Abstract
Antibody-dependent cellular cytotoxicity (ADCC), a key effector function for the clinical effectiveness of monoclonal antibodies, is triggered by the engagement of the antibody Fc domain with the Fcγ receptors expressed by innate immune cells such as natural killer (NK) cells and macrophages. Here, we fused cancer cell-binding peptides to the Fc domain of human IgG1 to engineer novel peptide-Fc fusion proteins with ADCC activity. The designed fusion proteins were expressed in human embryonic kidney 293T cells, followed by purification and characterization by western blots. One of the engineered variants (WN-Fc), bound with high affinity to a wide range of solid tumor cell lines (e.g., colon, lung, prostate, skin, ovarian, and mammary tumors). Treatment of cancer cells with the engineered peptide-Fc fusions in the presence of effector NK cells potentially enhanced cytotoxicity, degranulation, and interferon-γ production by NK cells when compared to cells treated with the Fc control. The presence of competing peptides inhibited NK cell activation. Furthermore, a bispecific peptide-Fc fusion protein activated NK cells against HER-1- and/or HER-2-expressing cancer cells. Collectively, the engineered peptide-Fc fusions constitute a new promising strategy to recruit and activate NK cells against tumor cells, a primary goal of cancer immunotherapy.
Introduction
One of the major problems in cancer chemotherapy is the toxic side effects of anticancer drugs designed to destroy dividing cells, including those found in healthy tissues.1,2 Such unwanted effects often result in dose reduction, treatment delay, or discontinuance of therapy. Ligands that recognize cancer-specific cell surface proteins have recently been highlighted as a promising strategy in cancer cell targeting.3,4 Coupling such ligands to effector molecules will generate “smarter” therapeutic agents that would have the potential to be both more effective and have fewer side effects. The need for targeted therapies was made obvious in clinical trials with monoclonal antibodies (mAbs) such as rituximab/Rituxam, cetuximab/Erbitux, and trastuzumab/Herceptin targeting CD20, epidermal growth factor receptor (EGFR/HER-1 or ErbB1), and HER-2, respectively.5 Notably, the activity of most approved mAbs used in cancer immunotherapy depends on the recruitment of Fc-receptor-bearing effector cells such as natural killer (NK) cells and macrophages.6–10 Although antibody therapy has led to important clinical advances, it has a number of limitations. For example, some patients do not respond at all, and in many malignancies, no antitumor antibodies that stimulate NK cell reactivity are yet available. Moreover, resistance has emerged as an important clinical issue.5 Given these challenges, the development of new targeted therapies against cancer is of critical importance.
The discovery of random peptide phage libraries facilitated screening large peptide libraries to isolate peptides that could bind to specific cell types such as cancer cells,11,12 thus eliminating the need for prior identification and purification of the target receptor(s). Furthermore, the strategy allows for selection of peptides that bind to their receptors in a native cellular context. We and others have applied this strategy to isolate peptides with good affinity and specificity for a variety of tumor types.13–19 For example, The LTVSPWY (LTV peptide) is part of a new generation of short targeting peptides that we have identified capable of binding and delivering therapeutics and imaging agents to cancer cells in vitro and in vivo.14,20–24 The LTV-peptide bound preferentially to HER-2-positive cancer cells.14,20 Urbanelli et al.17 also selected a peptide (MYWGDSHWLQYWYE), which specifically recognized HER-2-expressing cells. Moreover, high-affinity peptide ligands for identification and capture of lymphoma cells have been selected.18 The ability of the selected peptides to differentiate between tumor and normal cells make them ideal therapeutic targeting vectors as documented by several studies.24–29 Accordingly, incorporating cancer-cell-binding peptides selected from peptide phage libraries into the Fc domain of human IgG offers a new means for the development of targeted therapies and has the potential to broaden the diversity of tumor antigens that can be targeted for immunotherapy. Like antibodies, the fusion protein forms a homodimer due to the strong interactions of the CH3 domains. The homodimer structure stabilized by interchain disulfide bonds in the hinge regions provides some of the antibody properties, such as increased apparent affinity and serum half-life.30 In addition to their potential pharmacokinetic advantages, the production and manufacturing of peptide-Fc fusions, known as peptibodies,30–34 can be more efficient and less time consuming than those for full-length mAbs. Moreover, any unwanted toxicities directly related to peptide-Fc fusion concentration can be circumvented with synthetic peptides via competition binding to the receptor. Given their small size (64 kDa), peptide-Fc fusions are expected to have good tissue penetration as compared to antibodies (150 kDa).
By coupling cancer cell-binding peptides to the Fc domain of human IgG1, in this study, we have married the targeting specificities of four peptides with the effector function of antibodies such as the activation of NK cells and induction of antibody-dependent cellular cytotoxicity (ADCC). Our results show that the Fc domain of human IgG1 when fused to cancer cell-binding peptides can enhance NK cell activation. Like conventional mAbs, the peptide-Fc fusion proteins enhanced ADCC against cancer cells, suggesting their use as antitumor agents.
Results
Expression and characterization of the peptide-Fc fusion proteins
During the last decade, peptide phage libraries have been screened successfully to identify peptides for unknown receptors that are expressed preferentially and/or specifically on the surface of target cells, tissues, or organs.4 The peptide-encoding DNA sequences can be easily fused with any therapeutic gene. To this end, we generated peptide-Fc fusions consisting of short peptides and the Fc part of human IgG1 (Figure 1a). The selection of the peptides is based on their validation by several independent groups as cancer cell-binding peptides.14 The resulting peptide Fc fusion proteins were confirmed by DNA sequencing and then introduced into HEK293T cells using lipofectamine 2000 as described in the Materials and Methods. The constructs contain an IL-2 signal peptide for secretion in culture media. Transient expression of the WN, LTV, and MY peptide Fc-fusion proteins resulted in a significant secretion of the recombinant proteins into the culture media (Figure 1b, as a representative example).
Figure 1.
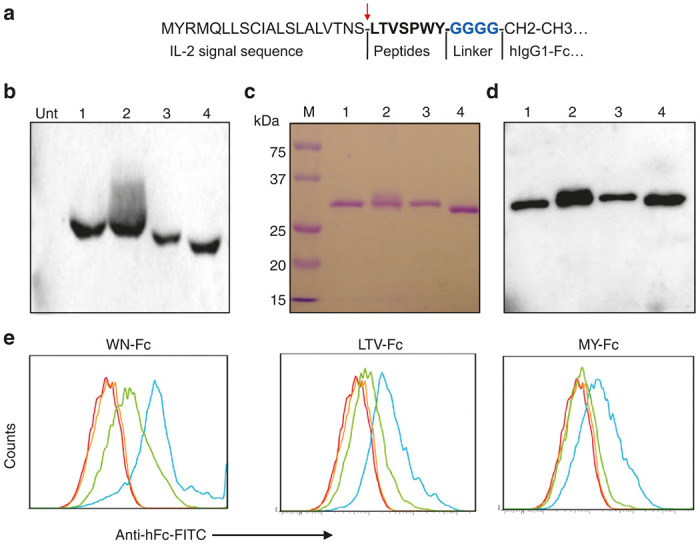
Generation and characterization of the peptide-Fc fusion proteins. (a) Schematic representation of peptide-Fc construction. The arrow indicates the cleavage site of IL-2 signal sequence. A glycine linker (in blue) was added to further increase the flexibility of the peptide moiety. (b) Peptide-Fc fusion expression in HEK293T cells. Subsequent to 48 hours of transfection time, culture supernatants (15 µl each) were analyzed by western blots using biotin-conjugated antihuman Fc antibody followed by streptavidin–HRP. Lanes 1 to 4 correspond to cells transfected with plasmid encoding WN-Fc, LTV-Fc, MY-Fc, and Fc control, respectively. Unt, supernatant from untransfeted cells. (c) Secreted peptide-Fc fusion proteins were purified with protein G affinity chromatography from culture supernatants and analyzed by SDS-PAGE under reducing conditions and then stained with Coomassie blue. (d) A duplicate gel was transferred to nitrocellulose membrane and processed as in b. Lanes 1 to 4 correspond to WN-Fc, LTV-Fc, MY-Fc, and Fc control fusion proteins, respectively. (e) Binding of the peptide-Fc fusion proteins to SKBR3. The cells were incubated with either peptide-Fc fusion proteins (blue histograms) or the Fc control (orange histograms) followed by FITC-conjugated antihuman Fc monoclonal antibody and analysis by flow cytometry. All molecules were tested at 10 µg/ml. In the case of competition experiments, the cells were incubated with the peptide (100 µg/ml) first and then with the corresponding peptide-Fc fusion protein (green histograms). Red histograms represent cells stained with only secondary antibody. Data are from one experiment and are representative of at least 10 independent experiments.
After having confirmed the expression, the peptide-Fc fusions were purified by protein G affinity chromatography and characterized by western blots. The purity of the engineered proteins was higher than 98% as measured by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunoblots (Figure 1c,d). As a result of glycosylation, the apparent molecular mass of recombinant human Fc is ~32 kDa in SDS-PAGE under reducing conditions. In contrast to the Fc control, all three peptide-Fc fusions bound to breast cancer cell line SKBR3 (Figure 1e, blue histograms). The WN- Fc, LTV-Fc, and MY-Fc fusions bound to SKBR3 cells with apparent affinities 15 ± 4, 90 ± 15, and 115 ± 18 nmol/l, respectively. The binding of the peptide-Fc fusion proteins to SKBR3 cells was inhibited by their corresponding synthetic peptides (green histograms), thereby confirming the binding specificity.
Activation of NK cells by peptide Fc-fusion proteins
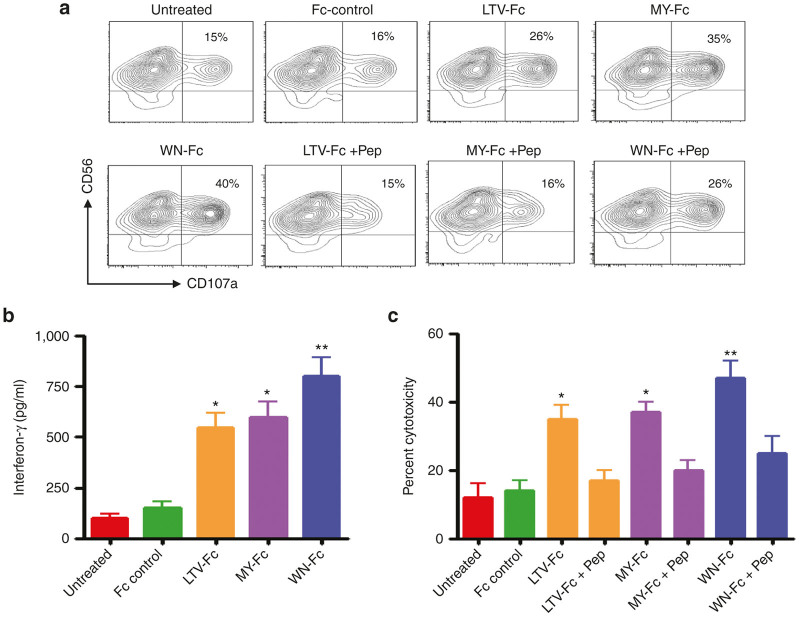
We next evaluated the ability of the engineered proteins to enhance NK cell activation against SKBR3 cells. The peptide-Fc fusion proteins significantly induced CD107a expression ranging from 26 to 40% of total NK cell population (Figure 2a; P < 0.05 compared to the Fc control). CD107a is a surrogate marker for granule mobilization, on resting and briefly activated NK cells. Usually CD107a is found in vesicle membranes, but during NK-target cell interaction, it is mobilized to the cell surface, and therefore, it can be quantified by flow cytometry. Notably, NK cell degranulation was inhibited by the corresponding synthetic peptides, which confirms that the binding was mediated through the interaction of the fused peptides with their receptors (Figure 2a, the three last panels). Moreover, the peptide Fc fusions induced the release of interferon-γ, which represents a second major effector mechanism by which NK cells contribute to antitumor immunity (Figure 2b).7
Figure 2.
Activation of NK cells and induction of ADCC. (a) NK cells (5 × 104) were cultured with SKBR3 cancer cells (105) in the presence or absence (untreated) of the indicated peptide-Fc fusion proteins (10 µg/ml each), and granule mobilization in gated NK cell population was analyzed by flow cytometry for CD107a expression on NK cells after 4 hours of incubation time in X-vivo 15 medium supplemented with IL-2 (10 ng/ml). The numbers in dot plots represent the percentage of CD107a-positive NK cells. Data are representative of four independent experiments. (b) Interferon-γ production was measured by ELISA of culture supernatants after 24 hours of incubation time. (c) ADCC assays were done with SKBR3 target cells incubated with NK cells at effector-to-target ratio of 20:1 in X-vivo 15 medium supplemented with IL-2. This ratio was selected from pilot experiments. The results in b and c are represented as means ± SD from triplicate determinations and are representative of at least three independent experiments using NK cells from different donors. Statistically significant differences between peptide-Fc fusion-treated cells and Fc control-treated cells are indicated by asterisks. *P < 0.05; **P < 0.02. Various colors are used to indicate the different molecules as indicated in x axis.
In addition to degranulation (CD107a surface display), we directly investigated the ADCC activity upon exposure of SKBR3 to the peptide-Fc fusions in the presence of NK cells. These analyses, which in contrast to experiments investigating NK cell degranulation (4–5 hours of assay time), were performed for 24 hours. Subsequently, lactate dehydrogenase release in culture supernatants upon cell lysis was analyzed. Coincubation with the peptide-Fc fusions increased ADCC against SKBR3 cells when compared to either untreated cells or cells treated with the Fc control (Figure 2c; P < 0.05). In the presence of NK cells, for example, the WN-Fc fusion was able to elicit 45 ± 6% cytotoxicity at an effector-to-target ratio of 20:1 (P < 0.02). The Fc control showed only 14 ± 3% cytotoxicity, which is comparable to that of untreated cells (SKBR3 + NK cells). Under our experimental conditions, the EC50 for the WN-Fc, MY-Fc, and LTV-Fc fusion proteins were around 20 ± 5, 45 ± 10, and 50 ± 12 nmol/l, respectively.
The WN-Fc-fusion protein is active against various cancer cell lines
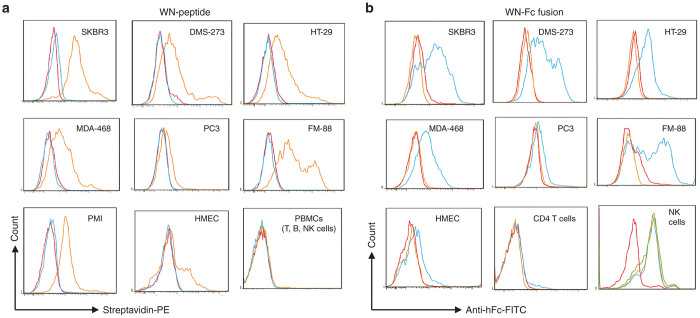
Given the strong binding of the WN-Fc fusion protein to SKBR3 and subsequent activation of NK cells, we set out to study its binding potency to other cancer cell lines. In parallel, we analyzed the binding of the synthetic peptide. By contrast to control peptide, the WN peptide bound to most solid cancer cell lines tested, but not peripheral blood lymphocyte population (B cells, T cells, and NK cells) (Figure 3a, orange histograms). Likewise, the corresponding WN-Fc-fusion protein bound to the tested cancer cell lines underlying its versatile use in cancer therapy (Figure 3b, blue histograms). Under our experimental conditions, the apparent affinities of the WN-Fc for SKBR3, DMS-273, HT29, MDA-468, and FM-88 cancer cell lines were comparable (15–20 nmol/l). No binding to blood CD4+ T cells was detected. As anticipated, all peptide-Fc fusion proteins displayed strong binding to NK cells via the interaction of the Fc domain with the activating Fcγ RIII a receptor (CD16) (Figure 3b, last panel). NK cells do not express inhibitory antibody receptors, underscoring a significant role in ADCC. Under our experimental conditions, the peptide and its Fc-fusion partner exhibited some binding to normal human mammary epithelial cells, although lower than that seen with cancer cells (Figure 3a,b). By contrast, the other engineered peptide-Fc fusions did not bind to human normal cells (data not shown). Collectively, the data suggest that the WN-Fc fusion protein recognizes a receptor that is common to many types of tumors.
Figure 3.
Representative examples of WN-peptide- and WN-Fc fusion-binding profiles. The indicated cell lines were stained with either the (a) WN peptide or the (b) WN-Fc fusion protein and then analyzed by flow cytometry. Freshly isolated NK cells were stained with the WN-Fc fusion (blue histogram), LTV-Fc fusion (green histogram), or Fc control (orange histogram). Red histograms represent cells stained with the secondary dectection agent. The data are from one experiment and are representative of at least five independent experiments. SKBR3, PMI, and MDA-468 are common breast cancer cell lines. DMS-273, small-cell lung cancer cell line; FM-88, melanoma cell line; HMEC, normal human mammary epithelial cells; HT-29, colon cancer cell line; PC3, prostate cancer cell line; PBMC, normal peripheral blood mononuclear cells.
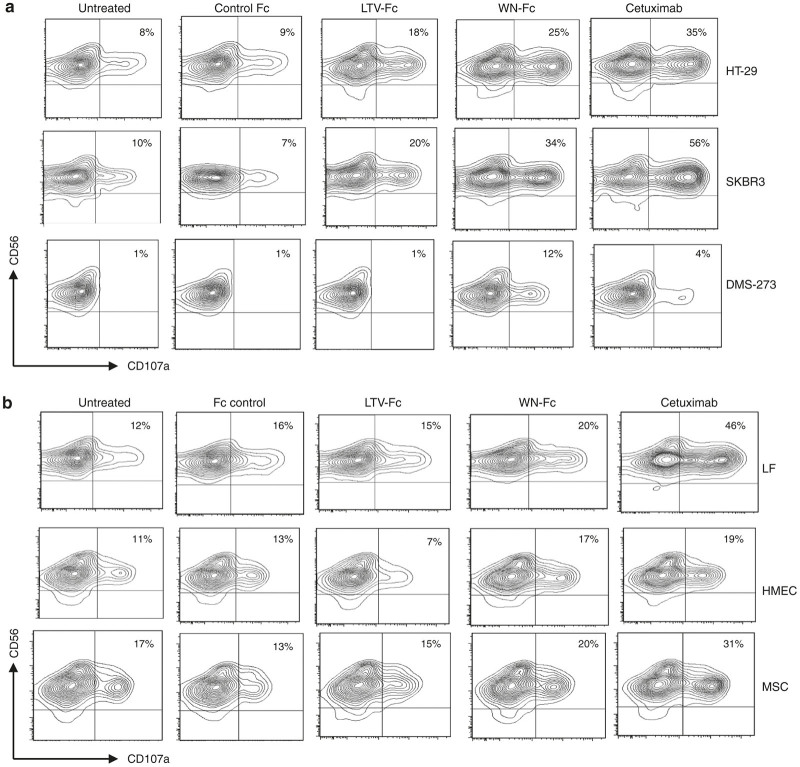
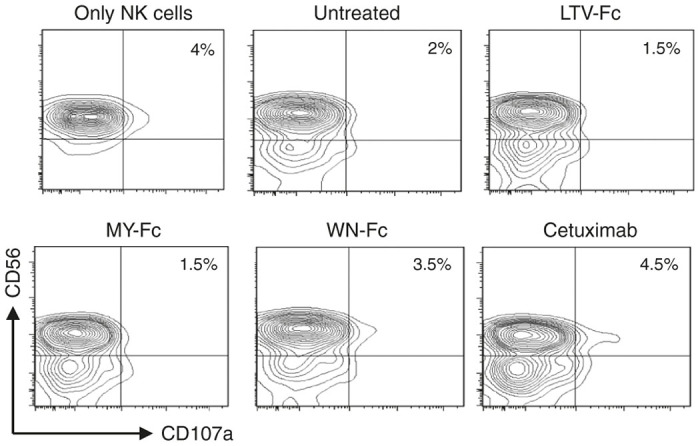
We next evaluated the ability of the WN-Fc fusion protein to activate NK cells against various solid tumor cell lines. In these experiments, SKBR3 (breast), HT-29 (colon), and DMS-273 (lung) were used as target cells. For comparison, we have included cetuximab, a human-mouse chimeric mAb that reacts with HER-1 and is currently US Food and Drug Administration approved for the treatment of certain types of cancer.35 Cetuximab is known to induce ADCC and receptor internalization.35 Notably, HER-1 is expressed by normal cells but overexpressed in a variety of tumor types, such as breast, gastric, colorectal, and ovarian tumors.36 The WN-Fc fusion protein enhanced NK cell activation as revealed by the upregulation of the CD107a marker (Figure 4a). Indeed, it was able to activate NK cells in the presence of target cells HT-29 (25 versus 9%; P < 0.05), SKBR3 (34 versus 7%; P < 0.05), and DMS-273 (12 versus 1%; P < 0.005) when compared to the Fc control. In the case of HT-29 and SKBR3, cetuximab showed higher activation potential than WN-Fc fusion protein (35 versus 25% and 56 versus 34%, respectively). However, the WN-Fc was more active against DMS-273 than cetuximab (12 versus 4%). With respect to SKBR3, the EC50 for the WN-Fc and cetuximab were 25 ± 3 and 9 ± 3 nmol/l, respectively. It should be noted that the effects of the different fusion proteins varied with NK cells of different donors, but in all cases, NK reactivity with peptide-Fc fusions was more pronounced than that with the Fc control. This supports the notion that NK reactivity is regulated by a balance of multiple activating and inhibitory signals that differ in individual patients.37
Figure 4.
Activation of NK cells. (a) NK cells (5 × 104) were incubated with various peptide-fusion proteins coated target cancer cells (105) in X-vivo 15 medium supplemented with IL-2 and then gated NK cell population was analyzed by flow cytometry for CD107a surface expression after 4 hours of incubation at 37 °C. All molecules were tested at 10 µg/ml. The numbers in dot plots represent the percentage of CD107a-positive NK cells. (b) Granule mobilization in the presence of normal allogenic cells. Experimental conditions are as in a. The same NK cell preparation was used in a and b panels. The data in a and b are from one single experiment and are representative of at least three independent experiments using NK cells from different donors.
We also determined whether the WN-Fc fusion protein affected NK cell reactivity against normal allogenic lung fibroblasts, human mammary epithelial cells and mesenchymal stem cells. A moderate enhancement of NK cell activation was observed when compared to the Fc control (Figure 4b), although significantly lower than that seen with cancer cells (P < 0.05). Among all the tested molecules, cetuximab gave the highest CD107a expression on NK in the presence of allogenic normal cells (Figure 4b, last panels). We further investigated the activation of NK cells against healthy autologous monocytes prepared from the same peripheral blood mononuclear cells as the NK cells. All tested constructs showed no significant effect on NK degranulation when compared to untreated cells (Figure 5).
Figure 5.
Granule mobilization in the presence of autologous normal cells. NK cells were added to autologous freshly isolated monocytes pretreated with the indicated molecules (10 µg/ml each) and then analyzed for CD107a surface expression after 4 hours of incubation at 37 °C. The data are from one single experiment and are representative of at least three independent experiments using NK cells from different donors.
Dual targeting of HER1 and HER2 by peptide-Fc fusions
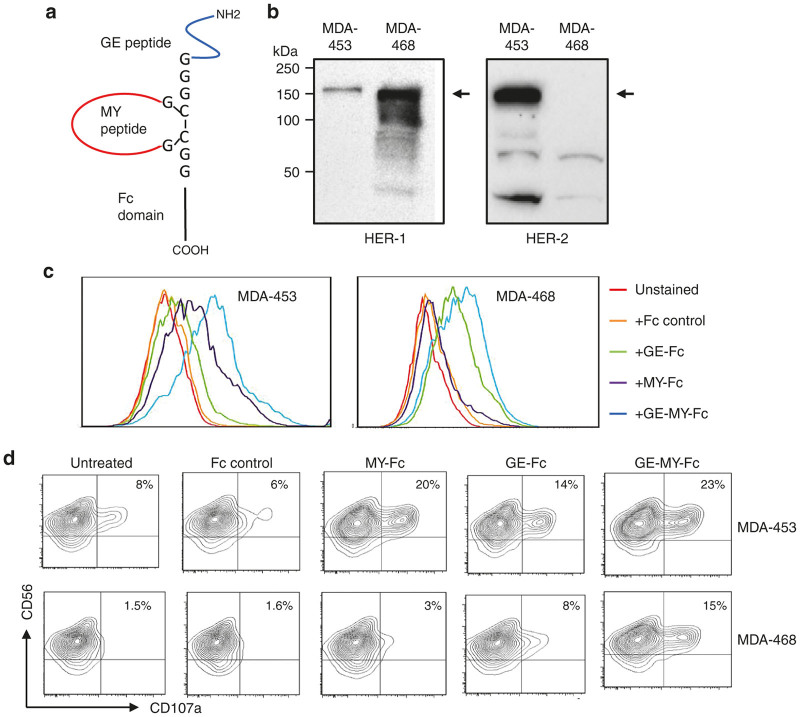
Next, we explored the possibility of engineering multiple specificities by including different peptide targeting sequences into a single Fc domain, an approach that will address the important issue of antigenic variability and escape in tumor immunotherapy. To this end, two validated peptides targeting HER-1 (GE-peptide) and HER-2 (MY peptide) were fused to the Fc-domain of human IgG1, expressed in HEK293T cells, and then protein G affinity purified as described in Figure 1b. The GE peptide was identified as HER-1 ligand and validated as a targeting moiety for HER-1 overexpressing tumors in vitro and in vivo.38 A schematic illustration of the fusion peptide is shown in Figure 6a. We introduced an artificial disulfide bridge into the MY peptide in order to reduce its interaction with the GE peptide. To test the specificity of the construct, two breast cancer cell lines MDA-453 and MDA-468 that express very low levels of HER-1 or HER-2, respectively, were used.39 As shown in Figure 6b, HER-2 protein was not detected in MDA-468, while MDA-453 expressed very low level of HER-1 when compared to MDA-468. The MY-Fc fusion protein showed significant binding to MDA-MB-453, but not to MDA-468 cells, which did not express HER-2 (Figure 6c, purple histograms). By contrast, the GE-Fc fusion protein bound more effectively to MDA-468 than MDA-453 (Figure 6c, green histograms). Interestingly, the dual peptide-Fc fusion protein bound to both cell lines (Figure 6c, blue histograms) with comparable apparent affinity (65 ± 10 nmol/l). In accordance with the binding profiles, the dual construct enhanced NK cell activation against MDA-453 (23 versus 6%) and MDA-468 (15 versus 1.6%) cell lines when compared to the Fc control (Figure 6d; P < 0.05), providing a new dual targeting opportunity for antibody-based therapy. In all experiments, MDA-453-coated peptide Fc fusions showed more sensitivity to NK cells than MDA-468.
Figure 6.
Bi-targeting HER-1 and HER-2 by peptide Fc fusions. (a) Schematic representation of the dual fusion peptide. Targeting peptides were separated by a glycine linker. (b) Western blot analysis of HER-1 and HER-2 expression in MDA-453 and MDA-468. The arrows indicate the immune-reactive bands. (c) Binding of the GE-, MY-, or GE-MY peptide-Fc fusions to breast cancer cell line MDA-453 and MDA-468. Cells were stained with the indicated fusion proteins and then analyzed by flow cytometry. (d) NK cells were incubated with various peptide-fusion coated target cells in X-vivo 15 medium supplemented with IL-2 and then gated NK cell population was analyzed by flow cytometry for CD107a expression after 4 hours of culture in X-vivo 15 medium supplemented with IL-2. All molecules were tested at 10 µg/ml. The numbers in dot plots represent the percentage of CD107a-positive NK cells. The data in c and d are from one single experiment and are representative of at least three independent experiments using NK cells from different donors.
The GE-MY peptide-Fc fusion induced ADCC in both cancer cell lines
After verifying the activation of NK cells by the GE-MY peptide-Fc fusion protein, we next investigated its ability to induce ADCC. In the presence of NK cells, the dual construct was able to elicit a significant ADCC in MDA-453 (35 ± 6%; P < 0.02) and MDA-468 (24 ± 2%; P < 0.02) when compared to the Fc control (Figure 7). Consistent with the receptor expression profiles, the parental MY-Fc fusion protein enhanced ADCC in only MDA-453. Although HER-1 expression was low in MDA-453 when compared to MDA-468, the GE-Fc fusion protein activated NK cells and induced a significant ADCC response against this cell line (18 ± 4%). These results are supportive of the possibility that peptide-Fc fusion-induced ADCC response could be elicited in cancer cells expressing low levels of target antigen. With respect to MDA-453, the EC50 for the dual construct and cetuximab were about 35 ± 5 and 25 ± 3 nmol/l, respectively. It should be noted that cetuximab is already optimized for clinical use (see Discussion).
Figure 7.
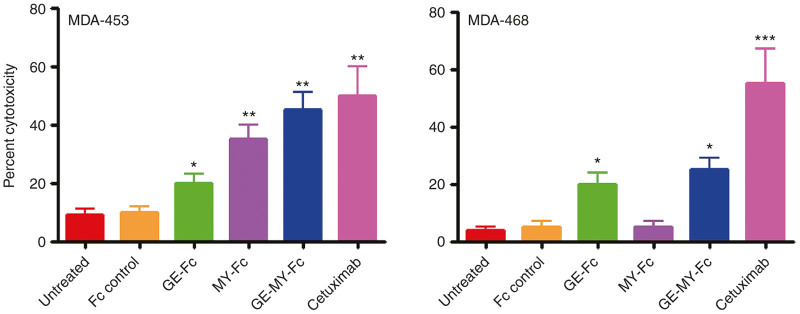
Induction of ADCC by the GE-MY peptide-Fc fusion. ADCC was evaluated with LDH release assay in which MDA-453 and MDA-468 (5 × 103/well) were incubated with the different peptide-Fc fusion proteins or cetuximab (30 nmol/l each) for 30 minutes at room temperature. After incubation, NK cells (105/well) were added to the wells and incubated at 37 °C for 24 hours in X-vivo medium supplemented with IL-2. Subsequently, LDH release in culture supernatants was quantified. The results are represented as means ± SD from triplicate determinations and are representative of three independent experiments using NK cells from different donors. Statistically significant differences between peptide-Fc fusion-treated cells and Fc control-treated cells are indicated by asterisks. *P < 0.05; **P < 0.02; ***P < 0.001. ADCC, antibody-dependent cellular cytotoxicity; LDH, lactate dehydrogenase.
Discussion
The development of new targeted therapies should represent a promising therapeutic strategy toward improving outcomes for cancer patients. In this study, we have demonstrated that fusion of a single copy of validated tumor-targeting peptides to Fc domain of human IgG1 can generate fusion partners that activate NK cells and enhance ADCC against various human tumor cell lines. Moreover, the engineered dual peptide-Fc variant constitutes a first step toward the targeting of tumors that coexpress multiple HER family members. It is of substantial value to be able to effectively target innate immune cells such as NK cells and macrophages to specific cell types by making use of peptides selected from phage display libraries.
Fc-fusion proteins have been used as research tools and more significantly as protein therapeutics. For example, etanercept/Enbrel, which is composed of the 75 kDa soluble extracellular domain of the TNF-α receptor II fused to the Fc domain of human IgG1, is the first successful example of using a soluble receptor-Fc fusion protein as a therapeutic drug.40,41 A peptide mimic of thrombopoietin fused to Fc (romiplostim, AMG-531) was the first peptide-Fc fusion (peptibody) developed for the treatment of chronic immune thrombocytopenia purpura.42 Trebananib (formerly known as AMG 386) is also a peptibody with dual specificity to angiopoietin (Ang)-1 and -2.43 AMG 386 neutralizes the binding of two soluble ligands, Ang-1 and Ang-2, to the Tie-2 receptor tyrosine kinase and inhibits tumor angiogenesis.44 More recently, Qin and colleages developed a peptibody that depleted myeloid-derived suppressor cells in tumor-bearing mice.33 Interestingly, the peptibody depleted myeloid-derived suppressor cells without affecting other proinflammatory cells, including dendritic cells and lymphocytes (T, B, and NK cells). To the best of our knowledge, the peptide-Fc fusions described in the present study are the first constructs reported to activate NK cells and enhance ADCC against tumor cells, thus providing a new tool for cancer immunotherapy. Furthermore, they can be used either as cancer detection or agent for radiotherapy after conjugation to a fluorescent probe or radionuclide, respectively. Also, attaching peptide-Fc fusions to chemotherapeutic agents can make them more powerful, and therefore, they will have positive impact on cancer patients.
Notably, cancer continues to remain a major health concern despite the progress in targeted therapies, mainly due to the development of resistance by cancer cells. Although multiple factors may contribute to the development of resistance to antibody therapy, several studies indicate that resistance is often associated with the downregulation of the antibody target and/or the involvement of other factors not targeted by the specific antibody.45 Hence, a therapeutic strategy targeting several factors might be superior from a conceptual point of view. Because most tumors coexpress multiple HER family members, identification of dual specific inhibitors may provide a therapeutic benefit to a broader patient population. Our data demonstrate that dual peptide-Fc fusion targeting HER-1 and HER-2 can be designed. The engineered protein induced ADCC in cell lines expressing HER-1 and/or HER-2. Moreover, the fusion protein was slightly more effective than the corresponding individual parental peptide Fc fusions. Similarly, a dual construct displaying the LTV and GE peptides showed a promising activity against several breast cancer cell lines (data not shown). Establishing the flexibility for dual targeting should pave the way to designing peptide-Fc fusion proteins with other dual specificities. Moreover, two or more specificities can be combined within single Fc domain to further improve efficacy, specificity, and reduce escape to antibody therapy.
With regard to toxicity, the specificity of a target antigen is an important issue. None of the used targeting peptides in this study bound to blood leukocytes, including T and B cells.4 As such, blood cytotoxicity is expected to be low. The LTV-Fc and MY-Fc fusion proteins showed selectivity toward HER-2-expressing cancer cells, whereas the GE-fusion protein exhibited specificity toward HER-1-expressing cancer cells. Treating normal human cells (e.g., lung fibroblasts, human mammary epithelial cells, and mesenchymal stem cells) with LTV-Fc, MY-Fc, GE-Fc, or GE-MY-Fc fusion protein had minimal effects on NK cell activation. We observed some binding of the WN-Fc fusion to normal cells, although weaker than that seen with cancer cells. However, it does not mean that the WN-Fc fusion will activate NK cells. Indeed, the WN-Fc receptor expression levels and many other immunoregulatory molecules such as NK inhibitory HLA class I will determine whether NK reactivity against these healthy cells will occur or not. Under our experimental conditions, the activation level of NK cells against normal cells in the presence of the WN-Fc fusion protein was less pronounced than that induced by cetuximab already approved for clinical use (Figure 4). Therefore, any potential toxicity is expected to be less pronounced than that induced by cetuximab.
It should be noted that the WN-fusion protein showed a strong binding to most solid tumor cell lines tested regardless of their HER-1 and/or HER-2 expression profiles, which supports its further consideration as a promising therapeutic agent in treatment of any form of tumors. Overall, the data suggest that the WN peptide recognizes a receptor that is common to many types of cancers, and therefore, it could make immunotherapy far more effective and perhaps cheaper since it can be produced by genetic engineering using eukaryotic or prokaryotic expression systems. It should be noted that any potential side effects upon application of the WN-Fc fusion to patients would likely be temporary in nature due to the expected pharmacokinetics of peptide-Fc fusion proteins.30 Indeed, the average plasma half-life of peptide-Fc fusions in humans ranges from 3 to 8 days, whereas that of antibodies is up to 4 weeks.46 Moreover, any unwanted toxicities related to the peptide-Fc fusion concentration can be reversed by the corresponding free synthetic peptide.
Although the designed peptide-Fc fusions activated NK cells against cancer cells, their effectiveness can be more enhanced by fusing two or more peptide sequences to the Fc domain of human IgG1 and/or addition of specifically designed flanking residues, spacers, or linker sequences. In addition, a number of engineered Fc variants showed significant enhancement in ADCC potency. These variants include the single mutants S239D and I332E and the double and triple mutants S239D/I332E and S239D/I332E/A330L.47,48 Also, the binding of IgG to Fcγ RIIIa was enhanced at least 10-fold by glycan that lacks the core fusosyl moiety.49,50 This increased binding to Fcγ RIIIa expressed by NK cells resulted in a substantial higher level of ADCC by all four IgG isotypes. The incorporation of such amino acid substitutions in our peptide-Fc fusions would increase their therapeutic potency. Indeed, some peptide-Fc fusions harboring the triple mutations strongly activated NK cells and inhibited tumor growth in vivo (Peng Q and Sioud M, manuscript in preparation).
While a number of selected peptides from phage libraries have been used for tumor imaging and/or drug delivery without knowledge of the cellular receptors, their clinical use will be further facilitated by the characterization of the binding receptors. Based on previous studies, the receptor for LTV and MY peptides is more likely HER-2 (refs. 18,19) and for GE peptide is HER-1 (ref. 38). We have performed a series of experiments (e.g., affinity capture) to identify the WN-peptide receptor, and the data suggest the involvement β3 integrin and other protein expressed on the cell surface of cancer cells. Hence, the binding may arise from the formation of a native conformational epitope involving the interaction of these protein partners. Such interaction is more likely to be lost upon the preparation of membrane proteins for affinity capture. Therefore, a combination of several cellular and molecular biology techniques will be needed to tackle this challenging question, which is under investigation.
In conclusion, the present data indicated that short peptides selected from phage display libraries can be turned into cancer cell killers via fusion to the Fc-domain of human IgG1. The engineered mono- or dual-specific peptide-Fc fusion proteins not only activated NK cells but also enhanced ADCC, which supports their further development as promising agents for cancer immunotherapy. Moreover, if antibodies could be engineered to recognize antigens that are common to many types of cancers, it could lead to a new versatile targeted cancer therapy. Hence, the WN-Fc fusion protein could be a candidate for such therapy.
Materials and Methods
Cell lines and culture conditions
Human cancer cell lines SKBR3, PM1, MDA-453, MDA-468, DMS-273, PC3, FM-88, and embryonic kidney HEK293T were purchased from the American Type Culture Collection. Cells were cultured in Roswell Park Memorial Institute medium 1640 (Sigma St Louis, MO) or Dulbecco’s Modified Eagle’s medium (Sigma St Louis, MO) supplemented with 10% heat-inactivated fetal calf serum and antibiotics. Normal human mammary epithelial cells and normal lung fibroblasts were purchased from Clonetics/BioWittaker (Allendale, NJ) and maintained in complete Mammary Epithelial Cell Growth medium (Lonza, Verviers, Belgium). Human peripheral blood mononuclear cells were prepared from buffy coats obtained from normal volunteers. Monocytes were enriched from peripheral blood mononuclear cells using plastic adherence, and purification was verified by phenotypic analysis of the surface marker CD14+. Bone marrow–derived mesenchymal stem cells were prepared from bone marrow aspirates as described previously.51 Approval for obtaining blood and bone marrow samples from normal volunteers was granted by Oslo University Hospital Ethics Committee.
Antibodies
Anti-CD107a and anti-CD56 antibodies were purchased from BD Pharmingen (San Diego, CA). Fluorescein isothiocyanate-conjugated mouse antihuman Fc and biotin-conjugated antihuman Fc mAbs were purchased from Sigma (St Louis, MO). Anti-HER-1 and anti-HER-2 antibodies were purchased from Santa Cruz (Dallas, TX). Anti-CD14, antimouse immunoglobulin G-, and antisheep IgG-horseradish peroxidase conjugates were purchased from Dako (Glostrup, Denmark). Streptavidin–horseradish peroxidase conjugates were obtained from Thermo Scientific (Rockford, IL).
Peptides, oligonucleotides, and cloning
Synthetic peptides were chemically synthesized and purified by high-performance liquid chromatography with >85% purity (GeneCust, Dudelange, Luxembourg). They were dissolved in dimethyl sulfoxide at 20 mg/ml and stored at −80 °C until use. LTV-peptide: LTVSPWY; WN-peptide: WNLPWYYSVSPT; MY-peptide: MYWGDSHWLQYWYE; GE-peptide: YHWYGYTPQNVI. The following overlapping DNA oligonucleotides encoding peptides were made and high-performance liquid chromatography purified by Eurofins Genomics (Ebersberg, Germany).
LTV peptide: 5′-AATTCGCTGACGGTGTCGCCTTGGTATGGTGGAGGCA-3′; 5′-GATCTGCCTCCACCATACCAAGGCGACACCGTCAGCG-3′
WN peptide: 5′-AATTCGTGGAATCTTCCTTGGTATTATAGCGTCAGTCCTACGGGTGGAGGCA-3′; 5′-GATCTGCCTCCACCCGTAGGACTGACGCTATAATACCAAGGAAGATTCCACG-3′
MY peptide: 5′-AATTCGATGTACTGGGGAGACTCTCACTGGCTGCAATACTGGTACGAGGGCGGCA-3′; 5′-GATCTGCCGCCCTCGTACCAGTATTGCAGCCAGTGAGAGTCTCCCCAGTACATCG-3′
GE-peptide: 5′-AATTCGTACCACTGGTACGGCTACACGCCACAAAATGTCATTGGCGGCGGCA-3′
5′-GATCTGCCGCCGCCAATGACATTTTGTGGCGTGTAGCCGTACCAGTGGTACG-3′
GE-MY peptide: 5′-AATTCGTACCACTGGTACGGCTACACGCCACAAAATGTCATTGGAGGAGGATGCGGAATGTACTGGGGAGACTCTCACTGGCTGCAATACTGGTACGAGGGATGCGGAGGAA-3′; 5′-GATCTTCCTCCGCATCCCTCGTACCAGTATTGCAGCCAGTGAGAGTCTCCCCAGTACATTCCGCATCCTCCTCCAATGACATTTTGTGGCGTGTAGCCGTACCAGTGGTACG-3′
GE-LTV peptide: 5′-AATTCGTACCACTGGTACGGCTACACGCCACAAAATGTCATTGGAGGAGGATGCGGACTGACGGTGTCGCCTTGGTATGGATGCGGAGGAA-3′; 5′-GATCTTCCTCCGCATCCATACCAAGGCGACACCGTCAGTCCGCATCCTCCTCCAATGACATTTTGTGGCGTGTAGCCGTACCAGTGGTACG-3′
Fc control peptide: 5′-AATTCGATATCGGCCATGGTTA-3′; 5′-GATCTAACCATGGCCGATATCG-3′
DNA oligonucleotides were annealed together to form double-stranded sequences with overhanging bases for EcoR1 and BglII restriction sites to allow cloning into EcoR1–BglII-cleaved pFuse-hIgG1-Fc2 vector in frame with IL-2 signal sequence and the Fc portion of human IgG1 (In vivoGen, San Diego, CA). Ligation mixtures were transformed into XL1 blue cells and plated on Zeocin LB plates. Plasmid DNAs were prepared from Zeocin-resistant clones and digested with EcoR1 and BglII restriction enzymes in order to verify the presence of the oligonucleotide inserts. Positive colonies were selected and then verified by DNA sequencing (Eurofins Genomics). The intracellular cleavage of the IL-2 signal peptide occurs after serine 20, just before the first amino acid of the cloned peptides, as indicated in Figure 1a.
Expression and purification of peptide-Fc fusion proteins
The peptide-Fc fusions were produced by transient transfection of the plasmids into HEK293T cells. Briefly, the cells were seeded into 75 cm2 tissue culture plates using 15 ml culture medium to reach 70–80% confluency after overnight culture. Next day, medium was completely replaced by DMEM supplemented with 5% v/v IgG stripped fetal calf serum and antibiotics to minimize co-purification of bovine IgG by protein G. Transfection was performed with lipofectamine 2000. A total of 30 µg plasmid DNA and 30 µl lipofectamine were separately diluted in 1.5 ml optimum medium, and both solutions were mixed and incubated for 30 minutes at room temperature. Thereafter, the mixtures were added to the cells and incubated for 48–72 hours. Peptide-Fc fusions were purified from culture supernatants by Protein G chromatography, and purity was determined by electrophoresis on 10% SDS-PAGE, followed by staining with Coomassie blue. Purified fractions were collected, pH adjusted to 7.5, and then stored at −80 °C until use.
Analysis of gene expression by western blots
Subsequent to 48 hours of transfection time with plasmid encoding peptide-Fc fusions, culture supernatants (15 µl) were separated by electrophoresis on 10% SDS-PAGE and electrotransfered to nitrocellulose membrane. After blocking in 5% milk in TBS/T (0.1% Tween) for 2 hours, membranes were incubated overnight at 4 °C with biotin-conjugated antihuman Fc mAb. Subsequently, the membranes were washed and then incubated with streptavidin–HRP conjugates. Immunoreactive proteins were detected using the enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK). HER-1 and HER-2 expression in MDA-468 and MDA-453 cancer cell lines were also analyzed by western blots. An equal amount of total protein extracts (30 µg) were separated by 10% SDS-PAGE, and immunoblotting was carried out using rabbit monoclonal anti-HER-2 and sheep monoclonal anti-HER-1 followed by HRP-conjugated antirabbit or antisheep IgG, respectively. Enhanced chemiluminescence detection reagent was used to visualize the protein bands.
Flow cytometry analysis and affinity determinations
The binding of the peptides and peptide-Fc fusion proteins to cancer and normal cells were analyzed by flow cytometry. In brief, aliquots of cells (105) were divided into conical 96-well microplate, washed with phosphate-buffered saline containing 1% fetal calf serum, and then incubated with the affinity-purified peptide-Fc fusions for 30 minutes on ice. After washing, cells were incubated with FITC-conjugated antihuman Fc for 30 minutes on ice, and then, the samples were analyzed by flow cytometry. Competition assays were performed by preincubating cancer cells with the peptides for 20 minutes on ice prior to staining with the peptide-Fc fusions. Binding of the synthetic WN-peptide and control peptide to cancer cells was performed as previously described.52 Briefly, the cells were incubated with biotin-conjugated peptides (10 µg/ml) for 30 minutes at 4 °C. After washing, they were incubated with streptavidin-phycoerythrin and then analyzed by flow cytometry. Sample acquisition was performed using a FACSCanto II (BD Biosciences, San Jose, CA), and mean fluorescence intensities were analyzed in Flow Jo software. Affinity measurements by enzyme-linked immunosorbent assay for cell surface antigens were calculated as described by Bator and Reading.53 Briefly, peptide-Fc fusions at various concentrations were mixed with cell suspensions incubated for 2 hours on ice with occasional mixing and were then pelleted by centrifugation. Supernatants were removed and retained for the quantification of unbound peptide-Fc fusions using the human IgG ELISA Quantification Kit (Bethyl Laboratories, Montgomery, TX). Bound peptide-Fc peptide concentrations are obtained by subtraction of unbound peptide-Fc fusions from the initial antibody concentrations, and these values are then used to construct Scatchard plots.
Degranulation of NK cells
NK cells were purified from buffy coats from healthy donors using NK cell isolation kit and auto MAC Pro Separator according to the manufacturer’s instructions (Miltenyi Biotec), and purification was verified by phenotypic analysis of the surface marker CD56. The purity of the cells was more than 96%. The target cells were seeded into 96-well plates at 2 × 104/100 µl DMEM supplemented with 10% (v/v) fetal calf serum and then incubated overnight at 37 °C. On the following day, the medium was replaced by X-vivo 15 medium (Lonza, Verviers, Belgium) supplemented with IL-2, and peptide-Fc fusions were added to the cells (5–10 µg/ml) and incubated at room temperature for 30 minutes. Subsequently, IL15-activated NK cells (5 × 104 in 100 µl X-vivo medium) along with PE-Cy5-conjugated mouse antihuman anti-CD107a (2 µl/well) and monensin (0.2 µl/well) were added, and the plates incubated at 37 °C. In some experiments nonbinding peptide-Fc fusions were removed prior to adding NK cells to target cells. Competition with the synthetic peptides (100 µg/ml) was performed by preincubating target cells with the peptides for 60 minutes prior to adding the corresponding peptide-Fc fusions (10 µg/ml). After incubation at 37 °C for 4 hours, the cells were harvested, washed, stained with FITC-conjugated mouse antihuman CD56 mAb, and then analyzed by flow cytometry. Sample acquisition was performed using a FACSCanto II (BD Biosciences), and mean fluorescence intensities were analyzed by Flow Jo software.
ADCC assay
The assay was conducted using the lactate dehydrogenase cytotoxicity kit based on the manufacturer’s instructions (Promega, Madison, WI). Target cells pretreated with the peptide-Fc fusions (10 µg/ml) were incubated with IL-15-activated NK cells at effector-to-target ratio of 20:1 for 24 hours at 37 °C in X-vivo 15 medium supplemented with IL-2. After incubation, cell supernatants were transferred to a 96-well plate to determine the amount of lactate dehydrogenase released. Percentage of cell lysis in the cytotoxicity assays was calculated as (experimental release − background release/maximum release − background release) × 100. The effector-to-target ratio 20:1 was determined from pilot experiments.
Statistical analysis
Statistical analysis was performed with a Student’s t-test. P values <0.05 were considered significant. Unpaired two-tailed t-test was used to assess differences in NK cell CD107a expression and ADCC activity.
Acknowledgments
This work was supported in part by the Gene Therapy Program at Oslo University Hospital and The Norwegian Cancer Society to M.S. We thank Anne Dybwad for critical reading of the manuscript. We also thank Qian Peng and Antoni Wiedlocha (Oslo University Hospital) for generous gift of cetuximab and anti-HER-1 antibody, respectively.
The authors declare no conflict of interest.
References
- Paci, A, Veal, G, Bardin, C, Levêque, D, Widmer, N, Beijnen, J et al. (2014). Review of therapeutic drug monitoring of anticancer drugs part 1–cytotoxics. Eur J Cancer 50: 2010–2019. [DOI] [PubMed] [Google Scholar]
- Mack, PC, Gandara, DR and Lara, PN Jr (2012). Efficacy and toxicity differences in lung cancer populations in the era of clinical trials globalization: the ‘common arm’ approach. Expert Rev Anticancer Ther 12: 1591–1596. [DOI] [PubMed] [Google Scholar]
- Bhutia, SK and Maiti, TK (2008). Targeting tumors with peptides from natural sources. Trends Biotechnol 26: 210–217. [DOI] [PubMed] [Google Scholar]
- Shadidi, M and Sioud, M (2003). Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat 6: 363–371. [DOI] [PubMed] [Google Scholar]
- Beck, A, Wurch, T, Bailly, C and Corvaia, N (2010). Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 10: 345–352. [DOI] [PubMed] [Google Scholar]
- Seidel, UJ, Schlegel, P and Lang, P (2013). Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 4: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier, E, Tomasello, E, Baratin, M, Walzer, T and Ugolini, S (2008). Functions of natural killer cells. Nat Immunol 9: 503–510. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn, F and Ravetch, JV (2008). Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8: 34–47. [DOI] [PubMed] [Google Scholar]
- Niwa, R, Sakurada, M, Kobayashi, Y, Uehara, A, Matsushima, K, Ueda, R et al. (2005). Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin Cancer Res 11: 2327–2336. [DOI] [PubMed] [Google Scholar]
- Mössner, E, Brünker, P, Moser, S, Püntener, U, Schmidt, C, Herter, S et al. (2010). Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115: 4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, GP and Petrenko, VA (1997). Phage display. Chem Rev 97: 391–410. [DOI] [PubMed] [Google Scholar]
- Kehoe, JW and Kay, BK (2005). Filamentous phage display in the new millennium. Chem Rev 105: 4056–4072. [DOI] [PubMed] [Google Scholar]
- Pasqualini, R and Ruoslahti, E (1996). Organ targeting in vivo using phage display peptide libraries. Nature 380: 364–366. [DOI] [PubMed] [Google Scholar]
- Shadidi, M and Sioud, M (2003). Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J 17: 256–258. [DOI] [PubMed] [Google Scholar]
- Oyama, T, Sykes, KF, Samli, KN, Minna, JD, Johnston, SA and Brown, KC (2003). Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett 202: 219–230. [DOI] [PubMed] [Google Scholar]
- Lo, A, Lin, CT and Wu, HC (2008). Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther 7: 579–589. [DOI] [PubMed] [Google Scholar]
- Urbanelli, L, Ronchini, C, Fontana, L, Menard, S, Orlandi, R and Monaci, P (2001). Targeted gene transduction of mammalian cells expressing the HER2/neu receptor by filamentous phage. J Mol Biol 313: 965–976. [DOI] [PubMed] [Google Scholar]
- McGuire, MJ, Samli, KN, Chang, YC and Brown, KC (2006). Novel ligands for cancer diagnosis: selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp Hematol 34: 443–452. [DOI] [PubMed] [Google Scholar]
- Wu, X, Huang, H, Wang, C, Lin, S, Huang, Y, Wang, Y et al. (2013). Identification of a novel peptide that blocks basic fibroblast growth factor-mediated cell proliferation. Oncotarget 4: 1819–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, XF, Birringer, M, Dong, LF, Veprek, P, Low, P, Swettenham, E et al. (2007). A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res 67: 3337–3344. [DOI] [PubMed] [Google Scholar]
- Florczak, A, Mackiewicz, A and Dams-Kozlowska, H (2014). Functionalized spider silk spheres as drug carriers for targeted cancer therapy. Biomacromolecules 15: 2971–2981. [DOI] [PubMed] [Google Scholar]
- Moreno, M, Zurita, E and Giralt, E (2014). Delivering wasp venom for cancer therapy. J Control Release 182: 13–21. [DOI] [PubMed] [Google Scholar]
- Gong, C, Pan, D, Qiu, F, Sun, P and Zhang, YH (2014). Selective DNA delivery to tumor cells using an oligoarginine-LTVSPWY peptide. PLoS One 9: e110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, H, Yang, J, Jin, H, Huang, C, Fu, J, Yang, F et al. (2011). Tetrameric far-red fluorescent protein as a scaffold to assemble an octavalent peptide nanoprobe for enhanced tumor targeting and intracellular uptake in vivo. FASEB J 25: 1865–1873. [DOI] [PubMed] [Google Scholar]
- Pastorino, F, Brignole, C, Di Paolo, D, Nico, B, Pezzolo, A, Marimpietri, D et al. (2006). Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Res 66: 10073–10082. [DOI] [PubMed] [Google Scholar]
- Zhan, C, Gu, B, Xie, C, Li, J, Liu, Y and Lu, W (2010). Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release 143: 136–142. [DOI] [PubMed] [Google Scholar]
- Guan, H, McGuire, MJ, Li, S and Brown, KC (2008). Peptide-targeted polyglutamic acid doxorubicin conjugates for the treatment of alpha(v)beta(6)-positive cancers. Bioconjug Chem 19: 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T, D’Souza, GG, Bedi, D, Fagbohun, OA, Potturi, LP, Papahadjopoulos-Sternberg, B et al. (2010). Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine (Lond) 5: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arap, W, Haedicke, W, Bernasconi, M, Kain, R, Rajotte, D, Krajewski, S et al. (2002). Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA 99: 1527–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, B and Sun, YN (2014). Pharmacokinetics of peptide-Fc fusion proteins. J Pharm Sci 103: 53–64. [DOI] [PubMed] [Google Scholar]
- Shimamoto, G, Gegg, C, Boone, T and Quéva, C (2012). Peptibodies: a flexible alternative format to antibodies. MAbs 4: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C (2009). Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr Opin Biotechnol 20: 692–699. [DOI] [PubMed] [Google Scholar]
- Qin, H, Lerman, B, Sakamaki, I, Wei, G, Cha, SC, Rao, SS et al. (2014). Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, KR, Pomerantz, SC, Li, J, Vafa, O, Naso, M, Strohl, W et al. (2015). Secretion of Fc-amidated peptide fusion proteins by Chinese hamster ovary cells. BMC Biotechnol 15: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga, J (2001). The EGFR as a target for anticancer therapy–focus on cetuximab. Eur J Cancer 37 (suppl. 4): S16–S22. [DOI] [PubMed] [Google Scholar]
- Hynes, NE and Lane, HA (2005). ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354. [DOI] [PubMed] [Google Scholar]
- Vivier, E, Raulet, DH, Moretta, A, Caligiuri, MA, Zitvogel, L, Lanier, LL et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science 331: 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z, Zhao, R, Wu, X, Sun, Y, Yao, M, Li, J et al. (2005). Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J 19: 1978–1985. [DOI] [PubMed] [Google Scholar]
- DeFazio-Eli, L, Strommen, K, Dao-Pick, T, Parry, G, Goodman, L and Winslow, J (2011). Quantitative assays for the measurement of HER1-HER2 heterodimerization and phosphorylation in cell lines and breast tumors: applications for diagnostics and targeted drug mechanism of action. Breast Cancer Res 13: R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey, D, Klareskog, L, Sasso, EH, Salfeld, JG and Tak, PP (2008). Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 117: 244–279. [DOI] [PubMed] [Google Scholar]
- Klareskog, L, van der Heijde, D, de Jager, JP, Gough, A, Kalden, J, Malaise, M et al. TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. (2004). Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363: 675–681. [DOI] [PubMed] [Google Scholar]
- Molineux, G and Newland, A (2010). Development of romiplostim for the treatment of patients with chronic immune thrombocytopenia: from bench to bedside. Br J Haematol 150: 9–20. [DOI] [PubMed] [Google Scholar]
- Herbst, RS, Hong, D, Chap, L, Kurzrock, R, Jackson, E, Silverman, JM et al. (2009). Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol 27: 3557–3565. [DOI] [PubMed] [Google Scholar]
- Karlan, BY, Oza, AM, Richardson, GE, Provencher, DM, Hansen, VL, Buck, M et al. (2012). Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol 30: 362–371. [DOI] [PubMed] [Google Scholar]
- Mukohara, T (2011). Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci 102: 1–8. [DOI] [PubMed] [Google Scholar]
- Keizer, RJ, Huitema, AD, Schellens, JH and Beijnen, JH (2010). Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 49: 493–507. [DOI] [PubMed] [Google Scholar]
- Lazar, GA, Dang, W, Karki, S, Vafa, O, Peng, JS, Hyun, L et al. (2006). Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 103: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, T, Niwa, R, Satoh, M, Akinaga, S, Shitara, K and Hanai, N (2009). Engineered therapeutic antibodies with improved effector functions. Cancer Sci 100: 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter, S, Birk, MC, Klein, C, Gerdes, C, Umana, P and Bacac, M (2014). Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 192: 2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, R, Shoji-Hosaka, E, Sakurada, M, Shinkawa, T, Uchida, K, Nakamura, K et al. (2004). Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res 64: 2127–2133. [DOI] [PubMed] [Google Scholar]
- Sioud, M, Mobergslien, A, Boudabous, A and Fløisand, Y (2010). Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol 71: 267–274. [DOI] [PubMed] [Google Scholar]
- Sioud, M, Skorstad, G, Mobergslien, A and Sæbøe-Larssen, S (2013). A novel peptide carrier for efficient targeting of antigens and nucleic acids to dendritic cells. FASEB J 27: 3272–3283. [DOI] [PubMed] [Google Scholar]
- Bator, JM and Reading, CL (1989). Measurement of antibody affinity for cell surface antigens using an enzyme-linked immunosorbent assay. J Immunol Methods 125: 167–176. [DOI] [PubMed] [Google Scholar]