Abstract
Background
Transcatheter aortic-valve implantation (TAVI) is a new therapeutic choice for treating aortic stenosis in patients considered high risk for surgery. This blooming therapeutic technique still requires evaluation of medium and long term outcome.
Method
We hereby report our results of the first 150 consecutive patients to receive TAVI implants in our population recruited from July 2009 to March 2013 in a retrospective and monocentric study. We analyzed long term morbidity and mortality criteria. We compared the apical and femoral approach results and researched predictors of cardiac mortality.
Results
The mean monitoring period was 387.62 days, mean Euroscore was 21.8, and mean Society of Thoracic Surgeons (STS) risk score was 9.2. The success rate for the procedure was 94.6 %. A total of 39 patients died. The mortality rates at the immediate perioperative point, 30 days, 1 year, and 2 years, were 4 %, 11.3 %, 22.7 %, and 26 %, respectively. As regards complications, there were 10 hemodynamic complications (6.6 %) and 20 vascular (13.3 %), 11 cardiac tamponades (7.4 %), eight mechanical (5.3 %), ten major hemorrhagic (6.7 %), 14 pulmonary (9.3 %), and 18 infectious complications (12 %). When comparing the rates of reported complications in terms of different approaches, we observed significantly more hemodynamic complications in the apical group (p = 0.049). Pulmonary complications were also significantly more common in cases of apical approach (p = 0.029). The majority of the patients reported clear functional improvement throughout their follow-up.
Conclusion
The results of the first 150 patients to receive the implant at the Nancy University Teaching Hospital (CHU Nancy) were consistent with findings in the literature. TAVI proved a credible and effective alternative to surgical valve replacement for patients at high risk during surgery.
Keywords: TAVI: Transcatheter aortic-valve implantation, Aortic stenosis, VARC: Valve Academic Research Consortium
Background
Calcified aortic stenosis is the most common type of valvular heart disease found among adults in Western industrialized countries [1]. The etiology for this disease is mainly degenerative, therefore those affected are primarily of an advanced age. Observational studies demonstrate that its prevalence increases significantly with age, from 1.5 % for the 64–74-year age group to 4.8 % for those aged 75 and over [1]. Since the first percutaneous transcatheter implantation of an aortic valve prosthesis [2], transcatheter aortic-valve implantation (TAVI) has become a valid alternative to surgical aortic valve replacement [3–6]. Our study analyzed the first 150 patients treated with TAVI at the university hospital of Nancy (CHU Nancy) and compared the results according to approach method. We were particularly focused on studying and analyzing the mortality rates at 31 days, 1 year, and 2 years, along with the demographic features of the patient population, procedural information, complications, clinical follow-up, and echocardiography results.
Methods
This study reports on the first 150 patients treated with TAVI at CHU Nancy from July 2009 to March 2013. This was a retrospective and monocentric study, yet does include prospective data gathered for the France 2 [5] and France TAVI registries.
Patients indicated for TAVI procedures were selected according to the guidelines of the Haute Autorité de Santé (HAS), the French national health authority, after the possibility of surgery was ruled out. Patient assessment was carried out at a multidisciplinary meeting, taking into account surgical risk scores (logistic Euroscore >20 % or Society of Thoracic Surgeons [STS] >10 %) and comorbidities. Each patient treated was fully informed and signed an informed consent form. The following information was collected before the procedure: demographic information, blood parameter values, and echocardiogram data. These same parameters were analyzed following surgery. The results were compared according to the type of approach used and the implantation success rate.
The effectiveness criteria consisted of the reduction in mean transaortic gradient and increase in aortic surface. The mortality rates were analyzed at several points: immediately perioperatively, at 30 days, 6 months, 1 year, 2 years, and 3 years. We also researched the predictive factors for cardiovascular mortality. Clinical follow-up, consisting of functional status according to the New York Heart Association [NYHA] classification, and echocardiography were carried out at the follow-up visits. Patient follow-up was performed at 6 months for all patients then at 1, 2, and even 3 years for the most elderly. This study involving the first 150 subjects thus extended over a period of 3 years and 7 months. We categorized the major complications in accordance with the Valve Academic Research Consortium (VARC) classification [7].
Statistical analysis
The statistical analysis was carried out using the SPSS 17.0 software for Windows (Chicago, Illinois). Quantitative variables were compared using paired and non-paired t-tests or by analysis of variance (ANOVA). Qualitative variables were compared using chi-squared tests or Fisher’s exact test. We investigated the entire patient population for predictive event survival factors by means of univariate Cox regression model. In order to determine if these were the result of independent predictive factors, for each test a value of p < 0.05 was considered significant and for each significant variable we stated the relative risk and 95 % confidence interval (CI). The survival curves were determined using the Kaplan-Meier method.
Results
The follow-up period extended from July 28th 2009 to July 3rd 2013, making a total follow-up period of 3 years and 9 months. In total, 150 patients were treated. The mean monitoring period was 387.62 days, with a median of 303.50 days (interquartile range [IQR]: 141.25-597.50).
The general features of the patient population have been presented in Table 1. Mean age at implantation was 82.6 years old. The mean Euroscore was 21.8 and mean STS was 9.2. All patients manifested symptoms. A study of the patient functional status revealed the following: 66 % exhibited Class III dyspnoea (NYHA); there was a high rate of coronary heart disease (46.7 %); both the femoral and apical approach groups were homogeneous.
Table 1.
Patient characteristics at baseline
| Characteristics | All patients (n = 150) | Transfemoral approach (n = 78) | Transapical approach (n = 72) | p |
|---|---|---|---|---|
| Male gender | 68 (45.3 %) | 35 (44.9 %) | 33 (45.8 %) | NS |
| Age (years) | 82.6 +/− 6.9 | 83.5 +/− 6.7 | 81.7 +/− 7.0 | NS |
| BMI | 25.4 +/− 4.1 | 24.9 +/− 4,2 | 24.9 +/− 4.1 | NS |
| Coronary artery disease | 70 (46.6 %) | 32 (41.0 %) | 38 (52.8 %) | NS |
| PCI | 43 (28.7 %) | 21 (27.0 %) | 22 (30.6 %) | NS |
| Previous myocardial infarction | 16 (10.7 %) | 7 (9.0 %) | 9 (12.5 %) | NS |
| Previous cardiac surgery | 31 (20.7 %) | 12 (15.4 %) | 19 (26.4 %) | 0.096 |
| CABG | 28 (18.7 %) | 10 (12.8 %) | 18 (25.0 %) | 0.056 |
| Mitral mechanical prosthesis | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NS |
| Aortic bioprosthesis | 2 (1.3 %) | 2 (2.6 %) | 0 (0 %) | NS |
| Aortic balloon valvuloplasty | 3 (2 %) | 1 (1.3 %) | 2 (2.8 %) | NS |
| Hypertension | 89 (59.3 %) | 47 (60.3 %) | 42 (58.4 %) | NS |
| Diabetes | 42 (28 %) | 24 (30.8 %) | 18 (25.0 %) | NS |
| Smoking | 46 (30.6 %) | 17 (21.8 %) | 29 (40.3 %) | 0.014 |
| Plasma creatinine (μmol/L) | 113.5 +/−115.2 | 113.4+/−127.6 | 114.0 +/−101.2 | NS |
| Renal dialysis | 3 (2 %) | 2 (2.6 %) | 1 (1.4 %) | NS |
| COPD | 24 (16 %) | 8 (10.3 %) | 16 (22.2 %) | 0.046 |
| Peripheral vascular disease | 25 (16.6 %) | 11 (14.1 %) | 14 (19.4 %) | NS |
| Cerebrovascular disease | 20 (13.3 %) | 12 (15.4 %) | 8 (11.1 %) | NS |
| Euroscore | 21.67 +/− 11.3 | 20.5 +/− 10.4 | 22.9 +/− 12.1 | NS |
| STS | 9.65 +/− 5.95 | 8.8 +/− 4.5 | 10.3 +/− 7.0 | NS |
| Acute pulmonary edema | 21 (14 %) | 6 (7.7 %) | 15 (20.8 %) | 0.020 |
| Heart failure | 39 (26 %) | 15 (19.2 %) | 24 (33.3 %) | 0.049 |
| Syncope | 6 (4 %) | 3 (3.8 %) | 3 (41.7 %) | NS |
| Angor pectoris | 11 (7.4 %) | 7 (9.0 %) | 4 (5.6 %) | NS |
The primary differences were the following: there were more patients with a history of heart and coronary bypass surgery in the apical group (p = 0.096 and p = 0.056, respectively); there were significantly more smokers and cases of chronic obstructive pulmonary disease (COPD) in the apical group (p = 0.014 and p = 0.046, respectively). In addition, with regard to symptomatology, there were significantly more patients presenting with acute pulmonary edema and cardiac decompensation in the apical group (p = 0.02 and p = 0.049, respectively).
The echographic features are presented in Table 2, the mean gradient and aortic orifice area were 52.8 mmHg and 0.6 cm2. Mean left ventricular ejection fraction (LVEF) was 52.8 %, 49.3 % of patients exhibited associated aortic insufficiency, 42 % had associated mitral regurgitation, and the mean systolic arterial pressure was 46.3 mmHg.
Table 2.
Echocardiographic characteristics at baseline
| Echocardiographic characteristics | All patients (n = 150) | Transfemoral approach (n = 78) | Transapical approach (n = 72) | p |
|---|---|---|---|---|
| Mean gradient (mmHg) | 52.8 +/− 11.5 | 52.8 +/− 11.2 | 52.8 +/− 11.8 | 0.626 |
| Maximal gradient (mmHg) | 83.7 +/− 16.1 | 85.2 +/− 15.7 | 82.2 +/− 16.5 | 0.064 |
| Vmax (m/s) | 4.3 +/− 0.8 | 4.6 +/− 0.7 | 4.7 +/− 0.7 | 0.678 |
| Aortic area (cm2) | 0.6 +/− 0.2 | 0.6 +/− 0.1 | 0.6 +/− 0.2 | 0.778 |
| LVEF (%) | 52.8 +/− 12.5 | 51.3 +/− 13.1 | 54.5 +/− 11.7 | 0.140 |
| Diastolic function E/E’ | 11.4 +/− 4.2 | 12.0 +/− 4.7 | 10.9 +/− 3.5 | 0.115 |
| Aortic annulus diameter (mm) | 22.4 +/− 2.7 | 22.1 +/− 3.1 | 22.8 +/− 2.2 | 0.089 |
| IVS (mm) | 12.4+/− 3.0 | 12.6 +/− 2.4 | 12.7 +/− 2.3 | 0.936 |
| Aortic regurgitation | 74 (49.3 %) | 36 (46.2 %) | 38 (52.8 %) | 0.137 |
| Grade 1 | 38 (26 %) | 22 (28.2 %) | 17 (23.6 %) | NS |
| Grade 2 | 34 (22 %) | 12 (15.3 %) | 21 (29.2 %) | NS |
| Grade 3 | 2 (1.3 %) | 2 (2.6 %) | 0 (%) | NS |
| Mitral regurgitation | 63 (42 %) | 27 (34.6 %) | 36 (50.0 %) | 0.128 |
| Grade 1 | 29 (19.3 %) | 12 (15.4 %) | 17 (23.6 %) | NS |
| Grade 2 | 31 (20.7 %) | 15 (19.2 %) | 16 (22.2 %) | NS |
| Grade 3 | 3 (2 %) | 0 (0 %) | 3 (4.2 %) | NS |
| PASP (mmHg) | 46.3 +/− 12.6 | 47.6 +/− 13.0 | 44.7 +/− 12.0 | 0.147 |
| TAPSE (mm) | 15 +/− 3 | 14.6 +/− 3.2 | 15.8 +/− 2.8 | 0.013 |
| Bicuspid aortic valve | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NS |
IVS interventricular septum, LVEF left ventricular ejection fraction, PASP pulmonary artery systolic pressure, TAPSE tricuspid annular plane systolic excursion
Perioperative results
A total of 78 patients were treated using the femoral route and 72 by the apical route. All operations were carried out under general anesthetic. Only 149 prostheses were implanted, as one patient died when anesthetized. Of all the prostheses, 137 were Edwards Sapien and 12 were CoreValve. The mean prosthesis diameter was 25.6 mm. The success rate for the procedure was 94.6 %, taking into account the six patients who died immediately during the operation and two failed procedures. The different reasons for death in the operating theatre were: one massive post-dilation aortic insufficiency, one cardiac arrest under anaesthetic, two ruptures of the aortic root after insertion of the prosthesis, and two cases of cardiac tamponade with refractory state of shock. The two failed procedure were one incorrect position of the valve because of undersizing of the prosthesis resulting in the migration of the valve into the left ventricle, treated by surgical conversion and aortic replacement, and one cardiac tamponade after insertion of the catheter via the apical approach. The mean length of hospital stay was 12.8 days +/− 10.7 days. Comparing the two approach routes, the apical group presented a significantly longer mean hospital stay length, at 15.5 days +/− 12.8 versus 10.3 days +/− 7.6 for the femoral group (p = 0.002).
Mortality rates
In total, 39 patients died. Our study found that six died in the operating theatre (4 %), 11 later within 31 days (11.3 %), 14 within six months (21.3 %), two at one year (22.7 %), and a further six had died by the 2-year point (26 %). There were no further deaths after 2 years of follow up and the mortality rate at both three and nine months was therefore 26 %. By the 2-year follow-up, 25 patients had died from cardiovascular causes (16.7 %) and 14 patients from non-cardiovascular causes (9.3 %). The different causes of mortality were: dislocation of the shoulder followed by confinement to bed, hepatocellular cancer, septic shock passing a kidney stone, colon cancer, two cases of postoperative failure to thrive syndrome, kidney failure after 5 months, hepatocellular failure with cirrhosis, confinement to the postoperative cerebrovascular accident (CVA) suite, rectorrhagia, cerebral lymphoma, and three unexplained causes at one year. Note that one patient died suddenly at home six months after discharge from the hospital, the diagnosis of sudden death was made; the patient did not have a pacemaker (Figs. 1 and 2).
Fig. 1.
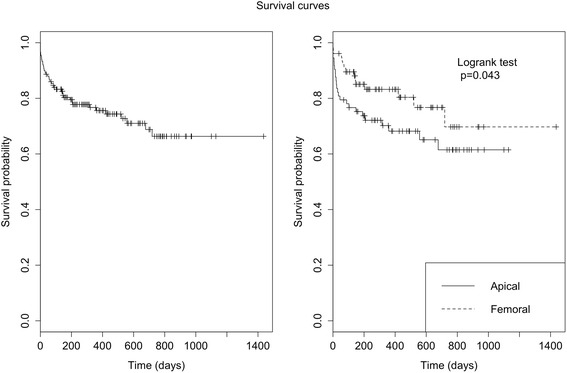
Presents the probability of survival in both the whole population and the population who died from cardiovascular diseases. Day 0 corresponds to the day of the TAVI procedure. We have not lost any of the patients to follow up. Survival rates reported at D1400 correspond to the retrospective time of the last included patient
Fig. 2.
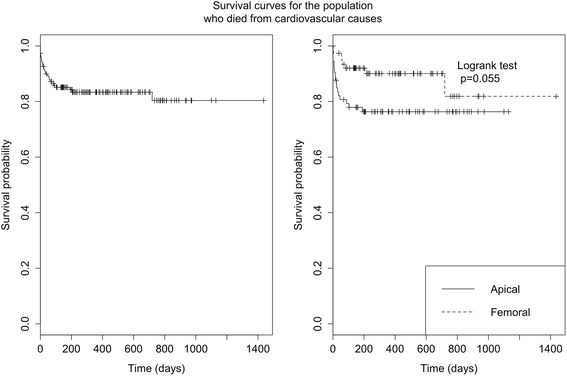
Compares mortality according to route of approach, with the overall mortality rate for the femoral route and the apical route at 14 % and 25 % at 6 months, 18 % and 30 % at 1 year, and 22 % and 39 % at 2 years, respectively
In our study, the probability of overall survival was higher for the femoral approach group than the apical group by a significant degree (p = 0.043). On comparing cardiovascular mortality according to the route of approach, the rates were 10 % vs. 21 % for the femoral and apical routes, respectively, at 6 months, 10 % vs. 21 % at 1 year, and 19 % vs. 25 % at 2 years. We found a non-significant trend towards an increased probability of survival in the femoral group compared to the apical group (p = 0.055).
Predictive factors of mortality
We found few predictive factors of cardiovascular mortality. In particular, the Euroscore and STS surgical risk assessment scores were not found to be significant. By analyzing single variables, we found the following predictive factors for cardiovascular mortality: preoperative mitral regurgitation ≥ Grade 2 (p = 0.024) and survival of major complications (=0.001). However, a tendency towards an increase in the cardiovascular mortality rate was found in the following contexts: where coronary disease was present (p = 0.07), in females (p = 0.053), with NYHA Class II or IV dyspnoea (p = 0.09), and with the apical approach (p = 0.08). On multivariate analysis, only the presence of a major complication was significantly predictive of cardiovascular mortality (p = 0.003). Detailed results are presented in Table 4.
Table 4.
Predictive factors of mortality (multivariate analysis)
| Characteristics | RR | 95 % CI | p |
|---|---|---|---|
| Female gender | 2.45 | [0.99–6.03] | 0.051 |
| Coronaropathy | 1.84 | [0.76–4.49] | 0.177 |
| NYHA Class 3 or 4 | 3.92 | [0.52–29.6] | 0.185 |
| Major complications | 9.13 | [2.12–39.4] | 0.003 |
| Transapical approach | 1.62 | [0.7–3.75] | 0.258 |
| Mitral regurgitation (≥ Grade 2) | 1.81 | [0.8–4.22] | 0.166 |
RR relative risk, CI confidence interval, NYHA New York Heart Association
Complications
The postoperative complications of the whole population have been classified and compared according to route of approach, presented in Table 5.
Table 5.
Postoperative complications
| Characteristics | All patients (n = 150) | Transfemoral approach (n = 78) | Transapical approach (n = 72) | p |
|---|---|---|---|---|
| Hemodynamic complications | 10 (6.6 %) | 2 (2.6 %) | 8 (11.1 %) | 0.049 |
| state of cardiogenic shock | 4 (2.7 %) | 0 (0 %) | 4 (5.6 %) | 0.035 |
| acute pulmonary edema | 5 (3.4 %) | 1 (1.3 %) | 4 (5.6 %) | NSS |
| multiple organ failure | 9 (6 %) | 2 (2.6 %) | 7 (9.7 %) | 0.065 |
| coronary obstruction | 1 (0.7 %) | 1 (1.3 %) | 0 (0 %) | NSS |
| massive aortic insufficiency | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| Vascular complications | 20 (13.3 %) | 11 (14.1 %) | 9 (12.5 %) | NSS |
| major vascular complications | 7 (4.7 %) | 4 (2.7 %) | 3 (4.2 %) | NSS |
| minor vascular complications | 2 (1.3 %) | 1 (1.3 %) | 1 (1.4 %) | NSS |
| mesenteric ischemia | 1 (0.7 %) | 1 (1.3 %) | 0 (0 %) | NSS |
| abdominal aortic aneurysm | 1 (0.7 %) | 1 (1.3 %) | 0 (0 %) | NSS |
| Tamponade | 11 (7.4 %) | 7 (9.0 %) | 4 (5.6 %) | NSS |
| Mechanical complications | 8 (5.3 %) | 2 (2.6 %) | 6 (8.3 %) | NSS |
| ventricular perforation | 2 (1.3 %) | 0 (0 %) | 2 (2.8 %) | NSS |
| VSD | 1 (0.7 %) | 1 (1.3 %) | 0 (0 %) | NSS |
| aortic root aneurysm | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| aortic fistula/right atrium | 1 (0.7 %) | 1 (1.3 %) | 0 (0 %) | NSS |
| mitral lesion | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| poor positioning of the valve | 2 (1.3 %) | 0 (0 %) | 2 (2.8 %) | NSS |
| surgical conversion | 3 (2 %) | 0 (0 %) | 3 (4.2 %) | NSS |
| Hemorrhagic complications | 55 (36.7 %) | 20 (25.7 %) | 33 (45.8 %) | NSS |
| major complications | 10 (6.7 %) | 3 (3.8 %) | 7 (9.7 %) | NSS |
| massive bleeding | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| major bleeding | 7 (4.7 %) | 3 (3.8 %) | 4 (5.6 %) | NSS |
| minor bleeding | 36 (24 %) | 14 (18.0 %) | 22 (30.6 %) | 0.070 |
| transfusion | 42 (28 %) | 15 (19.2 %) | 27 (37.5 %) | 0.013 |
| Cerebral complications | 10 (6.7 %) | 4 (5.1 %) | 6 (10.0 %) | NSS |
| Ischemic CVA | 9 (6 %) | 4 (5.1 %) | 5 (7.0 %) | NSS |
| Hemorrhagic CVA | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| Heart rhythm complications | ||||
| paroxysmal atrial fibrillation | 24 (16 %) | 13 (16.7.7 %) | 11 (15.3 %) | NSS |
| pacemaker implanted | 18 (12 %) | 12 (15.4 %) | 6 (8.3 %) | NSS |
| sudden death | 1 (0.7 %) | 0 (0 %) | 1 (1.4 %) | NSS |
| Acute kidney injury (AKI) | 15 (10 %) | 7 % (9.0 %) | 9 (12.5 %) | NSS |
| Stage 1 | 2 (1.3 %) | 1 (1.3 %) | 1 (1.4 %) | NSS |
| Stage 2 | 7 (4.7 %) | 3 (3.8 %) | 4 (4.2 %) | NSS |
| Stage 3 | 6 (4 %) | 3 (3.8 %) | 3 (2 %) | NSS |
| Pulmonary complications | 14 (9.3 %) | 2 (2.6 %) | 12 (8 %) | 0.029 |
| pleural effusion | 5 (3.3 %) | 1 (1.3 %) | 4 (5.6 %) | NSS |
| hemothorax | 1 (0.7 %) | 0 (0 %) | 1 (0.7 %) | NSS |
| pneumothorax | 3 (2 %) | 0 (0 %) | 3 (4.2 %) | NSS |
| Infectious complications | 18 (12 %) | 12 (15.4 %) | 6 (8.3 %) | NSS |
| Scarpa’s fascia infection | 11 (7.4 %) | 10 (12.8 %) | 1 (1.4 %) | 0.007 |
| thoracotomy infection | 4 (2.7 %) | 0 (0 %) | 4 (5.6 %) | 0.048 |
| endocarditis | 3 (2 %) | 2 (2.6 %) | 1 (1.4 %) | NSS |
CVA cerebrovascular accident, VSA ventricular septal defect, AKI Acute kidney injury
A comparison of the rates of reported complications in terms of approach method revealed significantly more hemodynamic complications among the apical group (p = 0.049). None of the patients who underwent surgery via the femoral route presented with a postoperative state of cardiogenic shock (p = 0.035). Seven patients who had surgery via the apical route presented with multiple organ failure, compared to two of those operated via the femoral route (p = 0.065).
On the other hand, we noted an insignificant difference in the level of minor bleeding (p = 0.07) for the femoral group. Transfusions were significantly more frequent in the apical approach group, with 18 % for the femoral approach versus 28 % for the apical approach (p = 0.013). Pulmonary complications were also significantly more frequent in the apical approach cases (p = 0.029). As regards infections, the rate of Scarpa’s fascia infection was significantly higher in cases using the femoral approach (p = 0.007) and thoracotomy infections were significantly higher for the apical approach (p = 0.048). Otherwise, a total of 18 pacemakers were fitted, 12/135 Edwards Sapien valves and 6/12 CoreValves. Finally, concerning the levels of renal insufficiency, these were divided into 3 stages depending on the severity of the case, using VARC classification (Stage 1 for an increase in creatininemia of 150 to 199 %, stage 2 for an increase of 200 % to 299 %, and stage 3 for an increase of more than 300 % or anuria for more than 12 h).
Follow-up
Patients exhibited significant improvement in dyspnea at 1 month (p < 0.001), this improvement still proved stable over time. The improvement in functional status was spectacular and there has been a clear improvement in the quality of life of our patients from the first month following the implantation, with over half presenting as NYHA Class I or II.
Echocardiographic results
The efficacy of TAVI was confirmed by this investigation, resulting in a drop in mean gradient of hemodynamic flow from 52.8 to 11.8 mmHg (p < 0.001), and the aortic surface increased from 0.6 to 1.6 cm2 (p < 0.001). These parameters remained stable throughout the follow-up period. The interindividual variability of the LVEF improved significantly over time (p = 0.001). Postoperative LVEF was 52.4 % on average, compared to 52.8 % prior to surgery. We observed a significant improvement in the ejection fraction at one month, increasing from 52.4 % to 54.6 % (p < 0.026), with this improvement proving stable over time.
There was no significant change in the diastolic function.
The systolic arterial pressure measurements significantly decreased from 46.3 to 40.8 mmHg (p < 0.001) and remained stable over time. Detailed results are presented in Table 6.
Table 6.
Echocardiographic postoperative results
| Echocardiographic characteristics | Preoperative | Postoperative | One month | Six months | One year | Two years |
|---|---|---|---|---|---|---|
| Population (n) | n = 150 | n = 144 | n = 125 | n = 101 | n = 52 | n = 25 |
| Mean gradient (mmHg) | 52.8 +/−11.5 | 11.8+/−4.9 | 11+/−4.6 | 11.5+/−5.2 | 11.9+/−4.4 | 11.2+/−3.8 |
| p < 0.001 | p < 0.001 | |||||
| Maximal gradient (mmHg) | 83.7 +/−16.1 | 21.5+/−8.1 | 22.1+/−18.8 | 21.4+/−7.7 | 20.5+/−5.6 | 19.6+/−4.3 |
| p < 0.001 | p < 0.001 | |||||
| Vmax (m/s) | 4.3 +/−0.8 | 2.1+/−0.4 | 1.7+/−0.5 | 1.7+/−0.6 | 1.6+/−0.5 | 1.7+/−0.5 |
| p < 0.001 | p < 0.001 | |||||
| Aortic area (cm2) | 0.6 +/−0.2 | 1.6+/−0.4 | 1.2+/−0.4 | 1.2+/−0.3 | 1.2+/−0.4 | 1.2+/−0.4 |
| p < 0.001 | p < 0.001 | |||||
| LVEF (%) | 52.8+/−12.5 | 52.4+/−12.1 | 54.6+/−10.4 | 55+/−10.7 | 54.4+/−10.9 | 52.8+/−12.5 |
| p = 0.002 | p = 0.026 | p = 0.056 | ||||
| Diastolic function E/E’ | 11.4+/−4.2 | 11.4+/−3.8 | 11.5+/−3.4 | 11.7+/−3.8 | 11.5+/−3.4 | 11.7+/−3.9 |
| NS | ||||||
| PAPS (mmHg) | 46.3+/− 12.6 | 40.8+/−12 | 41.7+/−10.7 | 41.5+/−10.5 | 40.5+/−11.2 | 38.8+/−6.4 |
| p = 0.006 | p < 0.001 | p = 0.078 | p = 0.045 | |||
| TAPSE (mm) | 15.2+/−3 | 13.8+/−3.2 | 15.4+/−3 | 15.1+/−2.9 | 14.8+/−3.4 | 14.2+/−3.2 |
| NS | ||||||
| Central aortic regurgitation | / | 4 (2.8 %) | 3 (2.4 %) | 2 (2.0 %) | 1 (1.9 %) | 0 (0 %) |
| NS | ||||||
| Paraprosthetic regurgitation | / | 74 (51.4 %) | 38 (30.4 %) | 14 (13.8 %) | 10 (19.2 %) | 8 (32.0 %) |
| p < 0.001 | ||||||
| Grade 1 | / | 35 (24.3 %) | 24 (19.2 %) | 6 (59.4 %) | 6 (11.5 %) | 6 (24.0 %) |
| Grade 2 | / | 39 (27.1 %) | 13 (10.6 %) | 8 (7.9 %) | 4 (7.6 %) | 2 (8 %) |
| Mitral regurgitation | 63 (42 %) | 83 (57.6 %) | 88 (70.6 %) | 72 (71.2 %) | 37 (71.1 %) | 19 (76.0 %) |
| Grade 1 | 29 (19.3 %) | 12 (8.3 %) | 49 (32.2 %) | 33 (32.6 %) | 15 (19.2 %) | 8 (32.0 %) |
| Grade 2 | 31 (20.7 %) | 71 (49.3 %) | 39 (31.2 %) | 39 (38.6 %) | 22 (42.3 %) | 11 (44.0 %) |
| Grade 3 | 3 (2 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) |
LVEF left ventricular ejection fraction, PASP pulmonary artery systolic pressure, TAPSE tricuspid annular plane systolic excursion
Discussion
The procedure success rate, defined as the correct deployment of the prosthesis, was 94.6 % in our study, in line with that of series reporting success rates with this procedure of over 90 % at the test centers.[8, 9] As we have detailed, we have kept the 6 patients who died in the operating theatre within the 5.4 % failure rate. Among the causes of implantation failure, one death was linked to the anaesthesia, a secondary one to aortic predilation, another secondary to inserting the catheter using the apical approach and the other causes were secondary to deployment of the prosthesis.
Otherwise, with regard to aortic leaks, it is currently established thinking that a paraprosthetic leak ≥ Grade 2 increases mortality from the 6th month following implantation [5, 10]. Our study reported accurate results, with a rate of 49.3 % for minor or moderate postoperative paraprosthetic leaks (35 % at Grade 1 and 39 % at Grade 2). By means of a comparison, we also searched the FRANCE 2 data, revealing 64.5 % aortic failure [5]. Moreover, aortic regurgitation remains a challenging pathology for the transapical approach [11]. Concerning the evolution of paraprosthetic aortic insufficiency, our study indeed found a tendency towards increasing the percentage of aortic insufficiency at 6 months, 1 year, then 2 years. Apart from statistical bias linked to the smaller population over time, this may be linked to a general cardiovascular aggravation, with a possible increase in left ventricular postload. An important point is the lack of major aortic insufficiency.
A large proportion of our study consisted of TAVI carried out using the apical approach, with 77 of the patients (51.3 %) treated using the femoral route and 72 (48 %) using the apical route. The usual distribution is more in favor of the femoral approach: 74 % of the patients in FRANCE 2 [5] and 69.5 % in PARTNER A [3] received the implant via the femoral route, compared with 19 % and 29.6 %, respectively, for the apical route. This significantly higher percentage for the apical route is due in part to the delay in marketing the Edwards 29 valve for the femoral route. Mortality at 6 months essentially results from extracardiac causes and is linked to comorbidities in elderly patients. This difference in mortality rates is probably explained by the increased use of the apical approach among our population, with a trend of increased mortality in this cohort. It has, in fact, been demonstrated that mortality rates are more significant in the apical approach than in the femoral one [12–15].
Patients treated via the transapical route are typically at higher risk. To date, there have been few studies comparing different devices or approaches. It should, nevertheless, be noted that all reports have indicated a learning curve effect on the success rate, incidence rate, and severity of the complications. Hemodynamic complications are a major cause of perioperative death [16]. These represent 24 % of deaths at the one month mark [16]. The significant difference in hemodynamic complications found in our study, greater in the femoral group (Table 3), also explains the difference in mortality rate between the two groups.
Table 3.
Implanted prostheses diameters
| Prosthesis diameter (mm) | All prostheses implanted (n = 149) | Transfemoral approach (n = 78) | Transapical approach (n = 72) | p |
|---|---|---|---|---|
| 23 | 52 (34.9 %) | 30 (38.5 %) | 22 (30.6 %) | 0.169 |
| 26 | 64 (42.9 %) | 37 (47.4 %) | 27 (37.5 %) | 0.154 |
| 29 | 25 (16.8 %) | 6 (7.7 %) | 21 (29.2 %) | <0.001 |
| 31 | 6 (4.0 %) | 5 (6.4 %) | 1 (1.4 %) | 0.083 |
The rate of acute kidney injury (AKI) in our study (10 %) were slightly lower than those reported in the literature.[17–19] Post-TAVI AKI was multifactorial, since preoperative renal function is a predictive factor independent of post-procedural AKI [19]. Note that the level of plasma creatinine in our cohort was 113.5 μmol/L +/−115.2, and of the six patients who suffered stage 3 AKI, none of them underwent dialysis apart from 3 patients who were receiving dialysis for a chronic condition already.
The rate of pulmonary complications was higher in the apical group compared with the femoral group, at 8 % vs. 1.3 % (p = 0.029). This constituted one of the most predominant causes of morbidity and mortality in the apical group. Nevertheless, we found no significant difference in rate of tamponade during the postoperative period when comparing the two approaches, namely 4.7 % in the apical group versus 2.7 % in the femoral group.
Pre-TAVI mitral regurgitation was identified in our study as a risk factor for mortality when Grade 2 or higher, as other studies have also observed [20–23]. The post-TAVI mitral regurgitation in our cohort showed a tendency to increase over time. The evolution of post-TAVI mitral regurgitation remains discordant, depending on the studies [3, 24, 25]. In our case, it can be explained by statistical bias linked to a reduction in population monitored over time, general age-linked cardiovascular deterioration, the tendency towards an increase in aortic insufficiency in our cohort or the high proportion of ischemic patients (46.6 %).On the other hand, the mechanism underlying the mitral regurgitation is an important factor [26]. The functional nature of the leak and presence of left ventricular failure are predictive factors for the reduction in mitral valve disease. Conversely, its organic nature, the dilatation of the left atrium, and the existence of pulmonary artery hypertension all suggest a lack of improvement [27].
Finally, the rate of major vascular complications was relatively low in our study, reported at 4.7 %. Taking into account minor vascular complications, the overall rate was 6 %. This could be as a result of the high percentage of apical approaches used. Vascular complications were found to remain a significant source of morbidity in the transfemoral route, with an incidence of 9.7 % [5]. Reduction in major vascular complications from 8 to 1 % has been demonstrated with the benefit of a more precise selection of patients, a completely percutaneous vascular approach, and advances in surgical techniques [28]. This demonstrated that the number of vascular complications and survival rate increases in parallel with increased experience of each center and over time [29].
Conclusions
TAVI has been confirmed as a credible alternative to surgical valve replacement and has become the first therapeutic choice for non-operable patients and a valid alternative for high-risk patients. The mid-term results of the first 150 TAVI procedures, conducted from 2009 to 2013 in our center, demonstrated mortality rates of 4 %, 11.3 %, 22.7 % and 26 % at the immediate perioperative point, 31 days, 1 year, and 2 years, respectively. The interindividual variability of LVEF improved significantly over time (p = 0.001). Our study revealed a trend towards increased probability of survival in the femoral group compared to the apical group. On comparing the rates of complications in terms of approach method, we observed that the patients treated through the transapical route, who are usually at higher risk, exhibited significantly more hemodynamic complications (p = 0.049) and more pulmonary complications (p = 0.029). These results underline the importance of a multidisciplinary decision concerning the choice of approach type.
Abbreviations
- AKI
acute kidney injury
- CI
confidence interval
- IQR
interquartile range
- OR
odds ratio
- STS
The Society of Thoracic Surgeons
- TA-AVI
transapical aortic valve implantation
- TAVI
transcatheter aortic valve implantation
- TF-AVI
transfemoral aortic valve implantation
- VARC
Valve Academic Research Consortium
Footnotes
Competing interests
To the best of our knowledge, no conflict of interest, financial or other, exists.
Authors’ contribution
All authors listed have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline. All authors read and approved the final manuscript.
Contributor Information
Alain Rougé, Email: alain.rouge@hotmail.com.
Olivier Huttin, Email: o.huttin@chu-nancy.fr.
Rumas Aslam, Email: aslam_rumas@hotmail.fr.
Thibaud Vaugrenard, Email: tvaugrenard@gmail.com.
Thomas Jouve, Email: tjouve@chu-grenoble.fr.
Michael Angioi, Email: michaelangioi@gmail.com.
Pablo Maureira, Email: jp.maureira@chu-nancy.fr.
References
- 1.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–4. doi: 10.1016/S0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–8. doi: 10.1161/01.CIR.0000047200.36165.B8. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. PARTNER Trial Investigators: Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. PARTNER Trial Investigators: Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 5.Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrié D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–15. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 6.Medtronic CoreValve ® System Demonstrates Positive Clinical Performance at Two Years in “Real World” ADVANCE Study.
- 7.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G-A, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European association of cardio-thoracic surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI) EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2008;4:193–9. doi: 10.4244/eijv4i2a36. [DOI] [PubMed] [Google Scholar]
- 9.de Heer LM, Kluin J, Stella PR, Sieswerda GTJ, Mali WPTM, van Herwerden LA, Budde RPJ. Multimodality imaging throughout transcatheter aortic valve implantation. Future Cardiol. 2012;8:413–24. doi: 10.2217/fca.12.19. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab M, Comberg T, Büttner HJ, El-Mawardy M, Chatani K, Gick M, Geist V, Richardt G, Neumann F-J, Segeberg-Krozingen TAVI. Registry: Aortic regurgitation after transcatheter aortic valve implantation with balloon- and self-expandable prostheses: a pooled analysis from a 2-center experience. JACC Cardiovasc Interv. 2014;7:284–92. doi: 10.1016/j.jcin.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Seiffert M, Bader R, Kappert U, Rastan A, Krapf S, Bleiziffer S, Hofmann S, Arnold M, Kallenbach K, Conradi L, Schlingloff F, Wilbring M, Schäfer U, Diemert P, Treede H. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv. 2014;7:1168–74. doi: 10.1016/j.jcin.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Rahnavardi M, Santibanez J, Sian K, Yan TD. A systematic review of transapical aortic valve implantation. Ann Cardiothorac Surg. 2012;1:116–28. doi: 10.3978/j.issn.2225-319X.2012.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walther T, Thielmann M, Kempfert J, Schroefel H, Wimmer-Greinecker G, Treede H, Wahlers T, Wendler O. One-year multicentre outcomes of transapical aortic valve implantation using the SAPIEN XTTM valve: the PREVAIL transapical study. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2013;43:986–92. doi: 10.1093/ejcts/ezs589. [DOI] [PubMed] [Google Scholar]
- 14.Wendler O, Walther T, Nataf P, Rubino P, Schroefel H, Thielmann M, Treede H, Thomas M. Trans-apical aortic valve implantation: univariate and multivariate analyses of the early results from the SOURCE registry. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2010;38:119–27. doi: 10.1016/j.ejcts.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 15.van der Boon RMA, Marcheix B, Tchetche D, Chieffo A, Van Mieghem NM, Dumonteil N, Vahdat O, Maisano F, Serruys PW, Kappetein AP, Fajadet J, Colombo A, Carrié D, van Domburg RT, de Jaegere PPT. Transapical versus transfemoral aortic valve implantation: a multicenter collaborative study. Ann Thorac Surg. 2014;97:22–8. doi: 10.1016/j.athoracsur.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 16.Khatri PJ, Webb JG, Rodés-Cabau J, Fremes SE, Ruel M, Lau K, Guo H, Wijeysundera HC, Ko DT. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med. 2013;158:35–46. doi: 10.7326/0003-4819-158-1-201301010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Koifman E, Segev A, Fefer P, Barbash I, Sabbag A, Medvedovsky D, et al. Comparison of acute kidney injury classifications in patients undergoing transcatheter aortic valve implantation: Predictors and long-term outcomes. Catheter Cardiovasc Interv. 2015. doi: 10.1002/ccd.26138. [Epub ahead of print] [DOI] [PubMed]
- 18.Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, Masson J-B, Gutiérrez MJ, Clavel M-A, Bertrand OF, Pibarot P, Rodés-Cabau J. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–74. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatani K, Abdel-Wahab M, Wübken-Kleinfeld N, Gordian K, Pötzing K, Mostafa AE, et al. Acute kidney injury after transcatheter aortic valve implantation: Impact of contrast agents, predictive factors, and prognostic importance in 203 patients with long-term follow-up. J Cardiol. 2015;66:514–19. [DOI] [PubMed]
- 20.Sannino A, Losi MA, Schiattarella GG, Gargiulo G, Perrino C, Stabile E, Toscano E, Giugliano G, Brevetti L, Franzone A, Cirillo P, Imbriaco M, Trimarco B, Esposito G. Meta-analysis of mortality outcomes and mitral regurgitation evolution in 4,839 patients having transcatheter aortic valve implantation for severe aortic stenosis. Am J Cardiol. 2014;114:875–82. doi: 10.1016/j.amjcard.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 21.D’Onofrio A, Gasparetto V, Napodano M, Bianco R, Tarantini G, Renier V, Isabella G, Gerosa G. Impact of preoperative mitral valve regurgitation on outcomes after transcatheter aortic valve implantation. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2012;41:1271–6. doi: 10.1093/ejcts/ezr236. [DOI] [PubMed] [Google Scholar]
- 22.Khawaja MZ, Williams R, Hung J, Arri S, Asrress KN, Bolter K, Wilson K, Young CP, Bapat V, Hancock J, Thomas M, Redwood S. Impact of preprocedural mitral regurgitation upon mortality after transcatheter aortic valve implantation (TAVI) for severe aortic stenosis. Heart Br Card Soc. 2014;100:1799–803. doi: 10.1136/heartjnl-2014-305775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarty T, Van Belle E, Jilaihawi H, Noheria A, Testa L, Bedogni F, Rück A, Barbanti M, Toggweiler S, Thomas M, Khawaja MZ, Hutter A, Abramowitz Y, Siegel RJ, Cheng W, Webb J, Leon MB, Makkar RR. Meta-analysis of the impact of mitral regurgitation on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2015;115:942–9. doi: 10.1016/j.amjcard.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Tzikas A, Piazza N, van Dalen BM, Schultz C, Geleijnse ML, van Geuns R-J, Galema TW, Nuis R-J, Otten A, Gutierrez-Chico J-L, Serruys PW, de Jaegere PP. Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2010;75:43–9. doi: 10.1002/ccd.22411. [DOI] [PubMed] [Google Scholar]
- 25.Almasood A, Ahmari SA, El-Shurafa H, Alotaibi M, Al KS, AlAbdallah M, Al-Moghairi A, Khushail AA, Al-Amri H. The change in mitral regurgitation severity after trans-catheter aortic valve implantation. J Saudi Heart Assoc. 2015;27:10–7. doi: 10.1016/j.jsha.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Aguilera J, Mesa-Rubio D, Ruiz-Ortiz M, Delgado-Ortega M, Villanueva-Fernández E, Romo-Peña E, Pan Álvarez-Ossorio M. Mitral regurgitation during transcatheter aortic valve implantation: the same complication with a different mechanism. J Invasive Cardiol. 2014;26:603–8. [PubMed] [Google Scholar]
- 27.Hekimian G, Detaint D, Messika-Zeitoun D, Attias D, Iung B, Himbert D, Brochet E, Vahanian A. Mitral regurgitation in patients referred for transcatheter aortic valve implantation using the Edwards Sapien prosthesis: mechanisms and early postprocedural changes. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2012;25:160–5. doi: 10.1016/j.echo.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, Lichtenstein S, Cheung A, Webb JG. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2011;78:977–84. doi: 10.1002/ccd.22961. [DOI] [PubMed] [Google Scholar]
- 29.Van Mieghem NM, Chieffo A, Dumonteil N, Tchetche D, van der Boon RMA, Buchanan GL, Marcheix B, Vahdat O, Serruys PW, Fajadet J, Carrié D, Colombo A, de Jaegere PPT. Trends in outcome after transfemoral transcatheter aortic valve implantation. Am Heart J. 2013;165:183–92. doi: 10.1016/j.ahj.2012.11.002. [DOI] [PubMed] [Google Scholar]


