Abstract
Background
Lung cancer is the leading cause of cancer-related death in China. Results from a randomized controlled trial using annual low-dose computed tomography (LDCT) in specific high-risk groups demonstrated a 20% reduction in lung cancer mortality.
Methods
A China national lung cancer screening guideline was developed by lung cancer early detection and treatment expert group appointed by the National Health and Family Planning Commission, based on results of the National Lung Screening Trial, systematic review of evidence related to LDCT screening, and protocol of lung cancer screening program conducted in rural China.
Results
Annual lung cancer screening with LDCT is recommended for high risk individuals aged 50–74 years who have at least a 20 pack-year smoking history and who currently smoke or have quit within the past five years. Individualized decision making should be conducted before LDCT screening. LDCT screening also represents an opportunity to educate patients as to the health risks of smoking; thus, education should be integrated into the screening process in order to assist smoking cessation.
Conclusions
A lung cancer screening guideline is provided for the high-risk population in China.
Keywords: China, low-dose computed tomography, lung cancer screening guideline
Introduction
Lung cancer is the leading cause of cancer-related death in the world. However, lung cancer mortality has steadily declined in some developed countries as a result of effective tobacco control and the improvement of early detection and treatment methods. In the United States (US), lung cancer mortality rates in men declined 36% between 1990 and 2011, and 11% between 2002 and 2011 in women.1 However, because of population age, high smoking prevalence, and serious air pollution, lung cancer in China has increased 465% during the past 30 years; since 2000, it has been ranked the highest among all types of cancer.2 During 2003–2005, the five-year survival rate of lung cancer in China was 19.5% and 11.2% in urban and rural areas, respectively.3
In 2003, with the increasing burden of cancer, the Chinese Ministry of Health issued a “Compendium of Cancer Prevention and Control in China (2004–2010).”4 Subsequently, a Chinese lung cancer early detection and treatment expert group issued a lung cancer screening guideline draft, authorized by the Ministry of Health.5 In view of the burden of lung cancer in China and excellent prognosis of lung cancer cases detected by low-dose computed tomography (LDCT), lung cancer screening in rural areas was included into the “Cancer Early Detection and Treatment Program of China” in 2009, supported by a public health special subsidy from the central government (although LDCT efficacy was not confirmed until 2011). Based on this program, an updated draft of the China National Lung Cancer Screening Guideline with LDCT was formulated to provide a screening protocol for the China National Lung Cancer Screening Program.6 In this program, early stage lung cancer in baseline and annual repeat screening accounted for 36.2% and 80.0% of all lung cancer cases, respectively. In 2011, the result of National Lung Screening Trial (NLST) was published and identified a 20% reduction in lung cancer–specific mortality, attributed to low dose computed tomography (LDCT) screening in a high-risk population.
In this article, a China national lung cancer screening guideline is proposed, based on NLST results and the draft of the LDCT Lung Cancer Screening Guideline in China. The article focuses on: (i) who should be screened by LDCT; and (ii) screening procedure. The benefits and harms of LDCT and effective implementation are also discussed.
Background
In China, lung cancer incidence and mortality has increased during the last few decades. According to three National Death Surveys of China conducted over the last 40 years, crude lung cancer mortality has significantly increased from 5.46/100 000 in 1973–1975 to 30.84/100 000 in 2004–2005, with a corresponding change in rank from the fifth to the first cause of cancer-related death.2 In 2010, a total of 605 946 new lung cancer cases were diagnosed in China, with a crude incidence of 46.08/100 000, and 486 555 lung cancer deaths occurred with a crude mortality of 37.00/100 000, accounting for 19.59% and 24.87% of all cancer diagnoses and deaths, respectively.7
The mortality and incidence of lung cancer in urban areas and in men has traditionally been higher than in rural areas and in women; however, the difference between urban and rural areas, and men and women is gradually reducing. During 1989–2008, the incidence rate ratio (IRR) between urban and rural areas remarkably decreased from 2.07 to 1.14, while the IRR between men and women reduced from 2.47 to 2.28.8 During the period 1996–2005, the average annual increase of total expenditure on inpatients of lung cancer in China reached to 16.15%.9 Lung cancer has become a nationwide public health issue, giving rise to an enormous burden on patients, healthcare professionals, and society.
Smoking cessation is the most important measure of primary prevention of lung cancer. In the US, reduced tobacco smoking during the period 1975–2000 could have potentially prevented approximately 32% of lung cancer deaths.10 In China, although many measures have been implemented in recent years, including enforcing smoke-free policies in public places and increasing taxes, heavy smoking has increased substantially; in 1993–2003, only 7.9% of current smokers in urban locations reported intending to quit.11 Therefore, any effects of smoking cessation on lung cancer mortality will not have an impact in the next 20–30 years. Outdoor and indoor air pollution is another important risk factor of lung cancer in China. Similar to tobacco control, it will take a long time to reduce outdoor air pollution and its negative health effect in China.
Chest X-ray and sputum cytological examinations are the most common lung cancer screening modalities. In the 1970s, four randomized controlled trials (RCTs) using chest X-ray and sputum cytology were conducted.12–15 These studies reported an increase in the detection of early stage lung cancer, more resectable cancers, and improved five-year survival, but no significant reduction in lung cancer mortality in the screened arm compared to the control arm. However, these results were controversial because of methodological defects. The effectiveness of chest X-ray screening was re-evaluated in the Prostate, Lung, Colorectal and Ovarian Cancer screening trial (PLCO), initiated in the 1990s. In the PLCO trial, no mortality benefit was demonstrated by chest X-ray screening.16
Since the 1990s, a number of uncontrolled studies have been conducted to evaluate the effectiveness of LDCT as a lung cancer screening tool. Results have shown that early stage lung cancer could be detected by LDCT, and patients with lung cancer detected by LDCT had excellent survival prospects.17 In light of the positive developments of LDCT screening, several prospective RCTs comparing LDCT with chest X–ray or usual care were launched in the US and Europe. NLST, the largest trial among these, reported a 20% reduction in lung cancer mortality after three rounds of LDCT screening, compared with chest X-ray.18 This was the first evidence from a RCT regarding the effectiveness of lung cancer screening.
Population-based lung cancer screening with chest X-ray and sputum cytology was conducted in occupational miners of the Yunnan Tin Corporation (YTC) in Gejiu City, Yunnan Provence, China. According to the nationwide mortality survey conducted in 1973–1975, this location had the highest lung cancer mortality rate in men in 2392 counties or cities in all of China.19 Compared with interval cases, screening-detected cases had earlier stage and better survival. However, as an uncontrolled screening program, the effectiveness of screening with chest X-ray and sputum cytology could not be evaluated. Recently, two government-sponsored LDCT lung cancer screening programs were initiated in rural and urban China.20,21
Materials and methods
A lung cancer early detection and treatment expert group developed this guideline. Panelists were selected and appointed by the National Health and Family Planning Commission and had multidisciplinary academic backgrounds, including medical oncology, thoracic surgery, radiology, pathology, and epidemiology. The guideline is based on: results of NLST, a systematic review of evidence related to LDCT screening, and the practice of lung cancer screening in China, particularly the protocol of a LDCT screening program in rural China.6,18,22 Guidelines of the American Association for Thoracic Surgery (AATS), US Preventive Services Task Force (UPSTF), the American Lung Association (ALA), the American College of Chest Physicians (ACCP), the American Cancer Society (ACS), and the National Comprehensive Cancer Network (NCCN) were referenced.23
To develop the foundation for development of an evidence-based clinical guideline, a systematic review from a collaboration of the ACS, ACCP, the American Society of Clinical Oncology (ASCO), and NCCN was conducted, following the 2011 NLST publication.22 Eight RCTs and 13 cohort studies were selected from 591 citations by literature search. Based on the selected studies, four key issues were reviewed: potential benefits of LDCT screening; potential harms of LDCT screening; patients likely to benefit; and effective screening settings. The expert panel consider that this comprehensive systematic review qualified as an evidence base for an LDCT screening guideline.
Primary results of the LDCT Lung Cancer Screening Program in rural China, launched in 2010, demonstrated that 36.2% and 80.0% of early lung cancer detection rates among high-risk individuals in baseline and annual repeat screening, respectively. The protocol of this program will be adopted to develop the China Lung Cancer Screening Guideline with LDCT.6
Results
Participant selection and screening interval
In the systematic review, the age for initiation and cessation of screening in RCTs ranged from 47–60 and 69–80 years, respectively, while the smoking exposure ranged from 15–30 pack-years, with the maximum time since quitting ranging from 10 to an unlimited number of years. Currently, there is no evidence to support a specific age to initiate or cease screening. The NLST provides the only evidence demonstrating the benefit of LDCT screening and the criteria included were: smokers with at least 30 pack-years of exposure, aged 55–74 years, who had quit smoking <15 years ago.
Several issues should be considered when defining an age interval for screening, including age-specific incidence and life expectancy. In China, lung cancer age-specific incidence is relatively low up to 45 years of age, but then dramatically increases, peaking at 80–84 years of age in both men and women. The rates in men were 76, 136, 212, 295, 409, 509, 561, and 486 per 100 000 in 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84, and 85 + age groups, respectively.7 In 2012, the average life expectancy in China was 75 years.24 In addition, the life expectancy at age 75 was nine years, thus, only half of the Chinese population are expected to live past the age of 84. Obviously, lung cancer risk increases with age. However, because of competing causes of death and decreased health status, it is difficult to weigh the benefits and potential harms related to LDCT screening in patients older than 74 years.
In the systematic review, screening was conducted annually in all RCTs, except the study by NELSON, which had planned screening at years one, two and four. All cohort studies conducted annual screening, except one biennial and one semiannual. However, no definitive evidence of a specific screening interval was found.
Based on these data, annual LDCT screening for lung cancer is recommend in persons aged 50–74 years with a minimum smoking history of 20 pack-years, who currently smoke or have quit smoking within the past five years. In regions with high lung cancer incidence caused by specific environmental or occupational carcinogens, annual screening is recommended. For example, persons aged 50–74 years, with at least 10 years exposure of domestic coal use in Xunwei City or occupational radon in Gejiu, Yunnan, are enrolled in the lung cancer screening program in rural China. Individuals who have a cancer history within the last five years (except for non-melanoma skin cancer, cervical carcinoma in situ, or localized prostate cancer), cannot tolerate possible lung cancer resection, or have a life-threatening disease, are not recommended for screening.
Low-dose computed tomography screening and nodule management
There are several differences in RCTs or cohort studies, including the definition of positive nodules, further work-up procedures for nodules with different characteristics (e.g. size, growth rate), and screening rounds (baseline or annual). However, how to define the optimal management protocol to discriminate between malignant and benign nodules is as yet undetermined. In the NLST, nodules measuring 4 mm or more received further work up.
The nodule management protocol adopted in this guideline was that used by LDCT lung cancer screening program in rural China, which demonstrated a high early detection rate.
Definition of positive screening scan
Nodules detected by LDCT are classified into two groups: definitely benign or calcified nodules and uncertain or non-calcified nodules. The latter groups were followed up according to characteristic and size of nodules.
Baseline screening: the result of CT scanning is positive if at least one solid or part-solid nodule ≥5 mm in diameter, or non-solid nodules ≥8 mm in diameter, or suspicious tracheal and/or bronchial lesions, are identified.
Repeat annual screening: any new non-calcified nodules or airway lesions are identified, or enlargement of a nodule is detected, compared with the baseline or last annual CT scan.
Nodule management and possible work-up
Nodule detected in baseline screening (Fig 1)
Figure 1.
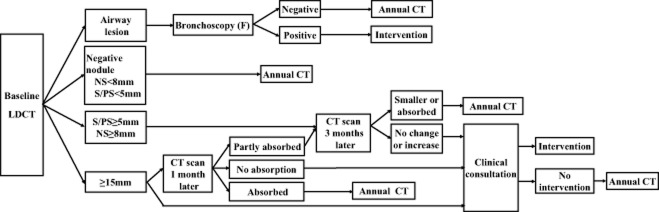
Nodule management guideline for baseline screening. CT, computed tomography; F, bronchoscopy; LDCT, low dose CT; S, solid nodules; PS, part solid nodules; NS, non solid nodules.
Solid or part-solid nodules <5 mm in diameter or non-solid nodules <8 mm in diameter enter into the next round of annual screening. Solid or part-solid nodules 5–14 mm in diameter or non-solid nodules 8–14 mm in diameter are followed-up by routine CT scanning three months later. If nodules continue to enlarge, clinical consultation by a senior clinical expert (a thoracic surgeon, radiologist, or pathologist) is recommended to determine the necessity of clinical intervention. In cases of no growth, a next round screening visit is recommended. For nodules 15 mm or larger, two pathways can be selected: immediate clinical consultation or a rescan after anti-inflammatory treatment. In the latter case, if the nodules are absorbed, participants enter into the next round screening, but if no changes in the nodules occur, clinical consultation is needed. If the nodules are partly absorbed, a further CT scan will be conducted, and based on changes to the nodules, different solutions are taken. Bronchoscopy is recommended if suspicious tracheal and/or bronchial lesions are detected.
Nodule detected in annual screening (Fig 2)
Figure 2.
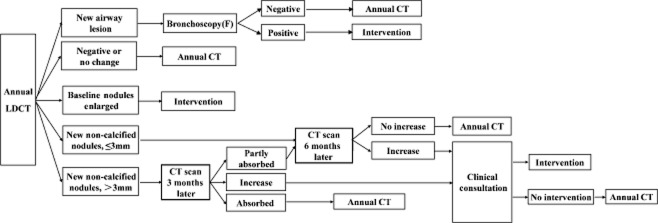
Nodule management guideline for annual screening. CT, computed tomography; F, bronchoscopy; LDCT, low dose CT
In patients with ≤3 mm new nodules, a rescan six months later is recommended. If the nodules continue to enlarge, patients will receive clinical consultation to determine the necessity of clinical intervention, otherwise they will receive a next round screening visit. For participants with >3 mm new nodules, a rescan three months later is recommended, and when necessary, anti-inflammatory treatment is provided in advance. If the nodules continue to enlarge, patients receive clinical consultation; if nodules are absorbed completely, patients wait for the next round of annual screening. However, if nodules are only partly absorbed, a second rescan six months later is provided; if the nodules grow, patients receive clinical consultation, otherwise they receive the next annual screening. When current images reveal significant enlargement or an increase to solid components compared with former corresponding images, clinical intervention is necessary. Bronchoscopy is recommended if suspicious tracheal and/or bronchial lesions are detected.
Integration of smoking cessation
It is well known that smoking cessation can reduce lung cancer risk. A number of studies have provided evidence that an abnormal finding via LDCT screening represents an opportunity for a health provider to initiate a conversation with a patient as to the benefits of ceasing smoking; however, whether such education can affect the smoking cessation rate has not been determined.25–28 On the other hand, results from a microsimulation model, in which cost per quality-adjusted life-year gained ($/QALY) were estimated, suggested that the cost-effectiveness of CT screening programs would be strongly influenced by smoking cessation rates among screening participants.29 ACCP guidelines also emphasize the need for smoking cessation.23 Thus, LDCT screening should not be viewed as an alternative to smoking cessation, instead, education as to the benefits of smoking cessation should be integrated into LDCT screening.
Shared decision making
While there are benefits to the mortality rate using LDCT screening, there are also adverse effects, including radiation risk, and false-positive results, which lead to further unnecessary and invasive procedures to procure diagnosis. Thus, it is import for eligible individuals to fully understand the benefits, limitations, and potential harms associated with LDCT screening. They should discuss these issues with physicians or healthcare providers before accepting any recommendation for LDCT screening.
Discussion
Most RCTs and cohort studies enrolled a high-risk population for screening according to age and smoking; however, increased evidence suggests that a risk prediction model might contribute to a refinement of selection criteria, such as the Bach, Spitz, and Liverpool models.30–32 The PCLOM2012 model had improved sensitivity and positive predictive values compared with NLST criteria, without any change in screening effect.33 The NCCN and AATS lung cancer screening guidelines include other factors for risk assessment.34 In the future, developing and validating a novel lung cancer prediction model is warranted to improve screening effect and reduce cost.
When individuals at high risk of lung cancer are recommended for LDCT lung cancer screening, potential harms exist.22 Most notably, a high false positive rate can lead to a large number of follow-up CTs; unnecessary and invasive biopsies with resulting morbidity and, in rare cases, mortality; anxiety; and waste of medical resources. In our recommendation, the criteria to suggest baseline screening includes nodules 5 mm in diameter, larger than the 4 mm in diameter recommended by the NLST; accordingly, the positive rate would be reduced to less than 20% without any loss in early lung cancer detection. It has been reported that predictive pools based on patient and nodule characteristics could be used to accurately estimate the probability that lung nodules detected on baseline screening are malignant.35 Volume double time based on annual screening might also be a valuable tool in the differentiation between benign and malignant pulmonary nodules; volumetric analysis was used in a Dutch-Belgium lung cancer screening trial (NELSON).36 We will trace the progress of these trials in order to revise the current screening guideline.
Radiation risk is another factor that needs to be considered, especially for individuals at a low risk of lung cancer. A preliminary modeling study suggested that in patients aged under 50, the mortality reduction from lung CT screening could not outweigh the radiation risk.37 Based on NLST data, approximately one cancer death may have been caused by radiation from CT per 2500 persons screened; thus, the benefit in preventing lung cancer death using the NLST was greater than the radiation risk. Technical improvements in CT scanners and settings will lead to lower doses; thus, accordingly, the radiation risk with CT screening may decrease.
Until now, the mortality benefit of LDCT screening has only been shown in an organized setting; therefore, opportunistic screening is not recommended. Similar to the NLST, most other RCTs and cohort studies involve academic medical centers, large hospitals, multidisciplinary teams, specific inclusion criteria, and nodule management protocols. However, it is unclear whether the NLST result can be generalized. Some organizations have suggested that demonstration projects should be implemented to evaluate how well LDCT screening can be implemented for different geographic regions or countries and to address the uncertainties associated with LDCT screening.23,38 In China, a demonstration program of an ongoing prospective, multi-centre, population-based lung cancer screening was initiated in 2010 to evaluate the feasibility of conducting population-based LDCT lung cancer screening in a Chinese context.20
Molecular markers may be helpful for identifying the high-risk population who will benefit most from LDCT screening and, thus, reduce the cost and screening-related harms. Additionally, the combination of molecular markers and LDCT in lung cancer screening may reduce high false-positive results.39 The NLST has established a biospecimen repository that includes blood, sputum, and urine samples collected serially during the screening course, which aims to develop and validate biomarkers in determining high risk individuals, distinguishing benign from malignant lung nodules detected by CT scan, and predicting tumor behavior.40 To date, numerous biomarkers, including gene methylation, micro-ribonucleic acid, and autoantibodies have been used for potential screening; however, most of these need prospective population-based validation. More research is warranted to translate biomarkers from discovery to clinical application.
Conclusion
Lung cancer is the leading cause of cancer-related death in China and has a poor prognosis. There is rigorous evidence to support the efficacy of LDCT in the early diagnosis of lung cancer. To maximize benefits and minimize harms, it is important that LDCT screening should be implemented in an organized manner, and individualized decision making should be conducted before acceptance of LDCT screening. The recommendations of this study will be revised with new evidence from the NLST, NELSON, and continuing trials, and the potential integration of biomarkers to augment LDCT screening of lung cancer.
Acknowledgments
This study was partly supported by the grants from the National High Technology Research and Development of China (No. 2012AA02A502) and National Project of Lung Cancer Early Diagnosis and Treatment of China (No. 201501002-1), the National Eleventh-Five-Year Key Task Projects of China (No.2006BAI02A01), and the China-Sweden International Scientific and Technological Cooperative Project (No. 09ZCDSF04100). We also thank the Cancer Foundation of China for organizing and implementing lung cancer screening programs in rural China.
Disclosure
No authors report any conflict of interest.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Ministry of Health People’s Republic of China. Report of the Third National Retrospective Survey of Death Causes in China. Beijing: Chinese Academy of Medical Sciences & Peking Union Medical College Press; 2008. pp. 49–59. [Google Scholar]
- Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003–2005: A population-based study. Int J Cancer. 2015;136:1921–1930. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- Ministry of Health People’s Republic of China. Compendium of cancer prevention and control in China (for year 2004–2010) China Cancer. 2004;14:65–68. (In Chinese.) [Google Scholar]
- Zhou Q, Wen Z, Wang Y. Screening, early detection and treatment of lung cancer. In: Zhiwei Dong, et al., editors. Screening, Early Detection and Treatment of Major Cancers in China. Beijing: Peking University Medical Press; 2004. pp. 154–176. [Google Scholar]
- Zhou Q, Qiao Y, Wu N, Huang Y, Wang G, Wang X. Protocol of Cancer Early Detection and Treatment (2011) Beijing: People’s Medical Publishing Press; 2011. Protocol of lung cancer early detection and treatment; pp. 172–209. Ministry of Health of the People’s Republic of China. [Google Scholar]
- Zheng R, Zeng H, Zhang S, et al. Lung cancer incidence and mortality in China, 2010. Thorac Cancer. 2014;5:330–336. doi: 10.1111/1759-7714.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Zheng R, Zhang S, Wu M, Chen W. Trend analyses on the differences of lung cancer incidence between gender, area and average age in China during 1989–2008. Chin J Lung Cancer. 2013;16:445–451. doi: 10.3779/j.issn.1009-3419.2013.09.02. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang Y, Guo B, et al. Status quo and issues of direct impatient cost of lung carcinoma in China. Chin Health Econ. 2007;26:59–62. [Google Scholar]
- Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst. 2012;104:541–548. doi: 10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Cai M, Gao J, Tang S, Xu L, Critchley JA. Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data. Bull World Health Organ. 2010;88:769–776. doi: 10.2471/BLT.09.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana RS, Sanderson DR, Woolner LB, et al. Screening for lung cancer. A critique of the Mayo Lung Project. Cancer. 1991;67(4 Suppl):1155–1164. doi: 10.1002/1097-0142(19910215)67:4+<1155::aid-cncr2820671509>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Frost JK, Ball WC, Jr, Levin ML, et al. Early lung cancer detection: Results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984;130:549–554. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest. 1984;86:44–53. doi: 10.1378/chest.86.1.44. [DOI] [PubMed] [Google Scholar]
- Kubík A, Polák J. Lung cancer detection. Results of a randomized prospective study in Czechoslovakia. Cancer. 1986;57:2427–2437. doi: 10.1002/1097-0142(19860615)57:12<2427::aid-cncr2820571230>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- International Early Lung Cancer Action Program Investigators. Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YG, Hu P, Jiang Y, et al. Association between sputum atypia and lung cancer risk in an occupational cohort in Yunnan, China. Chest. 2009;135:778–785. doi: 10.1378/chest.08-1469. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Fan Y, Wu N, et al. Demonstration program of population-based lung cancer screening in China: Rationale and study design. Thorac Cancer. 2014;5:197–203. doi: 10.1111/1759-7714.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai MSJ, Li N. Design and goal of urban cancer early diagnosis and treatment project in China. Chin J Prev Med. 2013;47:179–182. (In Chinese.) [Google Scholar]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: A systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. (Published errata appear in JAMA 2013; 309: 2212; JAMA 2012; 308: 1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e78S–92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2015. Global Health Observatory Data Repository: Life Expectancy Data by Country [Cited 16 Jul.] Available from URL: http://apps.who.int/gho/data/view.main.680.
- Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc. 2014;11:619–627. doi: 10.1513/AnnalsATS.201312-460OC. [DOI] [PubMed] [Google Scholar]
- Poghosyan H, Kennedy Sheldon L, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: A teachable moment? Cancer Nurs. 2012;35:446–475. doi: 10.1097/NCC.0b013e3182406297. [DOI] [PubMed] [Google Scholar]
- MacRedmond R, McVey G, Lee M, et al. Screening for lung cancer using low dose CT scanning: Results of 2 year follow up. Thorax. 2006;61:54–56. doi: 10.1136/thx.2004.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Hong WK, Amos CI, et al. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99:715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: An individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. (Published erratum appears in N Engl J Med 2013; 369 : 394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DM, Gietema H, de Koning H, et al. Nodule management protocol of the NELSON randomised lung cancer screening trial. Lung Cancer. 2006;54:177–184. doi: 10.1016/j.lungcan.2006.08.006. [DOI] [PubMed] [Google Scholar]
- de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: A comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field JK, Smith RA, Aberle DR, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol. 2012;7:10–19. doi: 10.1097/JTO.0b013e31823c58ab. [DOI] [PubMed] [Google Scholar]
- Hensing TA, Salgia R. Molecular biomarkers for future screening of lung cancer. J Surg Oncol. 2013;108:327–333. doi: 10.1002/jso.23382. [DOI] [PubMed] [Google Scholar]
- Patz EF, Jr, Caporaso NE, Dubinett SM, et al. National Lung Cancer Screening Trial American College of Radiology Imaging Network Specimen Biorepository originating from the Contemporary Screening for the Detection of Lung Cancer Trial (NLST, ACRIN 6654): Design, intent, and availability of specimens for validation of lung cancer biomarkers. J Thorac Oncol. 2010;5:1502–1506. doi: 10.1097/JTO.0b013e3181f1c634. [DOI] [PMC free article] [PubMed] [Google Scholar]


