Abstract
Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy (SHML), is an uncommon benign idiopathic lymphoproliferative disorder. The histologic hallmark of RDD is the finding of emperipolesis displayed by lesional histiocytes. While RDD most commonly affects lymph nodes, extranodal involvement of multiple organs has been reported, including the central nervous system (CNS). However, CNS involvement in RDD is rare and is not well characterized. As a result, therapeutic approaches to CNS involvement in RDD are not well established. Herein we report 6 cases of RDD with isolated CNS involvement and review the literature on RDD with CNS involvement. One of the presented cases exhibited intramedullary involvement of the spinal cord—a very rare form of RDD with CNS involvement.
INTRODUCTION
Rosai-Dorfman disease (RDD), or sinus histiocytosis with massive lymphadenopathy (SHML), is an uncommon benign lymphoproliferative disorder with an estimated incidence of approximately 100 cases per year in the United States.118 Initially described in 1969 as a distinct lymphoproliferative entity by pathologists Juan Rosai and Ronald Dorfman, RDD is histologically characterized by infiltration of lymph nodes or extranodal tissues by nonmalignant histiocytes exhibiting emperipolesis, a nondestructive phagocytosis of lymphocytes or erythrocytes, which represents the hallmark of this disease and is required for definitive diagnosis.93
RDD has been reported in all age groups and can involve any nodal or extranodal site, but most commonly presents as massive cervical lymphadenopathy in adolescents and young adults.118 Central nervous system (CNS) involvement has been reported, but is infrequent. It usually manifests as dura-based masses, with intraparenchymal involvement less common. Symptomatic intramedullary spinal cord involvement is extremely rare.1
Due to the rarity of CNS involvement by RDD, therapeutic approaches vary, and there are no uniform treatment recommendations. In localized presentations, surgical resection is usually the therapy of choice; however, resection is not always possible and other therapeutic modalities may be required for disease management.
Herein we present 6 cases of RDD with isolated CNS involvement diagnosed and treated at the University of Miami (n = 4) and Hebrew University-Hadassah Medical Center (n = 2). One of these cases exhibited intramedullary spinal cord involvement, which, to the best of our knowledge, was previously reported only twice.28,82 We also present a review of the literature on CNS involvement by RDD.
METHODS
Patients
We searched the pathology database at the University of Miami to identify all pathology specimens with a diagnosis of RDD and specifically selected patients who presented with CNS disease. A total of 4 patients were identified. In addition, we identified 2 patients with RDD presenting with CNS involvement at the Hebrew University-Hadassah Medical Center. Pathology slides of all patients were available for review and confirmation of RDD diagnosis. The study was approved by the institutional review boards of each institution.
Literature Review
We searched the English literature using PubMed (National Library of Medicine, Bethesda, MD) and SCOPUS (Elsevier) for the period 1970 to July 2013. For the PubMed search we used the terms Rosai-Dorfman disease, sinus histiocytosis with massive lymphadenopathy, and central nervous system, and for the SCOPUS search we used the keywords Rosai Dorfman and central nervous system. Relevant identified manuscripts in English were included, with specific attention to exclude repeated manuscripts from both sources. In addition, we reviewed references cited in all the collected manuscripts to identify additional reports of RDD presenting with CNS involvement.
Statistical Analysis
Comparison between tumor localization in isolated CNS RDD and systemic RDD with CNS involvement was performed with the chi-square test. P values less than 0.05 were considered statistically significant.
ILLUSTRATIVE CASE SUMMARIES
Case 1
A 32-year-old African American woman presented with worsening occipital headaches associated with photophobia and phonophobia. There were no neurologic deficits or peripheral lymphadenopathy on initial examination. Brain magnetic resonance imaging (MRI) demonstrated a 2.5-cm enhancing extraaxial mass originating from the clivus, causing significant brainstem compression (Figures 1A and 1B). The patient underwent 2 transsphenoidal biopsies that were nondiagnostic. Three months later she developed a left cranial nerve VI palsy and right hemiparesis, and a follow-up MRI showed enlargement of the previously described mass and a new dural rupture measuring 1.6 × 3.1 × 2.1 cm. Systemic workup for nodal/extranodal disease was negative. The patient underwent subtotal resection of the posterior fossa mass complicated by left hearing loss. Histologic examination revealed a vaguely nodular proliferation of large atypical histiocytes surrounded by a chronic lymphoplasmacytic inflammatory infiltrate (Figures 2A, B, and C). Many of the large histiocytes contained abundant amphophilic cytoplasm, and emperipolesis was readily apparent (Figures 2D and E). By immunohistochemistry, histiocytes were positive for CD68 and S100 and negative for CD1a (Figure 2F). Adjuvant localized radiation with a total dose of 4500 cGy in 180 cGy daily fractions resulted in clinical response, with improvement of the hemiparesis. Three years later, the patient presented with headaches, neck stiffness, dysarthria, and right hearing loss. A new MRI showed disease progression with an increase in the size of the original retroclival mass and a new large extraaxial lesion in the posterior fossa extending along the inferior margin of the cerebellar vermis down to the cervical spinal canal with associated mass effect (Figure 1C). The patient was locally re-irradiated (4500 cGy) followed by 1 cycle of adjuvant temozolomide 75 mg/m2, leading to resolution of headaches, neck stiffness, and dysarthria without improvement of hearing loss. Six years after the initial diagnosis, the patient’s symptoms remained stable, and follow-up MRI showed no disease progression.
FIGURE 1.
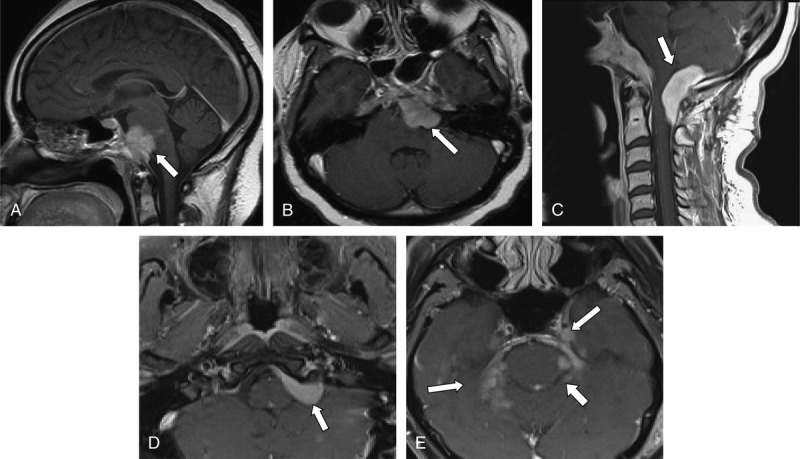
Neuroimaging findings in Cases 1 and 2. A, Sagittal postcontrast T1-weighted MRI showing a lobulated enhancing extraaxial mass (arrow) in the retroclival region with secondary mass effect over the ventral aspect of the pons and medulla oblongata in Case 1. B, Axial postcontrast T1-weighted MRI showing the same lobulated mass. C, Sagittal postcontrast T1-weighted MRI demonstrates an extraaxial homogeneously enhancing mass in the posterior fossa extending inferiorly to the foramen magnum and into the cervical canal with associated mass effect on the dorsal aspect of the cervicomedullary junction and cervical spinal cord in Case 1. D, Axial postcontrast T1-weighted MRI showing an elongated homogeneous enhancing extraaxial mass (arrow) in the left cerebellopontine angle with mild mass effect over the left aspect of the pons and anterior left cerebellum in Case 2. E, Axial postcontrast T1-weighted MRI demonstrating nodular enhancement along the dura and tentorium (arrows) in Case 2.
FIGURE 2.
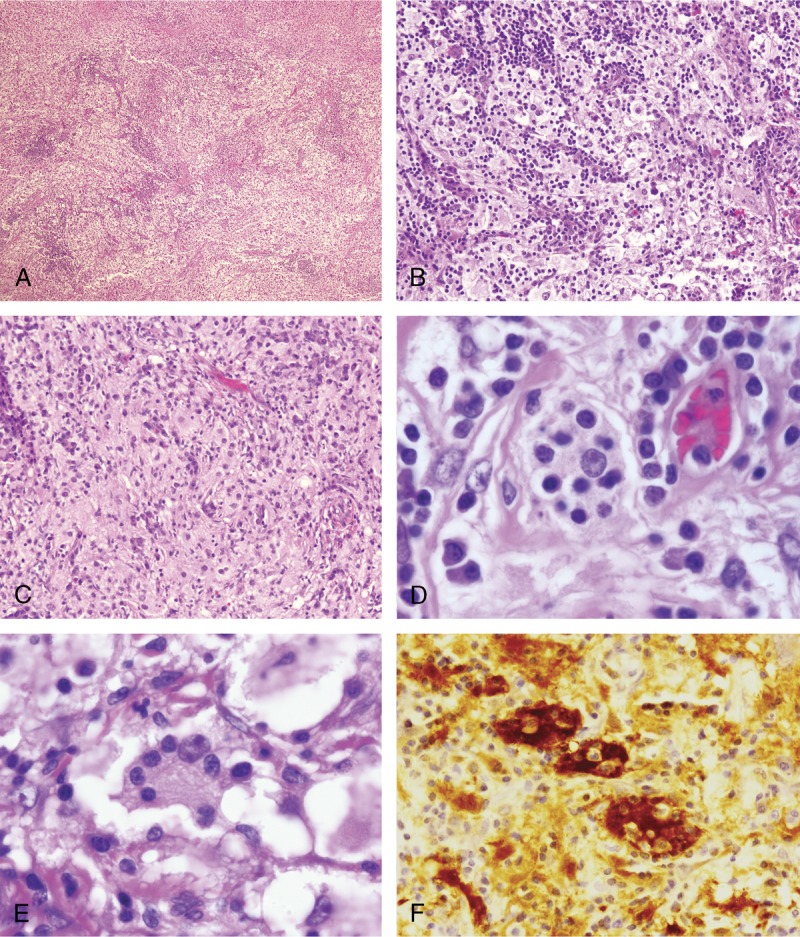
Pathologic findings in RDD involving the CNS. All cases in this series had similar morphologic features, and representative images are shown. A, B, and C, Biopsies contained an abnormal vaguely nodular polymorphous lymphohistiocytic infiltrate (hematoxylin and eosin; 4× magnification in A) composed of abundant loosely cohesive large histiocytes admixed with numerous small mature lymphocytes and plasma cells (hematoxylin and eosin; 10× magnification in B and C). D and E, Within the aggregates of histiocytes, the diagnostic feature of emperipolesis was identified in all cases (hematoxylin and eosin; 50× magnification). F, The cells are positive for S-100 protein by immunohistochemistry (hematoxylin and eosin; 20× magnification).
Case 2
A 51-year-old white man with a 20-year history of progressive hearing loss on both sides, with the left greater than the right, and previous radiologic diagnosis of left acoustic neuroma, presented with complaints of blurred vision, fatigue, weakness in 4 extremities, and sharp pain over both thighs. Except for bilateral hypoacusis, his examination was normal and there was neither lymphadenopathy nor organomegaly. Laboratory studies were unremarkable. Initial MRI of the brain showed bilateral dura-based masses involving the cavernous sinuses, internal auditory canals, cerebellopontine angles, and the foramen magnum (Figures 1D and 1E).
The patient underwent a left retrosigmoid craniotomy with partial resection of the left cerebellopontine angle and internal auditory canal masses. Histologic examination revealed a heterogeneous lymphohistiocytic cell infiltrate composed of abundant large histiocytes that were focally clustered and demonstrated copious amphophilic cytoplasm associated with numerous small mature lymphocytes and scattered plasma cells. Emperipolesis was readily apparent. By immunohistochemistry, histiocytes were positive for CD68 and S100 and negative for CD1a. Surgery was followed by localized radiotherapy, with a total dose of 3000 cGy in 200 cGy daily fractions. The patient had improvement of his blurry vision but with persistent bilateral hypoacusis. Over the next 7 months, the patient remained clinically and radiologically stable.
Case 3
Intracranial RDD was diagnosed in a 53-year-old white man at another institution 20 months before presentation to our outpatient clinic. He initially presented with headaches and was found to have meningeal lesions that were partially resected and led to the diagnosis of the RDD. Following surgery he was asymptomatic until about 3 months before his presentation to our clinic when he started to experience progressive unsteadiness, recurrent falls, and generalized body aches. Examination revealed 3/5 paresis with decreased muscle tone of both upper and lower extremities and ataxia. There was no evidence for lymphadenopathy or organomegaly. MRI of the brain showed enhancing lesions in the left cavernous sinus and right prepontine cistern with mass effect over the pons and pontomedullary junction. MRI of the cervical/thoracic spine showed diffuse intramedullary spinal cord signal abnormality with multiple areas of nodular cord enhancement, an extraaxial enhancing lesion at C5-C6, and a T2 hyperintensity at T1-2 and T5-6 levels (Figures 3A and 3B).
FIGURE 3.
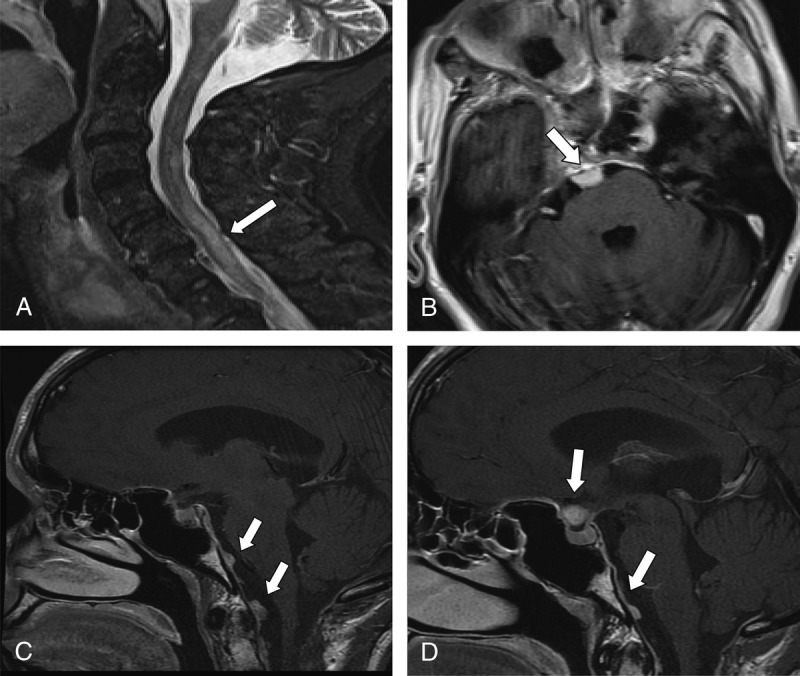
Neuroimaging findings in Cases 3 and 4. A, Sagittal postcontrast T2-weighted MRI showing an abnormal hyperintense intramedullary lesion throughout the cervical cord at C5-C6 (arrow) in Case 3. B, Axial postcontrast T1-weighted MRI demonstrating an extraaxial homogeneously enhancing mass (arrow) in the right prepontine cistern with mass effect on the ventral aspect of the pons in Case 3. C and D, Sagittal postcontrast T1-weighted MRI demonstrates multiple areas of nodular enhancement along the ventral aspect of the cervicomedullary junction consistent with dura-based lesion in Case 4.
Diagnostic lumbar puncture showed normal cerebrospinal fluid glucose and total protein levels without pleocytosis; immunoglobulin G of 10.7 mg/dL (0–6 mg/dL) and myelin basic protein of 2.9 ng/mL (normal: <4 ng/mL). Gram staining, bacterial cultures, acid-fast bacilli, and fungal smears were negative. The patient underwent left pterional craniotomy through a transsylvian and transpetrosal approach with partial resection of the mass. Histologic examination demonstrated a vaguely nodular heterogeneous histiocytic and lymphoplasmacytic proliferative lesion that consisted predominantly of large histiocytes and plasma cells separated by thick collagenous bundles. The histiocytes had vesicular nuclei with abundant amphophilic cytoplasm, and the characteristic finding of emperipolesis was easily identified. The lesional histiocytes were positive for CD163, CD68, and S-100 but negative for CD1a by immunohistochemistry. Therapy with dexamethasone (8 mg every 8 h) for 3 weeks was associated with significant side effects and no improvement in symptoms. For this reason the steroids were tapered off and radiation therapy was initiated to the entire spinal canal. The plan was to deliver a total of 3000 cGy, but after the patient received 2000 cGy he decided to discontinue radiation therapy due to lack of improvement.
Case 4
An 18-year-old African American man presented with progressive generalized weakness associated with episodic hand cramps. Examination revealed mild quadriparesis with preserved muscle tone and deep tendon reflexes and without peripheral lymphadenopathy or organomegaly. A brain MRI revealed an intracranial extraaxial lesion in the pontomedullary level extending to the upper cervical spine with a component of intraspinal infiltration. The patient underwent partial resection of the pontomedullary mass without complications. Histologic examination identified an atypical lymphohistiocytic infiltrate composed of numerous clustered large histiocytes that had abundant amphophilic cytoplasm and demonstrated definitive emperipolesis. The patient completed adjuvant localized radiation therapy (4500 cGy) as well as adjuvant systemic chemotherapy with vincristine, mercaptopurine (6-MP), prednisone, and methotrexate (MTX) for 2 years with partial remission of the disease and substantial improvement of quadriparesis. Seven years after the initial diagnosis he continues to be relatively asymptomatic with only mild residual neurologic deficits. However, follow-up MRI continued to demonstrate multiple areas of dural enhancement, both in the anterior and posterior cranial fossas, around the cervical spinal canal, the tentorium, within the right internal auditory canal, as well as a 5-mm rounded enhancing lesion in the suprasellar region (Figures 3C and 3D). Due to the lack of symptoms he was actively followed without specific treatment.
Case 5
A 38-year-old white man was incidentally diagnosed to have a tuberculum sellae mass on a follow-up head computed tomography (CT) performed because of chronic maxillary sinusitis. He was asymptomatic with normal neurologic examination, and his total body CT scan was unremarkable. On MRI, the homogenously enhancing extraaxial mass appeared to involve anteriorly both cavernous sinuses extending to the right clinoid process. In addition, there were 2 small asymptomatic dura-based masses, 1 in the left hemispheric convexity and another in the left foramen magnum. With the presumed diagnosis of multiple meningiomas, the patient underwent a complete macroscopic resection of the tuberculum sellae mass, demonstrating brain tissue infiltration by S100 and CD68 positive histiocytes with large nuclei, prominent nucleoli, and characteristic emperipolesis, establishing a diagnosis of RDD. The patient remained asymptomatic over the next year and had no recurrence or progression of the cranial lesions on repeat MRI studies. He was lost to follow-up after that time.
Case 6
A 60-year-old white man was evaluated for progressive cognitive deterioration over 1 year associated with mild right hemiparesis. A cerebral MRI demonstrated a homogenously enhancing extraaxial left parieto-occipital lesion, which was surgically completely resected. Histologic examination identified histiocytes that stained positively for S100 and negatively for CD68 and CD1a, confirming the diagnosis of RDD. Whole body CT showed no systemic involvement. Regular clinical and imaging follow-up over the next 10 years showed no signs of recurrence.
RESULTS AND DISCUSSION
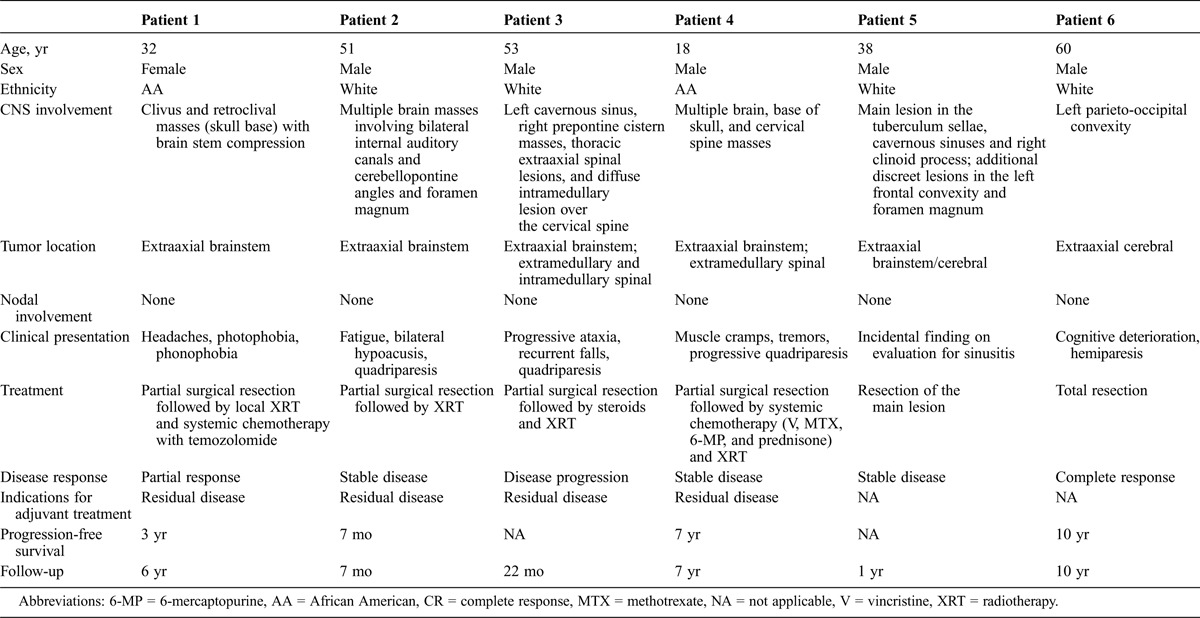
Table 1 presents the main clinical and imaging features of the 6 patients presented here with RDD with isolated CNS involvement. The disease was diagnosed at a mean age of 42 years (range, 18–60 yr), variably presenting with nonspecific headaches or location-related neurologic manifestations in 5 patients, and was incidentally diagnosed in the final patient. Five patients had isolated extraaxial tumors, intracranial (4/5) or intracranial and spinal (1/5), while 1 patient also had an additional spinal intramedullary lesion. The diagnosis was invariably based on the presence of histiocytic infiltrations with emperipolesis in surgically obtained tissue samples. Because 5 of the 6 patients had multiple intracranial or combined intracranial and spinal masses, complete surgical resection could be achieved only in 1 patient with a single intracranial lesion. Due to incomplete resection of the CNS masses, 4 patients received localized adjuvant radiotherapy, and 2 of these received systemic chemotherapy as well. Five patients responded to therapy, achieving stable disease with follow-up periods ranging from 7 months to 10 years. One additional patient who initially responded to surgery later experienced progressive disease without symptomatic response to localized radiotherapy or to adjuvant systemic steroids, and opted to continue with palliative therapy (Table 1). The follow-up of our patients is significantly longer than the follow-up of patients presented in the literature, thus demonstrating the natural history of the disease and disease course following treatment.
TABLE 1.
Characteristics, Treatment, and Clinical Outcome of 6 Patients With RDD With CNS Involvement
Extranodal involvement is observed in approximately 40% of patients diagnosed with RDD, but CNS involvement is very uncommon, with 210 cases reported in the English medical literature, including our 6 patients (Table 2).1,3–46,48–77,79–82,84,85,87,89–91,94–113,115–127 In 174 of the reported cases, RDD was isolated to the CNS, while in 36 patients it was part of a systemic disseminated disease (see Table 2). CNS involvement has been described in both adults and children, with a mean age at presentation of 39 years and a male prevalence (male: female ratio: 1.8: 1.0). RDD involving the CNS presents most commonly with dura-based extraaxial involvement of the cranium. Spinal cord involvement is exceedingly rare. Patients may present with a single or multiple masses. Adeleye et al1 found that between 1969 and 2009, a total of 111 cases of RDD involving the CNS were reported, with only 15 (14%) affecting the spine and 10 (9%) with both intracranial and spinal presentation. In our updated review of a total of 210 patients (including 6 patients presented herein), 167 (79.5%) presented with intracranial lesions, 24 (11.4%) with spinal involvement, and 19 (9.0%) with both intracranial and spinal involvement (see Table 2). Anatomical localization of the CNS lesions in RDD with isolated CNS involvement and systemic RDD with CNS involvement is shown in Table 2. Involvement of the spine was more common in the systemic RDD with CNS involvement (p = 0.017). In addition to our Case 3, we found only 2 previous cases of intramedullary spinal cord RDD in the literature. To our knowledge the first reported case of intramedullary RDD was described by Osenbach82 in 1996 in a 35-year-old man who presented with paraplegia associated with an intramedullary lesion in the thoracic spine detected by MRI. The mass was fully resected, and a diagnosis of intramedullary RDD was confirmed by pathologic studies.82 El-Molla et al28 recently reported the second case of intramedullary RDD, in a 76-year-old man with progressive right body hemiparesis and an intramedullary lesion involving the cervical spine, detected by MRI. The intraspinal tumor was confirmed to be RDD after surgical resection.28 While in these 2 patients the RDD presented as a localized intramedullary mass, in our Case 3 the spinal cord intramedullary mass was associated with additional extraaxial tumors.
TABLE 2.
Characteristics of CNS RDD Cases According to Type of Clinical Presentation, Present and Previous Reports*
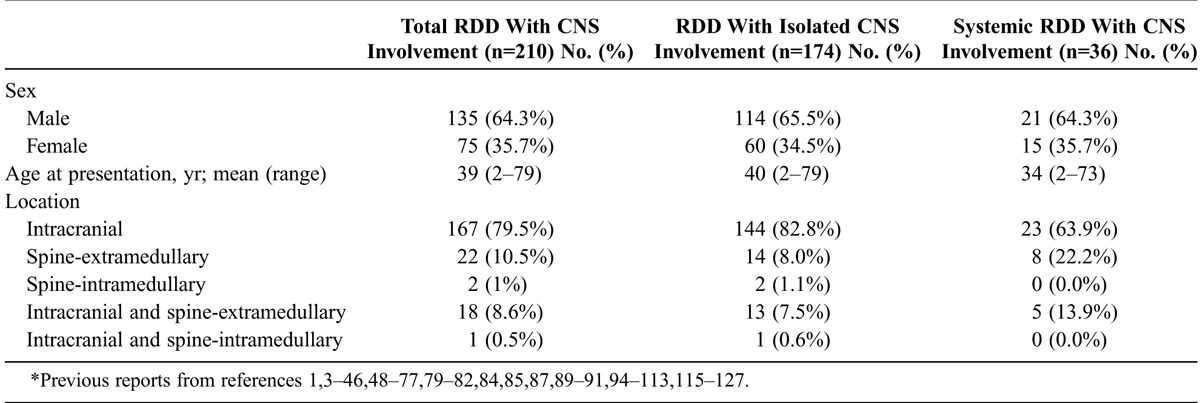
In patients with an isolated CNS presentation, constitutional symptoms are usually absent. The neurologic symptoms and findings depend on the localization of the lesions. Patients commonly present with headaches, seizures, focal neurologic deficits, and paraparesis or paraplegia when the spine is affected.
A review of the radiologic findings revealed that RDD may involve intracranial (82.8%) or spinal structures (9.1%), or both anatomic locations may be concurrently involved in only 14 (8.1%) patients. Because CNS involvement by RDD is an uncommon entity and diagnostic imaging findings mimic other more common lesions such as meningiomas, preoperative evaluation is challenging, and without biopsy the disease is commonly misdiagnosed.8,100,113,121 Spinal involvement by RDD occurs mostly in the cervical and thoracic regions; we found only 1 case report describing diffuse spinal involvement, including the lumbosacral area.8,45,84,108
On both CT and MRI these intramedullary lesions can present as dura-based masses in the vicinity of bone, therefore mimicking meningiomas, and can be localized to the anterior, middle, or posterior fossa as well as on the extradural compartment of the spine. Overall, both intramedullary and intraparenchymal lesions are very infrequent, especially when they present as isolated masses.1,45,84 Rarely, RDD presents as intraventricular lesions or lesions invading the dura, vascular sinuses, and bone.1,77,108 On CT scan, lesions of RDD can be seen as homogeneous hyperdense masses with marked contrast enhancement and can be associated with perilesional vasogenic edema, causing mass effect on adjacent structures or bone erosions. Characteristically, RDD masses do not have associated calcifications.1,35,69,92 MRI usually reveals single or multiple well-defined lesions that are isointense to the adjacent brain parenchyma on T1-weighted images and are strongly and homogeneously enhanced after gadolinium administration. On T2-weighted images, RDD lesions appear as heterogeneous hypo- or isointense masses similar to that of the contiguous dura.1,25,35,69,77,92,108 These characteristics can also be seen when lesions involve the spinal canal.8,28,45,84 The contrast T1-weighted MRI images of our cases demonstrated both intracranial and spinal homogeneously enhancing masses with an associated mass effect over surrounding structures.
Limited data exist on the applicability of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in RDD. Deshayes et al24 recently described the potential use of 18F-FDG PET/CT to diagnose relapsed intracranial RDD of the hypothalamus in a patient. Clearly, the role of PET/CT in the diagnosis and follow-up of RDD involving the CNS needs further exploration.
Since CNS involvement by RDD is rare, there are no prospective studies evaluating the best therapeutic approach for this disease, and most current approaches are based on case reports and small case series. Furthermore, in most reported cases the follow-up is short, not allowing assessment of long-term therapeutic effects. Because RDD is considered to be a benign, non-neoplastic lymphoproliferative disorder, treatment of RDD involving lymph nodes and extranodal areas outside the CNS is advised only in patients with symptomatic lesions or masses that are impeding the function of vital organs. In RDD patients without CNS involvement, complete spontaneous resolution is observed in about 20% of cases, and about 70% exhibit stable asymptomatic disease.30 Based on our experience and review of the literature, spontaneous resolution is not observed in RDD patients with CNS involvement.
Surgery is the preferred treatment for both diagnostic purposes and to obtain rapid improvement of neurologic symptoms in RDD patients with CNS involvement.1,21,52,78,121 Complete resection of the mass should be attempted, if feasible. Although complete resection is ideal, these masses can often adhere to surrounding critical structures, precluding complete surgical excision. In such cases, subtotal resection of target symptomatic lesions should be done to provide a pathologic diagnosis and symptomatic relief.21,121 If neurologic impairment is reversed after partial resection, a wait-and-watch approach can be implemented, since most patients achieve stable disease after partial excision of the mass, as was also done in some of the patients in the current study.21,47,121 If neurologic symptoms are persistent and/or the lesions are surrounding vital structures with potential to compromise their function, adjuvant therapies should be implemented, with localized radiotherapy being the preferred treatment. Both fractionated radiotherapy using doses of 2000–4500 cGy with 200 cGy/dose or stereotactic radiotherapy have been used.21,41,55 While many patients respond clinically, results vary and some patients do not show any improvement, as was also observed in our patient with intramedullary spinal cord involvement. Systemic therapy can be attempted in these patients. Patients with systemic RDD presenting with constitutional symptoms and/or symptomatic lymphadenopathy with rapid growth are commonly given a therapeutic trial of steroids. While some case reports suggest that dexamethasone might be superior to other steroids in this setting, there are no randomized trials to support this hypothesis.1,21,41 In cases with CNS involvement, steroid treatment has shown some therapeutic benefit in a few reported cases, but the evidence is insufficient to recommend corticosteroids as the mainstay treatment for this group of patients.1,21,41,47
While various chemotherapy regimens have been used to treat progressive systemic RDD, the responses are not uniform, and some of the therapies cannot be used in patients with CNS involvement because of reduced blood-brain barrier permeability. However, it is important to remember that extradural masses are not protected by the blood-brain barrier, and in patients with such masses there is no need to select agents that penetrate the blood-brain barrier. Overall, the literature on chemotherapy for RDD with CNS involvement is scarce, preventing the establishment of evidence-based therapeutic approaches. Pulsoni et al88 reviewed therapeutic outcomes of 12 patients with systemic RDD without CNS involvement who were treated with vinca alkaloids, anthracyclines, antimetabolites, and/or alkylating agents. Overall, therapeutic outcomes were disappointing. Only 2 patients achieved complete response, while the remaining patients had no response. Remarkably, both of the patients with complete response received MTX and 6-MP, and 1 of them obtained a rapid complete response, lasting for 36 months after failing therapy with etoposide. In the current report, Case 4 was also treated with MTX and 6-MP and achieved partial remission. Consequently, MTX with 6-MP may be useful in patients with CNS disease, as was also described in a report of spinal compression due to RDD.68
Newer therapies, including the tyrosine kinase inhibitor imatinib and the anti-CD20 monoclonal antibody rituximab, have also been used in systemic RDD with variable responses.2,83,86,114 Although these therapies showed clinical activity in classic RDD, the results cannot be extrapolated to patients with CNS involvement since none of the treated subjects had CNS disease.
In conclusion, CNS and especially intramedullary spinal cord involvement by RDD is rare, but this disease should always be considered in the differential diagnosis of dura-based and spinal lesions with progressive growth without evidence of calcification on neuroimaging. While randomized prospective data on therapeutic approaches in this patient population are lacking, based on our own experience and an updated literature review, patients with limited disease should undergo surgical resection that may be consolidated with radiotherapy. In cases with widespread CNS disease resistant to radiotherapy, steroids with or without MTX-based chemotherapy could be considered as a feasible active therapy. In the current series, 1 patient maintained prolonged disease control using MTX-based therapy; this was also reported previously by Tubbs et al.111 Rituximab was shown to be beneficial in systemic RDD, but its role in CNS disease is uncertain. Since histiocytes do not express CD20, its mechanism of action and efficacy in this disease are questionable. Due to the rarity of this entity, reports of a small patient series may provide additional valuable data on therapeutic approaches for these patients, but only multicenter international collaborations will eventually provide valuable data necessary to develop rational treatment strategies to improve the outcomes of these patients.
Footnotes
Abbreviations: 6-MP = mercaptopurine, CNS = central nervous system, CT = computed tomography, FDG = fluorodeoxyglucose, MRI = magnetic resonance imaging, MTX = methotrexate, PET = positron emission tomography, RDD = Rosai-Dorfman disease, SHML = sinus histiocytosis with massive lymphadenopathy.
Financial support and conflicts of interest: I.S.L. is supported by the National Institutes of Health [CA109335], Lymphoma Research Foundation, the Dwoskin and Recio Family Foundation grants. The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.Adeleye AO, Amir G, Fraifeld S, Shoshan Y, Umansky F, Spektor S. Diagnosis and management of Rosai-Dorfman disease involving the central nervous system. Neurol Res. 2010;32:572–578. [DOI] [PubMed] [Google Scholar]
- 2.Alqanatish JT, Houghton K, Bond M, Senger C, Tucker LB. Rituximab treatment in a child with Rosai-Dorfman disease and systemic lupus erythematosus. J Rheumatol. 2010;37:1783–1784. [DOI] [PubMed] [Google Scholar]
- 3.Amagasa M, Yuda F, Kojima H, Noshita N, Sato S. Natural course of lymphocytic infundibuloneurohypophysitis. Clin Neuropathol. 2001; 20: 229– 232. [PubMed] [Google Scholar]
- 4.Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-Dorfman disease isolated to the central nervous system: a report of 11 cases. Mod Pathol. 2001; 14: 172– 178. [DOI] [PubMed] [Google Scholar]
- 5.Antuna Ramos A, Alvarez Vega MA, Alles JV, Antuna Garcia MJ, Meilan Martinez A. Multiple involvement of the central nervous system in Rosai-Dorfman disease. Pediatr Neurol. 2012; 46: 54– 56. [DOI] [PubMed] [Google Scholar]
- 6.Asai A, Matsutani M, Kohno T, Fujimaki T, Tanaka H, Kawaguchi K, Koike M, Takakura K. Leptomeningeal and orbital benign lymphophagocytic histiocytosis. Case report. J Neurosurg. 1988; 69: 610– 612. [DOI] [PubMed] [Google Scholar]
- 7.Beros V, Houra K, Rotim K, Zivkovic DJ, Cupic H, Kosec A. Isolated cerebellar intraparenchymal Rosai-Dorfman disease—case report and review of literature. Br J Neurosurg. 2011; 25: 292– 296. [DOI] [PubMed] [Google Scholar]
- 8.Bhandari A, Patel PR, Patel MP. Extranodal Rosai-Dorfman disease with multiple spinal lesions: a rare presentation. Surg Neurol. 2006; 65: 308– 311. [DOI] [PubMed] [Google Scholar]
- 9.Bing F, Brion JP, Grand S, Pasquier B, Lebas JF. Tumor arising in the periventricular region. Neuropathology. 2009; 29: 101– 103. [DOI] [PubMed] [Google Scholar]
- 10.Brandsma D, Jansen GH, Spliet W, Van Nielen K, Taphoorn MJ. The diagnostic difficulties of meningeal and intracerebral plasma cell granulomas—presentation of three cases. J Neurol. 2003; 250: 1302– 1306. [DOI] [PubMed] [Google Scholar]
- 11.Breiner A, Dubinski W, Gray B, Munoz DG. A 63 year old woman with white matter lesions and pachymeningeal inflammation. Brain Pathol. 2013; 23: 225– 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchino JJ, Byrd RP, Kmetz DR. Disseminated sinus histiocytosis with massive lymphadenopathy: its pathologic aspects. Arch Pathol Lab Med. 1982; 106: 13– 16. [PubMed] [Google Scholar]
- 13.Camp SJ, Roncaroli F, Apostolopoulos V, Weatherall M, Lim S, Nandi D. Intracerebral multifocal Rosai-Dorfman disease. J Clin Neurosci. 2012; 19: 1308– 1310. [DOI] [PubMed] [Google Scholar]
- 14.Cao XY, Luan SH, Bao WM, Shen C, Yang BJ. Solitary intracranial Rosai-Dorfman disease: case report and literature review. J Int Med Res. 2011; 39: 2045– 2050. [DOI] [PubMed] [Google Scholar]
- 15.Castellano-Sanchez AA, Brat DJ. May 2003: 57-year-old-woman with acute loss of strength in her right upper extremity and slurred speech. Brain Pathol. 2003; 13: 641– 642. [PubMed] [Google Scholar]
- 16.Catalucci A, Lanni G, Ventura L, Ricci A, Galzio RJ, Gallucci M. A rare case of intracranial Rosai-Dorfman disease mimicking multiple meningiomas. A case report and review of the literature. Neuroradiol J. 2012; 25: 569– 574. [DOI] [PubMed] [Google Scholar]
- 17.Cavuoto K, Galor A, Dubovy SR, Gregori N, McCarthy M. Subconjunctival masses associated with central nervous system Rosai-Dorfman disease. Cornea. 2011; 30: 237– 240. [DOI] [PubMed] [Google Scholar]
- 18.Chan KW, Chow YY, Ghadially FN, Stansfeld AG, Woo CH. Rosai-Dorfman disease presenting as spinal tumor. A case report with ultrastructural and immunohistochemical studies. J Bone Joint Surg Am. 1985; 67: 1427– 1431. [PubMed] [Google Scholar]
- 19.Chen KT. Crush cytology of Rosai-Dorfman disease of the central nervous system. A report of 2 cases. Acta Cytol. 2003; 47: 1111– 1115. [DOI] [PubMed] [Google Scholar]
- 20.Clark WC, Berry AD., 3rd Extranodal sinus histiocytosis with massive lymphadenopathy: isolated central nervous system involvement mimicking meningioma. South Med J. 1996; 89: 621– 623. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SL, Jenrette JM. Rosai-Dorfman disease: management of CNS and systemic involvement. Clin Adv Hematol Oncol. 2012; 10: 199– 202. [PubMed] [Google Scholar]
- 22.Cunliffe CH, Fischer I, Monoky D, Law M, Revercomb C, Elrich S, Kopp MJ, Zagzag D. Intracranial lesions mimicking neoplasms. Arch Pathol Lab Med. 2009; 133: 101– 123. [DOI] [PubMed] [Google Scholar]
- 23.Deodhare SS, Ang LC, Bilbao JM. Isolated intracranial involvement in Rosai-Dorfman disease: a report of two cases and review of the literature. Arch Pathol Lab Med. 1998; 122: 161– 165. [PubMed] [Google Scholar]
- 24.Deshayes E, Le Berre JP, Jouanneau E, Vasiljevic A, Raverot G, Seve P. 18F-FDG PET/CT findings in a patient with isolated intracranial Rosai-Dorfman disease. Clin Nucl Med. 2013; 38: 50– 52. [DOI] [PubMed] [Google Scholar]
- 25.Di Rocco F, Garnett MR, Puget S, Pueyerredon F, Roujeau T, Jaubert F, Sainte-Rose C. Cerebral localization of Rosai-Dorfman disease in a child. Case report. J Neurosurg. 2007; 107: 147– 151. [DOI] [PubMed] [Google Scholar]
- 26.Dran G, Rasendrarijao D, Vandenbos F, Paquis P. Rosai-Dorfman disease causing spinal cord compression: case report. Neurosurgery. 2008; 62: E977– E978. [DOI] [PubMed] [Google Scholar]
- 27.El Majdoub F, Brunn A, Berthold F, Sturm V, Maarouf M. Stereotactic interstitial radiosurgery for intracranial Rosai-Dorfman disease. A novel therapeutic approach. Strahlenther Onkol. 2009; 185: 109– 112. [DOI] [PubMed] [Google Scholar]
- 28.El-Molla M, Mahasneh T, Holmes SE, Al-Khawaja D. Rare presentation of Rosai-Dorfman disease mimicking a cervical intramedullary spinal cord tumor. World Neurosurg. 2013;http://dx.doi.org/10.1016/j.wneu.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Fortea J, Compta Y, Valldeoriola F, Tolosa E, Rey MJ, Gaston F, Ribalta T. Fatal worsening of late-onset cerebellar ataxia with neuronal intranuclear inclusions due to superimposed meningeal Rosai-Dorfman disease. Mov Disord. 2008; 23: 1488– 1490. [DOI] [PubMed] [Google Scholar]
- 30.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990; 7: 19– 73. [PubMed] [Google Scholar]
- 31.Foucar E, Rosai J, Dorfman RF, Brynes RK. The neurologic manifestations of sinus histiocytosis with massive lymphadenopathy. Neurology. 1982; 32: 365– 372. [DOI] [PubMed] [Google Scholar]
- 32.Franco-Paredes C, Martin K. Extranodal Rosai-Dorfman disease involving the meninges. South Med J. 2002; 95: 1101– 1102. [PubMed] [Google Scholar]
- 33.Fukushima T, Yachi K, Ogino A, Ohta T, Watanabe T, Yoshino A, Katayama Y. Isolated intracranial Rosai-Dorfman disease without dural attachment—case report. Neurol Med Chir. 2011; 51: 136– 140. [DOI] [PubMed] [Google Scholar]
- 34.Gaetani P, Tancioni F, Di Rocco M, Rodriguez y Baena R. Isolated cerebellar involvement in Rosai-Dorfman disease: case report. Neurosurgery. 2000; 46: 479– 481. [DOI] [PubMed] [Google Scholar]
- 35.Geara AR, Ayoubi MA, Achram MC, Chamseddine NM. Rosai-Dorfman disease mimicking neurofibromatosis: case presentation and review of the literature. Clin Radiol. 2004; 59: 625– 630. [DOI] [PubMed] [Google Scholar]
- 36.Ghosal N, Murthy G, Visvanathan K, Sridhar M, Hegde AS. Isolated intracranial Rosai Dorfman disease masquerading as meningioma: a case report. Indian J Pathol Microbiol. 2007; 50: 382– 384. [PubMed] [Google Scholar]
- 37.Gies U, Gruia D, Lassmann H, Bergmann M. A case of rapidly progressive Rosai-Dorfman disease restricted to the central nervous system. Zentralbl Neurochir. 2005; 66: 142– 146. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths SJ, Tang W, Parameswaran R, Kelsey A, West CG. Isolated intracranial Rosai-Dorfman disease mimicking meningioma in a child. Br J Neurosurg. 2004; 18: 293– 297. [DOI] [PubMed] [Google Scholar]
- 39.Gupta DK, Suri A, Mahapatra AK, Mehta VS, Garg A, Sarkar C, Ahmad FU. Intracranial Rosai-Dorfman disease in a child mimicking bilateral giant petroclival meningiomas: a case report and review of literature. Childs Nerv Syst. 2006; 22: 1194– 1200. [DOI] [PubMed] [Google Scholar]
- 40.Gupta K, Bagdi N, Sunitha P, Ghosal N. Isolated intracranial Rosai-Dorfman disease mimicking meningioma in a child: a case report and review of the literature. Br J Radiol. 2011; 84: 138– 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hadjipanayis CG, Bejjani G, Wiley C, Hasegawa T, Maddock M, Kondziolka D. Intracranial Rosai-Dorfman disease treated with microsurgical resection and stereotactic radiosurgery. Case report. J Neurosurg. 2003; 98: 165– 168. [DOI] [PubMed] [Google Scholar]
- 42.Halelfadl S, Bougrine M, Fadli M, Elkettani F, Bellakhdar F. Rosai-Dorfman disease mimicking meningioma. Pan Arab J Neurosurg. 2007; 11: 89– 94. [Google Scholar]
- 43.Hargett C, Bassett T. Atypical presentation of sinus histiocytosis with massive lymphadenopathy as an epidural spinal cord tumor: a case presentation and literature review. J Spinal Disord Tech. 2005; 18: 193– 196. [DOI] [PubMed] [Google Scholar]
- 44.Hinduja A, Aguilar LG, Steineke T, Nochlin D, Landolfi JC. Rosai-Dorfman disease manifesting as intracranial and intraorbital lesion. J Neurooncol. 2009; 92: 117– 120. [DOI] [PubMed] [Google Scholar]
- 45.Hollowell JP, Wolfla CE, Shah NC, Mark LP, Whittaker MH. Rosai-Dorman disease causing cervical myelopathy. Spine. 2000; 25: 1453– 1456. [DOI] [PubMed] [Google Scholar]
- 46.Huang HY, Huang CC, Lui CC, Chen HJ, Chen WJ. Isolated intracranial Rosai-Dorfman disease: case report and literature review. Pathol Int. 1998; 48: 396– 402. [DOI] [PubMed] [Google Scholar]
- 47.Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002; 33: 1211– 1226. [DOI] [PubMed] [Google Scholar]
- 48.Jones MP, Rueda-Pedraza ME. Extranodal sinus histiocytosis with massive lymphadenopathy presenting as an intramedullary spinal cord tumor: a case report. Am J Hematol. 1997; 54: 253– 257. [DOI] [PubMed] [Google Scholar]
- 49.Juric G, Jakic-Razumovic J, Rotim K, Zarkovic K. Extranodal sinus histiocytosis (Rosai-Dorfman disease) of the brain parenchyma. Acta Neurochir. 2003; 145: 145– 149. [DOI] [PubMed] [Google Scholar]
- 50.Kaminsky J, Koerbel A, Mittelbronn M, Beschorner R, Ernemann U, Tatagiba M. Rosai-Dorfman disease involving the cranial base, paranasal sinuses and spinal cord. Clin Neuropathol. 2005; 24: 194– 200. [PubMed] [Google Scholar]
- 51.Kanner KA, Stmink A, Roth T, Gupa K, Nornan W. Rosai-dorfman disease mimicking parasagittal meningioma. Skull Base Surgery. 1999; 9: 17. [Google Scholar]
- 52.Kattner KA, Stroink AR, Roth TC, Lee JM. Rosai-Dorfman disease mimicking parasagittal meningioma: case presentation and review of literature. Surg Neurol. 2000; 53: 452– 457. [DOI] [PubMed] [Google Scholar]
- 53.Katz DS, Poe LB, Corona RJ., Jr Sinus histiocytosis with massive lymphadenopathy: a case of simultaneous upper respiratory tract and CNS disease without lymphadenopathy. AJNR Am J Neuroradiol. 1993; 14: 219– 222. [PMC free article] [PubMed] [Google Scholar]
- 54.Kayali H, Onguru O, Erdogan E, Sirin S, Timurkaynak E. Isolated intracranial Rosai-Dorfman disease mimicking meningioma. Clin Neuropathol. 2004; 23: 204– 208. [PubMed] [Google Scholar]
- 55.Kidd DP, Revesz T, Miller NR. Rosai-Dorfman disease presenting with widespread intracranial and spinal cord involvement. Neurology. 2006; 67: 1551– 1555. [DOI] [PubMed] [Google Scholar]
- 56.Kim GG, Friedel ME, Eloy JA, Jyung RW, Liu JK. Extensive multifocal Rosai-Dorfman disease involving the central nervous system and paranasal sinuses. Laryngoscope. 2011; 121: 234.21271566 [Google Scholar]
- 57.Kim M, Provias J, Bernstein M. Rosai-Dorfman disease mimicking multiple meningioma: case report. Neurosurgery. 1995; 36: 1185– 1187. [DOI] [PubMed] [Google Scholar]
- 58.Kitai R, Llena J, Hirano A, Ido K, Sato K, Kubota T. Meningeal Rosai-Dorfman disease: report of three cases and literature review. Brain Tumor Pathol. 2001; 18: 49– 54. [DOI] [PubMed] [Google Scholar]
- 59.Kitai R, Sato K, Kubota T, Kabuto M, Kawano H, Kobayashi H, Tsuji T. Meningeal sinus histiocytosis mimicking lymphoplasmacyte-rich meningioma. Case report. J Neurosurg. 1996; 84: 1051– 1054. [DOI] [PubMed] [Google Scholar]
- 60.Konishi E, Ibayashi N, Yamamoto S, Scheithauer BW. Isolated intracranial Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy). AJNR Am J Neuroradiol. 2003; 24: 515– 518. [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnamoorthy V, Parmar CF, Panikar D. Isolated intracranial Rosai Dorfman disease. Neurol India. 2011; 59: 443– 446. [DOI] [PubMed] [Google Scholar]
- 62.Kumar KK, Menon G, Nair S, Radhakrishnan VV. Rosai-Dorfman disease mimicking chronic subdural hematoma. J Clin Neurosci. 2008; 15: 1293– 1295. [DOI] [PubMed] [Google Scholar]
- 63.Kumar R, Giri PJ, Jaiswal A, Agarwal T, Pal L. Intracranial Rosai-Dorfman syndrome mimicking meningioma. Pan Arab J Neurosurg. 2008; 12: 70– 72. [Google Scholar]
- 64.Lauwers GY, Perez-Atayde A, Dorfman RF, Rosai J. The digestive system manifestations of Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy): review of 11 cases. Hum Pathol. 2000; 31: 380– 385. [DOI] [PubMed] [Google Scholar]
- 65.Le Guenno G, Galicier L, Uro-Coste E, Petitcolin V, Rieu V, Ruivard M. Successful treatment with azathioprine of relapsing Rosai-Dorfman disease of the central nervous system. J Neurosurg. 2012; 117: 486– 489. [DOI] [PubMed] [Google Scholar]
- 66.Leung JLY, Cheung JYL, Tan TC, Tang KW, Chan CM, Ho LC, Chan SCH. Carotid artery occlusion in a patient with intracranial Rosai-Dorfman disease. J Hong Kong Coll Radiol. 2003; 6: 211– 213. [Google Scholar]
- 67.Li Y, Sun H, Zhang Y, Liu W. Isolated intracranial Rosai-Dorfman disease presenting as mental deterioration. Clin Neurol Neurosurg. 2012; 114: 1070– 1073. [DOI] [PubMed] [Google Scholar]
- 68.Lohr HF, Godderz W, Wolfe T, Heike M, Knuth A, Meyer zum Buschenfelde KH, Dippold W. Long-term survival in a patient with Rosai-Dorfman disease treated with interferon-alpha. Eur J Cancer. 1995; 31a: 2427– 2428. [DOI] [PubMed] [Google Scholar]
- 69.Lou X, Chen ZY, Wang FL, Ma L. MR findings of Rosai-Dorfman disease in sellar and suprasellar region. Eur J Radiol. 2012; 81: 1231– 1237. [DOI] [PubMed] [Google Scholar]
- 70.Lu CH, Chang KC, Lee EJ, Chuang MT, Chang RS. Intracranial Rosai-Dorfman disease with unusual transcranial extension. J Neuroimaging. 2012; 22: 312– 315. [DOI] [PubMed] [Google Scholar]
- 71.Lu M, Guo DY. Leptomeningeal Rosai-Dorfman disease. J Neuroradiol. 2010; 37: 196– 197. [DOI] [PubMed] [Google Scholar]
- 72.Lungren MP, Petrella JR, Cummings TJ, Grant GA. Isolated intracranial Rosai-Dorfman disease in a child. AJNR Am J Neuroradiol. 2009; 30: E148– E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maiti TK, Gangadharan J, Mahadevan A, Arivazhagan A, Chandramouli BA, Shankar SK. Rosai-Dorfman disease presenting as cervical extradural lesion: a case report with review of literature. Neurol India. 2011; 59: 438– 442. [DOI] [PubMed] [Google Scholar]
- 74.McPherson CM, Brown J, Kim AW, DeMonte F. Regression of intracranial Rosai-Dorfman disease following corticosteroid therapy. Case report. J Neurosurg. 2006; 104: 840– 844. [DOI] [PubMed] [Google Scholar]
- 75.Miletic H, Rohling R, Stenzel W, Deckert M, Benz-Bohm G, Berthold F, Voges J. 8-year-old child with a lesion in the left nucleus lentiformis. Brain Pathol. 2008; 18: 598– 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirra SS, Tindall SC, Check IJ, Brynes RK, Moore WW. Inflammatory meningeal masses of unexplained origin. An ultrastructural and immunological study. J Neuropathol Exp Neurol. 1983; 42: 453– 468. [DOI] [PubMed] [Google Scholar]
- 77.Morandi X, Godey B, Riffaud L, Heresbach N, Brassier G. Isolated Rosai-Dorfman disease of the fourth ventricle. Case illustration. J Neurosurg. 2000; 92: 890. [DOI] [PubMed] [Google Scholar]
- 78.Morgan NV, Morris MR, Cangul H, Gleeson D, Straatman-Iwanowska A, Davies N, Keenan S, Pasha S, Rahman F, Gentle D, Vreeswijk MP, Devilee P, Knowles MA, Ceylaner S, Trembath RC, Dalence C, Kismet E, Koseoglu V, Rossbach HC, Gissen P, Tannahill D, Maher ER. Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet. 2010; 6: e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nalini A, Jitender S, Anantaram G, Santosh V. Rosai Dorfman disease: case with extensive dural involvement and cerebrospinal fluid pleocytosis. J Neurol Sci. 2012; 314: 152– 154. [DOI] [PubMed] [Google Scholar]
- 80.Natarajan S, Post KD, Strauchen J, Morgello S. Primary intracerebral Rosai-Dorfman disease: a case report. J Neurooncol. 2000; 47: 73– 77. [DOI] [PubMed] [Google Scholar]
- 81.Ng HK, Poon WS. Sinus histiocytosis with massive lymphadenopathy localized to the sella. Br J Neurosurg. 1995; 9: 551– 555. [DOI] [PubMed] [Google Scholar]
- 82.Osenbach RK. Isolated extranodal sinus histiocytosis presenting as an intramedullary spinal cord tumor with paraplegia. Case report. J Neurosurg. 1996; 85: 692– 696. [DOI] [PubMed] [Google Scholar]
- 83.Pagel JM, Lionberger J, Gopal AK, Sabath DE, Loeb K. Therapeutic use of rituximab for sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Am J Hematol. 2007; 82: 1121– 1122. [DOI] [PubMed] [Google Scholar]
- 84.Parmar V, Seward C, Huho A, Qian J, Gandhi R, Pilitsis JG. Rosai-Dorfman disease presenting as cervical radiculopathy. Clin Neurol Neurosurg. 2013; 115: 808– 810. [DOI] [PubMed] [Google Scholar]
- 85.Perras B, Petersen D, Lorch H, Fehm HL. Psychoneuroendocrine disturbances in a patient with a rare granulomatous disease. Exp Clin Endocrinol Diabetes. 2002; 110: 248– 252. [DOI] [PubMed] [Google Scholar]
- 86.Petschner F, Walker UA, Schmitt-Graff A, Uhl M, Peter HH. [“Catastrophic systemic lupus erythematosus” with Rosai-Dorfman sinus histiocytosis. Successful treatment with anti-CD20/rutuximab]. Dtsch Med Wochenschr. 2001; 126: 998– 1001. [DOI] [PubMed] [Google Scholar]
- 87.Petzold A, Thom M, Powell M, Plant GT. Relapsing intracranial Rosai-Dorfman disease. J Neurol Neurosurg Psychiatry. 2001; 71: 538– 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pulsoni A, Anghel G, Falcucci P, Matera R, Pescarmona E, Ribersani M, Villiva N, Mandelli F. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002; 69: 67– 71. [DOI] [PubMed] [Google Scholar]
- 89.Purav P, Ganapathy K, Mallikarjuna VS, Annapurneswari S, Kalyanaraman S, Reginald J, Natarajan P, Bapu KR, Balamurugan M. Rosai-Dorfman disease of the central nervous system. J Clin Neurosci. 2005; 12: 656– 659. [DOI] [PubMed] [Google Scholar]
- 90.Raslan OA, Schellingerhout D, Fuller GN, Ketonen LM. Rosai-Dorfman disease in neuroradiology: imaging findings in a series of 10 patients. AJR Am J Roentgenol. 2011; 196: W187– W193. [DOI] [PubMed] [Google Scholar]
- 91.Resnick DK, Johnson BL, Lovely TJ. Rosai-Dorfman disease presenting with multiple orbital and intracranial masses. Acta Neuropathol. 1996; 91: 554– 557. [DOI] [PubMed] [Google Scholar]
- 92.Robert EG, Fallon KB, Tender GC. Isolated Rosai-Dorfman disease of the sacrum. Case illustration. J Neurosurg Spine. 2006; 4: 425. [DOI] [PubMed] [Google Scholar]
- 93.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a pseudolymphomatous benign disorder. Analysis of 34 cases. Cancer. 1972; 30: 1174– 1188. [DOI] [PubMed] [Google Scholar]
- 94.Rotondo F, Munoz DG, Hegele RG, Gray B, Khatun N, Bonert M, Kovacs K. Rosai-Dorfman disease involving the neurohypophysis. Pituitary. 2010; 13: 256– 259. [DOI] [PubMed] [Google Scholar]
- 95.Russo N, Giangaspero F, Beccaglia MR, Santoro A. Intracranial dural histiocytosis. Br J Neurosurg. 2009; 23: 449– 454. [DOI] [PubMed] [Google Scholar]
- 96.Said R, Abi-Fadel F, Talwar J, Attallah JP, Dilawari A. Intracranial Rosai-Dorfman: a clinical challenge. Neurologist. 2011; 17: 117– 119. [DOI] [PubMed] [Google Scholar]
- 97.Sakai K, Koike G, Seguchi K, Nakazato Y. Sinus histiocytosis with massive lymphadenopathy: a case of multiple dural involvement. Brain Tumor Pathol. 1998; 15: 63– 69. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt S, Eich G, Hanquinet S, Tschappeler H, Waibel P, Gudinchet F. Extra-osseous involvement of Langerhans’ cell histiocytosis in children. Pediatr Radiol. 2004; 34: 313– 321. [DOI] [PubMed] [Google Scholar]
- 99.Scumpia AJ, Frederic JA, Cohen AJ, Bania M, Hameed A, Xiao PQ. Isolated intracranial Rosai-Dorfman disease with orbital extension. J Clin Neurosci. 2009; 16: 1108– 1109. [DOI] [PubMed] [Google Scholar]
- 100.Seyednejad F, Tubbs RS, Shoja MM, Daghigi MH, Oakes WJ. Presumed recurrence of intracranial Rosai-Dorfman disease as a cervical spine tumor. Acta Neurochir. 2007; 149: 425– 427. [DOI] [PubMed] [Google Scholar]
- 101.Shaver EG, Rebsamen SL, Yachnis AT, Sutton LN. Isolated extranodal intracranial sinus histiocytosis in a 5-year-old boy. Case report. J Neurosurg. 1993; 79: 769– 773. [DOI] [PubMed] [Google Scholar]
- 102.Shuangshoti S, Jr, Navalitloha Y, Sukpanichnant S, Unhasuta C, Shuangshoti S. Central nervous system involvement in Rosai-Dorfman disease: Report of a case with a review of the literature. Neuropathology. 1999; 19: 341– 346. [Google Scholar]
- 103.Sundaram C, Uppin SG, Prasad BC, Sahu BP, Devi MU, Prasad VS, Purohit AK. Isolated Rosai Dorfman disease of the central nervous system presenting as dural-based and intraparenchymal lesions. Clin Neuropathol. 2005; 24: 112– 117. [PubMed] [Google Scholar]
- 104.Symss NP, Cugati G, Vasudevan MC, Ramamurthi R, Pande A. Intracranial Rosai Dorfman disease: report of three cases and literature review. Asian J Neurosurg. 2010; 5: 19– 30. [PMC free article] [PubMed] [Google Scholar]
- 105.Tanboon J, Chaipipat M, Wattanasirmkit V, Wongtabtim W, Shuangshoti S, Bunyaratavej K. Squash cytology of Rosai-Dorfman disease in the sellar region. Acta Cytol. 2003; 47: 1143– 1144. [PubMed] [Google Scholar]
- 106.Tavangar SM, Mahta A, Haghpanah V, Larijani B. Extranodal Rosai-Dorfman disease involving the meninges in a 79-year-old man. Ann Saudi Med. 2006; 26: 474– 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Theeler BJ, Keylock JB, Yoest SM. Teaching NeuroImage: isolated intracranial Rosai-Dorfman disease mimicking a meningioma. Neurology. 2008; 70: e42. [DOI] [PubMed] [Google Scholar]
- 108.Toh CH, Chen YL, Wong HF, Wei KC, Ng SH, Wan YL. Rosai-Dorfman disease with dural sinus invasion. Report of two cases. J Neurosurg. 2005; 102: 550– 554. [DOI] [PubMed] [Google Scholar]
- 109.Tomio R, Katayama M, Takenaka N, Imanishi T. Complications of surgical treatment of Rosai-Dorfman disease: a case report and review. Surg Neurol Int. 2012; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trudel M. Dural involvement in sinus histiocytosis with massive lymphadenopathy. Case report. J Neurosurg. 1984; 60: 850– 852. [DOI] [PubMed] [Google Scholar]
- 111.Tubbs RS, Kelly DR, Mroczek-Musulman EC, Hammers YA, Berkow RL, Oakes WJ, Grabb PA. Spinal cord compression as a result of Rosai-Dorfman disease of the upper cervical spine in a child. Childs Nerv Syst. 2005; 21: 951– 954. [DOI] [PubMed] [Google Scholar]
- 112.Ture U, Seker A, Bozkurt SU, Uneri C, Sav A, Pamir MN. Giant intracranial Rosai-Dorfman disease. J Clin Neurosci. 2004; 11: 563– 566. [DOI] [PubMed] [Google Scholar]
- 113.Udono H, Fukuyama K, Okamoto H, Tabuchi K. Rosai-Dorfman disease presenting multiple intracranial lesions with unique findings on magnetic resonance imaging. Case report. J Neurosurg. 1999; 91: 335– 339. [DOI] [PubMed] [Google Scholar]
- 114.Utikal J, Ugurel S, Kurzen H, Erben P, Reiter A, Hochhaus A, Nebe T, Hildenbrand R, Haberkorn U, Goerdt S, Schadendorf D. Imatinib as a treatment option for systemic non-Langerhans cell histiocytoses. Arch Dermatol. 2007; 143: 736– 740. [DOI] [PubMed] [Google Scholar]
- 115.Walker RN, Nickles TP, Lountzis NI, Jacobs DL, Nawaz NK. Rosai-Dorfman disease with massive intracranial involvement: asymmetric response to conservative therapy. J Neuroimaging. 2011; 21: 194– 196. [DOI] [PubMed] [Google Scholar]
- 116.Wang E, Anzai Y, Paulino A, Wong J. Rosai-Dorfman disease presenting with isolated bilateral orbital masses: report of two cases. AJNR Am J Neuroradiol. 2001; 22: 1386– 1388. [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Gao X, Tang W, Jiang C. Rosai-Dorfman disease isolated to the central nervous system: a report of six cases. Neuropathology. 2010; 30: 154– 158. [DOI] [PubMed] [Google Scholar]
- 118.Warrier R, Chauhan A, Jewan Y, Bansal S, Craver R. Rosai-Dorfman disease with central nervous system involvement. Clin Adv Hematol Oncol. 2012; 10: 196– 198. [PubMed] [Google Scholar]
- 119.Woodcock RJ, Jr, Mandell JW, Lipper MH. Sinus histiocytosis (Rosai-Dorfman disease) of the suprasellar region: MR imaging findings—a case report. Radiology. 1999; 213: 808– 810. [DOI] [PubMed] [Google Scholar]
- 120.Wrzolek MA, Zagzag D. May 2002: 38-year-old man and 69-year-old woman with dural based masses. Brain Pathol. 2002; 12: 517– 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu M, Anderson AE, Kahn LB. A report of intracranial Rosai-Dorfman disease with literature review. Ann Diagn Pathol. 2001; 5: 96– 102. [DOI] [PubMed] [Google Scholar]
- 122.XiaoWen D, XueBin X, YuQing Y, Ting L. Intracranial Rosai-Dorfman disease: case report and literature review. Eur J Radiol Extra. 2010; 76: 75– 78. [Google Scholar]
- 123.Yetiser S, Cekin E, Tosun F, Yildirim A. Rosai-Dorfman disease associated with neurosensorial hearing loss in two siblings. Int J Pediatr Otorhinolaryngol. 2004; 68: 1095– 1100. [DOI] [PubMed] [Google Scholar]
- 124.Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006; 202: 165– 170. [DOI] [PubMed] [Google Scholar]
- 125.Zhang JT, Tian HJ, Lang SY, Wang XQ. Primary intracerebral Rosai-Dorfman disease. J Clin Neurosci. 2010; 17: 1286– 1288. [DOI] [PubMed] [Google Scholar]
- 126.Zhu F, Zhang JT, Xing XW, Wang DJ, Zhu RY, Zhang Q, Wang HT, Lang SY. Rosai-Dorfman disease: a retrospective analysis of 13 cases. Am J Med Sci. 2013; 345: 200– 210. [DOI] [PubMed] [Google Scholar]
- 127.Zhu H, Qiu LH, Dou YF, Wu JS, Zhong P, Jiang CC, Xu R, Wang XQ. Imaging characteristics of Rosai-Dorfman disease in the central nervous system. Eur J Radiol. 2012; 81: 1265– 1272. [DOI] [PubMed] [Google Scholar]


