Abstract
In transplantation, a major obstacle for graft acceptance in MHC-matched individuals is the mismatch of minor histocompatibility Ags. Minor histocompatibility Ags are peptides derived from polymorphic proteins that can be presented by APCs on MHC molecules. The APC subtype uniquely responsible for the rejection of minor Ag–mismatched grafts has not yet been identified. In this study, we examined graft rejection in three mouse models: 1) mismatch of male-specific minor Ags, 2) mismatch of minor Ags distinct from male-specific minor Ags, and 3) skin transplant. This study demonstrates that in the absence of pathogen-associated molecular patterns, Batf3-dependent dendritic cells elicit the rejection of cells and grafts expressing mismatched minor Ags. The implication of our findings in clinical transplantation may be significant, as minor Ag reactivity has been implicated in the pathogenesis of multiple allograft tissues.
Dendritic cells (DCs) are professional APCs with the capacity to initiate T cell–mediated immunity. In murine nonlymphoid and lymphoid tissues, there are two major forms of classical DCs: Batf3-dependent and Batf3-independent DCs (1). These DCs are distinguished by their cell surface expression for CD103/CD8/XCR1 and CD11b/CD4/SIRPα (2–4), as well as pattern recognition receptors, Ag presentation and processing, and, particularly, transcription factors involved in their development (Batf3 or IFN regulatory factor 4, respectively) (1, 5, 6). Others and we have demonstrated the selective ability of Batf3-dependent DCs but not Batf3-independent DCs to take up apoptotic cells (i.e., self), migrate to the draining lymph nodes (LNs) (Supplemental Fig. 1A), and there present exogenous cell-associated Ag peptides on MHC class I (i.e., cross-presentation) (4, 7–10), which can then be recognized by cognate CD8+ T cells. Subsequently, depending on the activation status of Ag-presenting DCs, proliferating Ag-specific CD8+ T cells can be instructed to develop into cytotoxic T cells (i.e., cross-priming) (11, 12). The induction of cytotoxic T cells by Batf3-dependent DCs has demarcated its beneficial roles in antiviral and antitumor immunity (1, 5, 12–15). However, detrimental roles for Batf3-dependent DCs have also been demonstrated in auto-immune diabetes (16). In this study, we investigated a new role for Batf3-dependent DCs in promoting the rejection of minor Ag-mismatched grafts.
Materials and Methods
Mice
C57BL/6 or BALB/c CD45.1 or CD45.2 mice aged 6–8 wk, 129SvEv/BL6 F2 (controls for 129SvEv/BL6 Batf3−/− F2 mice were kindly provided by Dr. Kenneth Murphy, Washington University), C57BL/6 Batf3−/−, BALB/c Batf3−/−, OT-I, OT-II, DO11.10, and CL4 mice were purchased from The Jackson Laboratory or Charles River Laboratories. Mice were housed in a specific pathogen-free environment at National Jewish Health (American Association for the Accreditation of Laboratory Animal Care accredited) and used in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Rejection model against male-specific minor Ags
Spleen and LNs were harvested from OT-I, OT-II, DO11.10, and CL4 mice. Two million cells were adoptively transferred (AT) i.v. into mice. On the next day, mice were immunized via the intranasal route with Ag 2 μg soluble OVA or 1 μg long influenza (flu) peptide in 50 μl PBS (12). To observe the acceptance or rejection of AT male T cells, mice were rechallenged at day 18 with 100 μg OVA or 10 μg flu peptide (i.e., recall response). Two days after the rechallenge, lung-draining LNs were examined for the presence or absence of AT male T cells.
Skin transplantation
BALB/c Batf3−/− male and female skins were transplanted onto syngeneic wild-type (WT) and Batf3−/− female mice. Full-thickness donor skin was acquired from the abdominal surface. Recipient mice were treated with buprenorphine and anesthetized with isoflurane. Graft beds were prepared on the left shoulder of recipient mice by excising skin equivalent to the size of the donor graft (~1 cm2). Grafts were held in place with Vetbond tissue adhesive glue (3M) and covered with Vaseline-coated gauze (Covidien) and triple antibiotic cream (Actavis Mid Atlantic). Finally, grafted area was wrapped with adhesive wrap (Fischer Scientific) and 500 μl saline was injected s.c. on the recipients’ flanks to aid in hydration and recovery. Skin grafts were assessed for acceptance or rejection up to 60 d after transplantation. Time of rejection was defined as the day when a necrotic donor graft had completely fallen off a recipient.
In vivo cytotoxic T cell assay
Soluble OVA (2 μg; 0.22-μm filtered, grade VII, Sigma-Aldrich) was delivered intranasally to promote expansion of male OT-I T cells. Ten days after immunization, target cells were labeled using 10 μM CFSE. Target cells were 107 (1:1) CFSE-labeled male CD45.2 and female CD45.1 splenocytes. Three days later, spleens were harvested and specific killing of AT congenic target cells was assessed.
Flow cytometry
Single-cell suspensions were stained for 30 min with the following mAbs: Pacific Blue–, eFluor 450–, or PerCP-Cy5.5–conjugated mAbs to CD8, CD4, and CD44; allophycocyanin- or PE-conjugated mAbs to CD45.1, CD45.2, and CD90.1; and FITC-conjugated mAbs Va2 or KJ1-26 (binds the TCR expressed on DO11.10). Analysis was performed on the BD LSR II and FlowJo (Tree Star, Ashland, OR).
Statistics
Statistical analysis was conducted using InStat and Prism software (GraphPad Software). Statistical tests were performed using two-tailed Student t test. A p value <0.05 was considered statistically significant.
Results and Discussion
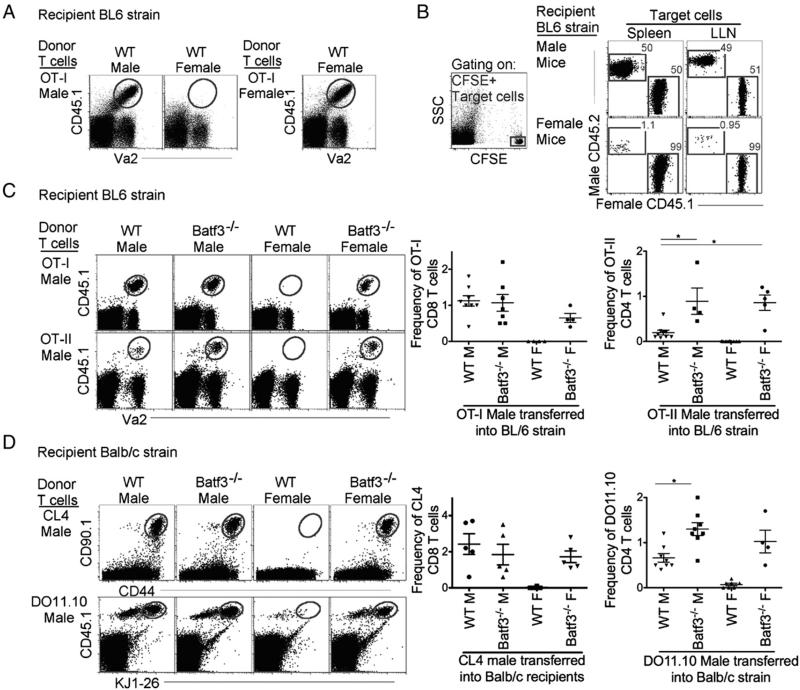
To determine whether Batf3-dependent DCs are required for rejection of minor Ag-mismatched grafts, we used a well-characterized system to study graft rejection. Grafted male cells and tissues are rejected in MHC-matched syngeneic female mice owing to the development of CTL against male-specific minor Ags (17). Therefore, we first illustrated the rejection of AT male lymphocytes in syngeneic WT female mice. Male CD45.1 OVA-specific CD8+ T cells (OT-I T cells) were i.v. injected into syngeneic CD45.2 WT male and female mice. One day after AT, the mice were immunized via the intranasal route with 2 μg soluble OVA. Delivery and trafficking of the Ag by two migratory DCs, Batf3-dependent and Batf3-independent DCs (Supplemental Fig. 1B) (18), initiated expansion of the male OT-I T cells in the lung-draining LNs. To observe the acceptance or rejection of male OT-I T cells, mice were rechallenged at day 18 with 100 μg OVA (i.e., recall response). Two days after the rechallenge, the lung-draining LNs were examined for the presence or absence of AT male OT-I T cells (schematic diagram, Supplemental Fig. 1C). As expected, there was no recall of the AT CD45.1 male OT-I T cells (i.e., the cells were rejected) in syngeneic WT female mice compared with syngeneic WT male mice (Fig. 1A). The rejection of male OT-I T cells in syngeneic WT female mice was not due to other mismatched minor Ags potentially present in the OT-I mouse strain, as the AT of female OT-I T cells were not rejected in syngeneic WT female mice (Fig. 1A).
FIGURE 1.
Rejection of male-specific minor Ags requires Batf3-dependent DCs. (A) Gated CD8+ T cells display the recall of AT CD45.1 male Va2+ OT-I T cells (gray gate) in WT male and female mice. (B) For in vivo CTL assays, male OT-I cells were AT into WT male and female mice 1 d prior to intranasal administration of 2 μg OVA, which expands male Ag-specific T cells, and 10 d later mice were i.v. administered (1:1) CFSE-labeled male (CD45.2+)/female (CD45.1+) target cells. Three days after target cell AT, cytotoxicity was assessed. (C) Gated CD8 (upper panels) or CD4 (lower panels) T cells display the recall of AT CD45.1 male Va2+ T cells (gray gate, OT-I [upper panels] and OT-II [lower panels]) in BL/6 WT and Batf3−/− male and female recipient mice. Scatter plots display frequency of OT-I and OT-II T cells from total CD8 and CD4 T cells. (D) Gated CD8 (upper panels) or CD4 (lower panels) T cells display the recall of AT CD45.1+KJ1-26+ or CD90.1+CD44+ male T cells (gray gate, CL4 [upper panels] and DO11.10 [lower panels]) from BALB/c WT and Batf3−/− male and female recipient mice. Scatter plot displays frequency of CL4 and DO11.10 T cells from total CD8 and CD4 T cells. In (B)–(D), data are representative of three independent experiments. *p < 0.05.
Next, to confirm that the absence of male cells in female mice after the recall response was due to the induction of endogenous male Ag-specific CTLs, we employed an in vivo CTL killing assay (17, 19). Ten days after the AT of male OT-I T cells and administration of the OVA Ag, CFSE-labeled target cells, WT CD45.2 male and CD45.1 female splenocytes, were i.v. delivered at a 1:1 ratio for the measurement of male Ag-specific killing. Three days later, in vivo CTL responses were assessed by measuring the killing of male target cells compared with female target cells (Fig. 1B). As expected, WT female mice that received male OT-I T cells displayed almost complete killing of male target cells compared with WT male mice, where no killing was observed (Fig. 1B). This experiment concurs with the well-established finding that cells expressing male Ag are rejected in female mice via the induction of endogenous male Ag-specific CTLs (19).
Next we examined whether Batf3−/− female mice (selectively lacking Batf3-dependent DCs) (1, 12, 13) reject AT male OT-I T cells as observed for WT female mice. However, unlike WT female mice, after rechallenge with Ag, male OT-I T cells were not rejected in Batf3−/− female mice, that is, the recall response was retained (Fig. 1C). The control experiment demonstrated that there was no rejection against female OT-I T cells AT into WT and Batf3−/− male and female mice (Supplemental Fig. 1D). Furthermore, similar to the findings for male Ag-specific CD8+ T cells, AT of male Ag-specific specific CD4+ T cells (CD45.1 OT-II T cells) were not rejected in Batf3−/− female mice but were rejected in WT female mice (Fig. 1C).
Because C57BL/6 female Batf3−/− mice lacked the ability to reject AT male cells we next investigated whether this finding was independent of mouse strain. BALB/c Batf3−/− mice were examined using the same experimental design as in Fig. 1A. However, instead of C57BL/6 Ag-specific T cells, BALB/c flu-specific CD8+ T cells (CL4) or OVA-specific CD4+ T cells (DO11.10) were AT into syngeneic WT or Batf3−/− male and female mice. As anticipated, male AT Ag-specific T cells were rejected in WT female BALB/c mice but not in WT or Batf3−/− male mice (Fig 1D), and in contrast to WT female mice, Batf3−/− female mice did not reject the male Ag-specific T cells. Note that in an allogeneic setting with major Ag mismatch, rejection of AT allogeneic cells occurred in both WT and Batf3−/− mice (Supplemental Fig. 2A). Overall, our data demonstrate that in both C57BL/6 and BALB/c female mouse strains, Batf3-dependent DCs are required for the rejection of minor Ag–mismatched grafts.
Another interesting observation with the AT of CD4+ T cell experiments was the enhanced recall of CD4+ T cells in Batf3−/− mice compared with WT mice (Fig. 1D). This finding is supported by an elegant study by Kim et al. (20) demonstrating that Batf3-independent DCs (DCs that preferentially presents Ag to CD4 T cells) substantially contribute to the development of memory T cells compared with Batf3-dependent DCs, hence resulting in a greater recall of CD4+ T cells in Batf3−/− mice compared with WT mice.
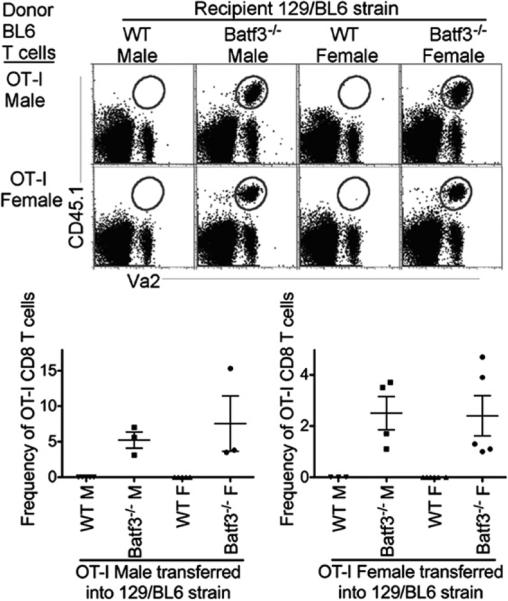
To further assess the requirement of Batf3-dependent DCs for the rejection of minor Ag-mismatched grafts, we examined rejection of minor Ags in F2 129SvEv/BL6 Batf3−/− mice and F2 129SvEv/BL6 WT mice, which were then continuously bred to each other. In this setting, although donor C57BL/6 OT-I T cells express the same MHC molecules as do recipient 129SvEv/BL6 mice, they contain multiple mismatched minor histocompatibility Ags that are distinct from the male-specific minor Ags (21). Using the experimental design from Fig. 1A, we AT male C57BL/6 OT-I T cells and observed rejection in both 129SvEv/BL6 WT male and female mice but not in 129SvEv/BL6 Batf3−/− male or female mice (Fig. 2). The difference in the rejection pattern observed for AT C57BL/6 OT-I male cells into 129SvEv/BL6 male mice compared with C57BL/6 male mice (Fig. 1C) was due to the mismatch of minor Ags outside of the H-Y locus. Similar to AT male OT-I T cells, AT C57BL/6 OT-I female cells were rejected in 129SvEv/BL6 WT male and female mice but not in 129SvEv/BL6 Batf3−/− male or female mice (Fig. 2). Thus, these data demonstrate that Batf3-dependent DCs are required for the rejection of AT cells expressing mismatched male-specific and/or male-nonspecific minor Ags.
FIGURE 2.
Rejection of mismatched minor Ags requires Batf3-dependent DCs. Experimental design was as described in Fig. 1A. Gated CD8 T cells display the recall of CD45.1+Va2+ AT male (upper) and female (lower) OT-I T cells (gray gate) from 129SvEv/BL6 WT and Batf3−/− male and female recipient mice. Scatter plots display frequency of OT-I T cells from total CD8 T cells. Data are representative of three independent experiments.
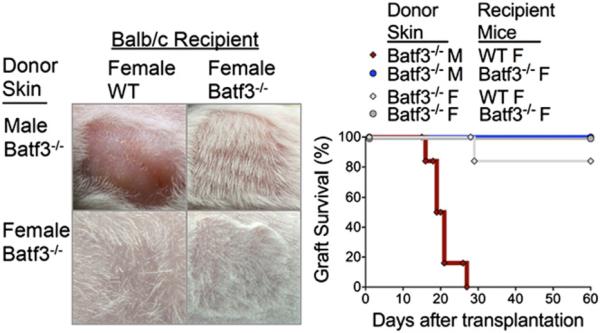
Next, using a skin transplant model (22), we assessed the requirement of Batf3-dependent DCs in skin graft rejection. BALB/c Batf3−/− male or female skin was transplanted onto syngeneic BALB/c WT or Batf3−/− female mice. Batf3−/− skin was used to assure the absence of Batf3-dependent DCs in the skin graft. As expected, male skin grafts were completely rejected by syngeneic WT female mice within 30 d after transplantation (Fig. 3). In striking contrast, male skin was completely accepted by Batf3−/− female mice (Fig. 3), and confirmed the absence of male Ag-specific CTL compared to WT female recipients (Supplemental Fig. 2B). Control mice demonstrated that transplanted female skin onto syngeneic WT and Batf3−/− female mice was accepted by 84% in WT mice and 100% in Batf3−/− mice. Thus far, our findings in the skin transplant model and AT T cells clearly demonstrate that Batf3-dependent DCs are required for rejection of minor Ag–mismatched grafts.
FIGURE 3.
Rejection of male skin grafts on female recipients requires Batf3-dependent DCs. Representative images show transplanted murine skin grafts from BALB/c Batf3−/− male and female donors onto BALB/c WT and Batf3−/− female recipients (day 60). Survival graph displays male Batf3−/− skin onto WT female (red diamond, 0 of 6) and Batf3−/− female (blue circle, 6 of 6) recipients. For controls, survival graph displays female Batf3−/− skin onto WT female (gray diamond, 5 of 6) and Batf3−/− female (gray circle, 6 of 6) recipients. Data represent two independent experiments.
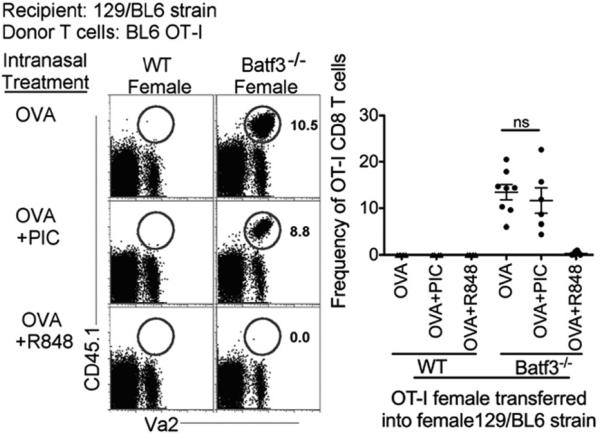
Lastly, we recently demonstrated that Batf3-independent DCs can acquire cross-presenting and cross-priming capabilities similar to Batf3-dependent DCs when the Batf3-independent DCs are stimulated with a TLR7 ligand (ssRNA, R848) but not TLR3 ligand (dsRNA, polyinosinic-polycytidylic acid [poly(I:C)], which selectively activates Batf3-dependent CD103+ DCs in tissue) (12). Therefore, we next examined whether TLR7-stimulated Batf3-independent DCs could promote rejection of minor Ag–mismatched grafts. Consistent with those previous findings, we show that when 129SvEv/BL6 Batf3−/− female mice were challenged with OVA in the presence or absence of poly(I:C), rejection of C57BL/6 OT-I female cells did not occur (Fig. 4). However, in the presence of the TLR7 ligand R848, complete rejection of AT C57BL/6 OT-I female cells was now observed in 129SvEv/BL6 Batf3−/− mice where Batf3-dependent DCs are absent (Fig. 4). Control mice, 129SvEv/BL6 WT female mice, displayed complete rejection of C57BL/6 OT-I female cells (Figs. 2, 4). Furthermore, single delivery of Ag with poly(I:C) or R848 does not promote the development of Batf3-dependent DCs in Batf3−/− mice (12). In conclusion, TLR7 stimulation bypasses the requirement for Batf3-dependent DCs to reject cells expressing mismatched minor Ags by altering the phenotype of Batf3-independent DCs to acquire the intrinsic characteristics of Batf3-dependent DCs. Overall, our study identifies Batf3-dependent DCs as the DC subtype required for rejection of minor Ag–mismatched grafts, in the absence of concurrent TLR7 stimulation. Our findings also indicate potential consequences of ssRNA viruses that should be considered if a therapeutic strategy were developed to eliminate Batf3-dependent DCs during transplantation.
FIGURE 4.
TLR7 ligand activates Batf3-independent DCs to promote rejection of mismatched minor Ags. Experimental design was as described in Fig. 1A. Plots display the recall of AT CD45.1 female C57BL/6 OT-I T cells (gray gate) from 129SvEv/BL6 WT and Batf3−/− female mice immunized with sOVA +/− poly(I:C) (20 μg) or R848 (50 μg). Scatter plot displays frequency of OT-I T cells from total CD8 T cells. Data are representative of three independent experiments.
The clinical implications of identifying the critical APC for presentation of mismatched minor Ags in transplantation are relevant, as it spans both HLA-matched and unmatched transplantation. This is underscored by the fact that several organs are not currently HLA matched to recipients because outcomes are minimally or not impacted compared with HLA-mismatched transplant recipients. Importantly, reactivity to several minor Ags has been implicated in the pathogenesis of acute cellular rejection, acute Ab-mediated rejection, and chronic rejection in multiple transplanted organs (23). In conclusion, perhaps developing depleting Abs against human APC-specific surface molecules such as Clec9a, DEC205, CD1c, or CD1a may improve transplant outcome.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL115334 (to C.V.J., S.M.A., and S.L.G.) and DK099187 (to R.G.G. and M.K.N.), Clinical and Translational Science Award Grant TL1 TR001081 (to M.K.N.), National Institute of Allergy and Infectious Diseases Training Grants T32 AI007405 (to A.N.D.) and AI18785 (to P.M.), and by National Institutes of Health Grants HL68864 and HL88138 (to P.M.H.).
Abbreviations used in this article
- AT
adoptively transferred
- DC
dendritic cell
- flu
influenza
- LN
lymph node
- poly(I:C)
polyinosinic-polycytidylic acid
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8a+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung SS, Fu SM, Rose CE, Jr., Gaskin F, Ju ST, Beaty SR. A major lung CD103 (aE)-b7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 3.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J. Exp. Med. 2011;208:1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 7.Contreras V, Urien C, Guiton R, Alexandre Y, Vu Manh TP, Andrieu T, Crozat K, Jouneau L, Bertho N, Epardaud M, et al. Existence of CD8alike dendritic cells with a conserved functional specialization and a common molecular signature in distant mammalian species. J. Immunol. 2010;185:3313–3325. doi: 10.4049/jimmunol.1000824. [DOI] [PubMed] [Google Scholar]
- 8.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 10.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD82 dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat. Rev. Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 12.Desch AN, Gibbings SL, Clambey ET, Janssen WJ, Slansky JE, Kedl RM, Henson PM, Jakubzick C. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat. Commun. 2014;5:4674. doi: 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8a+ conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 15.GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b2 but not plasmacytoid dendritic cells. J. Exp. Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavin MA, Dere B, Grandea AG, III, Hogquist KA, Bevan MJ. Major histocompatibility complex class I allele-specific peptide libraries: identification of peptides that mimic an H-Y T cell epitope. Eur. J. Immunol. 1994;24:2124–2133. doi: 10.1002/eji.1830240929. [DOI] [PubMed] [Google Scholar]
- 18.Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods. 2008;337:121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyznik AJ, Bevan MJ. The surprising kinetics of the T cell response to live antigenic cells. J. Immunol. 2007;179:4988–4995. doi: 10.4049/jimmunol.179.8.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8+ T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40:400–413. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 22.Ashman RB. Primary immune responses to H-Y in BALB/c-H-2k mice. Immunogenetics. 1983;18:125–129. doi: 10.1007/BF00368540. [DOI] [PubMed] [Google Scholar]
- 23.Dragun D, Catar R, Philippe A. Non-HLA antibodies in solid organ transplantation: recent concepts and clinical relevance. Curr. Opin. Organ Transplant. 2013;18:430–435. doi: 10.1097/MOT.0b013e3283636e55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.