Abstract
Aminolevulinic acid (ALA) is the first metabolite in the heme biosynthesis pathway in humans. In addition to the end product heme, this pathway also produces other porphyrin metabolites. Protoporphyrin (PpIX) is one heme precursor porphyrin with good fluorescence and photosensitizing activity. Because tumors and other proliferating cells tend to exhibit a higher level of PpIX than normal cells after ALA incubation, ALA has been used as a prodrug to enable PpIX fluorescence detection and photodynamic therapy (PDT) of lesion tissues. Extensive studies have been carried out in the past twenty years to explore why some tumors exhibit elevated ALA-mediated PpIX and how to enhance PpIX levels to achieve better tumor detection and treatment. Here we would like to summarize previous research in order to stimulate future studies on these important topics. In this review, we focus on summarizing tumor-associated alterations in heme biosynthesis enzymes, mitochondrial functions and porphyrin transporters that contribute to ALA-PpIX increase in tumors. Mechanism-based therapeutic strategies for enhancing ALA-based modalities including iron chelators, differentiation agents and PpIX transporter inhibitors are also discussed.
Keywords: aminolevulinic acid (ALA), protoporphyrin IX (PpIX), photodynamic therapy (PDT), tumor detection, fluorescence, heme biosynthesis
1. Introduction
Considered as pigments of life, porphyrins attract tremendous human curiosity about their chemistry, biosynthesis, role in homeostasis and pathogenesis, and potential as therapeutic agents. As a class of tetrapyrroles with highly conjugated heterocyclic structure, porphyrins typically have intense absorption of light in the visible range, giving the characteristic red color in animals (due to heme) and green color in plants (due to chlorophyll). It was nearly 150 years ago that hematoporphyrin (HP), a crude porphyrin extract from blood, was first shown to have fluorescence property and about 100 years ago that the fluorescence of HP was found useful for detecting tumors [1]. The photosensitizing property of porphyrins, the ability to convert absorbed light energy into the production of cytotoxic reactive species in the presence of oxygen, was first recognized in the 1900s using HP and extensively studied since the 1970s using partially purified HP preparations [2]. These included hematoporphyrin derivatives (HPD) and Photofrin, which ultimately led to the world-wide approval of Photofrin-mediated photodynamic therapy (PDT) [1].
Because all porphyrins are biosynthesized from aminolevulinic acid (ALA), an early precursor in the heme biosynthetic pathway that is found in nearly all mammalian cells, ALA can be used to boost the production of endogenous porphyrins for many diagnostic and therapeutic uses. Following the pioneer work by Malik [3], Kennedy and Pottier [4] and Moan and Peng [5] who showed enhanced ALA-mediated protoporphyrin IX (PpIX) accumulation in tumor cells and effective cell destruction after light illumination, ALA was rapidly established as a promising PDT agent. With proven effectiveness in eliminating unwanted cells, good selectivity and excellent cosmetic effect, ALA-PDT received world-wide approval in the late 1990s and has become a mainstream treatment in dermatology [6]. Its applications in managing other types of cancers and non-cancer diseases are being actively explored as well [7,8]. Not only is it a remarkable PDT agent, ALA is also a useful imaging probe. With a broad red fluorescence emission extending close to the near-infrared region, ALA-mediated PpIX fluorescence is being used to guide the resection of brain and bladder tumors with encouraging clinical outcomes [9,10].
The key to the successful use of ALA as a PDT and imaging agent lies in the preferential accumulation of PpIX in target cells following ALA administration. Extensive research has been performed to determine the molecular mechanism involved in enhanced ALA-PpIX in tumor cells. In this special issue on Advances in PDT, we would like to summarize research on this topic over the past two decades. We begin with an overview of the biosynthesis and transport of PpIX, and then summarize current understanding on the mechanism involved in preferential ALA-mediated PpIX synthesis and accumulation in tumors. Next, we discuss mechanism-based therapeutic strategies for enhancing ALA-based tumor detection and PDT. Finally, we end with perspectives on areas for future studies. For readers who are interested in the clinical applications of ALA and its derivatives, there are excellent recent reviews [7,11,12,13] including one on ALA-PpIX fluorescence-guided glioblastoma resection in this issue [13]. We focus this review on the molecular mechanism underlying elevated ALA-PpIX in tumors and mechanism-based therapeutic approaches for enhancing ALA-based modalities with the goal of encouraging further research in these important areas.
2. Biosynthesis and Transport of PpIX
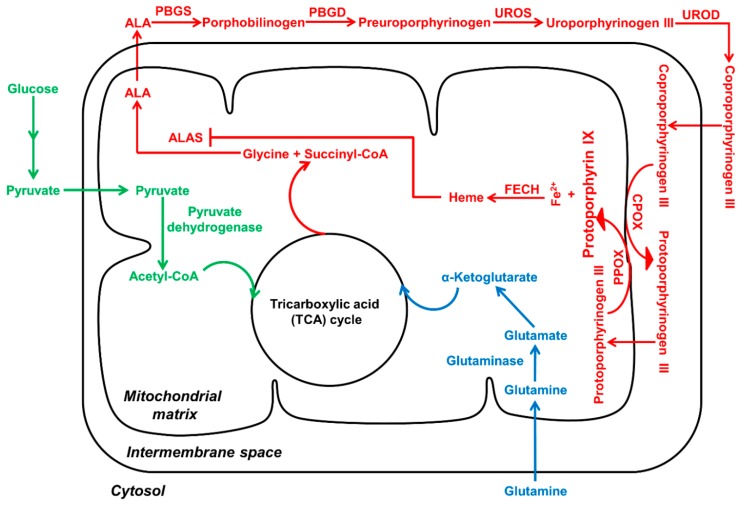
PpIX is a precursor to heme, the final product of the heme biosynthesis pathway (Figure 1). This pathway includes both mitochondrial and cytosolic processes catalyzed by a total of eight enzymes [14]. Seven enzymes are involved in PpIX synthesis and the last one converts PpIX to heme. The first enzymatic step in the heme biosynthesis pathway is the generation of ALA from glycine and succinyl coenzyme A by ALA synthase (ALAS) in the mitochondrion. ALA then migrates to the cytosol where two molecules of ALA are condensed to form porphobilinogen (PBG), the first monopyrrole in the pathway, in a reaction catalyzed by ALA dehydratase (ALAD), also known as porphobilinogen synthase (PBGS). Four molecules of PBG are connected to form hydroxymethylbilane (HMB), the first tetrapyrrole in the pathway, by porphobilinogen deaminase (PBGD), also known as hydroxymethylbilane synthase (HMBS). Linear tetrapyrrole HMB is closed to form cyclic uroporphyrinogen III by uroporphyrinogen III synthase (UROS). Decarboxylation of uroporphyrinogen III by uroporphyrinogen III decarboxylase (UROD) leads to coproporphyrinogen III. Coproporphyrinogen III is then transported back into mitochondria to undergo oxidative decarboxylation by coproporphyrinogen III oxidase (CPOX) to generate protoporphyrinogen III, which is further oxidized by protoporphyrinogen III oxidase (PPOX) to produce PpIX with aromatic chemical structure. PpIX is subsequently chelated with ferrous iron to form heme in mitochondria catalyzed by ferrochelatase (FECH) [14]. Interestingly, heme has essentially no fluorescence and photosensitizing activity, whereas PpIX possesses fluorescence and photosensitizing ability.
Figure 1.
Heme biosynthesis pathway (in red) connects with glucose (in green) and glutamine (in blue) metabolic pathways. Porphyrin synthesis converges with energy metabolism through TCA (tricarboxylic acid) cycle. Enhanced glycolysis and glutaminolysis in tumor cells may activate heme biosynthetic pathway to ensure energy production and avoid the accumulation of TCA metabolites.
Because free porphyrin metabolites such as PpIX and heme are prone to cause oxidative stress and cell damage, cells maintain a low level of free porphyrins through mechanisms including well-coordinated heme biosynthesis and degradation, rapid utilization of free heme for hemoprotein synthesis, and extracellular transport of excess free porphyrins [15]. Heme biosynthesis is negatively regulated by the intracellular level of heme, which inhibits heme biosynthesis by imposing negative feedback inhibition on the rate-limiting enzyme ALAS and stimulates heme degradation by promoting the expression of heme degradation enzyme heme oxygenase [14]. Free heme is often rapidly committed to the formation of many heme-containing proteins such as hemoglobin and cytochrome P450 enzymes. Excess PpIX, heme and other porphyrins are efficiently transported out of cells through various heme transporters [15]. Although being lipophilic in general, PpIX and other porphyrins are negatively-charged molecules that require membrane transporters to facilitate movement across cell membranes. Among all cell membrane transporters that have been identified for the transport of heme and other porphyrins, the ATP-binding cassette sub-family G (ABCG) 2 protein, also known as breast cancer resistance protein (BCRP), has the most well-defined role in PpIX extracellular transport [16].
3. Mechanisms Involved in Enhanced PpIX Production and Accumulation
Elevated PpIX fluorescence is commonly observed in a variety of tumor cells and tissues following ALA administration compared with normal counterparts [7], which provides the basis for using ALA as a prodrug for fluorescence detection and photodynamic targeting of tumors. Thus, why tumor cells and tissues exhibit enhanced PpIX production and accumulation becomes a fundamental question associated with the application of ALA-based modalities in oncology. Although this remains an open question, extensive studies have suggested that increased PpIX fluorescence in tumor cells is likely a result of multiple tumor-associated cellular alterations including alterations in heme biosynthetic enzymes, mitochondrial functions and porphyrin transporters.
3.1. Alterations in Heme Biosynthetic Enzymes
Because PpIX is a metabolite produced in the heme biosynthetic pathway, it is reasonable to assume that the enhanced level of PpIX in tumor cells after ALA incubation is due to changes in the expression or activity of heme biosynthesis enzymes in tumor cells. Comparing the expression or activity of heme biosynthesis enzymes between tumor and normal cells or tissues does reveal significant differences in some cases. However, conflicting results, often dependent on tumor type, are commonly seen in the literature.
ALAS gene expression was compared between micro-dissected tumor tissues from colorectal cancer patients and corresponding normal tissues by RT-PCR [17]. ALAS expression in tumor tissues was significantly lower than in normal tissues. However, both ALAS gene expression and protein level were found increased in HCC4017 non-small-cell lung cancer (NSCLC) cells compared with normal cells, and increased ALAS protein level was shown in a panel of human lung cancer xenograft tumor samples [18]. It should be pointed out that changes in ALAS expression and activity are not expected to affect ALA-mediated PpIX production because exogenous ALA bypasses this enzymatic step.
PBGD has been suggested to play an important role in elevated ALA-PpIX production in tumor cells based on the findings that increased PBGD expression or activity is associated with cell transformation [19,20], and the upregulation of PBGD enzymatic activity has been found in some cancer cells [21,22,23] and after ALA stimulation [24,25]. There are case studies suggesting the involvement of high PBGD gene expression or enzyme activity in the oncogenesis of cervical [26], prostate [27] and breast [28] cancers, and meningioma but not glioma [29]. Significantly higher than normal PBGD activity has been found in human bladder [22] and colon [23] cancer cells as well as in tissue samples from Barrett’s esophagus and esophageal cancer patients [21,30], although there is no significant difference in PBGD expression between human colorectal cancer and normal tissues [17]. Because increased PBGD activity and decreased FECH activity were detected in some esophageal tumor cells/tissues, the ratio of PBGD to FECH activity was proposed as an index to predict enhanced PpIX accumulation and cell sensitivity to ALA-PDT [21]. However, subsequent studies failed to demonstrate the predictive value of this index [22,23,30]. In addition, overexpression of PBGD does not result in increased PpIX production, suggesting a complex interplay between PBGD and other heme biosynthesis enzymes [25,31].
There is evidence showing the involvement of increased UROD gene expression or enzyme activity in tumor initiation and progression. UROD was highly expressed at an early stage, but not at a late stage, in Friend virus-induced erythroleukemia in mice [32]. Enhanced UROD activity together with increased porphyrin biosynthesis were detected in human breast tumor tissues compared with normal tissues [28]. UROD expression was significantly elevated in tumor biopsies from head and neck cancer patients [33]. In addition, patients with higher UROD expression were more likely to have shorter disease-free survival, suggesting the involvement of UROD in cancer progression. However, in human clear-cell renal carcinomas, there was no correlation between the UROD activity and the concentration of total porphyrins or the degree of malignancy [34].
As an enzyme responsible for converting PpIX to heme, FECH gene expression or enzyme activity has often been found reduced in a variety of tumor cells/tissues including liver [35], bladder [22], colorectal [17,23], esophageal, gastric and rectal cancers [17] compared with normal counterparts. Cell lines with reduced FECH level or activity tend to have a higher ALA-PpIX level while cells with increased FECH level or activity are more likely to exhibit a lower ALA-PpIX fluorescence [17,36]. Silencing FECH gene expression significantly increased ALA-PpIX fluorescence in human colon [17], urothelial [37], glioma [38] and breast [39] cancer cells and sensitized cells to ALA-PDT whereas overexpression of FECH reduced cell sensitivity to ALA-PDT by decreasing PpIX production [36]. Although reduced FECH expression/activity is often associated with enhanced ALA-PpIX in tumors, there are reports showing good ALA-PpIX production in tumors without reduced FECH activity [30,40,41], suggesting the involvement of other contributing factors.
3.2. Alterations in Mitochondrial Functions
The observation that PpIX is often accumulated in tumor cell mitochondria leads to a speculation that enhanced ALA-PpIX is related to mitochondrial alterations in tumor cells. Comparing mitochondrial content (as indicated by the fluorescence intensity of MitoTracker dye) and the activity of cytochrome c oxidase (a mitochondrial enzyme involved in oxidative phosphorylation) with ALA-PpIX level in different tumor cell lines indicates a correlation between ALA-PpIX level and mitochondrial content, but not with cytochrome c oxidase [41]. However, later studies involving more cell lines demonstrate that such a simple correlation between ALA-PpIX and mitochondrial content or the activity of certain mitochondrial enzymes often cannot be established [23,42,43].
Recent studies suggest that alterations in energy metabolism, particularly enhanced glycolysis, are involved in enhanced ALA-PpIX in tumor cells (Figure 1). Similar to PpIX/heme biosynthesis, glucose metabolism also has both cytosolic and mitochondrial processes. Succinyl-CoA, a metabolite produced in the glucose tricarboxylic acid (TCA) cycle in mitochondria, is one of two starting substrates for PpIX/heme synthesis. The crosstalk between glucose metabolism and porphyrin biosynthesis has been demonstrated by a recent finding that inactivation of TCA cycle activated heme biosynthesis in order to avoid the accumulation of TCA cycle metabolites and enable mitochondrial NADH production [44]. Because cancer cells often switch from the TCA cycle to aerobic glycolysis and glutamine for energy production [45], such metabolic reprogramming may lead to the accumulation of TCA cycle metabolites, which activates heme biosynthesis pathway to remove TCA metabolites. This may result in enhanced PpIX accumulation due to the saturation of FECH. Although more studies are needed to test this hypothesis, the connection between cancer cell metabolic reprogramming and ALA-PpIX accumulation is supported by another recent study where human glioma cells with mutated TCA cycle enzyme isocitrate dehydrogenase 1 (IDH1) exhibited enhanced ALA-PpIX as compared to cells with wide type IDH1 [46].
3.3. Alterations in Porphyrin Transporters
ALA-mediated PpIX synthesis depends on active ALA uptake and effective transport of different porphyrin metabolites between the cytosol and mitochondria by porphyrin transporters [15]. Porphyrin importers ensure porphyrin substrates are transported to the right intracellular site for porphyrin synthesis, while porphyrin exporters pump excess heme/porphyrins out of organelles or cells to maintain homeostasis. Theoretically, enhanced ALA-PpIX in tumor cells can be caused by factors including elevated ALA uptake, enhanced porphyrin importer activity and decreased PpIX exporter activity. Although enhanced ALA uptake has been shown in tumor cells with elevated PpIX [36], more studies demonstrate that the difference in ALA uptake between higher and lower PpIX producing cell lines is not significant [23,41,47] and the rate of ALA uptake is far greater than PpIX synthesis [48], indicating that ALA uptake does not appear to be a determining factor for enhanced ALA-PpIX in tumor cells.
One porphyrin transporter involved in PpIX synthesis is the ATP-binding cassette sub-family B member 6 (ABCB6) [49]. Originally identified as a transporter on the outer mitochondrial membrane, ABCB6 binds to various porphyrins including coproporphyrinogen III, PpIX and heme. Because it has the highest affinity to coproporphyrinogen III, ABCB6 is thought to be primarily involved in transporting coproporphyrinogen III into mitochondria for PpIX/heme synthesis [49]. Human glioma tumors show higher ABCB6 expression than normal brain tissues [50]. The notion that increased ABCB6 function plays an important role in enhanced ALA-PpIX levels in tumor cells is supported by the findings that human glioma tissues with higher ALA-PpIX fluorescence exhibit higher ABCB6 expression than glioma tissues with lower PpIX fluorescence, and ABCB6 overexpression significantly increases ALA-PpIX fluorescence in glioma cell lines [50]. However, ABCB6 has also been shown to localize to the cell membrane [51] and endoplasmic reticulum [52], and transport coproporphyrinogen III out of cells [53], suggesting that enhanced ABCB6 function on cell membrane could potentially reduce PpIX/heme level by decreasing the intracellular concentration of coproporphyrinogen III. The net effect of ABCB6 on ALA-PpIX level is likely dependent on the relative ABCB6 activity in the mitochondrial versus cell membrane.
As a drug efflux transporter responsible for cancer cell resistance to many anticancer agents, the ABCG2 transporter probably plays the most important role in the extracellular transport of PpIX. Knockdown of ABCG2 causes PpIX accumulation, particularly in mitochondria, and results in mitochondrial damage, indicating that PpIX is an endogenous substrate of ABCG2 [54,55]. The ABCG2 transporter is localized to both mitochondrial and cell membrane, which enables the extracellular transport of PpIX synthesized in mitochondria [56]. Increased ABCG2 activity has been shown to decrease intracellular PpIX level after ALA stimulation [16] and cell lines with high ABCG2 expressions or activities often exhibit reduced ALA-PpIX fluorescence [57]. In a recent study, we found that triple negative breast cancer cell lines have significantly reduced ALA-PpIX levels as compared with estrogen receptor (ER) positive and human epidermal growth factor receptor 2 (HER2) positive breast cancer cell lines because of elevated ABCG2 activity [58].
4. Therapeutic Strategies for Enhancing ALA-Based Tumor Detection and Therapy
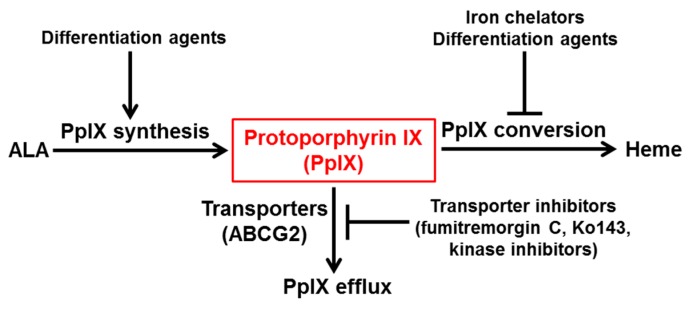
Although ALA-based modalities have been used in the clinic for detecting and targeting tumor tissues, its applications are limited by inadequate and heterogeneous PpIX production in tumor cells [59,60]. Thus, various therapeutic approaches have been proposed and evaluated to overcome these limitations. All enhancement approaches can be categorized into three therapeutic strategies, which are: enhancing PpIX synthesis, reducing PpIX conversion, and inhibiting PpIX efflux (Figure 2). Enhancing PpIX synthesis can be achieved by increasing the activity of enzymes involved in PpIX synthesis and the transport of porphyrin intermediates necessary for PpIX synthesis. Reducing PpIX conversion aims at inhibiting the bioconversion from PpIX to heme by removing the substrate ferrous ion required for the reaction and/or inhibiting the enzyme FECH that catalyzes the reaction. Inhibiting PpIX efflux is to prevent PpIX extracellular transport with inhibitors of PpIX transporters, the ABCG2 transporter in particular.
Figure 2.
Current therapeutic strategies for enhancing ALA-based tumor detection and therapy. These strategies include enhancing PpIX synthesis, reducing PpIX conversion and inhibiting PpIX efflux.
It is desirable for a therapeutic agent to enhance ALA-PpIX level through multiple mechanisms. For example, differentiation agent vitamin D is able to enhance PpIX synthesis by up-regulating CPOX and reduce PpIX conversion by down-regulating FECH [61]. It is important to point out that the therapeutic potential of some enhancement approaches has been validated by genetic approaches. For instance, transfection of mutated ALAS2 gene variants with increased enzymatic activity than the wild-type enzyme [62], as well as the CPOX-overexpressing vector [63], significantly increase PpIX production. Silencing FECH [17,38,39] and ABCG2 [55] gene expression has been shown to increase PpIX accumulation. Here we summarize the therapeutic agents that have been evaluated for enhancing ALA-based tumor detection and therapy with promising results.
4.1. Iron Chelators
Because ferrous iron is a substrate necessary for converting PpIX to heme by FECH, removal of ferrous iron by iron chelators prevents this conversion, resulting in PpIX accumulation. The non-specific metal ion chelator ethylenediamine tetraacetic acid (EDTA) was first used to enhance ALA-PpIX accumulation in leukemia cells [64]. A more potent and selective iron chelator deferoxamine (DFO) [65] was later found to increase ALA-PpIX accumulation and sensitivity to ALA-PDT in a variety of tumor cells and tissues [66,67,68,69,70,71]. However, in a small scale clinical study, DFO showed no enhancement of ALA-PpIX and PDT in patients with superficial basal cell carcinomas or Bowen’s disease, and was only able to increase ALA-PpIX in normal skin at a low, but not high, ALA dose [72].
CP94 (1,2-diethyl-3-hydroxypyridin-4-one hydrochloride) is another iron chelator that is superior to DFO in enhancing ALA-PpIX accumulation likely due to its lower molecular weight and higher lipophilicity, resulting in better tissue penetration [73]. CP94 is able to enhance ALA-PpIX accumulation in rat bladder urothelium [74] and colonic mucosa [75] in vivo. It increases PpIX levels in human skin [73,76], bladder [77] and glioma [76,78] cancer cells treated with ALA or its derivatives. The safety and effectiveness of CP94 in enhancing PDT with methyl-aminolevulinate (MAL) for basal cell carcinoma has also been demonstrated in a pilot clinical study [79], indicating the feasibility of adding iron chelators into clinical PDT protocols.
4.2. Differentiation Agents
Induction of keratinocyte differentiation by increasing calcium concentration in medium enhances ALA-PpIX production and cytotoxicity to ALA-PDT through increased ALA uptake, increased PpIX synthesis by elevating CPOX expression, and decreased PpIX efflux [80]. Following this early study, differentiation agents including methotrexate (MTX) and vitamin D have been extensively studied for enhancing ALA-PpIX production and ALA-PDT outcomes [81]. Vitamin D and MTX increase PpIX fluorescence and cytotoxicity to ALA-PDT in LNCaP prostate cancer cells by up-regulating CPOX and differentiation marker E-cadherin [63,82]. It appears that differentiation-induced CPOX up-regulation is a major contributing factor for enhancing ALA-PpIX production because CPOX over-expression alone is enough to increase PpIX fluorescence [63].
Both the active form of vitamin D (calcitriol) [61] and dietary vitamin D (cholecalciferol) [83] can enhance ALA-PpIX production and PDT-induced cell death in A431 tumor cells and tissues. Calcitriol has also been shown to enhance ALA-PpIX and PDT in the MDA-MB-231 breast tumor model [84] and is effective for detecting mouse skin tumors based on enhanced ALA-PpIX fluorescence [85]. Calcitriol treatment increases E-cadherin and Ki67 staining, up-regulates CPOX and down-regulates FECH, leading to ALA-PpIX accumulation [61]. Furthermore, it increases TNFα level, which may enhance extrinsic apoptosis induced by ALA-PDT [61]. The mechanism involved in calcitriol-induced CPOX up-regulation has been found to be related to the activation of transcription factor CCAAT enhancer binding proteins (CEBPs), which leads to the activation of its downstream CPOX gene transcription [86].
4.3. ABCG2 Transporter Inhibitors
The identification of PpIX as an endogenous substrate of ABCG2 transporter leads to the use of ABCG2 transport inhibitors to enhance ALA-PpIX fluorescence and PDT effects [16]. Increased ALA-PpIX accumulation in various tumor cell lines by inhibiting PpIX efflux has been demonstrated with ABCG2 inhibitors fumitremorgin C [16,87] and its less toxic and more selective analog Ko143 [57,58,88]. Because the activity of ABCG2 transporter depends on albumin, the effect of ABCG2 inhibitor on PpIX increase is particularly pronounced when cells are cultured in serum-containing medium and barely noticeable when serum-free medium is used. [87,89]. It is therapeutically important that some approved tyrosine kinase inhibitors including imatinib mesylate (Gleevec) [90] and gefitinib [91] are potent ABCG2 transporter inhibitors and effective in sensitizing tumor cells to ALA-PDT by increasing PpIX level. Moreover, the fact that ABCG2 inhibitor Ko143 is able to reduce PpIX fluorescence heterogeneity in tumor cells suggests that ABCG2 is involved in the intra-tumor heterogeneity of ALA-PpIX [58]. Finally, the effect of ABCG2 inhibitors on ALA-PpIX increase was observed only in cells with ABCG2 expression or activity, but not in cells lacking ABCG2 expression or activity, indicating the selectivity of this enhancement approach [57,58,90].
5. Conclusions and Future Perspectives
The past two decades have witnessed extensive research aiming at unraveling the mystery of enhanced ALA-PpIX in tumors and seeking therapeutic approaches to boost PpIX levels in tumors with insufficient PpIX accumulation. As a result of this intensive study, considerable knowledge has been obtained about various tumor-related pathological alterations that contribute to an increased level of PpIX in tumors following ALA stimulation. This knowledge has provided the foundation for the clinical application of ALA for detecting and targeting tumors, particularly in the skin, brain and bladder. It has also stimulated the exploration of mechanism-based therapeutic approaches to enhance ALA-based modalities, which has led to many encouraging preclinical and clinical results.
However, the molecular mechanism underlying enhanced ALA-PpIX in tumor cells remains elusive and those promising results are yet to be translated to clinical practice. To fulfill the potential of ALA-based modalities, more mechanistic basic research, well-designed clinical trials, and collaborative studies between bench scientists and clinicians are needed. In basic research, many cellular and functional alterations that contribute to an enhanced ALA-PpIX level in tumors have been identified. But we lack a comprehensive understanding of the underlying connection between these different pathological alterations and the relationship between these alterations and cell phenotypic changes (gaining survival advantage, metastatic potential, avoiding metabolic catastrophe etc.). More importantly, we do not know the cause of these pathological alterations in tumor cells. Is enhanced ALA-PpIX directly caused by oncogene activation in tumor cells? If so, what oncogenes are more likely to enhance tumor PpIX level and how? Obviously, answers to these fundamental questions will not only help us understand the biological meaning of increased ALA-PpIX level in tumors, but also have important implications in selecting appropriate patients for ALA-based modalities.
In clinical research, it is certainly encouraging to see promising results from pilot clinical studies where ALA-PpIX is used for diagnosing tumors other than glioblastoma, and iron chelators are able to improve PDT outcome. However, these clinical studies are limited by very small sample size and sometimes patients with mixed diseases. Well-designed large-scale clinical trials that will eventually convince the medical community and regulatory agencies to accept these new ALA-based therapeutic modalities are needed. Finally, in the field of oncology where personalized medicine is taking the lead, the importance, as well as the benefit, of collaborative research between bench scientists and clinicians cannot be overemphasized. ALA is certainly not good for all types of cancer nor for all patients with a certain type of cancer. Identifying the appropriate disease and patient subsets who will benefit the most from ALA-based modalities is probably the most important task facing researchers in this field in the next decade. Through more collaborative research between basic and clinical scientists, it is hoped that we will have more mechanistic understanding of ALA-based modalities and more patients will benefit from these modalities.
Acknowledgments
This study was supported in part by Research Scholar Grant RSG-10-035-01-CCE from the American Cancer Society (ACS).
Author Contributions
Xue Yang and Pratheeba Palasuberniam conducted literature review and summary. All authors contributed to manuscript writing and revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.O’Connor A.E., Gallagher W.M., Byrne A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009;85:1053–1074. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 2.Spikes J.D. Porphyrins and related compounds as photodynamic sensitizers. Ann. N. Y. Acad. Sci. 1975;244:496–508. doi: 10.1111/j.1749-6632.1975.tb41550.x. [DOI] [PubMed] [Google Scholar]
- 3.Malik Z., Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br. J. Cancer. 1987;56:589–595. doi: 10.1038/bjc.1987.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy J.C., Pottier R.H., Pross D.C. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J. Photochem. Photobiol. B. 1990;6:143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- 5.Peng Q., Moan J., Warloe T., Nesland J.M., Rimington C. Distribution and photosensitizing efficiency of porphyrins induced by application of exogenous 5-aminolevulinic acid in mice bearing mammary carcinoma. Int. J. Cancer. 1992;52:433–443. doi: 10.1002/ijc.2910520318. [DOI] [PubMed] [Google Scholar]
- 6.Zhao B., He Y.Y. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev. Anticancer Ther. 2010;10:1797–1809. doi: 10.1586/era.10.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nokes B., Apel M., Jones C., Brown G., Lang J.E. Aminolevulinic acid (ALA): Photodynamic detection and potential therapeutic applications. J. Surg. Res. 2013;181:262–271. doi: 10.1016/j.jss.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Szeimies R.M., Landthaler M., Karrer S. Non-oncologic indications for ALA-PDT. J. Dermatolog. Treat. 2002;13(Suppl. S1):S13–S18. doi: 10.1080/095466302317414654. [DOI] [PubMed] [Google Scholar]
- 9.Jichlinski P., Leisinger H.J. Photodynamic therapy in superficial bladder cancer: Past, present and future. Urol. Res. 2001;29:396–405. doi: 10.1007/s002400100215. [DOI] [PubMed] [Google Scholar]
- 10.Tetard M.C., Vermandel M., Mordon S., Lejeune J.P., Reyns N. Experimental use of photodynamic therapy in high grade gliomas: A review focused on 5-aminolevulinic acid. Photodiagn. Photodyn. Ther. 2014;11:319–330. doi: 10.1016/j.pdpdt.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Colditz M.J., Jeffree R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J. Clin. Neurosci. 2012;19:1471–1474. doi: 10.1016/j.jocn.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Colditz M.J., Leyen K., Jeffree R.L. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: Theoretical, biochemical and practical aspects. J. Clin. Neurosci. 2012;19:1611–1616. doi: 10.1016/j.jocn.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Eljamel S. 5-ALA fluorescence image guided resection of glioblastoma multiforme: A meta-analysis of the literature. Int. J. Mol. Sci. 2015;16:10443–10456. doi: 10.3390/ijms160510443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponka P. Cell biology of heme. Am. J. Med. Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Khan A.A., Quigley J.G. Control of intracellular heme levels: Heme transporters and heme oxygenases. Biochim. Biophys. Acta. 2011;1813:668–682. doi: 10.1016/j.bbamcr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robey R.W., Steadman K., Polgar O., Bates S.E. ABCG2-mediated transport of photosensitizers: Potential impact on photodynamic therapy. Cancer Biol. Ther. 2005;4:187–194. doi: 10.4161/cbt.4.2.1440. [DOI] [PubMed] [Google Scholar]
- 17.Kemmner W., Wan K., Ruttinger S., Ebert B., Macdonald R., Klamm U., Moesta K.T. Silencing of human ferrochelatase causes abundant protoporphyrin-IX accumulation in colon cancer. FASEB J. 2008;22:500–509. doi: 10.1096/fj.07-8888com. [DOI] [PubMed] [Google Scholar]
- 18.Hooda J., Cadinu D., Alam M.M., Shah A., Cao T.M., Sullivan L.A., Brekken R., Zhang L. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS ONE. :2013. doi: 10.1371/journal.pone.0063402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamet R., Leibovici L., Teitz Y., Schoenfeld N. Accelerated heme synthesis and degradation in transformed fibroblasts. Biochem. Med. Metab. Biol. 1990;44:175–180. doi: 10.1016/0885-4505(90)90058-9. [DOI] [PubMed] [Google Scholar]
- 20.Greenbaum L., Gozlan Y., Schwartz D., Katcoff D.J., Malik Z. Nuclear distribution of porphobilinogen deaminase (PBGD) in glioma cells: A regulatory role in cancer transformation? Br. J. Cancer. 2002;86:1006–1011. doi: 10.1038/sj.bjc.6600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinnen P., de Rooij F.W., van Velthuysen M.L., Edixhoven A., van Hillegersberg R., Tilanus H.W., Wilson J.H., Siersema P.D. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: A study in patients with (pre)malignant lesions of the oesophagus. Br. J. Cancer. 1998;78:679–682. doi: 10.1038/bjc.1998.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg R.C., Fickweiler S., Wolfbeis O.S., Knuechel R. Cell-type specific protoporphyrin IX metabolism in human bladder cancer in vitro. Photochem. Photobiol. 2000;72:226–233. doi: 10.1562/0031-8655(2000)072<0226:CTSPIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Krieg R.C., Messmann H., Rauch J., Seeger S., Knuechel R. Metabolic characterization of tumor cell-specific protoporphyrin IX accumulation after exposure to 5-aminolevulinic acid in human colonic cells. Photochem. Photobiol. 2002;76:518–525. doi: 10.1562/0031-8655(2002)076<0518:MCOTCS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Gibson S.L., Cupriks D.J., Havens J.J., Nguyen M.L., Hilf R. A regulatory role for porphobilinogen deaminase (PBGD) in δ-aminolaevulinic acid (δ-ALA)-induced photosensitization? Br. J. Cancer. 1998;77:235–242. doi: 10.1038/bjc.1998.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauder A., Feuerstein T., Malik Z. The centrality of PBGD expression levels on ALA-PDT efficacy. Photochem. Photobiol. Sci. 2011;10:1310–1317. doi: 10.1039/c1pp05085k. [DOI] [PubMed] [Google Scholar]
- 26.Goncalves T.L., Erthal F., Corte C.L., Muller L.G., Piovezan C.M., Nogueira C.W., Rocha J.B. Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clin. Biochem. 2005;38:1071–1075. doi: 10.1016/j.clinbiochem.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Neslund-Dudas C., Levin A.M., Rundle A., Beebe-Dimmer J., Bock C.H., Nock N.L., Jankowski M., Datta I., Krajenta R., Dou Q.P., et al. Case-only gene-environment interaction between ALAD tagSNPs and occupational lead exposure in prostate cancer. Prostate. 2014;74:637–646. doi: 10.1002/pros.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navone N.M., Polo C.F., Frisardi A.L., Andrade N.E., Battle A.M. Heme biosynthesis in human breast cancer—Mimetic “in vitro” studies and some heme enzymic activity levels. Int. J. Biochem. 1990;22:1407–1411. doi: 10.1016/0020-711X(90)90230-Z. [DOI] [PubMed] [Google Scholar]
- 29.Rajaraman P., Schwartz B.S., Rothman N., Yeager M., Fine H.A., Shapiro W.R., Selker R.G., Black P.M., Inskip P.D. δ-Aminolevulinic acid dehydratase polymorphism and risk of brain tumors in adults. Environ. Health Perspect. 2005;113:1209–1211. doi: 10.1289/ehp.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinnen P., de Rooij F.W., Terlouw E.M., Edixhoven A., van Dekken H., van Hillegersberg R., Tilanus H.W., Wilson J.H., Siersema P.D. Porphyrin biosynthesis in human barrett’s oesophagus and adenocarcinoma after ingestion of 5-aminolaevulinic acid. Br. J. Cancer. 2000;83:539–543. doi: 10.1054/bjoc.2000.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilf R., Havens J.J., Gibson S.L. Effect of δ-aminolevulinic acid on protoporphyrin IX accumulation in tumor cells transfected with plasmids containing porphobilinogen deaminase DNA. Photochem. Photobiol. 1999;70:334–340. doi: 10.1562/0031-8655(1999)070<0334:EOAAOP>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Misawa Y., Tojo A., Shibuya M. Isolation of genes highly expressed in early and late stages of friend virus-induced erythroleukemia in mice. Biochem. Biophys. Res. Commun. 1990;170:39–45. doi: 10.1016/0006-291X(90)91237-M. [DOI] [PubMed] [Google Scholar]
- 33.Ito E., Yue S., Moriyama E.H., Hui A.B., Kim I., Shi W., Alajez N.M., Bhogal N., Li G., Datti A., et al. Uroporphyrinogen decarboxylase is a radiosensitizing target for head and neck cancer. Sci. Transl. Med. :2011. doi: 10.1126/scitranslmed.3001922. [DOI] [PubMed] [Google Scholar]
- 34.Zawirska B. Uroporphyrinogen decarboxylase and porphyrins in the tissue of human clear-cell renal carcinoma and in its maternal renal cortex. Neoplasma. 1989;36:207–213. [PubMed] [Google Scholar]
- 35.Dailey H.A., Smith A. Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase. Biochem. J. 1984;223:441–445. doi: 10.1042/bj2230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohgari Y., Nakayasu Y., Kitajima S., Sawamoto M., Mori H., Shimokawa O., Matsui H., Taketani S. Mechanisms involved in δ-aminolevulinic acid (ALA)-induced photosensitivity of tumor cells: Relation of ferrochelatase and uptake of ALA to the accumulation of protoporphyrin. Biochem. Pharmacol. 2005;71:42–49. doi: 10.1016/j.bcp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Miyake M., Ishii M., Kawashima K., Kodama T., Sugano K., Fujimoto K., Hirao Y. siRNA-mediated knockdown of the heme synthesis and degradation pathways: Modulation of treatment effect of 5-aminolevulinic acid-based photodynamic therapy in urothelial cancer cell lines. Photochem. Photobiol. 2009;85:1020–1027. doi: 10.1111/j.1751-1097.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 38.Teng L., Nakada M., Zhao S.G., Endo Y., Furuyama N., Nambu E., Pyko I.V., Hayashi Y., Hamada J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer. 2011;104:798–807. doi: 10.1038/bjc.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X., Li W., Palasuberniam P., Myers K.A., Wang C., Chen B. Effects of silencing heme biosynthesis enzymes on 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy. Photochem. Photobiol. 2015;91:923–930. doi: 10.1111/php.12454. [DOI] [PubMed] [Google Scholar]
- 40.Hua Z., Gibson S.L., Foster T.H., Hilf R. Effectiveness of δ-aminolevulinic acid-induced protoporphyrin as a photosensitizer for photodynamic therapy in vivo. Cancer Res. 1995;55:1723–1731. [PubMed] [Google Scholar]
- 41.Gibson S.L., Nguyen M.L., Havens J.J., Barbarin A., Hilf R. Relationship of δ-aminolevulinic acid-induced protoporphyrin IX levels to mitochondrial content in neoplastic cells in vitro. Biochem. Biophys. Res. Commun. 1999;265:315–321. doi: 10.1006/bbrc.1999.1670. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs S.L., Chen B., O’Hara J.A., Hoopes P.J., Hasan T., Pogue B.W. Protoporphyrin IX level correlates with number of mitochondria, but increase in production correlates with tumor cell size. Photochem. Photobiol. 2006;82:1334–1341. doi: 10.1562/2006-03-11-RA-843. [DOI] [PubMed] [Google Scholar]
- 43.Cunderlikova B., Peng Q., Mateasik A. Factors implicated in the assessment of aminolevulinic acid-induced protoporphyrin IX fluorescence. Biochim. Biophys. Acta. 2013;1830:2750–2762. doi: 10.1016/j.bbagen.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Frezza C., Zheng L., Folger O., Rajagopalan K.N., MacKenzie E.D., Jerby L., Micaroni M., Chaneton B., Adam J., Hedley A., et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–228. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 45.Ward P.S., Thompson C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J.E., Cho H.R., Xu W.J., Kim J.Y., Kim S.K., Kim S.K., Park S.H., Kim H., Lee S.H., Choi S.H., et al. Mechanism for enhanced 5-aminolevulinic acid fluorescence in isocitrate dehydrogenase 1 mutant malignant gliomas. Oncotarget. 2015;6:20266–20277. doi: 10.18632/oncotarget.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson S.L., Havens J.J., Foster T.H., Hilf R. Time-dependent intracellular accumulation of δ-aminolevulinic acid, induction of porphyrin synthesis and subsequent phototoxicity. Photochem. Photobiol. 1997;65:416–421. doi: 10.1111/j.1751-1097.1997.tb08580.x. [DOI] [PubMed] [Google Scholar]
- 48.Nakanishi T., Ogawa T., Yanagihara C., Tamai I. Kinetic evaluation of determinant factors for cellular accumulation of protoporphyrin IX induced by external 5-aminolevulinic acid for photodynamic cancer therapy. J. Pharm. Sci. 2015;104:3092–3100. doi: 10.1002/jps.24462. [DOI] [PubMed] [Google Scholar]
- 49.Krishnamurthy P.C., Du G., Fukuda Y., Sun D., Sampath J., Mercer K.E., Wang J., Sosa-Pineda B., Murti K.G., Schuetz J.D. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- 50.Zhao S.G., Chen X.F., Wang L.G., Yang G., Han D.Y., Teng L., Yang M.C., Wang D.Y., Shi C., Liu Y.H., et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann. Surg. Oncol. 2013;20:4379–4388. doi: 10.1245/s10434-011-2201-6. [DOI] [PubMed] [Google Scholar]
- 51.Paterson J.K., Shukla S., Black C.M., Tachiwada T., Garfield S., Wincovitch S., Ernst D.N., Agadir A., Li X., Ambudkar S.V., et al. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443–9452. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- 52.Tsuchida M., Emi Y., Kida Y., Sakaguchi M. Human ABC transporter isoform B6 (ABCB6) localizes primarily in the Golgi apparatus. Biochem. Biophys. Res. Commun. 2008;369:369–375. doi: 10.1016/j.bbrc.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 53.Matsumoto K., Hagiya Y., Endo Y., Nakajima M., Ishizuka M., Tanaka T., Ogura S. Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: Tumor cell response to hypoxia. Photodiagn. Photodyn. Ther. 2015;12:45–51. doi: 10.1016/j.pdpdt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Jonker J.W., Buitelaar M., Wagenaar E., van der Valk M.A., Scheffer G.L., Scheper R.J., Plosch T., Kuipers F., Elferink R.P., Rosing H., et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. USA. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y.H., Chang H.M., Chang F.P., Shen C.R., Liu C.L., Mao W.Y., Lin C.C., Lee H.S., Shen C.N. Protoporphyrin IX accumulation disrupts mitochondrial dynamics and function in ABCG2-deficient hepatocytes. FEBS Lett. 2013;587:3202–3209. doi: 10.1016/j.febslet.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Kobuchi H., Moriya K., Ogino T., Fujita H., Inoue K., Shuin T., Yasuda T., Utsumi K., Utsumi T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS ONE. :2012. doi: 10.1371/journal.pone.0050082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barron G.A., Moseley H., Woods J.A. Differential sensitivity in cell lines to photodynamic therapy in combination with ABCG2 inhibition. J. Photochem. Photobiol. B. 2013;126:87–96. doi: 10.1016/j.jphotobiol.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Palasuberniam P., Yang X., Kraus D., Jones P., Myers K.A., Chen B. ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Sci. Rep. :2015. doi: 10.1038/srep13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stummer W., Tonn J.C., Goetz C., Ullrich W., Stepp H., Bink A., Pietsch T., Pichlmeier U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74:310–320. doi: 10.1227/NEU.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanick S.C., Davis S.C., Zhao Y., Hasan T., Maytin E.V., Pogue B.W., Chapman M.S. Dual-channel red/blue fluorescence dosimetry with broadband reflectance spectroscopic correction measures protoporphyrin IX production during photodynamic therapy of actinic keratosis. J. Biomed. Opt. :2014. doi: 10.1117/1.JBO.19.7.075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anand S., Wilson C., Hasan T., Maytin E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res. 2011;71:6040–6050. doi: 10.1158/0008-5472.CAN-11-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fratz E.J., Hunter G.A., Ferreira G.C. Expression of murine 5-aminolevulinate synthase variants causes protoporphyrin IX accumulation and light-induced mammalian cell death. PLoS ONE. :2014. doi: 10.1371/journal.pone.0093078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinha A.K., Anand S., Ortel B.J., Chang Y., Mai Z., Hasan T., Maytin E.V. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. Br. J. Cancer. 2006;95:485–495. doi: 10.1038/sj.bjc.6603273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanania J., Malik Z. The effect of EDTA and serum on endogenous porphyrin accumulation and photodynamic sensitization of human K562 leukemic cells. Cancer Lett. 1992;65:127–131. doi: 10.1016/0304-3835(92)90156-P. [DOI] [PubMed] [Google Scholar]
- 65.Richardson D., Ponka P., Baker E. The effect of the iron(III) chelator, desferrioxamine, on iron and transferrin uptake by the human malignant melanoma cell. Cancer Res. 1994;54:685–689. [PubMed] [Google Scholar]
- 66.Uehlinger P., Ballini J.P., van den Bergh H., Wagnieres G. On the role of iron and one of its chelating agents in the production of protoporphyrin IX generated by 5-aminolevulinic acid and its hexyl ester derivative tested on an epidermal equivalent of human skin. Photochem. Photobiol. 2006;82:1069–1076. doi: 10.1562/2005-12-04-RA-745. [DOI] [PubMed] [Google Scholar]
- 67.Uekusa M., Omura K., Nakajima Y., Hasegawa S., Harada H., Morita K.I., Tsuda H. Uptake and kinetics of 5-aminolevulinic acid in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2010;39:802–805. doi: 10.1016/j.ijom.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 68.Valdes P.A., Samkoe K., O’Hara J.A., Roberts D.W., Paulsen K.D., Pogue B.W. Deferoxamine iron chelation increases δ-aminolevulinic acid induced protoporphyrin IX in xenograft glioma model. Photochem. Photobiol. 2010;86:471–475. doi: 10.1111/j.1751-1097.2009.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J., Xia Y., Liu X., Jiang S., Xiong L. Desferrioxamine shows different potentials for enhancing 5-aminolaevulinic acid-based photodynamic therapy in several cutaneous cell lines. Lasers Med. Sci. 2010;25:251–257. doi: 10.1007/s10103-009-0721-0. [DOI] [PubMed] [Google Scholar]
- 70.Juzenas P., Juzeniene A., Moan J. Deferoxamine photosensitizes cancer cells in vitro. Biochem. Biophys. Res. Commun. 2005;332:388–391. doi: 10.1016/j.bbrc.2005.04.138. [DOI] [PubMed] [Google Scholar]
- 71.Fukuhara H I.K., Kurabayashi A., Furihata M., Fujita H., Utsumi K., Sasaki J., Shuin T. The inhibition of ferrochelatase enhances 5-aminolevulinic acid-based photodynamic action for prostate cancer. Photodiagn. Photodyn. Ther. 2013;10:399–409. doi: 10.1016/j.pdpdt.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Choudry K., Brooke R.C., Farrar W., Rhodes L.E. The effect of an iron chelating agent on protoporphyrin IX levels and phototoxicity in topical 5-aminolaevulinic acid photodynamic therapy. Br. J. Dermatol. 2003;149:124–130. doi: 10.1046/j.1365-2133.2003.05351.x. [DOI] [PubMed] [Google Scholar]
- 73.Pye A., Curnow A. Direct comparison of δ-aminolevulinic acid and methyl-aminolevulinate-derived protoporphyrin IX accumulations potentiated by desferrioxamine or the novel hydroxypyridinone iron chelator CP94 in cultured human cells. Photochem. Photobiol. 2007;83:766–773. doi: 10.1562/2006-05-30-RA-906. [DOI] [PubMed] [Google Scholar]
- 74.Chang S.-C., MacRobert A.J., Porter J.B., Brown S.G. The efficacy of an iron chelator (CP94) in increasing cellular protoporphyrin IX following intravesical 5-aminolaevulinic acid administration: An in vivo study. J. Photochem. Photobiol. B Biol. 1997;38:114–122. doi: 10.1016/S1011-1344(96)07441-6. [DOI] [PubMed] [Google Scholar]
- 75.Curnow A., McIlroy B.W., Postle-Hacon M.J., Porter J.B., MacRobert A.J., Bown S.G. Enhancement of 5-aminolaevulinic acid-induced photodynamic therapy in normal rat colon using hydroxypyridinone iron-chelating agents. Br. J. Cancer. 1998;78:1278–1282. doi: 10.1038/bjc.1998.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blake E., Allen J., Curnow A. An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection. Photochem. Photobiol. 2011;87:1419–1426. doi: 10.1111/j.1751-1097.2011.00985.x. [DOI] [PubMed] [Google Scholar]
- 77.Bech O., Phillips D., Moan J., MacRobert A.J. A hydroxypyridinone (CP94) enhances protoporphyrin IX formation in 5-aminolaevulinic acid treated cells. J. Photochem. Photobiol. B Biol. 1997;41:136–144. doi: 10.1016/S1011-1344(97)00095-X. [DOI] [PubMed] [Google Scholar]
- 78.Blake E., Curnow A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem. Photobiol. 2010;86:1154–1160. doi: 10.1111/j.1751-1097.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 79.Pye A., Campbell S., Curnow A. Enhancement of methyl-aminolevulinate photodynamic therapy by iron chelation with CP94: An in vitro investigation and clinical dose-escalating safety study for the treatment of nodular basal cell carcinoma. J. Cancer Res. Clin. Oncol. 2008;134:841–849. doi: 10.1007/s00432-008-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortel B., Chen N., Brissette J., Dotto G.P., Maytin E., Hasan T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br. J. Cancer. 1998;77:1744–1751. doi: 10.1038/bjc.1998.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anand S., Ortel B.J., Pereira S.P., Hasan T., Maytin E.V. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett. 2012;326:8–16. doi: 10.1016/j.canlet.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ortel B., Sharlin D., O’Donnell D., Sinha A.K., Maytin E.V., Hasan T. Differentiation enhances aminolevulinic acid-dependent photodynamic treatment of LNCaP prostate cancer cells. Br. J. Cancer. 2002;87:1321–1327. doi: 10.1038/sj.bjc.6600575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anand S., Rollakanti K.R., Horst R.L., Hasan T., Maytin E.V. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem. Photobiol. 2014;90:1126–1135. doi: 10.1111/php.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rollakanti K.R., Anand S., Maytin E.V. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015;4:633–642. doi: 10.1002/cam4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rollakanti K.R., Anand S., Davis S.C., Pogue B.W., Maytin E.V. Noninvasive optical imaging of UV-induced squamous cell carcinoma in murine skin: Studies of early tumor development and vitamin D enhancement of protoporphyrin IX production. Photochem. Photobiol. :2015. doi: 10.1111/php.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anand S., Hasan T., Maytin E.V. Mechanism of differentiation-enhanced photodynamic therapy for cancer: Upregulation of coproporphyrinogen oxidase by C/EBP transcription factors. Mol. Cancer Ther. 2013;12:1638–1650. doi: 10.1158/1535-7163.MCT-13-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogino T., Kobuchi H., Munetomo K., Fujita H., Yamamoto M., Utsumi T., Inoue K., Shuin T., Sasaki J., Inoue M., et al. Serum-dependent export of protoporphyrin IX by ATP-binding cassette transporter G2 in T24 cells. Mol. Cell. Biochem. 2011;358:297–307. doi: 10.1007/s11010-011-0980-5. [DOI] [PubMed] [Google Scholar]
- 88.Bebes A., Nagy T., Bata-Csorgo Z., Kemeny L., Dobozy A., Szell M. Specific inhibition of the ABCG2 transporter could improve the efficacy of photodynamic therapy. J. Photochem. Photobiol. B. 2011;105:162–166. doi: 10.1016/j.jphotobiol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 89.Desuzinges-Mandon E., Arnaud O., Martinez L., Huche F., Di Pietro A., Falson P. ABCG2 transports and transfers heme to albumin through its large extracellular loop. J. Biol. Chem. 2010;285:33123–33133. doi: 10.1074/jbc.M110.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu W., Baer M.R., Bowman M.J., Pera P., Zheng X., Morgan J., Pandey R.A., Oseroff A.R. The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin. Cancer Res. 2007;13:2463–2470. doi: 10.1158/1078-0432.CCR-06-1599. [DOI] [PubMed] [Google Scholar]
- 91.Sun W., Kajimoto Y., Inoue H., Miyatake S., Ishikawa T., Kuroiwa T. Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagn. Photodyn. Ther. 2013;10:42–50. doi: 10.1016/j.pdpdt.2012.06.003. [DOI] [PubMed] [Google Scholar]