.126.2.345. [DOI] [PubMed] [Google Scholar]
- 12.Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- 13.Schneider SQ, Bowerman B. beta-Catenin asymmetries after all animal/vegetal- oriented cell divisions in Platynereis dumerilii embryos mediate binary cell-fate specification. Dev. Cell. 2007;13:73–86. doi: 10.1016/j.devcel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Peter IS, Davidson EH. A gene regulatory network controlling the embryonic specification of endoderm. Nature. 2011;474:635–639. doi: 10.1038/nature10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaletta T, Schnabel H, Schnabel R. Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature. 1997;390:294–298. doi: 10.1038/36869. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–2695. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- 17.Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2007;104:3231–3236. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- 19.Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 20.Sokol SY. Maintaining embryonic stem cell pluripotency with Wnt signaling. Development. 2011;138:4341–4350. doi: 10.1242/dev.066209. [DOI] [PMC free article] [PubMed] [Google Scholar]