Abstract
Background
DNA repair gene polymorphisms could alter DNA repair capacity and therefore associate with tumor sensitivity to radiochemotherapy. This study assessed excision repair cross-complementing group 1 (ERCC1) C118T and X-ray cross-complementing group 1 (XRCC1) G399A single-nucleotide polymorphisms in esophageal patients for an association with sensitivity to radiation and chemotherapy.
Methods
Esophageal squamous cell carcinoma patients (n = 118) who relapsed after surgery were enrolled for assessment of ERCC1 C118T and XRCC1 G399A polymorphisms by direct DNA sequencing.
Results
The response rate of treatments was 48.30%: 14 complete response (CR, 11.86%), 43 partial response (PR, 36.44%), 49 stable disease (SD, 41.53%), and 12 progressive disease (PD, 10.17%). ERCC1 C118T was significantly associated with treatment response (C/T vs. C/C + T/T, odds ratio [OR] = 6.035, 95% confidence interval [CI]: 2.114–17.226, P = 0.001) after adjusting for other clinicopathological factors. Patients carrying the C/T genotype had significantly prolonged overall survival (OS) compared with C/C and T/T (median OS 43.00 vs. 27.00, P = 0.027). Multivariate Cox regression showed that a response was only an independent prognostic factor for OS (CR + PR vs. SD+PD, HR = 0.471 95% CI 0.269–0.826, P = 0.009). Grade III and IV adverse events occurred in 12 of 118 patients (10.17%). Only concurrent radiochemotherapy significantly increased these adverse events (OR = 26.529, 95% CI 2.312–304.389, P = 0.008).
Conclusion
ERCC1 C118T could be a predictive factor for the response to radiotherapy and chemotherapy, but not a prognostic factor for OS in esophageal cancer patients after surgery.
Keywords: Esophageal carcinoma, ERCC1, radiochemotherapy, single nucleotide polymorphism, XRCC1
Introduction
Esophageal cancer (EC) is the eighth most common cancer and sixth leading cause of cancer death in the world.1 As most EC patients are diagnosed at advanced stages of the disease, they are ineligible for curable surgery and are, thus, difficult to treat.1 Histologically, more than 90% of ECs are diagnosed as squamous cell carcinoma (SCC) in China and other developing countries, whereas two-thirds of EC diagnoses in western countries are adenocarcinomas. Compared to adenocarcinoma, esophageal SCC is more sensitive to radiation treatment.2 Injuries to the esophagus induced by radiation and tumor shrinkage after radio-chemical therapy vary between individuals. Thus, the outcomes of adjuvant chemotherapy after radiation for recurrent EC are still disappointing. In this regard, research on novel biomarkers could help predict the curability of individual radiotherapy in cases of recurrent EC.
Current radiotherapy and chemotherapy methods aim to damage tumor cell DNA in order to induce tumor cell apoptosis; growing evidence shows that the DNA repair mechanism is one of the crucial gene pathways potentially involved in individual sensitivity to chemotherapy and radiotherapy.3,4 DNA repair gene excision repair cross-complementing group 1 (ERCC1) is part of the nucleotide excision repair (NER) complex involved in the repair of platinum-induced interstrand and intrastrand DNA cross-links, while X-ray cross-complementing group 1 (XRCC1) is involved in the base excision repair (BER) system removing small lesions around damaged DNA bases.5,6 Previous studies have shown that altered expression or functional single nucleotide polymorphisms (SNPs) of these two genes can influence the sensitivity of different human cancers to radiation and chemotherapy through aberrant messenger ribonucleic acid (mRNA) expression, mRNA stability or influencing interactions with other repair proteins.7–9 Other studies have demonstrated that ERCC1 C118T and XRCC1 G399A polymorphisms were significantly associated with the outcome and survival of lung and colorectal cancer and head and neck SCC patients who were treated with cisplatin-based chemotherapy and radiotherapy.10–16 Thus, detection of ERCC1 and XRCC1 SNPs could be important in identifying patients who would benefit from radiotherapy and chemotherapy in clinical practice. Indeed, several studies have reported an association between the polymorphisms of DNA repair genes and sensitivity of cancer patients to neoadjuvant radiochemotherapy in Caucasian populations.17–20 However, to date, no study has demonstrated an association between ERCC1 and XRCC1 SNPs with responses to radiochemotherapy in EC patients. We therefore conducted a retrospective study to analyze the association between ERCC1 C118T and XRCC1 Arg399Gln polymorphisms and sensitivity to radiotherapy and chemotherapy in Chinese patients with recurrent EC.
Materials and methods
Study population
In this study we recruited a total of 118 patients with histologically diagnosed recurrent EC relapsed after surgery between January 2002 and December 2013 from the Cancer Center of Daping Hospital, The Third Military Medical University, Chongqing, China. Eligibility criteria included: (i) patients underwent radical esophagectomy of histologically confirmed squamous cell of the esophagus; and (ii) the age range of patients was 18–80, with a performance score from 0 to 2. Patients were considered to have relapsed during follow-up after surgery if any of the following criteria were met: (i) the short-diameter of mediastinal lymph nodes was greater than 1 cm assessed by radiographic scans, or the standardized uptake value was greater than 2.5 evaluated by positron emission tomography-computed tomography (PET-CT); (ii) neoplasms were detected in anastomotic stoma by gastrointestinal endoscopy with positive biopsy; and (iii) lesions in the trachea were observed by fiberoptic bronchoscopy. Out of 118 SCCs, 49 patients received adjuvant chemotherapy immediately after surgery, taxanes plus platinum in 40 cases and fluoropyrimidine plus platinum in nine cases, every three weeks for up to six cycles. Neoadjuvant chemotherapy prior to radiotherapy was delivered to 13 patients that were treated with paclitaxel plus platinum (n = 7) or fluoropyrimidine plus platinum (n = 6) for three weeks for up to three cycles. Forty-four patients were treated with concurrent radiochemotherapy of fluoropyrimidine plus platinum (n = 21) or taxanes plus platinum (n = 17) or irinotecan plus platinum (n = 6). Sixty patients were treated with adjuvant chemotherapy after radiotherapy with a regimen comprising taxanes plus platinum for three weeks for up to three cycles; only one patient received radiation therapy. All patients received radiotherapy through 8MV-X linear accelerator three-dimensional conformal radiation therapy or intensity-modulated radiation therapy according to target tumor lesions. The median radiation dose was 60 Gy (range: 45–66 Gy), dosed at a daily fraction of 1.8 to 2 Gy, five times per week. Blood samples were collected weekly for toxicity evaluation after radio-chemotherapy. One month after completion of radiotherapy, all patients were examined again with CT scans of the neck and chest in order to evaluate treatment response and toxicity. Written informed consent was obtained from each patient for biomarker analysis.
Response evaluation
The tumor response to therapy was evaluated by CT scan before and after completion of radiotherapy dose or adjuvant chemotherapy. Tumor response was defined based on the new Response Evaluation Criteria in Solid Tumors (version 1.1) as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). The objective response rate was defined by a combination of CR and PR.21
DNA extraction and genotyping
DNA was extracted from 2 ml peripheral limosis vein blood obtained before treatment using a QIAamp kit (Qiagen, Hilden, Germany). Gene polymorphisms were detected using polymerase chain reactions with the whole genome DNA as a template. The ERCC1 C118T primer sequences were 5′-AGGAGGGCCCTGTGGTTATC-3′ and 5′-AGGCTTCTCATAGAAC −3′, and XRCC1 G399A primer sequences were 5′-GATCACACCTAACTGGCATCTTC-3′ and 5′-CTGGGACCACCTGTGTTC-3′. The polymerase chain reaction products were then sequenced with a BigDye Terminator Sequencing kit (Applied Biosystems, Foster City, CA, USA) and ABI 3730XL DNA Analyzer (Applied Biosystems). The third nucleotide of ERCC1 118 codon and the second nucleotide of XRCC1 399 codon were read out. The analyses were performed without any knowledge of the clinical data.
Statistical analysis
The associations between SNP genotypes and different clinicopathological characteristics or tumor response were analyzed using the chi-square or Fisher exact test. The Hardy-Weinberg equilibrium was also evaluated by Fisher exact probability test. Univariate and multivariate logistic regressions were conducted to evaluate the association of objective response rate or III to IV grade adverse events with variant clinical factors and genotypes. A Kaplan–Meier curve and log-rank test were used to compare the disease-free survival (DFS) and overall survival (OS) in different clinicopathological characteristics and genotypes of ERCC1 C118T and XRCC1 Arg399Gln polymorphisms. Cox proportional hazards models were conducted to evaluate prognostic factors for OS. P < 0.05 was considered statistically significant for the two-sides. All statistical analysis was performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline patient characteristics
A total of 118 patients with recurrent esophageal SCC were enrolled in this study; the baseline characteristics of these patients are shown in Table 1. Specifically, among these 118 patients, 63.56% (75/118) had lymph node metastasis. After treatment, the outcomes were 11.86% (14/118) CR, 36.44% (43/118) PR, 41.53% (49/118) SD, and 10.17% (12/118) PD. The total response rate was 48.30%. Grade III and IV adverse events were observed in 12 patients (10.17%) (Table 1).
Table 1.
Baseline demographics and clinical characteristics of patients
| Characteristics | No. of patients (n = 118) | % |
|---|---|---|
| Gender | ||
| Female | 22 | 18.64 |
| Male | 96 | 81.36 |
| Age | ||
| ≤60 | 59 | 50.00 |
| >60 | 59 | 50.00 |
| Tumor stage | ||
| T1-T2 | 26 | 22.03 |
| T3-T4 | 92 | 77.97 |
| Lymph node metastasis | ||
| N0 | 43 | 36.44 |
| N1-N3 | 75 | 63.56 |
| Adjuvant chemotherapy after surgery | ||
| No | 69 | 58.47 |
| Yes | 49 | 41.53 |
| Neoadjuvant chemotherapy | ||
| No | 105 | 88.98 |
| Yes | 13 | 11.02 |
| Concurrent chemotherapy | ||
| No | 74 | 62.71 |
| Yes | 44 | 37.29 |
| Adjuvant chemotherapy | ||
| No | 58 | 49.15 |
| Yes | 60 | 50.85 |
| Treatment response | ||
| CR | 14 | 11.86 |
| PR | 43 | 36.44 |
| SD | 49 | 41.53 |
| PD | 12 | 10.17 |
| Adverse events after treatment | ||
| I-II | 106 | 89.83 |
| III-IV | 12 | 10.17 |
| Median | Range | |
| Age | 61 | 37–79 |
| Total Dose of Radiotherapy | 60 | 45–66 |
| Total Numbers of Chemotherapy Cycle | 2 | 0–10 |
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Association between clinical characteristics and polymorphisms of ERCC1 C118T and XRCC1 Arg399Gln
Of the 118 patients, 104 were analyzed for ERCC1 C118T polymorphisms and 73 for XRCC1 G399A, but only 59 patients were genotyped for both gene polymorphisms. There was no significant difference between this cohort of patients and the two subgroups of patients who were subjected to genotyping. Distribution of ERCC1 C118T and XRCC1 G399A genotypes and alleles in patients with EC are listed in Table 2. The distributions of both ERCC1 and XRCC1 genotypes were found to be in Hardy-Weinberg equilibrium.
Table 2.
Hardy-Weinberg equilibrium test
| Gene | No. of patients | % | Allele | % | χ2 | P |
|---|---|---|---|---|---|---|
| ERCC1 C118T | ||||||
| CC | 52 | 50.00% | C | 67.91 | 0.459 | 0.498 |
| CT | 41 | 39.42% | T | 30.29 | ||
| TT | 11 | 10.58% | ||||
| XRCC1 G399A | ||||||
| GG | 36 | 49.32% | G | 69.86 | 0.042 | 0.837 |
| GA | 30 | 41.10% | A | 30.14 | ||
| AA | 7 | 9.59% |
ERCC1, excision repair cross-complementing group 1; XRCC1, X-ray cross-complementing group 1.
Table 3 presents the distributions of different clinicopathological characteristics between wild type and variant genotypes. The ERCC1 C118T polymorphism was significantly associated with treatment response (χ2 = 13.410, P = 0.001). The heterogeneous variant C/T genotype indicated a therapy response in 68.30% (28/41), whereas, the response rate was only 36.54% (19/52) and 18.18% (2/11) in homogeneous C/C and T/T genotypes, respectively. Grade III-IV adverse events were more frequently observed with increasing variant T alleles of ERCC1 C118T. A significantly lower incidence of lymph node metastasis was associated with increasing variant alleles of the XRCC1 Arg399Gln polymorphism (χ2 = 8.028, P = 0.015). No statistically significant association was observed between ERCC1 C118T or XRCC1 Arg399Gln genotypes and other clinicopathological characteristics.
Table 3.
Association of clinicopathological characteristics with ERCC1 and XRCC1 polymorphisms
| Characteristics | ERCC1 C118T | χ2 | P | XRCC1 Arg/Gln | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | Arg/Arg | Arg/Gln | Gln/Gln | |||||
| Gender | ||||||||||
| Female | 13 | 7 | 1 | 1.521 | 0.529 | 8 | 6 | 4 | 3.993 | 0.128 |
| Male | 39 | 34 | 10 | 28 | 24 | 3 | ||||
| Age | ||||||||||
| ≤60 | 24 | 18 | 9 | 5.336 | 0.069 | 15 | 14 | 4 | 0.681 | 0.787 |
| >60 | 28 | 23 | 2 | 21 | 16 | 3 | ||||
| T-category | 2.095 | 0.293 | ||||||||
| T1-T2 | 12 | 8 | 4 | 1.510 | 0.448 | 4 | 6 | 2 | ||
| T3-T4 | 40 | 33 | 7 | 32 | 24 | 5 | ||||
| N-category | 8.028 | 0.015 | ||||||||
| N0 | 21 | 20 | 1 | 5.785 | 0.054 | 7 | 12 | 5 | ||
| N1-N3 | 31 | 21 | 10 | 29 | 18 | 2 | ||||
| Response | 1.748 | 0.407 | ||||||||
| SD+PD | 33 | 13 | 9 | 13.410 | 0.001 | 17 | 13 | 5 | ||
| CR+PR | 19 | 28 | 2 | 19 | 17 | 2 | ||||
| Adverse Events | 0.671 | 0.886 | ||||||||
| I-II | 48 | 37 | 7 | 5.975 | 0.047 | 32 | 25 | 6 | ||
| III-IV | 4 | 4 | 4 | 4 | 5 | 1 | ||||
CR, complete response; ERCC1, excision repair cross-complementing group 1; PD, progressive disease; PR, partial response; SD, stable disease.
Association of clinical characteristics and genotypes with tumor response rate
After univariate logistic analysis, patients with heterogeneous ERCC1 118 genotype C/T had a significantly higher response rate compared to those with homogeneous wild genotype C/C or homogeneous variant genotype T/T (odds ratio [OR] = 4.308 95% confidence interval [CI] 1.858–9.987, P = 0.001). All other clinicopathological factors, including gender, age, tumor node stage, and neoadjuvant, concurrent or adjuvant chemotherapy were not associated with disease outcome in these 104 patients.
Multivariate logistic regression analysis showed that ERCC1 C118T was significantly associated with treatment response (C/T vs. C/C + T/T, OR = 6.035, 95% CI: 2.114–17.226, P = 0.001) after adjusting for other clinicopathological factors. Similarly, patients treated with neoadjuvant chemotherapy also had a significantly higher response rate (OR = 18.100, 95% CI: 2.944–111.284, P = 0.002) (Table 4). However, in 73 patients, there was no significant association between the XRCC1 Arg399Gln polymorphism and treatment response in univariate or multivariate analyses. Furthermore, in a subset of 59 patients, after adjusting for other clinicopathological factors and the XRCC1 Arg399Gln polymorphism, ERCC1 C118T was the only predictive factor for response (C/T vs. C/C + T/T, OR = 7.950, 95% CI: 1.769–35.736, P = 0.007).
Table 4.
Results of multivariate logistic regression analyses for response rate and grade III-IV adverse events in 104 patients determined for ERCC1 C118T polymorphism
| Factors | ORR† | III-IV Grade AE† | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | HR | 95% CI | P | |
| Neoadjuvant chemotherapy (yes vs. no) | 18.1 | 2.944–111.284 | 0.002 | |||
| Concurrent chemotherapy (yes vs. no) | 26.529 | 2.312-304.389 | 0.008 | |||
| ERCC1 C118T (C/T vs. C/C+T/T) | 6.035 | 2.114–17.226 | 0.001 | |||
Age and the number of chemotherapy cycles were included in all regression analyses as continuous variables. AE, adverse events; CI, confidence interval; ERCC1, excision repair cross-complementing group 1; OR, odds ratio; ORR, objective response rate.
Association of clinical characteristics and genotypes with disease-free and overall survival
In the whole population of 118 patients, the median DFS and OS was 12.6 (95% CI 10.722–14.478) and 30 months (95% CI 24.609–35.391), respectively. No significant association was observed between the ERCC1 C118T polymorphism and DFS; however, a longer DFS tended to correlate with patients carrying at least one variant allele of XRCC1 Arg399Gln (mDFS: Arg/Arg vs Arg/Gln+Gln/Gln 11.00 vs. 19.00, P = 0.061) (Figs 1, 2).
Figure 1.
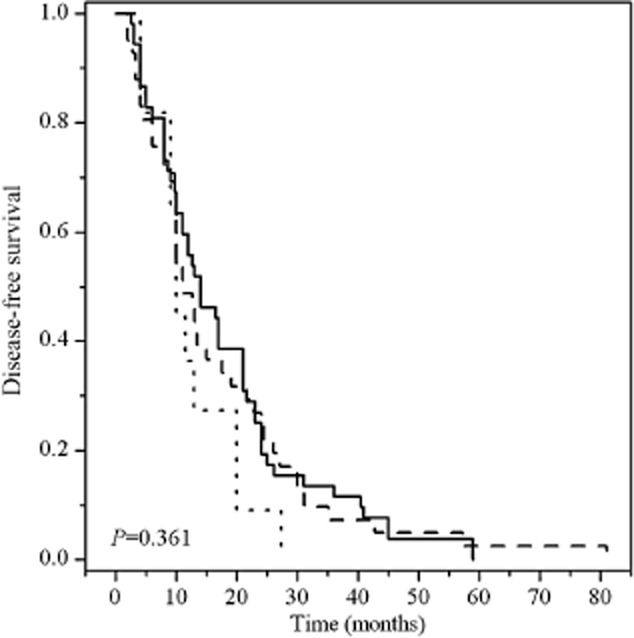
Kaplan–Meier survival curves of disease-free survival of postoperative esophageal cancer patients with three different genotypes of excision repair cross-complementing group 1 (ERCC1) C118T polymorphism. ERCC1 C118T:  , C/C;
, C/C;  , C/T;
, C/T;  , T/T.
, T/T.
Figure 2.
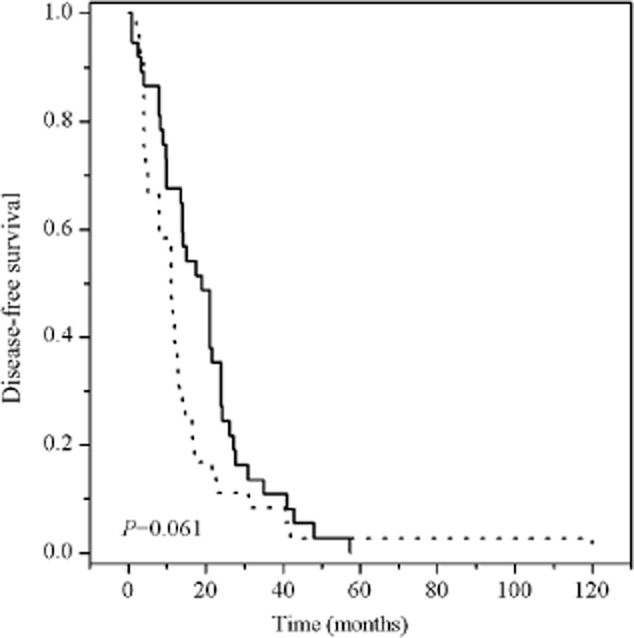
Kaplan–Meier survival curves of disease-free survival of postoperative esophageal cancer patients by X-ray cross-complementing group 1 (XRCC1) Arg399Gln. XRCC1 Arg399Gln:  , Arg/Arg;
, Arg/Arg;  , Arg/Gln+Gln/Gln.
, Arg/Gln+Gln/Gln.
Overall survival was related to lymph node metastasis, response, and the ERCC1 C118T polymorphism, although it was less obvious in the latter. Of the 118 patients, those who achieved CR and PR had longer OS than patients with SD and PD (median OS 39 vs. 20, P < 0.001) (Fig 3). Suggesting an association of ERCC1 C118T and response, patients carrying the C/T genotype had significantly prolonged OS compared with C/C and T/T (median OS 43 vs. 27, P = 0.027) (Fig 4). However, multivariate Cox regression showed that response was only a prognostic factor for OS (CR + PR vs. SD+PD HR = 0.471 95% CI 0.269–0.826, P = 0.009).
Figure 3.
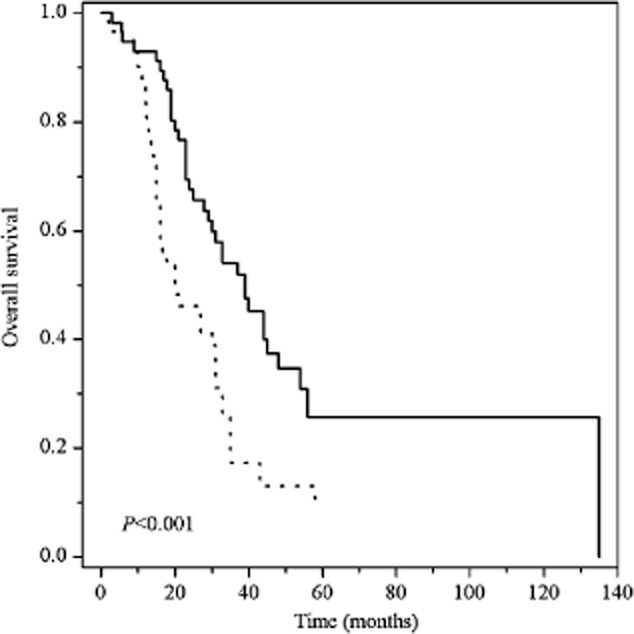
Kaplan–Meier survival curves of recurrent esophageal cancer patients grouped by response to radiotherapy and chemotherapy. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease. Response:  , SD+PD;
, SD+PD;  , CR + PR.
, CR + PR.
Figure 4.
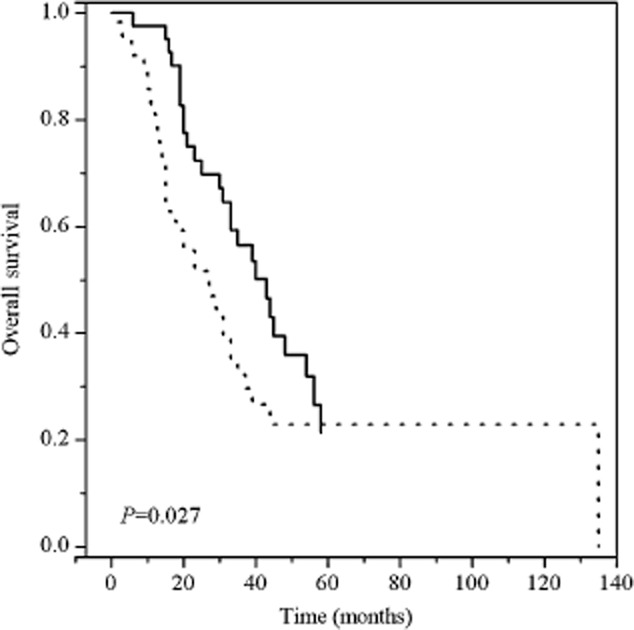
Kaplan–Meier survival curves of recurrent esophageal cancer patients grouped by excision repair cross-complementing group 1 (ERCC1) C118T polymorphism. ERCC1 C118T SNP:  , C/C + T/T;
, C/C + T/T;  , C/T.
, C/T.
Association of adverse events with clinicopathological characteristics and genotypes
Grade III and IV adverse events occurred in 12 of the 118 patients (10.17%). Eight patients experienced grade IV adverse events and died during radiotherapy (n = 3) or three months after completing radiotherapy (n = 5) as a result of uncontrolled hemoptysis. Six of these eight patients received a concurrent chemotherapy regimen of docetaxel plus cisplatin (TP). Overall, concurrent radiochemotherapy was associated with a greater incidence of grade III and IV adverse events (OR = 4.500, 95% CI 1.295–15.640, P = 0.018). Patients with ERCC1 T/T experienced significantly more frequent grade III and IV adverse events (OR = 6.857, 95% CI 1.389–33.853, P = 0.018) compared with C/C. However, in multivariable logistic analysis, only concurrent radiochemotherapy remained significantly associated with grade III and IV adverse events, after adjusting for other clinicopathological factors and polymorphisms (OR = 26.529, 95% CI 2.312–304.389, P = 0.008) (Table 4).
Discussion
In this study, we recruited 118 esophageal SCC patients after neoadjuvant, concurrent or adjuvant radiochemotherapy. The patients had CR (11.86%, 14/118), PR (36.44%, 43/118), SD (41.53%, 49/118), and PD (10.17%, 12/118). The total response rate was 48.30%, and grade III and IV adverse events were only observed in 12 patients (10.17%). Neoadjuvant chemotherapy and ERCC1 C118T SNPs were significantly associated with treatment response; however, there was no association of XRCC1 Arg399Gln genotypes with treatment response. ERCC1 C118T SNP was associated with OS in univariate analysis but not in multivariate Cox regression. Only response and concurrent radiochemotherapy were independent prognostic factors for OS and predictive factors for grade III-IV adverse events, respectively. The data from this study demonstrates that ERCC1 C118T could be a predictive factor for the response to radiotherapy and chemotherapy, but not a prognostic factor for OS in EC patients after surgery.
To date, ERCC1 C118T and C8092A have been extensively studied in different cancers. Several studies have shown that these two SNPs are associated with diverse clinical outcomes after systemic chemotherapy in various types of solid tumors.12,14,22 In the current study, we found that the ERCC1 118 C/T heterozygous variant genotype is associated with a significantly higher response rate to radiation and adjuvant chemotherapy in patients with recurrent EC than that of wild type and homozygous variant genotypes. Our data is consistent with that reported by Warnecke-Eberz et al., showing an association between the ERCC1 118 C/T polymorphism and response to neoadjuvant radiochemotherapy in patients with resected EC.17 In their study, the histopathological response rate in patients with the C/T genotype was 70%, whereas C/C and T/T genotype carriers only had response rates of 43% and 20%, respectively (P = 0.003).17 However, while Metzger et al. reported similar data, the OS advantage was not presented.18 Another study by Leichman et al. suggested that there was no association between ERCC1 C118T and a response to radiotherapy or chemotherapy for preoperative neoadjuvant radiochemotherapy of esophageal adenocarcinoma, but in contrast, the expression of ERCC1 mRNA in tumor tissues was indicated as a prognostic factor.4 Additionally, Bradburya et al. reported no association between the ERCC1 118 C/T genotype and OS or PFS in locally advanced EC patients after receiving adjuvant chemotherapy.19 However, in Chinese patients, Wang et al. demonstrated that ERCC1 C8092A SNP was significantly associated with a response to first-line chemotherapy of advanced esophageal carcinoma.23 These disparate results may be a result of the differences in genetic backgrounds of patients, tumor histopathology or heterogeneity of treatments.
In our study, the sensitivity of the ERCC1 118 CT genotype predicted a response rate of 68.30% in recurrent EC; however, the specificity was 66.67%, indicating that other factors also play a role in treatment response. This data is consistent with previous studies detecting tumor response through a single repair gene polymorphism.17,18 On the other hand, there was only a weak association between the ERCC1 C118T genotype and OS, as underlined in multivariate analysis. It was not significant after adjusting for other clinicopathological factors (C/T vs. C/C + T/T HR = 0.688 95% CI 0.382–1.237). This was consistent with the results reported by Warnecke-Eberz et al.18
A previous study demonstrated that the pathology complete response (pCR) was significantly associated with XRCC1 G399A in esophageal adenocarcinoma after neoadjuvant radiochemotherapy.24 Individuals with variant alleles (GA+AA) had significantly poorer pCR compared to wild-type carriers (OR = 2.75, 95% CI: 1.14–6.12).24 Another biomarker study, the Eastern Cooperative Oncology Group trial E1201, showed that only 6% of subjects with the variant allele (GA+AA) experienced a pCR compared to 28% of subjects with the GG genotype (OR = 5.37, P = 0.062).20 Although our study did not show an association between the XRCC1 Arg399Gln polymorphism and treatment response with OS (but an agreement with involved nodes), we did determine that patients carrying Arg/Arg had shorter DFS compared with other genotypes.
Tumor progression is a dynamic process and germ line mutations, or SNPs, are static indicators, which make them ineffective for reflecting dynamic tumor pathogenesis. In tumor tissues, gene expression is controlled by many factors rather than SNPs alone. Moreover, DNA damage repair is a multiple enzyme process. All of these factors contribute to the equivocal association between a single SNP and clinical outcomes.10,13 For example, a recent study showed that the ERCC1 C118T polymorphism did not lead to altered cellular ERCC1 protein expression, suggesting that other causative variants or haplotypes linked to ERCC1 C118T might account for this clinical association.25 As Lambrechts et al. suggested, a single marker for certain treatment is unlikely to exist, rather multiple markers might be needed for accurate response prediction.26
Our data demonstrated that treatment-related adverse events were tolerable (only 12 Grade III and IV adverse events occurred in 118 patients). However, there was no association between ERCC1 and XRCC1 polymorphisms and toxicity. Multivariate analysis showed that the incidence of grade III and IV esophagitis and pneumonitis in patients who received concurrent radiochemotherapy was significantly higher than those who received radiotherapy alone. This was more obvious in the six cases treated with a TP regimen of concurrent chemotherapy. Grade IV esophago-tracheal fistula was accompanied with hemoptysis (n = 4) and radiation pneumonitis (n = 2). This finding could be explained by delayed repair as a result of rapid shrinkage of the tumor lesion. Liew et al. demonstrated that a taxane-contained regimen of concurrent chemotherapy could lead to serious radiation pneumonitis in non-small cell lung cancer patients.27 This phenomenon suggests that a TP regimen must be used with caution in cases of concurrent radiochemotherapy.
Our current study does have some limitations. For example, the patient sample was small and genotyping data was not available for all patients. The treatment was clearly heterogeneous. However, to the best of our knowledge, this is the first study that has explored an association between the ERCC1 C118T polymorphism and treatment outcome in recurrent EC in a Chinese population.
Conclusions
Our results may provide evidence for individualized treatment and predictive factors of short-term efficacy for radiation and chemotherapy in recurrent esophageal SCC patients. It is recommended that future studies investigate the use of combined multiple polymorphism sites in order to create an accurate predictive score system.
Acknowledgments
We would like to thank Dr. Shiheng Zhang of the Cancer Center, Daping Hospital and Research Institution of Surgery, The Third Military Medical University for technical assistance. This study was supported in part by a grant from Wu Jieping Medical Foundation (#320.6750.12177).
Disclosure
No authors report any conflict of interest.
Supporting Information
Additional Supporting Informationmay be found in the online version of this article at the publisher's website:
Table S1 Results of multivariate Cox regression of overall survival in 73 patients detected with the XRCC1 Arg399Gln polymorphism.
Table S2 Results of multivariate Cox regression of overall survival in 59 patients detected with both the ERCC1 C118T and XRCC1 Arg399Gln polymorphisms.
Table S3 Results of multivariate logistic regression of grade III-IV adverse events in 73 patients detected with the XRCC1 Arg399Gln polymorphism.
Table S4 Results of multivariate logistic regression of grade III-IV adverse events in 59 patients detected with both the ERCC1 C118T and XRCC1 Arg399Gln polymorphisms.
References
- Kanai M, Matsumoto S, Nishimura T, et al. Retrospective analysis of 27 consecutive patients treated with docetaxel/nedaplatin combination therapy as a second-line regimen for advanced esophageal cancer. Int J Clin Oncol. 2007;12:224–227. doi: 10.1007/s10147-007-0666-x. [DOI] [PubMed] [Google Scholar]
- Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- Kim MK, Cho KJ, Kwon GY, et al. Patients with ERCC1-negative locally advanced esophageal cancers may benefit from preoperative chemoradiotherapy. Clin Cancer Res. 2008;14:4225–4231. doi: 10.1158/1078-0432.CCR-07-4848. [DOI] [PubMed] [Google Scholar]
- Leichman LP, Goldman BH, Bohanes PO, et al. S0356: A phase II clinical and prospective molecular trial with oxaliplatin, fluorouracil, and external-beam radiation therapy before surgery for patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4555–4560. doi: 10.1200/JCO.2011.36.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1:227–235. [PubMed] [Google Scholar]
- Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase beta in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18:48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier J, Boige V, Miquel C, et al. ERCC1 codon 118 polymorphism is a predictive factor for the tumor response to oxaliplatin/5-fluorouracil combination chemotherapy in patients with advanced colorectal cancer. Clin Cancer Res. 2005;11:6212–6217. doi: 10.1158/1078-0432.CCR-04-2216. [DOI] [PubMed] [Google Scholar]
- Chen P, Wiencke J, Aldape K, et al. Association of an ERCC1 polymorphism with adult-onset glioma. Cancer Epidemiol Biomarkers Prev. 2000;9:843–847. [PubMed] [Google Scholar]
- Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA. XRCC1 polymorphisms: Effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res. 1999;59:2557–2561. [PubMed] [Google Scholar]
- Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol. 2004;22:2594–2601. doi: 10.1200/JCO.2004.08.067. [DOI] [PubMed] [Google Scholar]
- Liao WY, Shih JY, Chang GC, et al. Genetic polymorphism of XRCC1 Arg399Gln is associated with survival in non–small-cell lung cancer patients treated with gemcitabine/platinum. J Thorac Oncol. 2012;7:973–981. doi: 10.1097/JTO.0b013e31824fe98c. [DOI] [PubMed] [Google Scholar]
- Cheng J, Ha M, Wang Y, et al. A C118T polymorphism of ERCC1 and response to cisplatin chemotherapy in patients with late-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2012;138:231–238. doi: 10.1007/s00432-011-1090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzo A, Graziano F, Loupakis F, et al. Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFOX-4 chemotherapy. J Clin Oncol. 2007;25:1247–1254. doi: 10.1200/JCO.2006.08.1844. [DOI] [PubMed] [Google Scholar]
- Páre L, Marcuello E, Altés A, et al. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Br J Cancer. 2008;99:1050–1055. doi: 10.1038/sj.bjc.6604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Li Q, Qiu W, et al. Genetic polymorphism of XRCC1 correlated with response to oxaliplatin-based chemotherapy in advanced colorectal cancer. Pathol Oncol Res. 2012;18:1009–1014. doi: 10.1007/s12253-012-9536-6. [DOI] [PubMed] [Google Scholar]
- Quintela-Fandino M, Hitt R, Medina PP, et al. DNA-repair gene polymorphisms predict favorable clinical outcome among patients with advanced squamous cell carcinoma of the head and neck treated with cisplatin-based induction chemotherapy. J Clin Oncol. 2006;24:4333–4339. doi: 10.1200/JCO.2006.05.8768. [DOI] [PubMed] [Google Scholar]
- Warnecke-Eberz U, Vallböhmer D, Alakus H, et al. ERCC1 and XRCC1 gene polymorphisms predict response to neoadjuvant radiochemotherapy in esophageal cancer. J Gastrointest Surg. 2009;13:1411–1421. doi: 10.1007/s11605-009-0881-z. [DOI] [PubMed] [Google Scholar]
- Metzger R, Warnecke-Eberz U, Alakus H, et al. Neoadjuvant radiochemotherapy in adenocarcinoma of the esophagus: ERCC1 gene polymorphisms for prediction of response and prognosis. J Gastrointest Surg. 2012;16:26–34. doi: 10.1007/s11605-011-1700-x. [DOI] [PubMed] [Google Scholar]
- Bradbury PA, Kulke MH, Heist RS, et al. Cisplatin pharmacogenetics, DNA repair polymorphisms, and esophageal cancer outcomes. Pharmacogenet Genomics. 2009;19:613–625. doi: 10.1097/FPC.0b013e32832f3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Catalano PJ, Murphy KM, et al. Genetic variation in DNA-repair pathways and response to radiochemotherapy in esophageal adenocarcinoma: A retrospective cohort study of the Eastern Cooperative Oncology Group. BMC Cancer. 2011;11:176. doi: 10.1186/1471-2407-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Chen C, Wang F, Wang Z, et al. Polymorphisms in ERCC1 C8092A predict progression-free survival in metastatic/recurrent nasopharyngeal carcinoma treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;72:315–322. doi: 10.1007/s00280-013-2196-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Li X, et al. Genetic polymorphisms of ERCC1 and their effects on the efficacy of cisplatin-based chemotherapy in advanced esophageal carcinoma. Oncol Rep. 2011;25:1047–1052. doi: 10.3892/or.2011.1170. [DOI] [PubMed] [Google Scholar]
- Wu X, Gu J, Wu TT, et al. Genetic variations in radiation and chemotherapy drug action pathways predict clinical outcomes in esophageal cancer. J Clin Oncol. 2006;24:3789–3798. doi: 10.1200/JCO.2005.03.6640. [DOI] [PubMed] [Google Scholar]
- Gao R, Reece K, Sissung T, Reed E, Price DK, Figg WD. The ERCC1 N118N polymorphism does not change cellular ERCC1 protein expression or platinum sensitivity. Mutat Res. 2011;708:21–27. doi: 10.1016/j.mrfmmm.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberchts D, Claes B, Delmar P, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: An analysis of data from the AViTA and AVOREN randomized trials. Lancet Oncol. 2012;13:724–733. doi: 10.1016/S1470-2045(12)70231-0. [DOI] [PubMed] [Google Scholar]
- Liew MS, Sia J, Starmans MH, et al. Comparison of toxicity and outcomes of concurrent radiotherapy with carboplatin/paclitaxel or cisplatin/etoposide in stage III non-small cell lung cancer. Cancer Med. 2013;2:916–924. doi: 10.1002/cam4.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Results of multivariate Cox regression of overall survival in 73 patients detected with the XRCC1 Arg399Gln polymorphism.
Table S2 Results of multivariate Cox regression of overall survival in 59 patients detected with both the ERCC1 C118T and XRCC1 Arg399Gln polymorphisms.
Table S3 Results of multivariate logistic regression of grade III-IV adverse events in 73 patients detected with the XRCC1 Arg399Gln polymorphism.
Table S4 Results of multivariate logistic regression of grade III-IV adverse events in 59 patients detected with both the ERCC1 C118T and XRCC1 Arg399Gln polymorphisms.


