Abstract
Background
It has been shown that 14-3-3 σ serves as a tumor suppressor gene, and is downregulated in various tumor tissues. However, the role of 14-3-3 σ during the initiation and progression of lung squamous cell carcinoma (SqCC) is not well understood.
Methods
The expression status of 14-3-3 σ in archival tissue samples from 40 lung SqCC patients (36 with normal bronchia, 19 squamous metaplasia, and 17 dysplasia/carcinoma in situ, in their tissue samples) was examined by immunohistochemical analysis. The proliferation rate and tumor formation ability of the H520 cell transfected with 14-3-3 σ was tested with methyl thiazolyl tetrazolium assay and nude mice subcutaneous injection, respectively.
Results
In the normal bronchial epithelia, 14-3-3 σ was highly expressed, whereas it was significantly decreased in precancerous and cancerous tissues. Compared with matched invasive cancer tissues, the expression level of 14-3-3 σ in squamous metaplasia was significantly higher (P = 0.049), while that in dysplasia/carcinoma in situ showed no significant changes (P = 0.135). Statistical analysis showed that the expression level of 14-3-3 σ in tumor tissue was associated with the differentiation grade of the tumor (P = 0.001) and the prognosis of the patient (P = 0.003). The overexpression of 14-3-3 σ significantly suppressed the proliferation of H520 cells in vitro and in vivo.
Conclusion
The inactivation of 14-3-3 σ may be a very early event in tumorigenesis and could facilitate the initiation and progression of lung SqCC in a sustainable way.
Keywords: 14-3-3 σ, lung squamous cell carcinoma, precancerous lesion, prognosis
Introduction
Lung cancer is one of the most common causes of cancer death in the world.1 To conquer this fatal disease, we need to better understand the molecular events involved in lung carcinogenesis, especially those arising in early stages. Squamous cell carcinoma (SqCC) is a large subset of lung cancer. Similar to most epithelial malignancies, lung SqCC is believed to arise after a series of progressive histopathological and genetic changes.2 For example, the serial bronchial mucosal abnormalities (through squamous metaplasia to dysplasia and then carcinoma in situ [CIS]) could be precursors for invasive lung cancer.3 Knowledge about the sequence and multiple stepwise accumulations of genetic abnormalities associated with histopathological progression is essential to develop novel strategies for early detection and even the prevention of lung cancer.
The 14-3-3 family of proteins are highly conserved in eukaryotic organisms. In humans, this family consists of seven members: β, γ, ε, η, σ, τ, and ζ. These proteins serve as regulators or molecular supervisors in cells, thereby playing an important role in signal transduction, stress response, and malignant transformation, among others. Unlike other family members that are expressed in most tissues, 14-3-3 σ (also referred to as stratifin) is only expressed in epithelial cells. In normal epithelial cells, 14-3-3 σ is an important means for p53 to suppress tumorigenesis.4 When DNA damage occurs in a cell, p53 and p21 are activated immediately, cooperating to increase the expression level of 14-3-3 σ. 14-3-3 σ combines with cell division cycle protein 2 (cdc2)-cyclin, which results in cdc2-cyclin B1 remaining in the cytoplasm rather than entering the nucleus. The cell is thereby kept in the G2 phase instead of entering the M phase.5 The cells remaining in the G2 phase then have time for DNA repair, while those that cannot be repaired undergo apoptosis, thereby suppressing malignant cellular transformation.
Certain studies have shown that 14-3-3 σ, being a tumor suppressor gene, has lower expression levels in various tumor tissues, including oral squamous cell carcinoma, breast, lung, and other similar cancers, and that its low expression may result in a poorer clinical prognosis for certain types of tumor patients.6–13 However, the role of 14-3-3 σ during cancer initiation and progression is not well understood. The conclusions of these studies have often been inconsistent. According to Shiba-Ishii et al., for adenocarcinoma in situ of the lung, a type of small lung adenocarcinoma (2 cm in diameter or less), patients have an extremely favorable prognosis, and the expression level of 14-3-3 σ is lower than in invasive adenocarcinoma.14 Additionally, functional studies have shown that high expression levels of 14-3-3 σ may enhance the proliferative ability of lung adenocarcinoma cells. Studies of prostate cancer have shown that 14-3-3 σ is generally highly expressed in normal prostate epithelium, while in the prostatic intraepithelial neoplasia stage, the expression level of 14-3-3 σ begins to decrease significantly (i.e. 14-3-3 σ has poor or no expression in 90% of the samples).15 The situation is even more dramatic for invasive adenocarcinomas, in which 97% of the samples exhibit poor or no expression of 14-3-3 σ.
To characterize the expression level alterations of 14-3-3 σ associated with the initiation and progression of lung SqCC, the present study used immunohistochemical (IHC) analysis to investigate the expression of 14-3-3 σ in the tumor tissues of SqCC of the lung and related cell lines. Furthermore, the significance of 14-3-3 σ expression in stepwise tumorigenesis of human bronchial epithelium was evaluated, and the effect of 14-3-3 σ overexpression on cell proliferation and tumorigenesis was assayed in vitro and in vivo, respectively.
Methods
Patients and tissue samples
Formalin-fixed, paraffin-embedded (FFPE) tissue samples derived from 40 patients with primary SqCC of the lung were used for IHC analysis. All patients were surgically treated at the Liaoning Cancer Hospital & Institute, China, from June 2001 to December 2004, and none received any adjuvant systemic therapy. After surgery, patients were followed up for one to eight years, or until death. Most of the cases included normal bronchia (36 in 40 cases), and some contained precancerous lesions in addition to the invasive tumor in their tissue samples. All tissue sections were diagnosed and reconfirmed by two experienced pathologists independently, according to the World Health Organization/International Association for the Study of Lung Cancer classification system.2 Histopathological diagnoses for precancerous lesions were categorized as squamous metaplasia (19 in 40 cases) or dysplasia/CIS (17 in 40 cases). Representative images of the precancerous and cancerous lesions are shown by hematoxylin and eosin staining in Figure S1. The clinical features of all of the patients are presented in Table S1.
Immunohistochemistry analysis
Immunohistochemical staining was performed using a standard streptavidin-biotin-peroxidase complex method with a mouse monoclonal antibody against human 14-3-3 σ (1:100, Neomarkers, catalog #MS-1185-P, Rockford, IL, USA).16 Phosphate buffered saline was used, instead of a primary antibody against 14-3-3 σ, as a negative control. The nuclei were counter stained with hematoxylin. Two experienced pathologists independently scored immunostaining results. The immunoreactivity scoring (IRS) criteria was graded according to the following scale: 0, no staining; 1 +, weak diffuse cytoplasmic staining (with stronger staining in <25% of the cells); 2, moderate cytoplasmic staining in 25–50% of the cells; and 3, intense staining in >50% of the cells.16 The samples ranked as IRS 0 and 1 were defined as low, and IRS 2 and 3 were considered high expression.
Immunoblotting
The expression of 14-3-3σ in cell lines was examined by Western blotting probed with the antibody used in IHC and diluted 1:1000, using β-actin (Sigma, St. Louis, MO, USA) as a loading control.
Gene cloning and plasmid construction
14-3-3 σ full-length transcript was amplified from a pool of human lung cDNAs (primers: 5′-ATAAGCTTCCAGAGC-CATGGAGA-3′ and 5′-CACGTGGCTCTGGGGCTCCTG-3′) cloned in pcDNA3.1/mycHis(-)B (Invitrogen, Carlsbad, CA, USA).17
Cell lines, cell culture and transfection
The human lung cancer cell line H520 obtained from American Type Culture Collection (Manassas, VA, USA) was used in this study. The cells were grown in RPMI 1640 medium (Life Technologies, Grand Island, NY, USA) containing 10% fetal calf serum and maintained in a humidified incubator with 5% CO2 at 37°C. Plasmid transfection was performed using Lipofectamine 2000 (Invitrogen). 14-3-3 σ in pcDNA3.1/mycHis(-)B, or the empty vector were transfected into H520 (hereafter referred to as H520-14-3-3 σ and H520-vector, respectively). The stable clones were selected with 800 μg/mL geneticin (Invitrogen). Clones expressing the highest levels of 14-3-3 σ were chosen for subsequent experiments.
Cell proliferation assay
Cell growth rate was measured by the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using the CellTiter 96 Non-Radioactive Cell Kit (Promega, Madison, WI, USA). The assay was performed at 24-hour intervals for seven days, as previously described.18 The cells were dislodged by incubation with trypsin-ethylenediaminetetraacetic acid and counted each day with a hemocytometer. The trypan blue dye (Sigma-Aldrich, St Louis, MO, USA) exclusion method was used to identify viable cells. This experiment was repeated three times.
Tumorigenicity in nude mice
Female BALB/c athymic mice (nu/nu, 4 weeks old, n = 10) were housed under aseptic conditions and fed in accordance with the guidelines of the Animal Center of the Liaoning Cancer Institute. Mice were subcutaneously injected with H520-14-3-3σ (n = 5 mice) and H520-vector (n = 5 mice) (5 × 106 cells per mouse).
Statistical analysis
All calculations were performed with SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA). The statistical analysis of IHC data was performed using a Mann-Whitney U test for comparison of two independent groups, a Kruskal-Wallis test for comparison among more than two groups, and a Wilcoxon signed rank test for the paired comparison of IRS. Spearman’s rank-order correlation was applied to reveal the association between the expression level of 14-3-3 σ and the clinical features of the patients. Survival curves were determined by the Kaplan–Meier method, and examined by the log-rank test. All comparisons were two tailed, and P < 0.05 was considered to be significant.
Results
Expression of 14-3-3 σ in the tissue samples derived from lung cancer patients
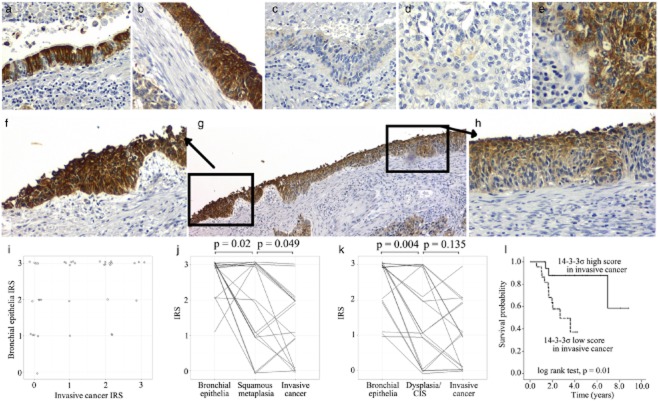
The IHC staining of 14-3-3 σ was detected predominantly in the cytoplasm, as shown in Figure 1a-h. The expression status of 14-3-3 σ in cancerous or precancerous tissues is summarized in Table 1, and the detailed IRS of each patient is listed in Table S1. Overall, with the progress of pathological changes, the expression level of 14-3-3 σ was significantly reduced (Kruskal-Wallis test, P < 0.001). Compared to the matched bronchial epithelia (IRS mean 2.33 ± 0.89), a significantly lower expression of 14-3-3 σ was observed in invasive cancer tissues (Wilcoxon signed ranks test, P < 0.001, IRS mean 1.17 ± 1.06). Figure 1i indicates the relationship between the tumor tissue IRS and the matched bronchial epithelia. In 19 samples with squamous metaplasia, a Wilcoxon signed rank test showed that the expression level of 14-3-3 σ was significantly higher in bronchial epithelia (IRS mean 2.74 ± 0.56) than in squamous metaplasia (IRS mean 1.89 ± 1.20; P = 0.02, Fig 1j) on the same slice and was significantly higher in squamous metaplasia than in invasive cancer (IRS mean 1.37 ± 1.01; P = 0.049). In 17 samples with dysplasia/CIS found on their slices, a Wilcoxon signed rank test showed that the expression level of 14-3-3 σ was significantly higher in bronchial epithelia (IRS mean 2.50 ± 0.75) than both in dysplasia/CIS (IRS mean 1.44 ± 1.12) (P = 0.004) and in invasive cancer (IRS mean 1.00 ± 0.97); there was no significant difference between the latter two (P = 0.135). Figure 1f-h illustrates the stepwise precancerous lesions derived from a patient with lung SqCC, in which it can be seen that the expression of 14-3-3 σ decreases from squamous metaplasia (Fig. 1f) to dysplasia (Fig. 1h).
Figure 1.
The 14-3-3 σ expression status in precancerous and cancerous lesions of patients with lung squamous cell carcinoma (SqCC) and its prognostic value. Expression of 14-3-3 σ observed by immunohistochemical (IHC) staining for tissues with (a) normal bronchial epithelia; (b) squamous metaplasia; (c) dysplasia; and (d-e) invasive cancer. (f-h) 14-3-3 σ IHC staining on the tissue section containing the stepwise precancerous lesions derived from a patient with lung SqCC. (f) Squamous metaplasia; (h) dysplasia; (g) original image of (f) and (h). (i) The relationship between immunoreactivity scoring (IRS) of invasive cancer (x-axis) and normal bronchial epithelia (y-axis) in the same patient. Each patient was depicted with a circle. In order to avoid over-plotting, small random disturbance was added to the real value. (j) and (k) The relationship among IRS of normal bronchial epithelia, (j) squamous metaplasia or (k) dysplasia/carcinoma in situ, and invasive cancer of the same patients. In these panels, each line represented a patient, with small random disturbance added to the y-axis to avoid over-plotting. (l) Survival analysis of 40 patients with lung SqCC stratified by the IRS of their invasive cancer. 14-3-3 σ high score, IRS 2–3; 14-3-3 σ low score, IRS 0–1.
Table 1.
Immunohistochemical staining for 14-3-3σ in different histological types of tissues
| Histological classification | Sample No. | 14-3-3 σ IRS (%) | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Bronchial epithelia | 36 | 1 (2.8) | 7 (19.4) | 7 (19.4) | 31 (58.4) | P < 0.001† |
| Squamous metaplasia | 19 | 3 (15.8) | 5 (26.2) | 2 (10.5) | 9 (47.5) | |
| Dysplasia/carcinoma in situ | 18 | 5 (27.8) | 5 (27.8) | 3 (16.6) | 5 (27.8) | |
| Invasive cancer | 40 | 14 (35.0) | 8 (20.0) | 11 (27.5) | 7 (17.5) | |
Bronchial epithelia versus squamous metaplasia versus dysplasia/carcinoma in situ versus invasive cancer (P-value from Kruskal-Wallis test). IRS, immunoreactivity scoring.
14-3-3 σ expression is associated with tumor grade
As shown in Table 2, the Kruskal-Wallis test revealed a significant difference between the 14-3-3 σ IRS of the tumors displaying different degrees of differentiation (P = 0.001). The mean value of 14-3-3 σ IRS for the well, moderately, and poorly differentiated tumors was 2.71 ± 0.49, 1.11 ± 0.96, and 0.80 ± 1.01, respectively. The Spearman’s rank-order correlation statistics revealed a significantly negative association between the 14-3-3 σ IRS and differentiation grade of the tumors (rs = (0.506, P = 0.001). However, the association between the 14-3-3 σ expression level and the stage of lung SqCC patients was not significant (P = 0.858).
Table 2.
Correlations between 14-3-3σ expression in cancer tissues and the clinicopathologic variables of the patients
| Clinical variables | Sample No. | 14-3-3 σ IRS in cancer tissue (%) | P-value | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Stage | P = 0.855† | |||||
| I | 20 | 6 (30.0) | 5 (25.0) | 5 (25.0) | 3 (15.0) | |
| II-III | 20 | 8 (40.0) | 3 (15.0) | 6 (30.0) | 4 (20.0) | |
| Grade | P = 0.001‡ | |||||
| Well | 7 | 0 (0) | 0 (0) | 2 (28.6) | 5 (71.4) | |
| Moderate | 18 | 6 (33.3) | 5 (27.8) | 6 (33.3) | 1 (5.6) | |
| Poor | 15 | 8 (53.3) | 3 (20.0) | 3 (20.0) | 1 (6.7) | |
| Risk | P = 0.003§ | |||||
| Low risk | 13 | 1 (7.7) | 2 (15.4) | 6 (46.1) | 4 (30.8) | |
| High risk | 11 | 5 (45.5) | 4 (36.4) | 2 (18.1) | 0 (0) | |
Stage, I versus II-III (P-value from Mann-Whitney U test).
Differentiation grade, well versus moderate versus poor (P-value from Kruskal-Wallis test).
Risk, low risk versus high risk (P-value from Mann-Whitney U test). IRS, immunoreactivity scoring.
Association of low levels of 14-3-3 σ protein in tumor tissue from patients with poor prognosis
Patients who survived longer than four years (n = 13), and those who expired within 2.5 years after surgery (n = 11) were defined as patients with low and high-risk disease, respectively. The expression levels of 14-3-3 σ in tumor tissues were significantly different between these two groups of patients (Mann-Whitney U test, P = 0.003) with a mean IRS value for low and high risk patients of 2 ± 0.91 and 0.64 ± 0.81, respectively. Furthermore, survival analysis revealed that the patients with low IHC scores (IRS = 0 or 1) had a significantly unfavorable prognosis compared with those with high IHC scores (IRS = 2 or 3) (P = 0.01, as shown in Fig. 1l).
Proliferation and tumorigenicity inhibition properties of 14-3-3 σ
We established H520-14-3-3 σ, the clone of H520 that stably expresses 14-3-3 σ (Fig. 2a). H520-14-3-3 σ grew slower than the vector-transfected control cells, as determined by MTT assay (Fig. 2b). The difference of the mean OD (570–630 nm) of H520-14-3-3 σ and that of the H520-vector at the seventh day was 0.56 (1.18 vs. 1.74), with a Mann-Whitney rank sum test showing significance (P < 0.001). To further test the tumorigenic inhibition potential of 14-3-3 σ in vivo, H520-14-3-3 σ and H520-vector cells were subcutaneously injected into nude mice. Three days later, neoplasms were observed in all of the control mice (n = 5), but only in two of the mice injected with H520-14-3-3 σ (n = 5). Tumors were weighed 30 days after the injection. The tumor weights of the H520-14-3-3 σ and H520-vector groups were significantly different (Student t-test, P = 0.005), with mean values of 0.389 g and 0.695 g, respectively (Fig. 2c).
Figure 2.
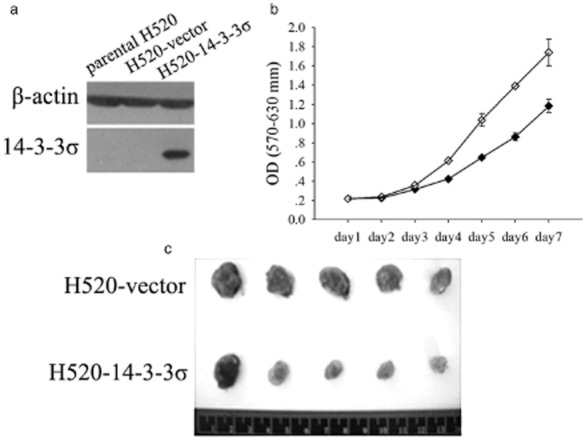
Overexpression of 14-3-3 σ inhibited the proliferation and tumorigenicity of squamous cell carcinoma (SqCC). (a) Immunoblot of untransfected H520 cells (parental H520), H520 cells transfected with empty vector (H520-vector), and H520 cells transfected with 14-3-3 σ (H520-14-3-3 σ). Blots were probed with monoclonal mouse β-actin antibody to control for protein loading and transfer. (b) The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of H520 cell clones in (a), except for parental H520. Means and 95% confidence intervals (error bars, calculated from the 8 replicates) are shown. (c) Tumors in nude mice inoculated with H520 colonies stably transfected with vector or 14-3-3 σ.  , H520-14-3-3 σ;
, H520-14-3-3 σ;  , H520-vector.
, H520-vector.
Discussion
As an important regulator involved in processes such as cell cycle regulation, apoptosis and the like, 14-3-3 σ is considered the most tumor-related member of the 14-3-3 family, and may serve as a tumor suppressor gene by suppressing the genesis/development of malignant tumors.19 In the present study, it was shown that 14-3-3 σ has no or low expression in the tumor tissues of most lung SqCC patients (IRS = 0 or 1, n = 22 out of 40). Patients with no/low expression of 14-3-3 σ in their tumor tissues are inferior to other patients (IRS = 2 or 3) in terms of both tumor differentiation grade and clinical prognosis, indicating that the absence of 14-3-3 σ may cause the biological behavior of the tumor tissues in these patients to be more aggressive. In vitro experiments have confirmed that 14-3-3 σ is capable of suppressing the proliferative capacity of lung cancer cell lines, and in vivo experiments have further confirmed the tumor-suppressing power of 14-3-3 σ.
More importantly, we investigated sequential precancerous lesions available from the same group of patients with SqCC. Statistical analysis with respect to the tissue sections containing the stepwise precancerous lesions has shown a significantly higher expression of 14-3-3 σ in normal epithelium than in squamous metaplasia and in dysplasia/CIS. For both the latter cases, the expression level of 14-3-3 σ in squamous metaplasia is significantly higher than in invasive cancer; while as dysplasia/CIS progresses to the invasive stage, 14-3-3 σ has no significant changes. This indicates that the inactivation of 14-3-3 σ may be a very early event in the tumorigenesis of lung SqCC, which occurs even before bronchial epithelial cells show atypia.
14-3-3 σ is downregulated in various human malignancies, including lung SqCC, as shown in our work. Moreover, Shiba-Ishii et al. reported a significantly higher expression of 14-3-3 σ in lung adenocarcinoma than in lung adenocarcinoma in situ.14 They also indicated that 14-3-3 σ facilitates the cell proliferation capacity of lung adenocarcinoma. Osada et al. found a different DNA hypermethlation frequency in small cell and non-small cell lung cancer cell lines.9 These results suggest that 14-3-3 σ might be a context-dependent gene, and its involvement in lung tumorigenesis might be in a histological type-specific manner.9,14
Conclusion
Data derived from this study have shown that the aberrant expression of 14-3-3 σ may play an important role, not only in progression of SqCC, but also in malignant transformation of respiratory epithelium. It might serve as a biomarker for an early warning of lung tumorigenesis, and reversing the 14-3-3 σ protein expression level might become a potential therapeutic method in the future.
Acknowledgments
This research was supported by grants from the Natural Science Foundation of Liaoning Province (20102120), the Key Scientific and Technological Project of Shenyang City (F10-149-9-40), and the Liaoning BaiQianWan Talents Program (20102120). We thank Dr. Yi Ren, Liang Zhang, Jijia Li, Zhuang Tong, Dan Yang and Xue Qiao for their logistical support of this study.
Disclosure
No authors report any conflict of interest.
Supporting Information
Additional Supporting Informationmay be found in the online version of this article at the publisher's website:
Figure S1 Hematoxylin and eosin staining of the precancerous and cancerous lesions, also shown in Figure 1. (a) Dysplasia/carcinoma in situ; (b) squamous metaplasia; (c) invasive cancer.
Table S1 Clinical information and 14-3-3 σ immunoreactivity scoring of enrolled patients.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004. [Google Scholar]
- Meert AP, Verdebout JM, Martin B, Ninane V, Feoli F, Sculier JP. Epidermal growth factor receptor expression in pre-invasive and early invasive bronchial lesions. Eur Respir J. 2003;21:611–615. doi: 10.1183/09031936.03.00064902. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Lodygin D, Hermeking H. The role of epigenetic inactivation of 14-3-3sigma in human cancer. Cell Res. 2005;15:237–246. doi: 10.1038/sj.cr.7290292. [DOI] [PubMed] [Google Scholar]
- Bhawal UK, Sugiyama M, Nomura Y, Kuniyasu H, Tsukinoki K. Loss of 14-3-3 sigma protein expression and presence of human papillomavirus type 16 E6 in oral squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:1055–1059. doi: 10.1001/archotol.134.10.1055. [DOI] [PubMed] [Google Scholar]
- Luo J, Feng J, Lu J, et al. Aberrant methylation profile of 14-3-3 sigma and its reduced transcription/expression levels in Chinese sporadic female breast carcinogenesis. Med Oncol. 2010;27:791–797. doi: 10.1007/s12032-009-9287-8. [DOI] [PubMed] [Google Scholar]
- Osada H, Tatematsu Y, Yatabe Y, et al. Frequent and histological type-specific inactivation of 14-3-3sigma in human lung cancers. Oncogene. 2002;21:2418–2424. doi: 10.1038/sj.onc.1205303. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki T, Akahira J, et al. 14-3-3sigma in endometrial cancer – a possible prognostic marker in early-stage cancer. Clin Cancer Res. 2005;11:7384–7391. doi: 10.1158/1078-0432.CCR-05-0187. [DOI] [PubMed] [Google Scholar]
- Ravi D, Chen Y, Karia B, et al. 14-3-3 sigma expression effects G2/M response to oxygen and correlates with ovarian cancer metastasis. PLoS One. 2011;6(1):e15864. doi: 10.1371/journal.pone.0015864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Trope CG, Suo Z, et al. The clinicopathological and prognostic impact of 14-3-3 sigma expression on vulvar squamous cell carcinomas. BMC Cancer. 2008;8:308. doi: 10.1186/1471-2407-8-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Liu CZ, Tao LY, et al. The clinicopathological and prognostic impact of 14-3-3 protein isoforms expression in human cholangiocarcinoma by immunohistochemistry. Asian Pac J Cancer Prev. 2012;13:1253–1259. doi: 10.7314/apjcp.2012.13.4.1253. [DOI] [PubMed] [Google Scholar]
- Shiba-Ishii A, Kano J, Morishita Y, Sato Y, Minami Y, Noguchi M. High expression of stratifin is a universal abnormality during the course of malignant progression of early-stage lung adenocarcinoma. Int J Cancer. 2011;129:2445–2453. doi: 10.1002/ijc.25907. [DOI] [PubMed] [Google Scholar]
- Cheng L, Pan CX, Zhang JT, et al. Loss of 14-3-3sigma in prostate cancer and its precursors. Clin Cancer Res. 2004;10:3064–3068. doi: 10.1158/1078-0432.ccr-03-0652. [DOI] [PubMed] [Google Scholar]
- Li M, Xiao T, Zhang Y, et al. Prognostic significance of matrix metalloproteinase-1 levels in peripheral plasma and tumour tissues of lung cancer patients. Lung Cancer. 2010;69:341–347. doi: 10.1016/j.lungcan.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Neupane D, Korc M. 14-3-3sigma modulates pancreatic cancer cell survival and invasiveness. Clin Cancer Res. 2008;14:7614–7623. doi: 10.1158/1078-0432.CCR-08-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ma J, Zheng H, et al. Overexpression of OLC1, cigarette smoke, and human lung tumorigenesis. J Natl Cancer Inst. 2008;100:1592–1605. doi: 10.1093/jnci/djn379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Wen YY, Chen CH, Lozano G, Lee MH. 14-3-3 sigma positively regulates p53 and suppresses tumor growth. Mol Cell Biol. 2003;23:7096–7107. doi: 10.1128/MCB.23.20.7096-7107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Hematoxylin and eosin staining of the precancerous and cancerous lesions, also shown in Figure 1. (a) Dysplasia/carcinoma in situ; (b) squamous metaplasia; (c) invasive cancer.
Table S1 Clinical information and 14-3-3 σ immunoreactivity scoring of enrolled patients.