Abstract
Hepatic hydrothorax is an important and difficult-to-manage complication of cirrhosis and portal hypertension. Here, we aimed to study its clinical features and natural history. Complete clinical data, including outcomes, were abstracted from hospital records of patients with cirrhosis and ascites admitted to University of Texas Southwestern University teaching hospitals from January 2001 to July 2012. Hepatic hydrothorax was diagnosed based on currently accepted clinical characteristics of the disease, including a known diagnosis of cirrhosis, the presence of portal hypertension, pleural fluid analysis, and the absence of primary cardiopulmonary disease.
Seventy-seven of 495 (16%) hospitalized cirrhotic patients with pleural effusion (28 female; mean age, 52 yr) met the criteria for diagnosis of hepatic hydrothorax. Resting dyspnea and cough were the most prominent presenting symptoms, occurring in 34% and 22% of patients, respectively. Pleural effusions were most often right-sided (56/77; 73%), followed by left-sided only (13/77; 17%) and bilateral effusions (8/77; 10%); 7 (9%) patients did not have detectable ascites. The mean Model for End-Stage Liver Disease (MELD) score at presentation was 16. The serum to pleural fluid albumin gradient (SPAG) was ≥1.1 in all 48 patients in whom it was measured. Most patients (64/77; 83%) were managed with diuretics and/or thoracentesis, while 8 (10%) underwent transjugular intrahepatic portosystemic shunt (TIPS) and 5 (7%) underwent liver transplant. A total of 44 of 77 (57%) patients died during a mean follow-up of 12 months. The average time from presentation to death for all patients was 368 days, while for those after TIPS it was 845 days. No deaths were reported in the liver transplant group. The data indicate that a substantial number of patients with hepatic hydrothorax had what may be considered atypical presentations, including left-sided only effusions, or pleural effusion without ascites. Here, we propose that the term “serum to pleural fluid albumin gradient (SPAG)” be used to describe the gradient between serum and pleural fluid albumin levels and suggest that not only is it consistent with the portal hypertensive pathophysiology of hepatic hydrothorax, but also it is a useful criterion for diagnosis of hepatic hydrothorax. Finally, the overall outcome of hepatic hydrothorax was extremely poor, except in those undergoing TIPS or liver transplantation.
INTRODUCTION
Hepatic hydrothorax is an important complication of cirrhosis and portal hypertension. It has been historically described in patients with a large transudative pleural effusion (typically >500 mL), in whom a primary cardiopulmonary or malignant process has been excluded.17,31 Hepatic hydrothorax has been reported to occur in an estimated 4%–12% of patients with cirrhosis.4,17,31,36 The most commonly reported clinical manifestations of hepatic hydrothorax include symptoms associated with the complications of decompensated cirrhosis and may also include those associated with the pleural effusion, such as pleuritic symptoms: cough and shortness of breath.4,17 Hepatic hydrothorax is classically believed to present as an isolated right-sided pleural effusion, although bilateral effusions have been reported rarely.10,31,33 It is thought to be most common in patients with cirrhosis and severe ascites, but there are reports of hydrothorax in patients with little to no ascites.6,10,14,15,35
The management of hepatic hydrothorax typically includes medical management with diuretics and sodium restriction and therapeutic thoracentesis as needed.4,21,30,33 Dietary sodium restriction and diuretics are favored for long-term management or for treatment of mild pleural fluid accumulation, while therapeutic thoracentesis is commonly performed for acute relief of symptoms.4,21,33 However, transjugular intrahepatic portosystemic shunts (TIPS), liver transplant, and surgical repair of diaphragmatic defects have also been advocated in some patients, typically those with refractory disease.4,9,21,22,27,33 Further, TIPS has been suggested as an ideal approach to bridge patients to liver transplantation.36
We conducted the current study to better understand the natural history of hepatic hydrothorax, including clinical manifestations, objective findings, and outcomes. After carefully defining the criteria for a diagnosis of hepatic hydrothorax, we identified 77 patients, and herein describe their clinical phenotype and outcomes.
PATIENTS AND METHODS
The current study was a retrospective cohort investigation of patients admitted to the University of Texas Southwestern teaching hospitals, University of Texas Southwestern University Hospital (UH) and Parkland Memorial Hospital (PMH) from January 2001 to July 2012. Institutional review board approval was received from both institutions prior to data collection. Patients were identified by matching International Classification of Diseases, 9th revision (ICD-9) codes for chronic liver disease and cirrhosis (ICD-9: 571.0–571.3, 571.40–571.41) and pleural effusion (ICD-9: 511.8, 511.9) using electronic medical records. Patient data, comorbidities, reported respiratory symptoms, radiologic tests, and pleural and peritoneal fluid analysis were abstracted. In patients with multiple admissions before, during, and after the time period noted above, the earliest admission data were included in the study.
Patients included in the study met the following criteria: 1) known diagnosis of cirrhosis, either biopsy-proven or based on the presence of clinical complications in the setting of a clinical scenario consistent with cirrhosis; 2) known diagnosis of pleural effusion based on plain film or computed tomography imaging; 3) pleural fluid consistent with the known characteristics of hepatic hydrothorax and not considered to be consistent with the presence of infection, malignancy, or other known chronic disease (outlined below); 4) no history of primary cardiopulmonary disorder, including but not limited to congestive heart failure; 5) evidence of portal hypertension as established by the presence of esophageal varices, portal hypertensive gastropathy, ascites, portal vein thrombosis, or an elevated hepatic venous pressure gradient (HVPG). Patients were excluded if they had known primary pulmonary malignancy or metastatic pulmonary lesions or tuberculosis, or were incidentally found to have a clinically insignificant pleural effusion, which was not evaluated by thoracentesis (Figure 1).
FIGURE 1.

Patients. Using an ICD 9-CM based search of patients with cirrhosis and pleural effusion as in Methods, a total of 495 patients were identified. Of those patients, 77 met the criteria for diagnosis of hepatic hydrothorax as outlined in Methods.
Pleural fluid was characterized as being consistent with hepatic hydrothorax in patients with cirrhosis when the pleural fluid analysis was categorized as a transudate without evidence of primary cardiopulmonary disease, primary malignancy or metastatic disease, or active pulmonary infection. A transudate was defined as having either a pleural fluid to serum protein ratio of ≤0.50, a pleural fluid to serum lactate dehydrogenase (LDH) ratio of ≤0.6, or a pleural fluid LDH <143 units/L, which is two-thirds of the upper limit of normal for serum LDH (214) at our institutions.12,23 Patients were excluded in the setting of known infection, positive Gram stain, culture, or glucose <65 mg/dL.4,10,31
Clinical data abstracted included patient demographics (age, sex, and race), presenting clinical features and symptoms, extensive laboratory data, and imaging that focused on laterality and sizing of pleural effusions and amount of ascites. The size of ascites was differentiated into the following categories based on predefined criteria assigned as previously described by the International Ascites Club25 as follows: 1) minimal to small (only detectable by imaging studies), 2) moderate (symmetrical abdominal distension seen on physical exam), 3) large (gross ascites with significant abdominal distension), and 4) none.
Laboratory data abstracted included the following: 1) hemogram (white blood cell count [WBC], hematocrit, and platelets); 2) serum chemistries (creatinine, LDH, and total protein); 3) liver studies (albumin, total bilirubin, and international normalized ratio [INR]); 4) pleural fluid (pH, glucose, LDH, albumin, total protein, and cell count); and 5) ascites fluid (albumin, total protein, and cell count).
Treatment modalities and deaths were identified via the electronic medical record as well as public death records. Three main treatment modalities were identified: 1) diuretics and/or therapeutic thoracentesis alone, 2) TIPS, and 3) liver transplant. The average presentation time to death was calculated based on the date of first hospital admission to UH/PMH to the date of recorded death. The hospital admission to UH/PMH was recorded as either the initial presentation with hepatic hydrothorax or was identified as a subsequent admission.
Causes of death were classified into 8 categories, based on clinical judgment according to the information provided by electronic medical records, as follows: gastrointestinal bleeding, cardiac or respiratory failure, renal failure, liver failure or cirrhosis complications, sepsis, multiorgan dysfunction syndrome, terminal malignancy, or other using predefined definitions as previously reported.1,2,7,18,34,38 Gastrointestinal bleeding (also hemorrhage) was defined as ongoing bleeding in the setting of multiple transfusions. Respiratory failure was defined as evidence of primary respiratory diseases with need for mechanical ventilation. Renal failure or hepatorenal syndrome was defined as dialysis requirement with no urine output. Sepsis was defined as sepsis syndrome, including positive blood cultures, hypotension, and fever or hypothermia. Multiorgan dysfunction syndrome was defined as the presence of altered organ function in acutely ill patients such that homeostasis could not be maintained without intervention. We (RB and DCR) adjudicated the cause of death in a blinded fashion (without knowledge of the primary physician’s conclusion as to the cause of death), and agreed on the cause of death in all cases that could be determined. The cause of death was able to be clearly determined in 16 of 44 (36%) cases, because many patients died out of the hospital, and records surrounding the immediate cause of death were not available.
RESULTS
Demographics
During the study period, a total of 495 patients with cirrhosis and pleural effusion were identified. Of those, 77 patients met the criteria for diagnosis of hepatic hydrothorax as defined in the Methods (see Figure 1). Men were more commonly found to have hepatic hydrothorax than women, and the average age was 52 years (Table 1). Hispanics and non-Hispanic whites were the most commonly represented ethnicities at 45% and 51%, respectively; we note that despite a substantial population of African American patients cared for at both PMH and UH,5,28 there was only 1 African American patient (1%) in the entire study cohort.
TABLE 1.
Demographics

Among the patients with cirrhosis and pleural effusion who were excluded from the analysis, there were 16% African Americans, consistent with the population of African Americans in these hospitals.5,28 Alcohol was the most common cause of cirrhosis, followed closely by hepatitis C (see Table 1).
Clinical Presentation
At the time of presentation, most patients typically had multiple complaints, with the most commonly reported symptoms being dyspnea at rest (34%), cough (22%), nausea (11%), and pleuritic chest pain (8%) (Table 2). Twenty percent of all patients included a nonpulmonary complaint, while 4 of 77 (5%) had only nonpulmonary complaints. Nearly three-quarters of patients (56 of 77; 73%) were found to have right-sided only pleural effusions. Left-sided only effusions were found in 13 (17%) and bilateral effusions in 8 (10%) patients (Figure 2). The majority (71%) of pleural effusions were defined as large in size, according to radiologic criteria, with evidence of compression atelectasis (see Table 2). For most patients (53 of 77; 69%), the index hospital admission to UH/PMH was the initial presentation with hepatic hydrothorax.
TABLE 2.
Clinical Presentation and Imaging Results

FIGURE 2.

Comparison of pleural fluid locality. The physical location of (chest) pleural fluid in 77 patients in the cohort is shown in the diagram.
Ascites was present in the vast majority of patients (70 of 77). Classified by size according to predefined criteria,25 13% of patients had large ascites, nearly one-third of patients had moderate ascites, and most patients had minimal or small ascites. Seven (9%) patients did not have detectable ascites (see Table 2 and Figure 3). Five percent of patients had portal venous thrombosis.
FIGURE 3.
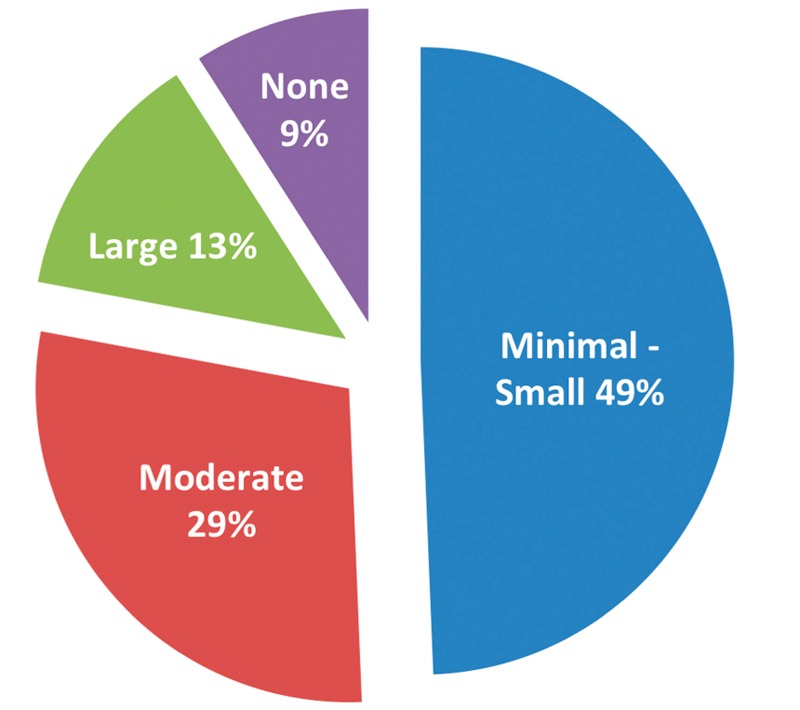
Comparison of ascites size and prevalence. The prevalence of ascites and recorded size of ascites is shown in 77 patients in the cohort.
Laboratory Features
Blood, pleural fluid, and peritoneal fluid were obtained routinely (Tables 3 and 4). Laboratory data were notable for the absence of leukocytosis in most patients; the mean WBC was 8.0 × 109/L. Anemia and thrombocytopenia were typical. The serum LDH was minimally elevated, while serum albumin levels were typically very depressed. Nearly all patients had evidence of substantial liver dysfunction, including elevated bilirubin levels and PT-INRs. Creatinine levels were often abnormal (mean, 1.2 mg/dL; median, 0.82 mg/dL). The mean Model for End-Stage Liver Disease (MELD) score was 16 (range, 3-47), reflecting underlying liver dysfunction. Most patients were classified as either Child-Pugh Class B (50%) or Class C (47%) at the time of presentation (see Table 3).
TABLE 3.
Laboratory Data (Blood)
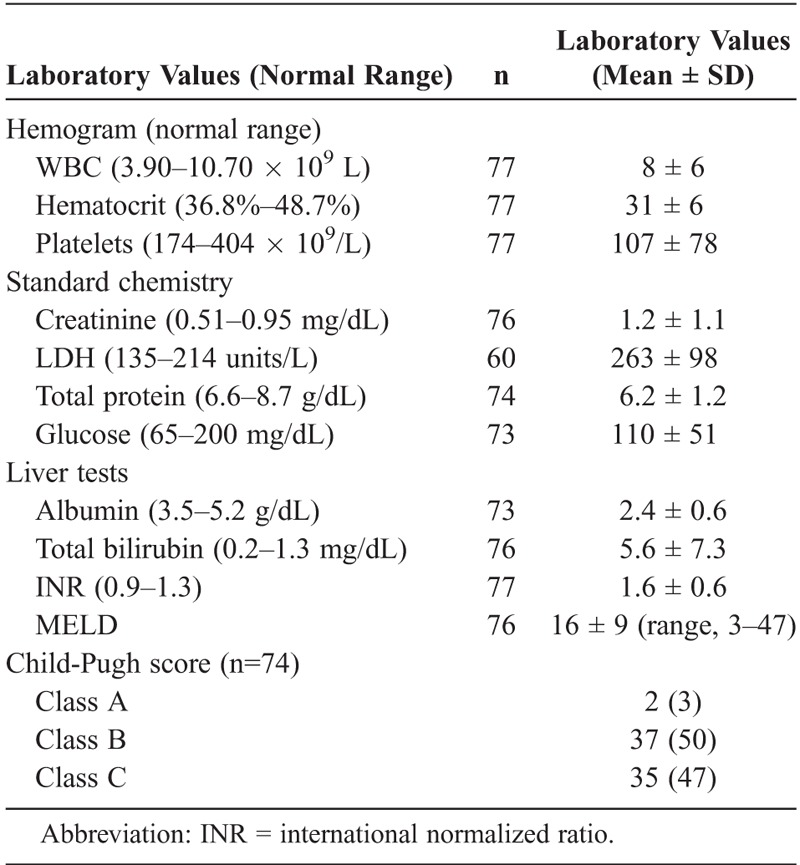
TABLE 4.
Laboratory Data (Pleural Fluid)
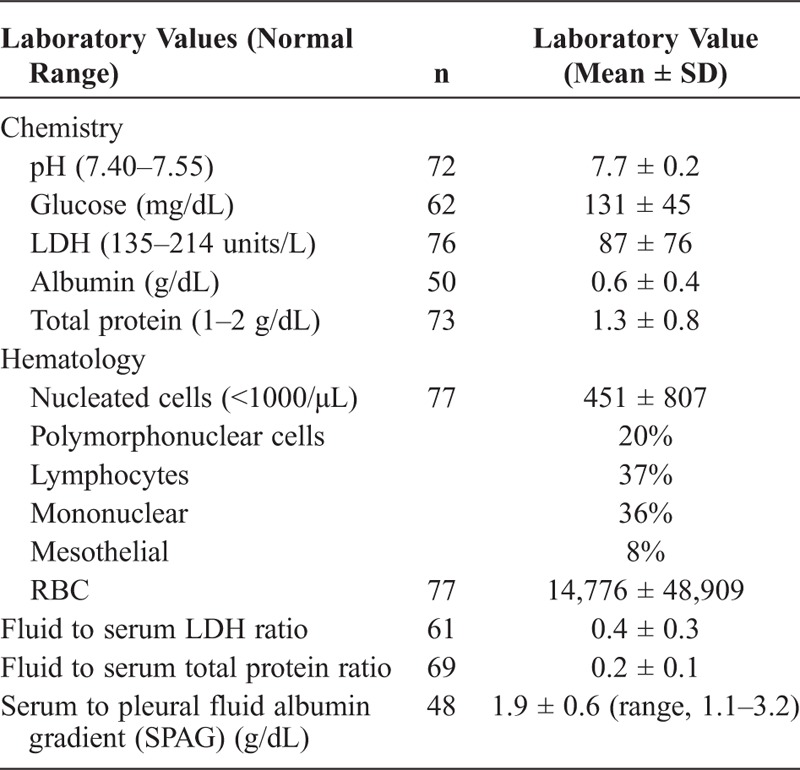
Pleural fluid studies demonstrated transudative features in effusions (see Table 4), including a relatively high pH (7.7 ± 0.2) and glucose (131 mg/dL ± 45), and low LDH (87 units/L ± 76). Albumin and total protein levels were typically low in pleural fluid (see Table 4 and Figure 4). The serum to pleural fluid albumin gradient (SPAG) mirrored the serum to ascites fluid albumin gradient (SAAG). Simultaneous serum albumin and pleural fluid was available for 48 patients and was greater than or equal to 1.1 g/dL in all patients (mean, 1.9 g/dL; range, 1.1–3.2) (Figure 5). Of note, in patients with cirrhosis and pleural effusion not consistent with hepatic hydrothorax as outlined in the Methods, the SPAG was varied, and was usually below 1.1 g/dL, particularly for patients with infection or malignancy (see Figure 5). SAAG was also available in 16 patients, and was greater than 1.1 g/dL in all patients. Seven patients had SPAG and SAAG performed simultaneously; their mean values were comparable (SPAG, 2.1 ± 0.7 and SAAG, 2.4 ± 0.6 g/dL). The average total pleural fluid protein for these patients was 1.2 ± 0.5 g/dL (see Table 4).
FIGURE 4.
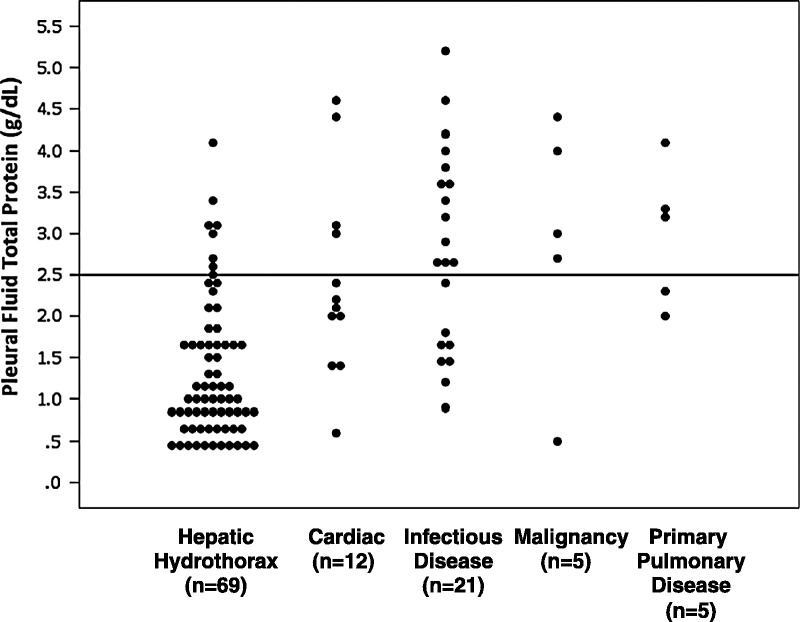
Pleural fluid total protein in patients with cirrhosis and hepatic hydrothorax or cirrhosis and other causes of pleural effusion. Each patient’s serum fluid total protein level is represented by a single dot. Values are segregated according to the cause of pleural effusion, shown in the x axis. Units are g/dL and a horizontal line is placed at a value of 2.5 g/dL for reference (n = 69).
FIGURE 5.
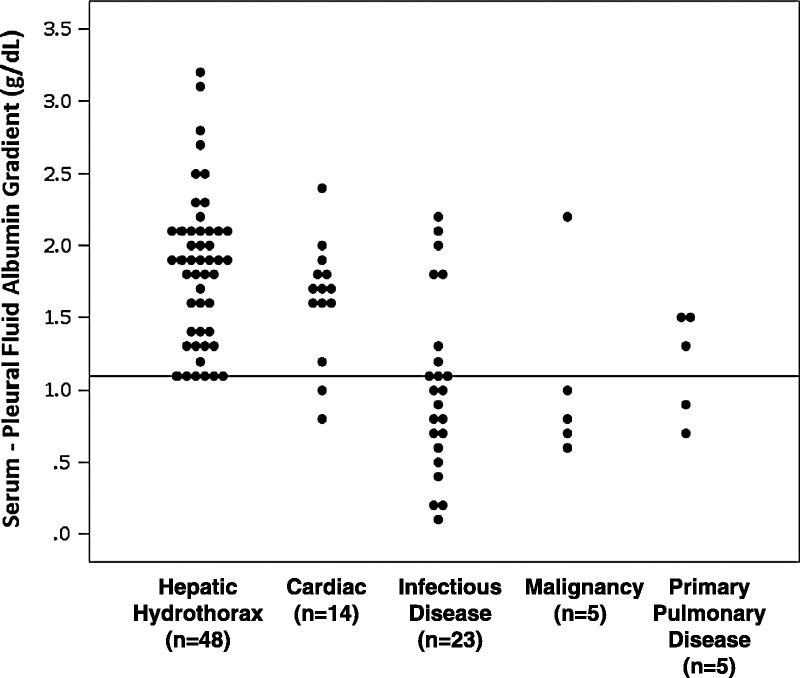
SPAG in patients with cirrhosis and hepatic hydrothorax or cirrhosis and other causes of pleural effusion. Each patient’s SPAG value is represented by a single dot (that is, for patients with hepatic hydrothorax, n = 48). Levels are segregated according to the cause of pleural effusion, shown in the x axis. Units are g/dL and a horizontal line is placed at a value of 1.1 g/dL for reference.
The average total pleural fluid protein for all patients was 1.3 ± 0.8 g/dL (see Table 4). In 62 of 69 patients (90%), the pleural fluid total protein was ≤2.5 g/dL (see Figure 4). In comparison, patients with effusions not characterized as hepatic hydrothorax had much higher levels of total protein in the pleural fluid: approximately two-thirds of patients with cardiac-mediated effusions had a total protein level <2.5 g/dL, 38% of those with infectious disease, 20% with malignancy, and 40% of patients with primary pulmonary disease (see Figure 4). In the 7 patients (10%) with pleural fluid protein >2.5 g/dL (mean, 3.1; max, 4.1 g/dL), there was an elevation in the mean pleural fluid red blood cell (RBC) count (17,310/μL). The mean pleural fluid LDH for these patients was 110 ± 46 units/L, and the mean pleural WBC was 605 ± 558/μL. All 7 patients had a SPAG ≥1.1 g/dL (mean, 1.8 ± 0.7 g/dL). Of note, these patients with elevated pleural fluid protein were also on diuretics at time of presentation.
Nucleated cells were common in pleural fluid; the cell count differential typically included more lymphocytes and mononuclear cells than polymorphonuclear cells. A large of number of RBCs was present in many patients (see Table 4).
Treatment and Outcome
Medical management was the most common therapeutic approach (64 of 77; 83%) and included treatment with sodium restriction, diuretics, and/or therapeutic thoracentesis. A total of 8 patients (10%) underwent TIPS, and 5 (7%) underwent liver transplant. The indication for TIPS was refractory ascites and/or refractory hepatic hydrothorax. In all, 44 of 77 (57%) patients died during a mean follow-up of 12 months (Table 5). The mean duration from initial presentation to death in the 44 patients was 368 days, and the median survival was 167 days. In the 66 patients who were followed until death or to a minimum of 1 year, 39 patients survived; and, in the 33 patients who were followed until death or to a minimum of 2 years, 24 patients survived. Among the entire cohort, 5 patients were lost to follow-up within 1 year, 3 patients were lost to follow-up within 2 years, and 4 patients were lost after 2 years of follow-up. Among the various treatment groups, survival was shortest in patients receiving diuretics and/or thoracentesis (mean, 321 days after initial presentation); patients who had undergone TIPS lived an average of 845 days; and those with liver transplant were all still alive at the time of the study (the mean follow-up for these patients was 1896 days; see Table 5). Among the cohort, 69 of 77 (90%) patients were alive 30 days from index hospital admission: mean MELD, 14 (3–46); 62 of 77 (81%) patients were alive after 30 days using from the most recent admission to the hospital (that is, thus including those with multiple admissions): mean MELD, 14 (3–49). The 90-day survival was 74% (57 of 77) in those after the last admission (mean MELD, 14; 3–49).
TABLE 5.
Comparison of Treatment Modalities

In patients in whom the specific cause of death was able to be ascertained (n = 16; 36%), hepatic hydrothorax was considered to be directly related to death in 3 patients (19%). In the patients who died from respiratory failure (2 Child-Pugh Class C and 1 Class B), the mean MELD score for these patients was 16, and they died an average of 105 days after presentation. Other known causes of mortality included multiorgan dysfunction syndrome, sepsis, renal failure, respiratory failure, and gastrointestinal hemorrhage (Table 6).
TABLE 6.
Causes of Death

DISCUSSION
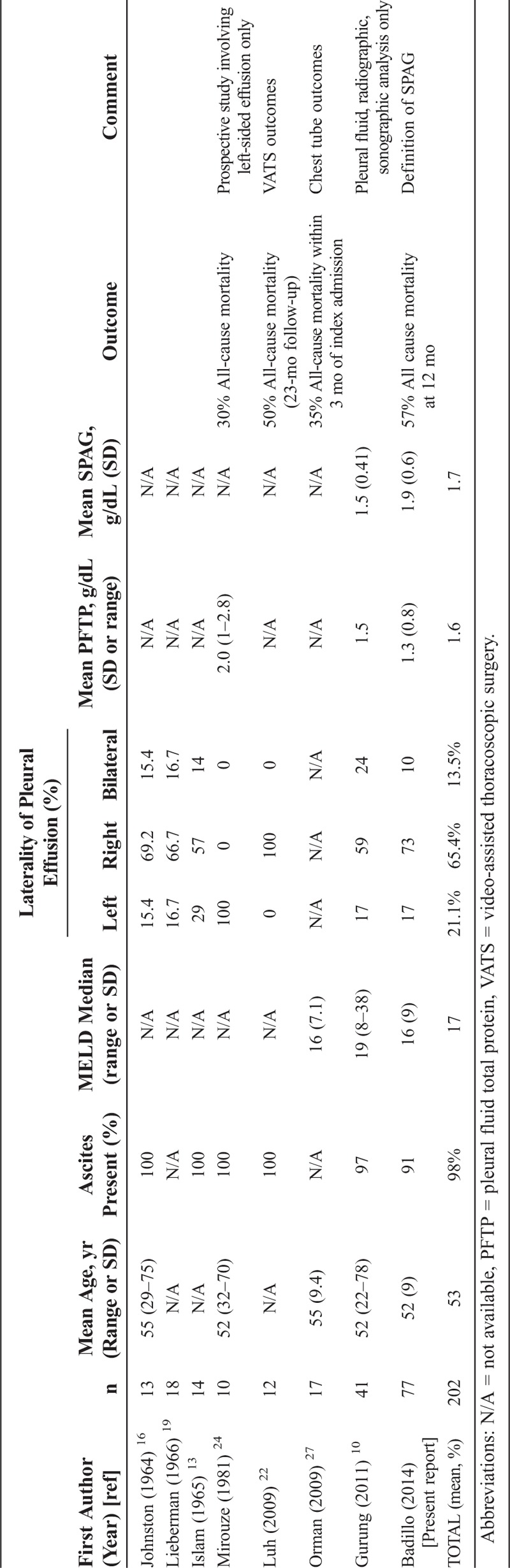
In this study, we identified a large number of patients with cirrhosis and one or more pleural effusions; approximately 16% of which were due to hepatic hydrothorax according to the criteria outlined in the Methods. The current study is comparable to previous literature on the topic of hepatic hydrothorax; we provide a large cohort with comprehensive analysis of the demographic, characteristics, laboratory evaluation and outcome data in this unique patient population (Table 7).13,16,19,22,24,27,37 The clinical presentation was in general consistent with that reported previously.4,10 However, we identified a substantial number of patients with hepatic hydrothorax who had what might be considered atypical presentations, including several patients with left-sided only effusions, bilateral pleural effusions, and nearly 10% with pleural effusion without ascites. Notably, we also found that the prognosis of patients with hepatic hydrothorax was poor.
TABLE 7.
Hepatic Hydrothorax, Previous and Present Reports
Based on our data, we suspect that patients with hepatic hydrothorax may have worse outcomes than would be predicted based on MELD model predictions alone. The mean MELD score of the entire cohort at time of index admission was 16. Additionally, patients with hepatic hydrothorax who died from respiratory failure (2 Child-Pugh Class C and 1 Class B) had low MELD scores (mean, 16), and they died an average of 105 days after presentation, a much shorter survival period than would be predicted based on MELD alone (a MELD score between 10 and 19 is expected to confer a 3-month mortality of approximately 6.0%-7.7%.26,39) Additionally, most telling was that the 90-day survival after the most recent admission was 74% (57 of 77) despite a mean MELD score of 14, suggesting a poorer survival than predicted based on MELD. While these data suggest that some patients with hepatic hydrothorax die not of liver failure, but of complications arising from their pulmonary disease, we would emphasize that we were not able to ascertain the specific cause of death in all patients that died. Thus, based on this cohort, we would urge caution in drawing firm conclusions about the most likely reasons for death in patients with hepatic hydrothorax.
The pathophysiology underlying hepatic hydrothorax remains incompletely understood.4,6,14,15,31,35 The most common view is that the pleural effusion arises from the passage of peritoneal fluid into the pleural cavity through varied size diaphragmatic defects assisted by negative intrathoracic pressure resulting from inspiration.4,31 This mechanism presupposes that in situations where there is no ascites, there is complete equilibrium between the amount of ascites produced and that present in the pleural space; this is consistent with previous reports.4,6,14,31,33
We found that management of hepatic hydrothorax was similar to management of portal hypertensive ascites, including medical management with diuretics and sodium restriction, therapeutic removal of fluid, TIPS, and liver transplant.4,9,21,33,40 Some reports have documented successful treatment of refractory hepatic hydrothorax in patients who do not meet criteria for TIPS with video-assisted thoracoscopic surgery (VATS) repair of diaphragmatic defects.22,33 However, this highly specialized approach was not attempted in any of our patients. Previous reports have commented on the use of chest tube drainage, largely emphasizing its ineffectiveness (see Table 7).11,20,27 Notably, we did not identify any patient in our cohort who underwent this treatment—this is consistent with the clinical practice at our institutions, where chest tubes are discouraged in this clinical condition. Only 13 (17%) patients in our study underwent TIPS or liver transplantation, despite the fact that outcomes seemed to be better with these approaches (31% mortality with TIPS or transplantation vs. 63% mortality for medical therapy alone).
The overall outcome of patients in our cohort was poor, with approximately half of patients dying within 1 year of presentation. We found that patients who underwent TIPS or liver transplantation had substantially longer periods of survival. While it is attractive to speculate therefore that TIPS and/or liver transplantation may be better treatment options than medical management, we urge caution in interpretation of these data. First, only a small number of patients underwent TIPS or transplant (15% of the total cohort). Second, it is possible that patients with a more favorable prognosis were intentionally selected for TIPS or liver transplantation (see Table 5). For example, the MELD scores for patients managed with TIPS were lower (mean, 12; range, 7–28) than patients treated with medical management alone (mean, 16; range, 4–46). Further, selection of patients for transplant is extremely complicated, and while patients with advanced liver disease are by definition selected, transplant candidates typically have few comorbid conditions. Thus, drawing conclusions based on small numbers of patients retrospectively may lead to inaccurate conclusions.
We propose here that the term “serum to pleural fluid albumin gradient (SPAG)” be used to describe the gradient between serum and pleural fluid for albumin. In addition, although a previous study revealed that pleural fluid protein and albumin levels were higher than we found,32 the SPAG was at least 1.1 g/dL in 100% of patients reported here (see Figure 5), and that SPAG was similar to the SAAG in essentially all patients where data were available. Thus, as with SAAG, SPAG is also consistent with the portal hypertensive pathophysiology of hepatic hydrothorax. Further, in patients without hepatic hydrothorax, the SPAG was typically below the 1.1 g/dL threshold. Therefore, we speculate that this test is useful in both the understanding of the pathogenesis of hepatic hydrothorax and the analysis of patients with cirrhosis and pleural effusion. Future studies should investigate SPAG in patients with pleural effusion without hepatic hydrothorax.
In the 7 patients with high total pleural fluid protein values, all had high pleural fluid RBC counts. Thus, it is possible that these RBCs may have elevated the total fluid protein levels. We were unable to identify studies outlining the prevalence or significance of RBCs in hepatic hydrothorax, and the few studies that report RBC pleural fluid findings include a range of 60–60,800/μL.10 Also, all 7 patients were on diuretics at the time of presentation, consistent with other reports of patients with hepatic hydrothorax who had elevated pleural fluid protein.3,8,10,23,29
An interesting finding of our study was that hepatic hydrothorax was largely restricted to 2 major ethnic groups, non-Hispanic whites and Hispanics. In contrast, African Americans were under-represented, despite the fact that cirrhosis occurs commonly in African American patients, including in patients at our hospitals.28 We are unable to explain this apparent discrepancy in the prevalence of hepatic hydrothorax. While these data lead us to speculate that there could be a predisposition to diaphragmatic defects in certain racial groups, we would emphasize that this point is speculative, and that future studies conducted in racially diverse populations could help address this issue.
We recognize limitations of this study. First, we found that some patients were lost to follow-up. This could have biased the reported outcome data such that outcomes were actually worse overall than reported here. However, based on our clinical experience, we do not feel that the reasons for lack of follow-up were systematically related to a specific clinical situation (such as resolution or worsening of disease), and thus, we believe that the follow-up available for the patients in whom it was present was likely representative and reflected the natural history of hepatic hydrothorax. It should be pointed out that patients were included in this study only by meeting rigorous and specific guideline criteria from pleural fluid analyses and clinical features. Thus, it is possible that some patients with hepatic hydrothorax may not have been included in the analysis, and therefore we have likely underestimated the prevalence of hepatic hydrothorax in patients with cirrhosis and pleural effusions.
In summary, we have described a large cohort of patients with hepatic hydrothorax in which the presentation was often characterized by a constellation of hepatic and pulmonary symptoms, although the pulmonary picture dominated the clinical picture in some patients. We also identified a substantial number of patients with atypical presentations, including isolated left pleural effusion or pleural effusion without ascites. We speculate that it is essential to make an early and accurate diagnosis so as to direct specific therapy. Outcomes in this cohort were generally poor, with the best outcomes found in patients undergoing TIPS or liver transplantation. Future studies, likely requiring multiple centers, should rigorously assess the different diagnostic criteria and treatment modalities typically used in these patients.
ACKNOWLEDGMENTS
The authors thank Dr. John Jacobs Jr. for thoughtful discussion, provoking their curiosity in this topic, and initiating this study.
Footnotes
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
Abbreviations: LDH = lactate dehydrogenase, MELD = Model for End-Stage Liver Disease, PMH = Parkland Memorial Hospital, RBC = red blood cell, SAAG = serum to ascites fluid albumin gradient, SPAG = serum to pleural fluid albumin gradient, TIPS = transjugular intrahepatic portosystemic shunts, UH = University of Texas Southwestern University Hospital, UTSW = University of Texas Southwestern, WBC = white blood cell count.
REFERENCES
- 1.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013; 57: 753– 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E, Day CP, George J. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011; 54: 1208– 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess LJ, Maritz FJ, Taljaard JJ. Comparative analysis of the biochemical parameters used to distinguish between pleural transudates and exudates. Chest. 1995; 107: 1604– 1609. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004; 20: 271– 279. [DOI] [PubMed] [Google Scholar]
- 5.Dallas County Community Health Dashboard Parkland Health & Hospital System. [Parkland Hospital Webpage] 2009 [cited 2012]; Available from: http://www.parklandhospital.com/whoweare/at_a_glance/community_health_dashboard.html.
- 6.Doraiswamy V, Riar S, Shrestha P, Pi J, Alsumrain M, Bennet-Venner A, Kam J, Klukowicz A, Miller R. Hepatic hydrothorax without any evidence of ascites. ScientificWorldJournal. 2011; 11: 587– 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003; 124: 1016– 1020. [DOI] [PubMed] [Google Scholar]
- 8.Gonlugur U, Gonlugur TE. The distinction between transudates and exudates. J Biomed Sci. 2005; 12: 985– 990. [DOI] [PubMed] [Google Scholar]
- 9.Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, III, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997; 25: 1366– 1369. [DOI] [PubMed] [Google Scholar]
- 10.Gurung P, Goldblatt M, Huggins JT, Doelken P, Nietert PJ, Sahn SA. Pleural fluid analysis and radiographic, sonographic, and echocardiographic characteristics of hepatic hydrothorax. Chest. 2011; 140: 448– 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris K, Chalhoub M. The use of a PleurX catheter in the management of recurrent benign pleural effusion: a concise review. Heart Lung Circ. 2012; 21: 661– 665. [DOI] [PubMed] [Google Scholar]
- 12.Hassan T, Al-Alawi M, Chotirmall SH, McElvaney NG. Pleural fluid analysis: standstill or a work in progress? Pulmonary Med. 2012; 2012: 716235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam N, Ali S, Kabir H. Hepatic hydrothorax. British J Dis Chest. 1965; 59: 222– 227. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez-Saenz M, Venero J, Castro J, Herrerias JM, Garrido M. Hepatic hydrothorax without ascites: a rare form of a common complication. J R Soc Med. 1990; 83: 747– 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John S, Paul MP, Murthy UK. An unusual presentation of cirrhotic pleural effusion in a patient with no ascites: a case report. Cases J. 2009; 2: 6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston RF, Loo RV. Hepatic hydrothorax; studies to determine the source of the fluid and report of thirteen cases. Ann Intern Med. 1964; 61: 385– 401. [DOI] [PubMed] [Google Scholar]
- 17.Kinasewitz GT, Keddissi JI. Hepatic hydrothorax. Curr Opin Pulm Med. 2003; 9: 261– 265. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y, Miyakawa Y, Kumada H. Natural history of compensated cirrhosis in the Child-Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol. 2006; 78: 459– 465. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman FL, Hidemura R, Peters RL, Reynolds TB. Pathogenesis and treatment of hydrothorax complicating cirrhosis with ascites. Ann Intern Med. 1966; 64: 341– 351. [DOI] [PubMed] [Google Scholar]
- 20.Liu LU, Haddadin HA, Bodian CA, Sigal SH, Korman JD, Bodenheimer HC, Jr, Schiano TD. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004; 126: 142– 148. [DOI] [PubMed] [Google Scholar]
- 21.Liu WL, Kuo PH, Ku SC, Huang PM, Yang PC. Impact of therapeutic interventions on survival of patients with hepatic hydrothorax. J Formos Med Assoc. 2010; 109: 582– 588. [DOI] [PubMed] [Google Scholar]
- 22.Luh SP, Chen CY. Video-assisted thoracoscopic surgery (VATS) for the treatment of hepatic hydrothorax: report of twelve cases. J Zhejiang Univ Sci B. 2009; 10: 547– 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangaraj M, Kumari S, Nanda R, Pattnaik MR, Mohapatra PC. Pleural fluid MDA and serum-effusion albumin gradient in pleural effusion. Indian J Clin Biochem. 2008; 23: 81– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirouze D, Juttner HU, Reynolds TB. Left pleural effusion in patients with chronic liver disease and ascites. Prospective study of 22 cases. Dig Dis Sci. 1981; 26: 984– 988. [DOI] [PubMed] [Google Scholar]
- 25.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003; 38: 258– 266. [DOI] [PubMed] [Google Scholar]
- 26.Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol. 2012; 2012: 318627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009; 3: 582– 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimi RS, Elliott AC, Rockey DC. Altered mental status in cirrhosis: etiologies and outcomes. J Investig Med. 2013; 61: 695– 700. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Candeira S, Fernandez C, Martin C, Sanchez-Paya J, Hernandez L. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med. 2001; 110: 681– 686. [DOI] [PubMed] [Google Scholar]
- 30.Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010; 59: 988– 1000. [DOI] [PubMed] [Google Scholar]
- 31.Roussos A, Philippou N, Mantzaris GJ, Gourgouliannis KI. Hepatic hydrothorax: pathophysiology diagnosis and management. J Gastroenterol Hepatol. 2007; 22: 1388– 1393. [DOI] [PubMed] [Google Scholar]
- 32.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992; 117: 215– 220. [DOI] [PubMed] [Google Scholar]
- 33.Sawant P, Vashishtha C, Nasa M. Management of cardiopulmonary complications of cirrhosis. Int J Hepatol. 2011; 2011: 280569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlichting P, Christensen E, Fauerholdt L, Poulsen H, Juhl E, Tygstrup N. Main causes of death in cirrhosis. Scand J Gastroenterol. 1983; 18: 881– 888. [DOI] [PubMed] [Google Scholar]
- 35.Serrat J, Roza JJ, Planella T. Hepatic hydrothorax in the absence of ascites: respiratory failure in a cirrhotic patient. Anesth Analg. 2004; 99: 1803– 1804, table of contents. [DOI] [PubMed] [Google Scholar]
- 36.Siddappa PK, Kar P. Hepatic hydrothorax. Trop Gastroenterol. 2009; 30: 135– 141. [PubMed] [Google Scholar]
- 37.Strauss RM, Boyer TD. Hepatic hydrothorax. Semin Liver Dis. 1997; 17: 227– 232. [DOI] [PubMed] [Google Scholar]
- 38.Wang SB, Wang JH, Chen J, Giri RK, Chen MH. Natural history of liver cirrhosis in south China based on a large cohort study in one center: a follow-up study for up to 5 years in 920 patients. Chin Med J (Engl). 2012; 125: 2157– 2162. [PubMed] [Google Scholar]
- 39.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003; 124: 91– 96. [DOI] [PubMed] [Google Scholar]
- 40.Wilputte JY, Goffette P, Zech F, Godoy-Gepert A, Geubel A. The outcome after transjugular intrahepatic portosystemic shunt (TIPS) for hepatic hydrothorax is closely related to liver dysfunction: a long-term study in 28 patients. Acta Gastroenterol Belg. 2007; 70: 6– 10. [PubMed] [Google Scholar]


