Abstract
Background
Central airway obstruction related to endobronchial malignancy is one of the most difficult oncological complications and requires efficient palliative intervention.
Methods
Fifty-three consecutive patients with unresectable endobronchial malignancy receiving bronchoscopic cryotherapy as palliative treatment were retrospectively reviewed. Efficiency was evaluated by the improvement of performance status (PS), and the best achievement of tumor removal was assessed as complete or partial removal.
Result
Patients’ PS after cryotherapeutic tumor removal improved from the baseline PS (P = 0.006). In multivariate logistic regression analysis, the compression part of the tumor (odds ratio [OR] 0.42; 95% confidence interval [CI] 0.23∼0.75, P = 0.004) and the thin tumor stalk (OR 87.86; 95% CI 2.31∼3337.37, P = 0.016) were independent predictors of complete tumor removal. Tumors larger than 9.3 cm, including compression and invasion parts, had the highest odds of being only partially removed (positive predictive value [PPV]: 88.2%, likelihood ratio [LR]+: 10.49); tumors smaller than 9.3 cm were likely to be completely removed (negative predictive value [NPV]: 80.6%, LR−: 0.34). After cryotherapy, re-obstruction was significantly associated with non-squamous cell carcinoma (65.7 vs. 16.7%, P = 0.001) and patients who had longer overall survival (11.7 vs. 1.5 months, P < 0.001). Odds of tumor re-obstruction increased 2.28-fold (PPV: 81.6%, LR+: 2.28) beyond two months; the odds decreased by 81% (NPV: 73.3%, LR−: 0.19) within two months.
Conclusion
Debulking of a tumor using cryotherapy is a useful palliative treatment for endobronchial obstruction secondary to a variety of malignancies.
Keywords: Bronchoscopic, cryotherapy, endobronchial, malignancy
Introduction
Endobronchial malignancy associated with central airway obstruction is one of the most difficult oncological complications and often results in severe respiratory symptoms. Regardless of where the primary tumor originates, those who suffer from central airway invasion or metastasis from the primary tumor often experience cough, hemoptysis, and respiratory distress that requires efficient palliative intervention.1–3
Effective palliative practices involve laser ablation, photodynamic therapy, and brachytherapy; each has its advantages and limitations. Bronchoscopy-assisted cryotherapy featuring tumor removal via a rapid freezing technique serves as another option. Previous reports of cryotherapeutic palliation for lung cancer with central airway obstruction reported positive outcomes in terms of improvement in the patients’ pulmonary function and an increase in distance in the six-minute walking test.4,5 However, this improvement applies to a select population that can tolerate exercise assessment. Whether it also applies to other populations that involve a wider range of disease situations, in terms of different performance status (PS) and diversity of tumor types, is not well understood.
The development of central airway obstruction as an advanced oncological complication usually presents a course of progressive involvement. Most tumors affect the patients’ respiratory condition initially through external compression of the lumen. Lumen invasion subsequently ensues, imposing an increasing respiratory burden via progressive obstruction.6–8 The greater scale of the tumor burden, in terms of the compression and invasion parts, is frequently associated with a worse PS and an increasing difficulty of performing bronchoscopic cryotherapy. In line with this point, how the tumor burden affects the achievement of cryotherapeutic tumor removal is less clearly understood. Most of the existing studies have elaborated the association of successful cryotherapeutic tumor removal and improved quality of life; however, there is a gap in the knowledge regarding the predictive factors that influence the achievement of this goal.4,5
As cryotherapy is essentially an intervention of a palliative nature, the sustainability of tumor removal through its use is a key concern. Some patients that suffer from malignancy-related central airway obstruction do not succumb quickly to their underlying disease. Therefore, questions regarding the factors associated with tumor re-obstruction and at what point after cryotherapy clinicians should raise the concern of re-obstruction remain, issues that are inadequately addressed in previous reports.
In this study, we investigated the efficacy of cryotherapeutic tumor removal as a palliative treatment for malignant endobronchial obstruction in a patient cohort. The clinical factors associated with the achievement of this goal are specifically evaluated and the variables associated with tumor re-obstruction are also addressed.
Materials and methods
Inclusion of the study population
Between 2007 and 2011, consecutive patients with unresectable endobronchial malignancy who received bronchoscopic cryotherapy as a part of palliative treatment were retrospectively reviewed. This study was conducted at the Chang Gung Memorial Hospital, a university-affiliated hospital in Taiwan; the hospital’s institutional review board independently approved the study (IRB: 100-3211B). All patients provided informed consent prior to bronchoscopic cryotherapy.
Endobronchial tumor assessment
Chest contrast-enhanced computed tomography (CT) with 5 mm collimation, including transverse and coronal planes, was performed on all subjects to assess the target tumor directly causing the airway obstruction. The components of the relationship between the target tumor and the airway were identified as the compressive part outside the airway and the invasive part inside the airway. The long axis under the transverse plane of the chest CT was used to measure the compressive part of the tumor. The invasive part of the tumor was measured by serial examination of transverse plans to the segment of obstruction and assisted by direct measurement on bronchoscopy whenever possible (Fig 1). Tumor obstruction of the airway to the extent that the flexible bronchoscope was unable to pass through was defined as total obstruction; otherwise, the extent was recorded as partial obstruction.
Figure 1.

The measurement of the long-axis of the compressive part is indicated by the black double-head arrow, while the yellow arrow indicates a thin tumor stalk affixing to the trachea (a) Tracing up the segment of tracheal obstruction on transverse planes reveals the tip of the invasive part as a representative image (b, yellow arrow head), in this case three planes equivalent to 1.5 cm. (c) Direct measurement of the invasive part could be assisted by bronchoscopy from the root of the tumor stalk in this case, as shown by the blue arrow. (d) The invasive part of the tumor (blue arrowhead) was still free from recurrence on the bronchoscopy follow-up at four weeks after tumor removal.
Bronchoscopic cryotherapy technique
A cryo-probe (ERBOKRYO CA, Erbe, Marietta, GA, USA) with carbon dioxide as cryogen was used with a temperature of approximately −70°C achieved at the probe tip. The probe was inserted via the bronchoscope so as to have direct contact with the mass. Cryotherapy was started with −70°C CO2 from 20 to 60 seconds at the lesion site, and the mass was detached outward by the bronchoscope. Whenever patients experienced desaturation, intractable bleeding or severe cough and were unable to tolerate the practice, the operator terminated the procedure. If the tumor was not completely removed in a single procedure, the operator clinically evaluated subsequent procedures based on each patient’s condition and tolerance. The best achievement of tumor removal was recorded as complete removal, with the airway totally free from tumor obstruction; otherwise, the best achievement was recorded as partial removal. All study patients underwent a sedation procedure that was performed with intravenous midazolam or propofol with a bispectral index for consciousness monitoring.9,10 Local anesthesia with 2% xylocaine solution was also routinely administered during bronchoscopy.
Assessment of efficacy and outcome
Change in an individual’s Eastern Cooperative Oncology Group (ECOG) performance status (PS) was used to evaluate the efficacy of palliation. Patients’ PS at baseline and after tumor removal was recorded for this assessment. After intervention, all study patients underwent follow-up with a chest CT scan every eight weeks or bronchoscopy study as needed, whenever they experienced symptoms associated with suspected endobronchial tumor progression. Individuals with documented tumor progression from their previous best achievement of tumor removal were defined as having tumor re-obstruction. Overall survival (OS) was calculated from the day of cryotherapeutic tumor removal.
Statistical analysis
All group variables were analyzed by Fisher’s exact test with contingency tables, and the Mann-Whitney U test was used for analysis of continuous variables. Paired ranked data were analyzed with the Wilcoxon matched-pairs signed-rank test. The receiver operating characteristic (ROC) curve was used to determine the performance of binary classifiers, and diagnostic performance was calculated by standard definition. All analyses were conducted using SSPS software (SPSS v. 13.0; SPSS Inc., Chicago, IL, USA) and all reported P values were 2-sided, with a P value <0.05 considered statistically significant.
Results
Baseline characteristics of study subjects
Fifty-three patients with unresectable endobronchial malignancy were included in the analysis (Table 1). The median age was 67 years, and 39 (73.6%) of the patients were male. Thirty-seven (69.8%) patients had primary lung cancer and 16 (30.2%) had metastasis; 30 (56.6%) patients had an ECOG PS of 0–1 and 23 (43.4%) had an ECOG PS of 2–3. A histology of squamous cell carcinoma was noted in 27 (50.9%) patients; 10 (18.9%) had adenocarcinoma; six (11.3%) non-small cell carcinoma not otherwise specified; three (5.7%) small cell carcinoma; four (7.5%) sarcoma; one (1.9%) renal cell carcinoma; one (1.9%) lymphoma; and one (1.9%) had endometrial carcinoma. The median size of the compression and invasion part of the tumor was 5.5 cm and 2.0 cm, respectively. Nine (17.0%) patients had a tumor in the trachea, 28 (52.8%) in the main bronchi, and 16 (30.2%) in the lobar bronchi. After tumor removal, chemotherapy was given as a platinum-based doublet 25 (47.2%) or singlet 12 (22.6%) regimen, and 16 (30.2%) patients received best supportive care alone. The median OS for the study cohort was 13.0 (9.5–18.8) months.
Table 1.
Baseline patient characteristics
| Variables | No, (%) |
|---|---|
| Age, year, median (range) | 67 (49.5–76.0) |
| Male gender | 39 (73.6) |
| Baseline ECOG PS | |
| 0–1 | 30 (56.6) |
| 2–3 | 23 (43.4) |
| Histology | |
| Squamous cell carcinoma | 27 (50.9) |
| Adenocarcinoma | 10 (18.9) |
| NSCLC-NOS | 6 (11.3) |
| Small cell carcinoma | 3 (5.7) |
| Sarcoma | 4 (7.5) |
| Renal cell carcinoma | 1 (1.9) |
| Lymphoma | 1 (1.9) |
| Endometrial carcinoma | 1 (1.9) |
| Tumor origin | |
| Primary lung cancer | 37 (69.8) |
| Metastasis | 16 (30.2) |
| Tumor size, cm, median (range) | |
| Compression part | 5.5 (4.1–7.2) |
| Invasion part | 2.0 (1.5–3.0) |
| Site of tumor | |
| Trachea | 9 (17.0) |
| Main bronchi | 28 (52.8) |
| Lobar bronchi | 16 (30.2) |
| Chemotherapy | |
| Platinum-based doublet | 25 (47.2) |
| Singlet | 12 (22.6) |
| BSC alone | 16 (30.2) |
| Overall survival | 13.0 (9.5–18.8) |
| Total patients | 53 (100) |
BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC-NOS, non-small cell lung cancer not otherwise specified.
Efficacy of cryotherapeutic tumor removal
Each patient’s ECOG PS at baseline and after cryotherapeutic tumor removal was analyzed by the Wilcoxon matched-pairs signed-rank test. The results showed that in three of 13 (23.1%) patients the ECOG PS of 3 improved to 2, in five of 10 (50.0%) patients the ECOG PS of 2 improved to 1, and no change in ECOG PS was seen for 30 patients whose baseline ECOG PS was 1 (Fig 2a, P = 0.006). Among patients whose tumor was totally removed, in three of seven (42.9%) patients the ECOG PS was 3 improved to 2, and in two of five (40.0%) the ECOG PS was 2 improved to 1 (Fig 2b, P = 0.037). Among those whose best achievement of tumor removal was partial, six patients’ ECOG PS of 3 remained unchanged, and three of five patients’ (60.0%) ECOG PS of 2 improved to 1 (Fig 2c, P = 0.149).
Figure 2.

Change in performance status (PS) is illustrated by the number of subjects with each PS at baseline and after cryotherapy. (a) In all patients, cryotherapy improved PS compared to baseline PS (P = 0.006). (b) Improvement in PS after cryotherapy was significant for individuals experiencing total tumor removal (P = 0.037). (c) Improvement in PS for individuals experiencing partial removal was not significant (P = 0.149). ECOG: Eastern Cooperative Oncology Group. P-value was calculated using the Wilcoxon matched-pairs signed-ranks test. (a)  , N = 10;
, N = 10;  , N = 3;
, N = 3;  , N = 5;
, N = 5;  , N =
, N =  5, N = 30; (b)
5, N = 30; (b)  , N = 4;
, N = 4;  , N = 3;
, N = 3;  , N = 3;
, N = 3;  , N = 2;
, N = 2;  , N = 19; (c)
, N = 19; (c)  , N = 6;
, N = 6;  , N = 2;
, N = 2;  , N = 3;
, N = 3;  , N = 11.
, N = 11.
Factors associated with the achievement of tumor removal
Variables associated with complete or partial removal of the endobronchial tumor were analyzed (Table 2). In univariate analysis, factors significantly associated with complete tumor removal were the smaller compression (4.3 vs. 7.2 cm, P < 0.001) and invasive parts of the tumors (1.9 vs. 3.0 cm, P = 0.003), the thin tumor stalk (38.7 vs. 4.5%, P = 0.008), and partial obstruction of the airway (38.7 vs. 9.1%, P = 0.025). In the multivariate logistic regression model, the compression part of the tumor (odds ratio [OR] 0.42; 95% confidence interval [CI], 0.23∼0.75, P = 0.004) and thin tumor stalk (OR 87.86; 95% CI, 2.31∼3337.37, P = 0.016) were independent predictors, but the invasive part of the tumor (ORs 0.41; 95% CI, 0.15∼1.08, P = 0.070) had a tendency toward significance (Table 3).
Table 2.
Predictive factors associated with complete or partial tumor removal
| Variables | Complete | Partial | P-value |
|---|---|---|---|
| No. (%) n = 31 | No. (%) n = 22 | ||
| Age, year, median (range) | 61 (49.0–72.0) | 71 (48.8–77.0) | 0.376* |
| Male gender | 22 (71.0) | 17 (77.3) | 0.755 |
| Baseline ECOG PS | |||
| 0–1 | 19 (61.3) | 11 (50.0) | |
| 2–3 | 12 (38.7) | 11 (50.0) | 0.574 |
| Tumor origin | |||
| Primary lung cancer | 13 (41.9) | 8 (36.4) | |
| Metastasis | 18 (58.1) | 14 (63.6) | 0.779 |
| Site of tumor | |||
| Trachea | 8 (25.8) | 1 (4.5) | |
| Non-trachea | 23 (74.2) | 21 (95.5) | 0.064 |
| Main carina invasion | |||
| Yes | 5 (16.1) | 8 (36.4) | |
| No | 26 (83.9) | 14 (63.6) | 0.114 |
| Tumor size, cm, median (range) | |||
| Compression part | 4.3 (3.5–6.1) | 7.2 (5.5–8.7) | <0.001* |
| Invasion part | 1.9 (1.5–2.2) | 3.0 (2.0–4.0) | 0.003* |
| Tumor stalk | |||
| Thin stalk | 12 (38.7) | 1 (4.5) | |
| Broad base | 19 (61.3) | 21 (95.5) | 0.008 |
| Extent of obstruction | |||
| Complete | 19 (61.3) | 20 (90.9) | |
| Partial | 12 (38.7) | 2 (9.1) | 0.025 |
| Presence of lung collapse | |||
| Yes | 15 (48.4) | 18 (81.8) | |
| No | 16 (51.6) | 4 (18.2) | 0.021 |
Data analyzed using Mann-Whitney U test. ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 3.
Logistic regression analysis of the factors associated with complete tumor removal
| Variables | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Tumor size (cm) | |||
| Compression part | 0.42 | 0.23–0.75 | 0.004 |
| Invasion part | 0.41 | 0.15–1.08 | 0.070 |
| Tumor stalk | |||
| Thin stalk | 87.86 | 2.31–3337.37 | 0.016 |
Tumor size as discriminator between complete and partial tumor removal
The ROC curve and its area under the curve (AUC) were constructed using tumor size to discriminate between complete and partial tumor removal. Discriminatory power in terms of the compression (AUC: 0.80; 95% CI, 0.68∼0.92, P = 0.001), and invasive parts of the tumor (AUC: 0.74; 95% CI, 0.60∼0.88, P = 0.003), and the summation of both (AUC: 0.85; 95% CI, 0.74∼0.96, P < 0.001), was significant based on AUC measurement (Fig 3a). A standard analysis of the predictive performance at different cut-off levels using the summation of the compression and invasion parts of the tumors is summarized in Table 4. For a summation greater than 9.3 cm, the odds of having partial tumor removal as the best achievement increased 10.49-fold (sensitivity: 68.2%, likelihood ratio [LR]+: 10.49, positive predictive value [PPV]: 88.2%), but a summation of less than 9.3 cm decreased these odds by 66% (specificity: 93.5%, LR−:0.34, negative predictive value [NPV]: 80.6%). This specific cut-off is demonstrated in a plot in which the summation of the compression and invasion parts of the tumors was compared between the patients having complete and partial tumor removal as their best achievement (Fig 3b).
Figure 3.
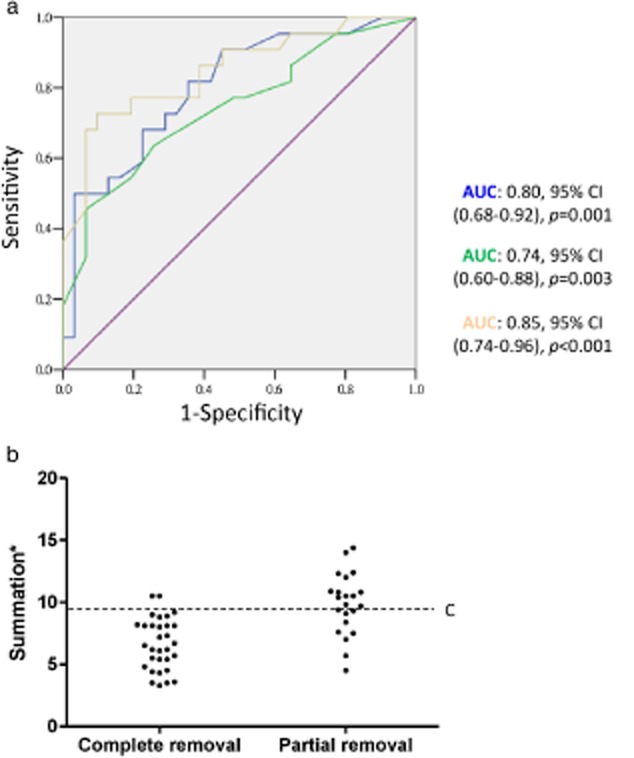
(a) The receiver operating characteristic curve is plotted using tumor size, in terms of the invasion part (blue), compression part (green), and summation of both (yellow) to discriminate the best achievement of tumor removal. Discriminatory power was best using a summation of both based on area under the curve (AUC) measurement. (b) The distribution of tumor size with respect to the best achievement of tumor removal as complete or partial removal. The cut-off (C) represents 9.3 cm, corresponding to the likelihood ratio [LR]+: 10.49, positive predictive value [PPV]: 88.2%. *: summation of the size of the compression and invasion parts of the endobronchial tumor. Tumor size:  , invasion part;
, invasion part;  , compression part;
, compression part;  , summation*;
, summation*;  , reference line. CI, confidence interval.
, reference line. CI, confidence interval.
Table 4.
Sensitivity, specificity, positive and negative predictive value and likelihood ratio at different cut-off levels
| Cut-off level summation* (cm) | Sensitivity | % Specificity | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|
| 5.6 | 95.5 | 35.5 | 51.2 | 91.7 | 1.48 | 0.13 |
| 6.9 | 90.9 | 54.8 | 58.8 | 89.5 | 2.01 | 0.17 |
| 7.6 | 81.8 | 61.3 | 60.0 | 82.6 | 2.11 | 0.29 |
| 8.3 | 77.3 | 80.6 | 73.9 | 83.3 | 3.98 | 0.28 |
| 9.3 | 68.2 | 93.5 | 88.2 | 80.6 | 10.49 | 0.34 |
| 10.5 | 45.5 | 93.5 | 83.3 | 70.7 | 7.00 | 0.58 |
Summation of invasion and compression part of tumors. LR+, positive likelihood ratio; LR−, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Factors associated with recurrence of tumor obstruction
Variables associated with the presence or absence of re-obstruction by the endobronchial tumor were analyzed (Table 5). Factors significantly associated with re-obstruction were non-squamous cell carcinoma (65.7 vs. 16.7%, P = 0.001) and patients who had longer OS (11.7 vs. 1.5 months, P < 0.001) after cryotherapy. To determine a minimal time for the assessment of re-obstruction to be suggested, the ROC curve and its AUC were constructed using OS to discriminate the presence or absence of tumor re-obstruction. Discriminatory power (AUC: 0.69; 95% CI, 0.51∼0.86, P = 0.027) was significant based on AUC measurement (Fig 4). Analysis of predictive performance using OS at different cut-off levels was summarized (Table S1), and a cut-off level was set at 2.0 months. For those who survived longer than 2.0 months, the odds of tumor re-obstruction increased 2.28-fold (sensitivity: 88.6%, LR+: 2.28, PPV: 81.6%), whereas in those who survived less than 2.0 months, the odds decreased by 81% (specificity: 61.1%, LR−: 0.19, NPV: 73.3%).
Table 5.
Factors associated with tumor re-obstruction
| Variables | With re-obstruction | Without re-obstruction | P-value |
|---|---|---|---|
| No. (%) n = 35 | No. (%) n = 18 | ||
| Age, year, median (range) | 65 (47.0–75.0) | 70.5 (52.0–76.3) | 0.499* |
| Male gender | 25 (71.4) | 14 (77.8) | 0.784 |
| Tumor origin | |||
| Primary lung cancer | 16 (45.7) | 5 (27.8) | |
| Metastasis | 19 (54.3) | 13 (72.2) | 0.247 |
| Histology | |||
| SCC | 12 (34.3) | 15 (83.3) | |
| Non-SCC | 23 (65.7) | 3 (16.7) | 0.001 |
| Site of tumor | |||
| Trachea | 3 (8.6) | 6 (33.3) | |
| Non-trachea | 32 (91.4) | 12 (66.7) | 0.050 |
| Previous tumor removal | |||
| Complete | 21 (60.0) | 10 (55.6) | |
| Partial | 14 (40.0) | 8 (44.4) | 0.777 |
| Tumor size, cm, median (range) | |||
| Compression part | 5.5 (4.2–6.8) | 5.2 (3.3–7.8) | 0.948* |
| Invasion part | 2.0 (1.6–3.2) | 2.0 (1.5–3.0) | 0.670* |
| Tumor stalk | |||
| Thin stalk | 8 (22.9) | 5 (27.8) | |
| Broad base | 27 (77.1) | 13 (72.2) | 0.743 |
| Chemotherapy | |||
| Yes | 27 (77.1) | 10 (55.6) | |
| No | 8 (22.9) | 8 (44.4) | 0.125 |
| Overall Survival (months) | 11.7 (4.2–19.5) | 1.5 (0.6–6.8) | <0.001* |
Data analyzed using Mann-Whitney U test. SCC, squamous cell carcinoma.
Figure 4.

The receiver operating characteristic curve is plotted using overall survival to discriminate whether tumor re-obstruction was observed. The discriminatory power was significant based on area under the curve measurement. AUC, area under the curve; CI, confidence interval.
Discussion
In this study, we showed that cryotherapeutic tumor removal served as an efficient palliative intervention for malignant endobronchial obstruction. External compression imposed by a smaller tumor burden and tumors with a thin stalk were found to be associated with better success in this practice. Patients with non-squamous cell carcinoma and those who survived longer had a higher risk of tumor re-obstruction.
The efficacy of endobronchial tumor removal via cryotherapy has been evaluated frequently by improvement in pulmonary function or increased distance in the six-minute walking test. However, patients that qualified for these tests in previous studies were a subset of subjects with better PS, rather than all potential candidates suitable for this palliative treatment. This fact partly stemmed from the unsettled controversy about whether an aggressive palliative treatment of malignant endobronchial tumor obstruction did any good for individuals with a poor PS. Our study, in which 43% of patients had PS 2–3, provides positive evidence, showing an improvement in PS after cryotherapeutic tumor removal.
However, this improvement in PS, which was more significant in patients with total removal than in those with partial removal, highlights the fact that clinical factors are urgently needed for clinicians to single out patients that would most likely benefit from this practice. We found that an endobronchial tumor with a thin stalk was a positive factor for complete tumor removal. This was not surprising; first, because it provided the bronchoscopist a specific point to approach, thus the tumor could be detached by damaging only a limited amount of tissue in the airway. Second, the thin stalk region of the tumor might contain fewer vessels than broad stalk tumors; therefore, tumor bleeding would be easier to manage.
Of interest, a larger compression part of a tumor, compared to the invasion part, was found to be more influential in tumor removal. In these individuals, the higher partial removal as the best achievement could possibly be a result of poorer tolerance during bronchoscopic intervention. From the pathophysiological perspective, a large primary lung cancer tumor burden or metastasis is frequently associated with pulmonary endothelial dysfunction, such as pulmonary thromboemboli, that underlies the patient’s ventilation-perfusion (V/Q) mismatch.11,12 Lying down for a prolonged period during the bronchoscopic procedure could result in profound hypoxemia by aggravating the V/Q mismatch via gravity-dependent blood redistribution to the thoracic cavity. On the other hand, a large compressive tumor often impedes venous blood return to the heart, which subsequently increases the risk of hemodynamic instability with sedative drug infusion during the procedure.3,13,14 These situations possibly account for the increasing difficulty of achieving complete removal of a tumor with a larger compression part. However, by analyzing the ROC curve, we were able to set the threshold at 9.3 cm in terms of the summation of the size of the compression and invasion parts of a tumor, as 93.5% of subjects achieving complete tumor removal had tumors smaller than this cut-off (specificity: 93.5%). Although the percentage of the tumor partially removed that was larger than this cut-off was not the highest (sensitivity: 68.2%) the LR+ was 10.49. Because most of the patients in this situation have limited options for palliative treatment, our study suggests a criterion as inclusive as possible to encompass potential candidates.
Being a palliative treatment, the sustainability of cryotherapeutic tumor removal as measured by tumor re-obstruction is a key concern. A tumor of a non-squamous histology was found to be associated with more re-obstruction in the current study. Previous studies have reported that there was a certain variation in the efficiency of achieving freezing-induced cell death across different cell types. This finding could stem from the varied anti-apoptosis ability, different morphological structure of cancerous tissue, and varied susceptibility to cell dehydration and intracellular ice formation in response to freezing.15–19 There were lower recurrences of chemotherapy after recanalization in this study (50.0 vs. 73.0%, P = 0.125), although the result was not statistically significant. A similar result was also noted in the Kaplan–Meier curve of time to re-obstruction comparing subjects receiving versus not receiving chemotherapy (6.2 vs. 4.0 months, P = 0.268; Fig S1). These findings justified the need for a prospective study to clarify this issue. Longer survival after cryotherapeutic treatment is another factor related to the occurrence of re-obstruction. We suggest this assessment at two months, as 88.6% of subjects had re-obstruction beyond this time point (sensitivity: 88.6%, LR+: 2.28). Although the percentage without re-obstruction before this time point was not the highest (specificity: 61.1%), the negative LR was the lowest (LR-: 0.19), thus clinicians can make a trade-off between maximizing the probability of detecting re-obstruction and minimizing the unnecessary intervention.
The limitation of the current study is its inherent bias as a retrospective study. The indications, timing, and process of the treatment were not as standardized as in prospective studies. However, appropriate criteria were set to address these biases, although at the expense of a smaller sample size. In addition, although the improvement of PS after cryotherapy was noted only in eight (15.1%) patients, all of them were spared the use of non-invasive ventilator and intensive care unit admission. Furthermore, an extra 30 (56.6%) subjects were exempted from receiving rigid bronchoscopy.
Conclusion
In conclusion, this study suggests that cryotherapy is a helpful palliative treatment to deal with endobronchial obstruction secondary to a wide range of malignancies in patients with diverse PS.
Disclosure
No authors report any conflict of interest.
Supporting Information
Additional Supporting Informationmay be found in the online version of this article at the publisher's website:
Figure S1 Kaplan–Meier curve of time to re-obstruction after recanalization. Group receiving chemotherapy (6.2 months) had a longer time to re-obstruction (P = 0.268), than the group without chemotherapy (4.0 months); however not statistically significant.
Table S1 Sensitivity, specificity, positive and negative predictive value, and likelihood ratio at different cut-off levels.
References
- Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- Kvale PA, Selecky PA, Prakash UB. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):368S–403S. doi: 10.1378/chest.07-1391. [DOI] [PubMed] [Google Scholar]
- McMahon CC, Rainey L, Fulton B, Conacher ID. Central airway compression. Anaesthetic and intensive care consequences. Anaesthesia. 1997;52:158–162. doi: 10.1111/j.1365-2044.1997.64-az0066.x. [DOI] [PubMed] [Google Scholar]
- Homasson JP. Endoscopic palliation of tracheobronchial malignancies. Thorax. 1991;46:861. doi: 10.1136/thx.46.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Maiwand MO, Nath AR, Lockwood P, Lloyd MH, Saab M. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax. 1990;45:509–513. doi: 10.1136/thx.45.7.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoglu S, Ucan ES, Celik G, et al. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis. 2005;22:587–591. doi: 10.1007/s10585-005-5787-x. [DOI] [PubMed] [Google Scholar]
- Coriat R, Diaz O, de la Fouchardière C, Desseigne F, Négrier S. Endobronchial metastases from colorectal adenocarcinomas: Clinical and endoscopic characteristics and patient prognosis. Oncology. 2007;73:395–400. doi: 10.1159/000136794. [DOI] [PubMed] [Google Scholar]
- Kiryu T, Hoshi H, Matsui E, et al. Endotracheal/endobronchial metastases: Clinicopathologic study with special reference to developmental modes. Chest. 2001;119:768–775. doi: 10.1378/chest.119.3.768. [DOI] [PubMed] [Google Scholar]
- Lin TY, Lo YL, Hsieh CH, et al. The potential regimen of target-controlled infusion of propofol in flexible bronchoscopy sedation: A randomized controlled trial. PLoS ONE. 2013;8(4):e62744. doi: 10.1371/journal.pone.0062744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YL, Lin TY, Fang YF, et al. Feasibility of bispectral index-guided propofol infusion for flexible bronchoscopy sedation: A randomized controlled trial. PLoS ONE. 2011;6(11):e27769. doi: 10.1371/journal.pone.0027769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R, MacCallum P. Treatment and secondary prevention of venous thromboembolism in cancer. Br J Cancer. 2010;102(Suppl. 1):S17–23. doi: 10.1038/sj.bjc.6605601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es N, Bleker SM, Di Nisio M. Cancer-associated unsuspected pulmonary embolism. Thromb Res. 2014;133(Suppl. 2):S172–178. doi: 10.1016/S0049-3848(14)50028-X. [DOI] [PubMed] [Google Scholar]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: Continuing professional development. Can J Anaesth. 2011;58:853–859. doi: 10.1007/s12630-011-9539-x. 860–7. [DOI] [PubMed] [Google Scholar]
- Béchard P, Létourneau L, Lacasse D, Côté D, Bussières JS. Perioperative cardiorespiratory complications in adults with mediastinal mass: Incidence and risk factors. Anesthesiology. 2004;100:826–834. doi: 10.1097/00000542-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Clarke DM, Robilotto AT, Rhee E, et al. Cryoablation of renal cancer: Variables involved in freezing-induced cell death. Technol Cancer Res Treat. 2007;6:69–79. doi: 10.1177/153303460700600203. [DOI] [PubMed] [Google Scholar]
- Ismail M, Morgan R, Harrington K, Davies J, Pandha H. Enhancing prostate cancer cryotherapy using tumour necrosis factor related apoptosis-inducing ligand (TRAIL) sensitisation in an in vitro cryotherapy model. Cryobiology. 2009;59:207–213. doi: 10.1016/j.cryobiol.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Rui J, Tatsutani KN, Dahiya R, Rubinsky B. Effect of thermal variables on human breast cancer in cryosurgery. Breast Cancer Res Treat. 1999;53:185–192. doi: 10.1023/a:1006182618414. [DOI] [PubMed] [Google Scholar]
- Zhang A, Xu LX, Sandison GA, Zhang J. A microscale model for prediction of breast cancer cell damage during cryosurgery. Cryobiology. 2003;47:143–154. doi: 10.1016/j.cryobiol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Balasubramanian SK, Wolkers WF, Bischof JC. Membrane hydration correlates to cellular biophysics during freezing in mammalian cells. Biochim Biophys Acta. 2009;1788:945–953. doi: 10.1016/j.bbamem.2009.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan–Meier curve of time to re-obstruction after recanalization. Group receiving chemotherapy (6.2 months) had a longer time to re-obstruction (P = 0.268), than the group without chemotherapy (4.0 months); however not statistically significant.
Table S1 Sensitivity, specificity, positive and negative predictive value, and likelihood ratio at different cut-off levels.


