Abstract
Background
Micro ribonucleic acid (miR-101) can regulate the expression of cyclooxygenase-2 (COX-2) and participate in the pathogenesis of malignant tumors. This study investigates the effects of miRNA-101 and COX-2 in lung cancer and the impact of miR-101 on the proliferation and invasion of human lung cancer A549 cell line.
Methods
The expression of miR-101 in 20 separate lung cancer tissues was detected by real time polymerase chain reaction; COX-2 expression was also detected. A549 cells were transfected with miR-101 or negative control oligonucleotide duplex mimic (miR-NC). In vivo tumorigenesis abilities were detected in localized human lung cancer xeno-transplant models in BALB/c nude mice.
Results
MiR-101 expression was significantly lower and the level of COX-2 significantly higher in lung cancer tissues than in adjacent parenchyma (2.918 ± 1.006 vs. 5.953 ± 1.976, P = 0.001; 0.887 ± 0.260 vs. 0.355 ± 0.156, P = 0.001, respectively). Correlation analysis revealed that miR-101 negatively correlated with COX-2 in lung cancer tissues (R = −0.596, P = 0.002). Compared with A549-miR-NC cells, the expression of COX-2 was significantly decreased in A549 cells transfected with miR-101 (P < 0.001). The proliferation of A549 cells was markedly inhibited after transfection of miR-101. The in vivo tumor growth of A549 cells transfected with miR-101 was significantly slower than wide type A549 cells.
Conclusion
MiR-101 expression is decreased in lung cancer, inducing an increase in COX-2 level. Enforced expression of miR-101 can remarkably reduce the cell proliferation and invasion ability of lung cancer cells.
Keywords: COX-2, lung cancer, miRNA-101
Introduction
The mortality of lung cancer is higher than in all other types of malignant tumors.1 Surgery is the first alternative and the most effective method for the treatment of lung cancer; however, at diagnosis, a considerable number of patients are no longer eligible for surgery. Therefore, further clarifying the mechanism of lung cancer is extremely important for developing new treatment methods and improving the prognosis of lung cancer.
Micro ribonucleic acids (miRNAs) are a class of approximately 22 nucleotide-long, endogenously expressed, highly conserved noncoding RNAs with important regulatory functions in cell proliferation, apoptosis, and metastasis.2–4 Computational and experimental outcomes predict that approximately 50% of miRNA genes are located in cancer-related genomic regions.5,6 MiRNAs are thought to regulate cancer development as tumor suppressors. Therefore, miRNAs may be ideal targets of cancer treatment.
Cyclooxygenase 2 (COX-2) is a key rate-limiting enzyme that catalyzes the biosynthesis of prostaglandins from arachidonic acid.7 Recent research results have indicated that COX-2 could induce tumor angiogenesis and is correlated with hematogenous metastasis of tumors.8 Therefore, COX-2 is an attractive target for regulating cancer cell growth. Chakrabarty et al. have proven that the human miR-101 duplex mimic (miR-101) can regulate the expression of COX-2.9 Although miR-101 has been reported to target COX-2 in various tumors, such as endometrial serous adenocarcinomas, and colon and gastric cancers, the role of miR-101 in lung cancer growth is unclear.10–12 In this study, we explore the role of miR-101 in lung cancer development.
Materials and methods
Patients and tissue samples
Lung cancer tissues and adjacent parenchyma lung tissues were obtained from 20 patients (13 men, 7 women) who were diagnosed with lung cancer and underwent primary surgical resection between January and December 2014 at the Tianjin Medical University General Hospital. The median age of patients was 59 years (range, 44–67). All samples were reviewed and diagnosed by two independent pathologists. The ethical committee of Tianjin Medical University General Hospital approved the current research. Informed consent was obtained from all patients involved.
Cell lines and culture
A lung cancer cell line (A549) was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in Dulbecco’s Modified Eagle’s Medium (Gibco, Life Technologies, Grand Island, NY, USA), with 10% fetal bovine serum, 50 U/mL of penicillin, and 50 mg/mL of streptomycin, at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Transfection of micro ribonucleic acid (miRNA)
The human miR-101 duplex mimic and negative control oligonucleotide duplex mimic (miR-NC) were designed and provided by GenePharma (Shanghai, China). RNA oligonucleotides were transfected using LipofectamineTM2000 (Invitrogen, San Diego, CA, USA). After 24 hours of transfection, cells were used for subsequent experiments.
Real-time polymerase chain reaction
Total RNA from the frozen tissue specimens and cultured cells was isolated using the TRIzol kit (Invitrogen) following the manufacturer’s instructions. The cDNA Synthesis kit (TaKaRa, Tokyo, Japan) was then used to reverse transcribe and amplify the RNA. Real-time polymerase chain reaction (RT-PCR) was performed using QuantiFast SYBR Green PCR mixture (Invitrogen). The specific primers of miR-101 were: forward: 5′-TACAGTACTGTGATAACTGAA-3′, reverse: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTTCAGT-3′. The U6 small nuclear RNA was amplified as a loading control. The primers for this U6 internal control were purchased from Invitrogen. Data were shown as fold change (2-ΔΔCt) and analyzed using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Experiments were repeated three times.
Western blotting
Cell lysates were prepared from tissue specimens or cells, washed twice in cold phosphate buffered saline, and lysed for 20 minutes using radioimmunoprecipitation assay buffer (Thermo Fisher Scientific, Waltham, MA, USA), with protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN, USA). The protein concentration was determined using Bradford’s reagent (BioRad, Hercules, CA, USA). An equal amount of protein (50 μg) was loaded in sodium dodecyl sulfate-polyacrylamide gels and then transferred onto nitrocellulase membranes. The membranes were blocked with 2% bovine serum albumin in tris-buffered saline and Tween-20, containing 0.1% Tween-20 for one hour at room temperature, and probed with anti-COX-2 (1:500, ab15191) and anti-β-Actin antibodies (1:500, ab8227). Horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:2500, Beyotime) were added. Blots were visualized using a chemiluminescence reagent (Millipore, Temecula, CA, USA) in a Syngene Gel Documentation System using GeneSnap software (Syngene, Cambridge, UK).
Colony formation assay
Colony formation assay was used for evaluating cell proliferation and growth. A549, A549-miR-NC, and A549-miR-101 cells were seeded at a density of 100 cells per well in six-well tissue culture plates. The colonies that formed after nearly 14 days were stained with 0.1% trypan blue in 50% ethanol. Any colony containing more than 10 cells was considered a viable clonogenic cell. At least three independent experiments were conducted with triplicate test samples. The formula: Cloning efficiency = Colony formation/Cells seeded × 100% was used.
Methyl thiazolyl tetrazolium assay
The methyl thiazolyl tetrazolium (MTT) method was used to estimate cell viability. Cells were plated at an initial density of 104 cells per well in 96-well cell culture plates and allowed to grow for six days. MTT (Beyotime Inst., Biotech, Haimen, China) was added to each well, followed by incubation for four hours at 37°C. After removing the media, dimethyl sulfoxide was added to each well for solubilizing the formazan that had formed. After 30 minutes at room temperature, the plates were scanned spectrophotometrically with a microplate reader, set at 490 nm for measuring the absorbance.
Human tumor xenografts in athymic (nude) mice
Six-week-old, male BALB/c athymic nude mice (nu/nu) were obtained from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). Mice were housed in temperature-controlled rooms with a 12-hour alternating light–dark cycle. The mice were separated into two groups: A549-NC and A549-miR-101. Each group contained five mice. Approximately 5 × 106 cells were injected subcutaneously into the left and right lower backs of mice, respectively. The mice were housed for six weeks. During the experimental period, tumor volume was measured once a week. The length and width of the tumor were regularly measured and the volume was calculated based on the following formula: Volume = length × width2/2. Guidelines for the humane treatment of animals were followed as approved by the Tianjin Medical University General Hospital Animal Care and Use Committee.
Statistical analysis
All statistical analyses were carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation and were evaluated using independent t-test or one-way analysis of variance, as appropriate. Differences were considered significant at P < 0.05.
Results
MiR-101 expression in lung cancer tissue and adjacent parenchyma
To test whether the expression of miR-101 decreased during lung cancer development, we measured the relative expression of miR-101/U6 in the lung cancer tissue and adjacent parenchyma of 20 patients. The results of quantitative RT-PCR analysis revealed that the expression of miR-101/U6 was significantly lower in lung cancer tissue than that in adjacent parenchyma (2.918 ± 1.006 vs. 5.953 ± 1.976, P = 0.001) (Fig 1).
Figure 1.
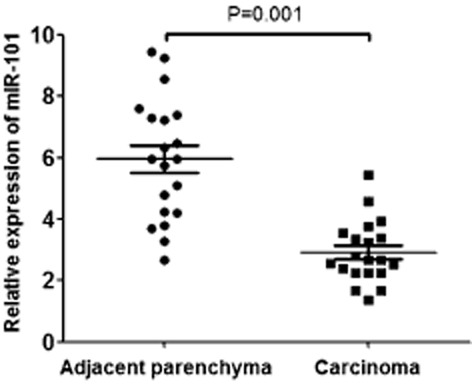
Micro ribonucleic acid (miR)-101 expression in lung cancer tissue and adjacent parenchyma. miR-101 expression was calculated as the ratio of miR-101/U6. The results of quantitative real-time polymerase chain reaction RT-PCR analysis displayed that the expression of miR-101/U6 was significantly lower in lung cancer tissue than in adjacent parenchyma.
Cyclooxygenase-2 (COX-2) level in lung cancer tissue and adjacent parenchyma
Because the expression of miR-101 decreased during lung cancer development, we measured the relative level of COX-2/β-Actin in the lung cancer tissue and adjacent parenchyma of the 20 patients. The results of Western-blotting revealed that the level of COX-2/β-Actin was significantly higher in lung cancer tissue than in adjacent parenchyma (0.887 ± 0.260 vs. 0.355 ± 0.156, P = 0.001) (Fig 2).
Figure 2.
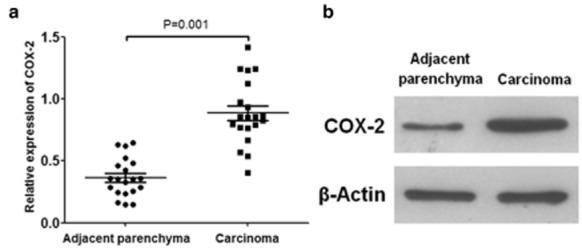
Cyclooxygenase-2 (COX-2) level in lung cancer tissue and adjacent parenchyma. (a) COX-2 level was calculated as the ratio of COX-2/β-Actin. The results of Western-blotting displayed that the level of COX-2/β-Actin was significantly higher in lung cancer tissue than in adjacent parenchyma. (b) Representative images of the Western-blotting of COX-2 and β-Actin.
Correlation of miR-101 expression and COX-2 level in lung cancer tissues
As it had been proven that miR-101 could regulate the expression of COX-2, we analyzed the correlation of miR-101 expression and COX-2 level in lung cancer tissues. As shown in Figure 3, we found that the expression of miR-101 negatively correlated with the level of COX-2 in lung cancer tissues (R = −0.596, P = 0.002).
Figure 3.
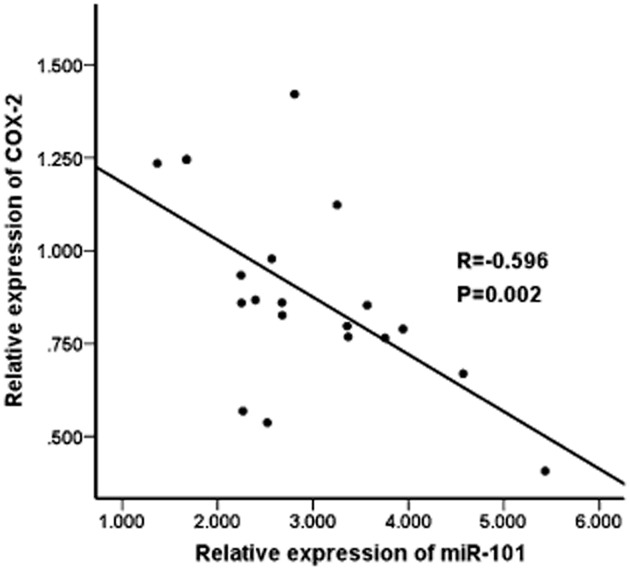
Correlation of the expression of micro ribonucleic acid (miR)-101 and the level of cyclooxygenase-2 (COX-2) in lung cancer tissues.
Effect of enforced expression of miR-101 on the COX-2 levels of A549 cells
The ratio of transfection was about 60%. As shown in Figure 4, the relative level of COX-2/β-Actin in A549-miR-NC cells was 1.579 ± 0.185, while the relative level of COX-2/β-Actin in A549-miR-101 cells was 0.367 ± 0.089 (P < 0.001). Therefore, the relative level of COX-2/β-Actin in A549 cells was attenuated by transfection of miR-101.
Figure 4.
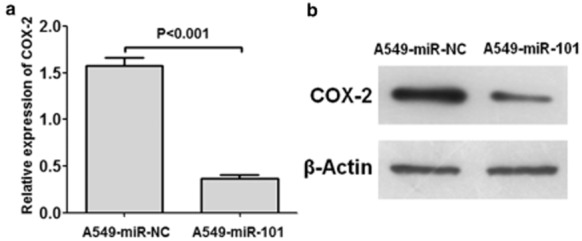
Effect of enforced expression of micro ribonucleic acid (miR)-101 on the cyclooxygenase-2 (COX-2) levels of A549 cells. (a) Levels of COX-2/β-Actin of A549-negative control oligonucleotide duplex mimic (miR-NC) and A549-miR-101 cells. The relative level of COX-2/β-Actin of A549 cells was attenuated by transfection of miR-101. (b) Representative images of the Western-blotting of COX-2 and β-Actin.
Effect of enforced expression of miR-101 on the proliferation of A549 cells in vitro
As shown in Figure 5a, cloning efficiency was comparable in A549 and A549-miR-NC cells. By the second week, cloning efficiencies were as follows: A549 cells 66.1 ± 6.8%, A549-miR-NC cells 68.2 ± 10.2% (P = 0.642). The cloning efficiency of A549-miR-101 cells (39.8 ± 10.2%) by the second week was significantly lower than that of A549-miR-NC cells (P = 0.002). Therefore, the cloning efficiency of A549 cells was significantly attenuated by transfection of miR-101.
Figure 5.
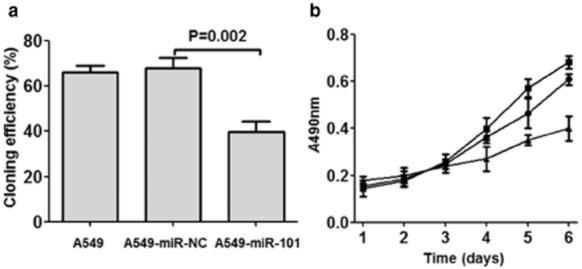
Effect of enforced expression of micro ribonucleic acid (miR)-101 on the proliferation of A549 cells in vitro. (a) Cloning efficiency of A549, A549-negative control (NC), and A549-miR-101 cells. The cloning efficiency was comparable in A549 cells and in A549-miR-NC, while the cloning efficiency was significantly attenuated by transfection of miR-101. (b) Growth ability of A549, A549-NC, and A549-miR-101 cells. The growth ability was comparable in A549 and in A549-miR-NC cells, while the growth ability was significantly attenuated by transfection of miR-101.  , A549;
, A549;  , A549-miR-NC;
, A549-miR-NC;  , A549-miR-101.
, A549-miR-101.
The growth abilities of cells were determined by MTT assay. As shown in Figure 5b, growth ability was comparable in A549 and A549-miR-NC cells. By the sixth day, the cell viability of A549 cells was 0.609 ± 0.025, while the cell viability of A549-miR-NC cells was 0.682 ± 0.026 (P = 0.531, compared with A549 cells). The cell viability of A549-miR-101 cells by the sixth day was 0.401 ± 0.052 (P = 0.003, compared with A549-miR-NC cells). Therefore, the growth ability of A549 cells was significantly attenuated by transfection of miR-101.
Effect of enforced expression of miR-101 on the growth of A549 cells in vivo
The effect of enforced expression of miR-101 on the growth of A549 cells was further tested in a tumor xenograft animal model (Fig 6). Two weeks after subcutaneous inoculation of A549-miR-NC or A549-miR-101 cells into BALB/c athymic nude mice, each cell inoculation developed into a solid tumor xenograft. The growth curve of tumor xenografts showed that enforced expression of miR-101 slowed tumor growth. By week four, the tumor volume of A549-miR-101 was lower than A549-miR-NC cells (206 ± 64 mm3 vs. 795 ± 255 mm3, P = 0.003). By week six, the average tumor volume of A549-miR-101 cells was 864 ± 237 mm3, while the average tumor volume of A549-miR-NC cells was 2157 ± 598 mm3.
Figure 6.
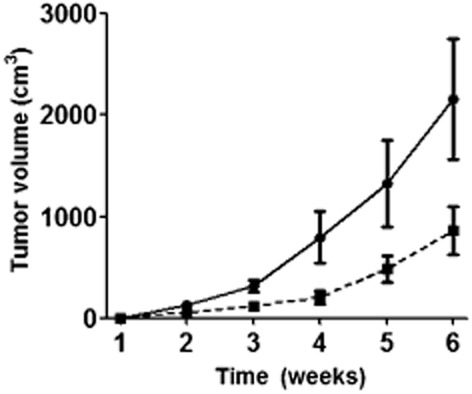
Effect of enforced expression of micro ribonucleic acid (miR)-101 on the growth of A549 cells in vivo. Solid line: the growth curve of tumor xenografts of A549- negative control (NC) cell. Dotted line: the growth curve of tumor xenografts of A549-miR-101 cell. The tumor volume of A549-miR-101 was lower than that of A549-miR-NC cells since week 4.  , A549-miR-NC;
, A549-miR-NC;  , A549-miR-101.
, A549-miR-101.
Discussion
Recent studies have revealed that cancer-associated miRNAs play important roles in tumorigenesis and may serve as diagnostic and prognostic biomarkers of various cancers.13–15 Increasing evidence indicates that miRNA deregulation has frequently been observed in the proliferation, differentiation, apoptosis, metastasis, and drug resistance of cancer cells.16–19 Although the precise function of individual miRNAs has not been characterized, biochemical and genetic studies have revealed that miRNAs regulate a variety of biological processes, such as cell proliferation, apoptosis, development, and differentiation.20
Lung cancer is the most common type of malignant tumor and the leading cause of cancer-related mortality worldwide. Any significant increase in tumor mass must be preceded by an increase in vascular supply to deliver nutrients and oxygen to the tumor.21 Therefore, angiogenesis plays an important role in the process of cancer invasion and metastasis. COX-2 is an inducible enzyme that catalyzes arachidonic acid to prostaglandins during inflammatory and tumorigenic stages. Many studies have revealed that COX-2 might be involved in angiogenesis.22–24 Recent clinical epidemiological studies have demonstrated the preventive effect of COX inhibitors on cancer.25 Thus, COX-2 is a critical target that has implications for both the prevention and treatment of cancer. To date, the mechanism underlying the role of COX-2 in tumor angiogenesis has been unclear. The most important substance that is regulated by COX-2 is vessel endothelial growth factor (VEGF), a highly specific mitogen of vessel endothelial cells.26 It has been reported that COX-2 might interact with VEGF. The regulatory effect of COX-2 on VEGF production and tumor angiogenenesis suggests that COX-2 expression might be an upstream event in tumor angiogenesis.27
There is strong evidence that miR-101 plays the role of tumor suppressor in various cancers. Predictions based on PicTar and TargetScan DNA analysis software suggest that COX-2 messenger RNA 3-untranslated region is a target gene of miR-101.28 The depressed expression of miR-101 would result in elevated expression of its target gene, COX-2, and the promotion of tumorigenesis. Although several studies have reported that miR-101 can inhibit cancer cell growth via blocking COX-2 expression, the role of miR-101 in COX-2 regulation of lung cancer growth has not been fully elucidated. In this study, we explored the role of miR-101 in the development and process of lung cancer. We found that the expression of miR-101 was significantly lower and the level of COX-2 was significantly higher in lung cancer tissue than that in adjacent parenchyma. Correlation analysis showed that the expression of miR-101 negatively correlated with the level of COX-2 in lung cancer tissues. Furthermore, we found that enforced expression of miR-101 decreased the level of COX-2 of A549 cells and inhibited the proliferation of cells in vitro and in vivo. Our data showed that miR-101 expression was significantly reduced in lung cancer, which may play a critical role in the increase of COX-2 and the development of lung cancer.
There are some limitations to our study. Our study demonstrated that miR-101 plays a tumor suppressor role in lung cancer. However, this fact does not preclude the possibility that miR-101 has other targets that have similar cellular functions to COX-2. Zheng et al. reported that enforced expression of miR-101 in hepatocellular carcinoma cells simultaneously decreased enhancer of zeste homolog 2 (EZH2), COX2, stathmin 1, and rho-associated protein kinase 2.29 MiR-101 has also been reported to target other genes, such as mammalian target of rapamycin, zinc finger E-box binding homeobox 1 (ZEB1), ZEB2, sex determining region Y-box 9, and Ras-related C3 botulinum toxin substrate 1.30–34 Cho et al. reported that miR-101 inhibits lung cancer invasion through the regulation of EZH2, which plays a part in the trimethylation of H3K27 and leads to the silencing of tumor-suppressor genes.30 This suggests that miR-101 modulates multiple steps, such as proliferation, adhesion, and migration of tumor cells in lung cancer. Thus, miR-101 might be a powerful anti-cancer therapeutic modality by repressing multiple molecular targets. This needs to be investigated in future.
Conclusion
In summary, we demonstrated that the expression of miR-101 decreases in lung cancer, and enforced expression of miR-101 could remarkably reduce the cell proliferation and invasion ability of lung cancer cells. This study provides new insight into the role of miR-101 in lung cancer and shows the promise of miR-101 as a potential molecular target for lung cancer.
Disclosure
No authors report any conflict of interest.
References
- Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30:93–98. doi: 10.1055/s-0033-1342949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Lin C, Huang F, Zhang YJ, Tuokan T, Kuerban G. Roles of MiR-101 and its target gene Cox-2 in early diagnosis of cervical cancer in Uygur women. Asian Pac J Cancer Prev. 2014;15:45–48. doi: 10.7314/apjcp.2014.15.1.45. [DOI] [PubMed] [Google Scholar]
- Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. (Published erratum appears in 2004; : 631) [DOI] [PubMed] [Google Scholar]
- Wu AW, Gu J, Li ZF, Ji JF, Xu GW. COX-2 expression and tumor angiogenesis in colorectal cancer. World J Gastroenterol. 2004;10:2323–2326. doi: 10.3748/wjg.v10.i16.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, van den Berg A, Huitema S, Gouw AS, Molema G, de Jong KP. Correlation of microRNA-16, microRNA-21 and microRNA-101 expression with cyclooxygenase-2 expression and angiogenic factors in cirrhotic and noncirrhotic human hepatocellular carcinoma. PLoS ONE. 2014;9(4):e95826. doi: 10.1371/journal.pone.0095826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104:15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Zhang M. COX2 regulation of breast cancer bone metastasis. Oncoimmunology. 2013;2(3):e23129. doi: 10.4161/onci.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Ruan HJ, He XJ, et al. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qi F, Cao Y, Chen M, Zu X. Down-regulated microRNA-101 in bladder transitional cell carcinoma is associated with poor prognosis. Med Sci Monit. 2014;20:812–817. doi: 10.12659/MSM.890300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu KL, Chari R, Lockwood WW, Lam S, Lam WL. miR-101 DNA copy loss is a prominent subtype specific event in lung cancer. J Thorac Oncol. 2011;6:1594–1598. doi: 10.1097/JTO.0b013e3182217d81. [DOI] [PubMed] [Google Scholar]
- Shen Q, Bae HJ, Eun JW, et al. MiR-101 functions as a tumor suppressor by directly targeting nemo-like kinase in liver cancer. Cancer Lett. 2014;344:204–211. doi: 10.1016/j.canlet.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Xu Y, An Y, Wang Y, et al. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2013;29:2019–2024. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- Zhan P, Qian Q, Yu LK. Prognostic value of COX-2 expression in patients with non-small cell lung cancer: A systematic review and meta-analysis. J Thorac Dis. 2013;5:40–47. doi: 10.3978/j.issn.2072-1439.2013.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Wang M, Jin C, Qi Q. miR-101 sensitizes A549 NSCLC cell line to CDDP by activating caspase 3-dependent apoptosis. Oncol Lett. 2014;7:461–465. doi: 10.3892/ol.2013.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li X, Yang Q, et al. The role of microRNA in human lung squamous cell carcinoma. Cancer Genet Cytogenet. 2010;200:127–133. doi: 10.1016/j.cancergencyto.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Varambally S, Cao Q, Mani RS, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322:1695–1699. doi: 10.1126/science.1165395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki N, Abroun S, Soleimani M, et al. Involvement of microRNA in T-cell differentiation and malignancy. Int J Hematol Oncol Stem Cell Res. 2015;9:33–49. [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park W, Ryu H, Jeon NL. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics. 2014;8(5):054102. doi: 10.1063/1.4894595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Gao Q, Jiang W. Relationship between tumour angiogenesis and expression of cyclo-oxygenase-2 and vascular endothelial growth factor-A in human renal cell carcinoma. J Int Med Res. 2015;43:110–117. doi: 10.1177/0300060514545799. [DOI] [PubMed] [Google Scholar]
- Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, Agarwal R. Silibinin modulates TNF-α and IFN-γ mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2012;51:832–842. doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Hwang SH, et al. Dual inhibition of cyclooxygenase-2 and soluble epoxide hydrolase synergistically suppresses primary tumor growth and metastasis. Proc Natl Acad Sci U S A. 2014;111:11127–11132. doi: 10.1073/pnas.1410432111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Bechi P, et al. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–1347. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- Leung WK, To KF, Go MY, et al. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317–1322. [PubMed] [Google Scholar]
- Hao Y, Gu X, Zhao Y, et al. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev Res (Phila) 2011;4:1073–1083. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Liao YJ, Cai MY, et al. Systemic delivery of microRNA-101 potently inhibits hepatocellular carcinoma in vivo by repressing multiple targets. PLoS Genet. 2015;11(2):e1004873. doi: 10.1371/journal.pgen.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HM, Jeon HS, Lee SY, et al. MicroRNA-101 inhibits lung cancer invasion through the regulation of enhancer of zeste homolog 2. Exp Ther Med. 2011;2:963–967. doi: 10.3892/etm.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Cogdell D, Hu L, et al. MiR-101 suppresses the epithelial-to-mesenchymal transition by targeting ZEB1 and ZEB2 in ovarian carcinoma. Oncol Rep. 2014;31:2021–2028. doi: 10.3892/or.2014.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Guan H, Li H, et al. MiR-101 inhibits cell proliferation by targeting Rac1 in papillary thyroid carcinoma. Biomed Rep. 2014;2:122–126. doi: 10.3892/br.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Shao NN, Fan L, Ma XC, Pu FF, Shao ZW. Effect of microRNA-101 on proliferation and apoptosis of human osteosarcoma cells by targeting mTOR. J Huazhong Univ Sci Technolog Med Sci. 2014;34:889–895. doi: 10.1007/s11596-014-1369-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo X, Xiong L, et al. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362–4370. doi: 10.1016/j.febslet.2012.10.053. [DOI] [PubMed] [Google Scholar]


