Abstract
Negative arterial to end-tidal Pco2 differences ((a-ET)Pco2) have been reported in normoxia. To determine the influence of blood temperature on (a-ET)Pco2, 11 volunteers (21 ± 2 years) performed incremental exercise to exhaustion in normoxia (Nx, PIo2: 143 mmHg) and hypoxia (Hyp, PIo2: 73 mmHg), while arterial blood gases and temperature (ABT) were simultaneously measured together with end-tidal Pco2 (PETco2). After accounting for blood temperature, the (a-ET) Pco2 was reduced (in absolute values) from −4.2 ± 1.6 to −1.1 ± 1.5 mmHg in normoxia and from −1.7 ± 1.6 to 0.9 ± 0.9 mmHg in hypoxia (both P < 0.05). The temperature corrected (a-ET)Pco2 was linearly related with absolute and relative exercise intensity, VO2, VCO2, and respiratory rate (RR) in normoxia and hypoxia (R2: 0.52–0.59). Exercise CO2 production and PETco2 values were lower in hypoxia than normoxia, likely explaining the greater (less negative) (a-ET)Pco2 difference in hypoxia than normoxia (P < 0.05). At near-maximal exercise intensity the (a-ET)Pco2 lies close to 0 mmHg, that is, the mean Paco2 and the mean PETco2 are similar. The mean exercise (a-ET)Pco2 difference is closely related to the mean A-aDO2 difference (r = 0.90, P < 0.001), as would be expected if similar mechanisms perturb the gas exchange of O2 and CO2 during exercise. In summary, most of the negative (a-ET)Pco2 values observed in previous studies are due to lack of correction of Paco2 for blood temperature. The absolute magnitude of the (a-ET)Pco2 difference is lower during exercise in hypoxia than normoxia.
Keywords: Blood temperature, gas exchange, gradient, hypoxia, Pco2
Introduction
The alveolar-to-arterial Po2 difference (A-aDO2, PAo2-Pao2) increases with exercise intensity in humans (Holmgren and Linderholm 1958; Dempsey et al. 1984), and to a greater extent in hypoxia than in normoxia (Torre-Bueno et al. 1985; Schaffartzik et al. 1992; Wagner 1992; Calbet et al. 2008). In contrast, both positive and negative arterial to end-tidal Pco2 ((a-ET)Pco2) values have been reported during exercise (Forster 1977; Gurtner 1977; Piiper 1986). It has been postulated that negative (a-ET)Pco2 differences could be in part due to measurement artifacts, such as loss of CO2 from blood samples, dilution with heparin solutions present in syringes, and underestimation of lung temperature (Scheid and Piiper 1980; Piiper 1986).
Although the (a-ET)Pco2 has been studied in healthy exercising humans (Whipp and Wasserman 1969; Jones et al. 1979; Robbins et al. 1990; Williams and Babb 1997) and patients with lung disease (Luft et al. 1979; Mahler et al. 1985; Liu et al. 1995), in none of these studies was the (a-ET)Pco2 calculation corrected to account for the increase of lung blood temperature during exercise.
Due to the high diffusivity of CO2, mean alveolar Pco2 (PAco2) is similar to the end capillary Pco2 in well-ventilated and perfused alveoli and hence, similar to Paco2 (Cerretelli and Di Prampero 1987). However, mean PAco2 and PETco2 may fluctuate differently during the respiratory cycle (Hlastala 1972), both at rest and during exercise (Dubois et al. 1952; Johnson et al. 2011). One of the main factors influencing PETco2 and hence (a-ET)Pco2, is the respiratory rate (Dubois et al. 1952; Hlastala 1972; Johnson et al. 2011). Compared to normoxia, during submaximal exercise in hypoxia pulmonary ventilation is increased by a combined elevation of tidal volume (VT) and respiratory rate (RR) (Paterson et al. 1987; Lundby et al. 2004; Calbet and Lundby 2009). In theory, for a given VO2, (a-ET)Pco2 should increase with greater ventilation. However, the effect of severe hypoxia on exercise (a-ET)Pco2 has not been assessed.
Therefore, the primary aim of this study was to examine the impact of: (1) blood temperature correction; and (2) severe hypoxia on the (a-ET)Pco2 difference during exercise in healthy subjects.
We hypothesized that: (1) correcting for blood temperature will reduce the absolute value of the (a-ET)Pco2 difference; and (2) the absolute value (a-ET)Pco2 difference will be lower during exercise in severe hypoxia than in normoxia, due to a greater impairment of pulmonary gas exchange during exercise in hypoxia.
Methods
General overview
This study was part of a larger project including several experiments designed to address the mechanisms limiting whole-body exercise performance in humans with assessment of central and local hemodynamics combined with measurements of oxygen transport, and pulmonary and muscle gas exchange (Calbet et al. 2015; González-Henriquez et al. 2015; Morales-Alamo et al. 2015). On the first visit to the laboratory, anthropometric measures and body composition analysis were performed. Thereafter, subjects reported to the laboratory on separate days to complete different incremental tests to exhaustion (see Respiratory variables below) in normoxia and hypoxia (Lode Excalibur Sport 925900, Groningen, The Netherlands). Subjects were requested to refrain from ingesting caffeine- and taurine-containing drinks and from exercise 24 h before the experiments.
Subjects
Eleven healthy men participated in these studies. Their mean ± SD age, height, weight, percentage of body fat, and maximal oxygen uptake (VO2max) were 21.5 ± 2.0 years, 173.8 ± 8.0 cm, 72.3 ± 9.3 kg, 16.1 ± 4.9%, and 3.621 ± 0.326 L min−1, respectively. Before any experimental procedure, subjects received full oral and written information about the experiments. The study was performed in accordance with the Helsinki Declaration and was approved by the Ethical Committee of the University of Las Palmas de Gran Canaria (CEIH-2010-01).
Catheterization and preparation for the experiments
Both femoral veins and one femoral artery were catheterized under local anesthesia (2% lidocaine), as previously reported (Calbet et al. 2006). In the right femoral vein, a 16G catheter was inserted 3-cm below inguinal ligament and advanced 12–13 cm distally (Arrow ES-04306). This catheter was used for saline ice-cold injection to measure the leg blood flow (LBF) by thermodilution (Andersen and Saltin 1985). In the same femoral vein, a thermodilution catheter (PV2014L16N, Pulsion Medical Systems AG, Munich, Germany) was inserted 2 cm below the inguinal ligament and advanced 12 cm cranially. This catheter was used to measure the temperature of the blood in the femoral vein. The same type of catheter was also inserted into the right femoral artery and used to measure blood pressure and femoral artery blood temperature. A final 20G catheter was inserted into the contralateral femoral vein from 2 cm below the inguinal ligament and advanced 12 cm in the direction toward the heart (Arrow ES-04150), and used for sampling femoral vein blood. All catheters were doubly sutured to the skin at the insertion point.
The two thermistors were connected to the temperature conditioning and processing boxes (Flemming Jessen Engineering, Copenhagen, Denmark), and the right femoral artery and vein catheters to blood pressure bridge amplifiers (ML-117, ADInstruments, Sydney, Australia).
An electrocardiogram (ECG) was displayed on a monitor during catheterization and the rest of the experimental procedures for safety reasons. The ECG, blood pressure, and the temperature registered by the thermistor, as well as the infusate temperature were recorded simultaneously with the data acquisition system (Power Lab ML880, ADInstruments, Bella Vista, Australia).
Exercise protocol
On the experimental day, subjects reported to the laboratory at 07.00 in fasted conditions. After catheterization, subjects were assigned randomly to either an incremental exercise test until volitional exhaustion in normoxia (PIo2: ∼143 mmHg) or hypoxia (PIo2: ∼73 mmHg, Altitrainer200, SMTEC, Switzerland). The test in normoxia started at 80 W with load increments of 30 W every 2 min. The test in hypoxia started at 60 W with load increments of 20 W every 2 min until exhaustion (Exh1). At exhaustion, the subjects were rapidly switched to breathe room air (normoxia) and were requested to continue exercising at the same load for 2 min, and then the load was increased by 20 W every 2 min until exhaustion (Exh2). The tests were separated by 90 min rest. After the second test, a lunch break and a 120 min resting period were followed. Thereafter, the incremental exercise in hypoxia was repeated.
Blood sampling
Blood samples were drawn simultaneously from the arterial and venous femoral catheters over a 10-sec period during the last minute of the step of each workload. The sampling period was then aligned with the respective respiratory data, assuming a circulating time of ∼10 sec (Calbet and Boushel 2015). Blood gases and hemoglobin concentrations were determined immediately after collection (ABL90, Radiometer, Copenhagen, Denmark). Uncorrected blood gases were expressed at 37°C. Arterial blood gasses and pH were corrected for blood temperature, using the arterial thermistor. Arterial Po2 and pH were corrected using Severinghaus equations (Severinghaus 1979), while Pco2 was corrected, using the equation Pco2tc = Pco2(37)*(10^0.021*(T-37)) according to Siggaard-Andersen (Siggaard-Andersen 1974), where Pco2tc is the temperature-corrected Pco2, Pco2(37) is the Pco2 measured at 37°C, and T is the arterial blood temperature.
Respiratory variables
Respiratory variables were recorded continuously with a metabolic cart (Vmax N29; Sensormedics, California), calibrated prior to each test according to the manufacturer instructions with high-grade calibration gases (Carburos Metálicos, Las Palmas de Gran Canaria). Respiratory variables were analyzed breath-by-breath and averaged every 10 sec during the incremental exercise tests. Then, the respiratory data were aligned with the appropriate blood sample, assuming a 10-sec shift between pulmonary gas exchange and arterial blood gases.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD) unless otherwise stated. Random-effects regression models were applied for data analysis. The random intercepts and slopes models were compared. The random intercepts models fit better into the data in all cases. Intercept and experimental error were assumed to have a Gaussian distribution. The model was estimated, using the restricted maximum likelihood method. For the goodness of fit, the conditional Nakagawa and Schielzeth’s R2GLMM was used (Nakagawa and Schielzeth 2013). In addition, near-maximal exercise (a-ET)Pco2 values were compared between normoxia and hypoxia, using a paired Student t-test. The relationship between the mean (a-ET)Pco2 and the mean A-aDO2 was tested with linear regression analysis. P ≤ 0.05 was considered significant. Analysis was performed using a commercially available software package (SPSS version 15.0, SPSS, Inc., Chicago, Illinois) and The R Project for Statistical Computing version 3.2.0.
Results
The mean responses of the respiratory variables to both exercise conditions are reported in Table1. The mean of all PETco2 measured values (submaximal and maximal exercise conditions) was 3.2 ± 2.3 mmHg higher than Paco2 (37.7 ± 5.8 mmHg and 33.5 ± 4.2 mmHg, respectively, P < 0.01). After temperature correction, the mean Paco2 increased to 34.9 ± 4.3 mmHg, consequently the (a-ET)Pco2 was increased from −3.2 ± 2.3 to −1.8 ± 2.1 mmHg (P < 0.01). This correction of the Paco2 value for arterial blood temperature accounted for 44% of the measured (a-ET)Pco2. The effect of the temperature correction on the magnitude of the (a-ET)Pco2 was greater during exercise in normoxia than hypoxia, and increased with exercise intensity (Table2). After accounting for blood temperature the (a-ET)Pco2 was increased from −4.2 to −1.1 mmHg in normoxia, and from −1.7 to 0.9 mmHg in hypoxia (Table2).
Table 1.
Respiratory variables and arterial blood pH during exercise in normoxia (PIo2: ∼143 mmHg) and hypoxia (PIo2: ∼73 mmHg)
| n | Intensity (%Wpeak) | Intensity (watts) | VO2 (L·min−1) | VCO2 (L·min−1) | VE (L·min−1) | RR (breaths·min−1) | VT (mL) | PETco2 (mmHg) | PETo2 (mmHg) | Paco2 (mmHg) | Paco2tc (mmHg) | Arterial pH tc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normoxia | |||||||||||||
| Mean | 9 | 28.5 | 82.2 | 1.58 | 1.39 | 35.9 | 21.1 | 1738 | 43.7 | 94.1 | 39.0 | 39.48 | 7.393 |
| SD | 1.7 | 6.7 | 0.14 | 0.24 | 6.9 | 4.7 | 301 | 3.7 | 5.9 | 3.8 | 3.73 | 0.022 | |
| Mean | 6 | 69.4 | 215 | 3.18 | 3.37 | 88.4 | 37.3 | 2366 | 43 | 102.1 | 38.6 | 40.37 | 7.304 |
| SD | 1.3 | 25.1 | 0.26 | 0.28 | 11.4 | 3.6 | 217 | 4 | 3.1 | 3.1 | 3.29 | 0.031 | |
| Mean | 11 | 78.3 | 225.5 | 3.25 | 3.55 | 99.4 | 39.7 | 2516 | 40.4 | 105.5 | 35.6 | 37.72 | 7.282 |
| SD | 2 | 34.5 | 0.38 | 0.48 | 13.4 | 6.2 | 215 | 2.5 | 2 | 1.7 | 2.2 | 0.038 | |
| Mean | 11 | 89.2 | 256.4 | 3.54 | 4.03 | 124.7 | 48.1 | 2594 | 37.1 | 109.7 | 32.8 | 34.99 | 7.259 |
| SD | 1.0 | 36.7 | 0.31 | 0.4 | 17.7 | 4.8 | 258 | 3.2 | 3.1 | 1.6 | 1.82 | 0.044 | |
| Mean | 9 | 100 | 290 | 3.62 | 4.24 | 143.2 | 59.1 | 2445 | 35.3 | 112.6 | 31.7 | 34.18 | 7.221 |
| SD | 0 | 42.4 | 0.39 | 0.47 | 19.4 | 7.3 | 374 | 2.1 | 3 | 2.9 | 3.06 | 0.041 | |
| Hypoxia | |||||||||||||
| Mean | 9 | 44.4 | 82.2 | 1.62 | 1.8 | 55.5 | 26.6 | 2118 | 34.4 | 43.2 | 32.3 | 32.69 | 7.441 |
| SD | 5.0 | 6.7 | 0.18 | 0.14 | 6.9 | 4.6 | 303 | 3.2 | 0.8 | 1.9 | 2.04 | 0.035 | |
| Mean | 7 | 70.7 | 125.7 | 1.96 | 2.37 | 79.2 | 36.7 | 2176 | 31.6 | 45.8 | 30.3 | 31.05 | 7.391 |
| SD | 4.8 | 27.6 | 0.31 | 0.48 | 18.7 | 9.4 | 222 | 3.2 | 2.2 | 2.5 | 2.42 | 0.04 | |
| Mean | 7 | 79.2 | 150 | 2.3 | 2.92 | 98.8 | 41.7 | 2372 | 30.4 | 47.4 | 29.6 | 30.55 | 7.335 |
| SD | 2.6 | 30 | 0.3 | 0.45 | 20.1 | 8.2 | 171 | 3.3 | 2.2 | 2.9 | 2.83 | 0.047 | |
| Mean | 6 | 88.8 | 160 | 2.33 | 2.95 | 104.7 | 48.4 | 2167 | 28.5 | 49 | 28.2 | 29.39 | 7.317 |
| SD | 1.3 | 21.9 | 0.22 | 0.43 | 12.3 | 4.6 | 205 | 2 | 1.4 | 2.1 | 2.03 | 0.064 | |
n = number of subjects.
Table 2.
Intensity (%Wpeak), arterial blood temperature (°C) and arterial-to-end-tidal Pco2 difference ((a-ET)Pco2) (mmHg) during exercise in normoxia (PIo2: ∼143 mmHg) and hypoxia (PIo2: ∼73 mmHg) without and with blood temperature correction (tc)
| Intensity (%Wpeak) | Arterial temperature (°C) | (a-ET)Pco2 (mmHg) | (a-ET)Pco2 (tc) (mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | Range | Mean | SD | Range | Mean | SD | Range |
| Normoxia | |||||||||||
| 28.5 | 1.7 | 9 | 37.3 | 0.5 | 36.4–38.0 | −4.8 | 1.8 | (−8.1) to (−2.8) | −4.2 | 1.6 | (−7.1) to (−2.3) |
| 69.4 | 1.3 | 6 | 37.9 | 0.4 | 37.4–38.6 | −4.4 | 1.4 | (−6.2) to (−2.9) | −2.6 | 1.3 | (−4.2) to (−0.9)* |
| 78.3 | 2.0 | 11 | 38.2 | 0.5 | 37.5–39.0 | −4.8 | 1.8 | (−8.0) to (−1.9) | −2.7 | 1.8 | (−6.2) to (0.8)* |
| 89.2 | 1.0 | 11 | 38.4 | 0.5 | 37.5–39.1 | −4.3 | 2.4 | (−9.6) to (−0.3) | −2.1 | 2.2 | (−6.5) to (2.2)* |
| 100.0 | 0.0 | 9 | 38.5 | 0.4 | 37.9–39.3 | −3.6 | 1.6 | (−5.6) to (−0.8) | −1.1 | 1.5 | (−3.4) to (0.8)* |
| Hypoxia | |||||||||||
| 44.4 | 5.0 | 9 | 37.2 | 0.4 | 36.4–37.7 | −2.0 | 1.8 | (−4.9) to (0.8) | −1.7 | 1.6 | (−4.1) to (0.1) |
| 70.7 | 4.8 | 7 | 37.5 | 0.5 | 36.8–38.1 | −1.3 | 1.3 | (−2.4) to (1.1) | −0.6 | 1.3 | (−2.3) to (1.6) |
| 79.2 | 2.6 | 7 | 37.7 | 0.3 | 37.1–38.0 | −0.8 | 1.0 | (−2.6) to (0.8) | 0.1 | 1.0 | (−1.2) to (1.7)* |
| 88.8 | 1.3 | 6 | 37.9 | 0.2 | 37.6–38.2 | −0.3 | 0.8 | (−1.7) to (0.4) | 0.9 | 0.9 | (−0.6) to (2.0)* |
n = number of subjects per intensity.
P < 0.05 vs. uncorrected (a-ET)Pco2.
Random-effects regression analyses between (a-ET)Pco2 and respiratory variables are shown in Table3. After temperature correction, (a-ET)Pco2 was linearly related to absolute and relative exercise intensity, VO2, VCO2, and RR in normoxia and hypoxia (Table3). In normoxia, there was also a linear relationship between (a-ET)Pco2 with VT and A-aDO2tc. The intercept of the linear relationship between (a-ET)Pco2 and the absolute load was significantly higher in hypoxia than in normoxia, while the slopes were similar. Likewise, for a given respiratory rate, (a-ET)Pco2 was higher in hypoxia than in normoxia (Table3). Since the intercepts and slopes of the linear relationship between (a-ET)Pco2 and the relative intensity were not significantly different between normoxia and hypoxia, a combined random-effects regression equation (eq. 1) was generated:
| 1 |
Table 3.
Random-effects regression analysis between the arterial-to-end-tidal Pco2 (mmHg) difference ((a-ET)CO2) corrected for arterial blood temperature, exercise intensity and respiratory variables during exercise in normoxia (PIo2: ∼143 mmHg) and hypoxia (PIo2: ∼73 mmHg)
| Normoxia | Hypoxia | Normoxia (P values) | Hypoxia (P values) | Nox vs. Hyp | Nox vs. Hyp | R 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept ± SE | Slope ± SE | Intercept ± SE | Slope ± SE | I | S | I | S | Ic | Sc | ||
| Intensity (w) | −5.364 ± 0.669 | 0.013 ± 0.003 | −2.712 ± 1.021 | 0.018 ± 0.008 | 0.000 | 0.000 | 0.009 | 0.020 | 0.025 | 0.54 | 0.54 |
| Intensity (%Wpeak) | −5.531 ± 0.655 | 0.04 ± 0.008 | −4.436 ± 1.085 | 0.058 ± 0.015 | 0.000 | 0.000 | 0.000 | 0.000 | 0.38 | 0.30 | 0.56 |
| VO2 (L·min−1) | −6.18 ± 0.855 | 1.195 ± 0.268 | −4.771 ± 1.538 | 2.147 ± 0.745 | 0.000 | 0.000 | 0.003 | 0.005 | 0.42 | 0.23 | 0.52 |
| VCO2 (L·min−1) | −5.555 ± 0.717 | 0.905 ± 0.197 | −3.412 ± 1.222 | 1.212 ± 0.48 | 0.000 | 0.000 | 0.006 | 0.013 | 0.12 | 0.55 | 0.53 |
| RER | −10.742 ± 2.018 | 7.652 ± 1.871 | −4.132 ± 3.867 | 3.027 ± 3.203 | 0.000 | 0.000 | 0.29 | 0.35 | 0.13 | 0.21 | 0.48 |
| RR (breaths·min−1) | −6.15 ± 0.648 | 0.087 ± 0.015 | −3.677 ± 1.002 | 0.087 ± 0.026 | 0.000 | 0.000 | 0.000 | 0.001 | 0.036 | 0.99 | 0.59 |
| VT (l) | −5.259 ± 1.523 | 1.136 ± 0.638 | −2.034 ± 3.016 | 0.705 ± 1.351 | 0.001 | 0.08 | 0.50 | 0.60 | 0.33 | 0.77 | 0.35 |
| A-aDO2 tc (mmHg) | −4.313 ± 0.684 | 0.158 ± 0.057 | −2.269 ± 2.081 | 0.116 ± 0.128 | 0.000 | 0.008 | 0.28 | 0.37 | 0.33 | 0.75 | 0.40 |
Nox, normoxia; Hyp, hypoxia; I, Intercept; S, slope; Ic, comparison of intercepts between normoxia and hypoxia; Sc, comparison of slopes between normoxia and hypoxia; HR, heart rate; VO2, oxygen uptake; VCO2, CO2 production; RER, respiratory exchange ratio; RR, respiratory rate; VT, tidal volume; PETco2, end-tidal Pco2; A-aDO2, alveolar-to-arterial oxygen pressure difference; tc, temperature corrected.
The intercept SE was 0.714 (P < 0.001) and slope SE 0.009 (P < 0.001).
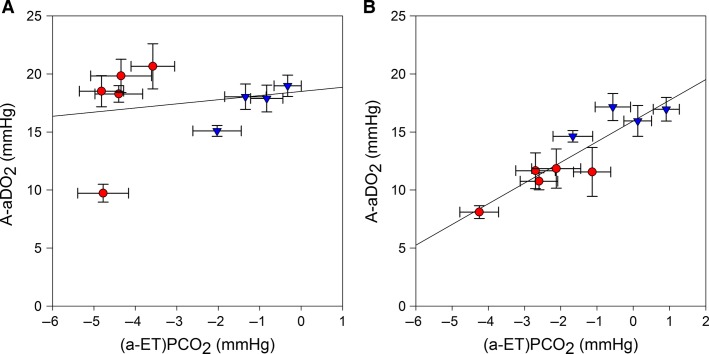
There was a close relationship between the mean (a-ET)Pco2 and the mean A-aDO2 when both FIO2 conditions were analyzed conjointly as follows:
| 2 |
(r = 0.90, EES = 1.45 mmHg, n = 9, each point representing the mean of 6–11 observations, P < 0.001) (Fig.1).
Figure 1.
Relationship between alveolar-to-arterial O2 pressure difference (A-aDO2) and alveolar-to-end-tidal CO2 pressure difference ((a-ET)Pco2); (A) without correction of arterial blood gases for blood temperature and (B): after blood gases correction for blood temperature (A-aDO2 = 15.96 + 1.79 × (a-ET) Pco2; r = 0.90, EES = 1.45 mmHg, n = 9, each point representing the mean of 6–11 observations, P < 0.001). Error bars represent the standard error of the mean.
Discussion
In this study, we have shown that most of the negative (a-ET)Pco2 value is due to a lack of correction of Paco2 for blood temperature, and that a near maximal exercise intensity the mean (a-ET)Pco2 should be lying close to 0 mmHg in healthy humans. Moreover, we have demonstrated that in healthy humans, the temperature corrected (a-ET)Pco2 increases linearly with absolute and relative exercise intensity, VO2, VCO2, and RR in normoxia and hypoxia, with similar slopes in normoxia and severe hypoxia. Consequently, at the same absolute exercise intensity, the (a-ET)Pco2 is higher in hypoxia (i.e., less negative) than in normoxia. We have also shown that at a similar respiratory rate, (a-ET)Pco2 is higher in hypoxia than in normoxia indicating that factors other than, or in addition to, the respiratory rate or tidal volume should explain the greater (a-ET)Pco2 observed in hypoxia.
Impact of temperature correction on the (a-ET)Pco2 difference
Since PETco2 overestimates Paco2 at all exercise intensities, the derived (a-ET)Pco2 has negative values as previously reported in young (Jones et al. 1979; Robbins et al. 1990; Liu et al. 1995; Williams and Babb 1997) and elderly men (St Croix et al. 1995). This study reveals the importance of correcting Paco2 for lung blood temperature has for the accurate determination of the (a-ET)Pco2. In fact, this correction alone explains ∼70% of the negative (a-ET)Pco2 at maximal exercise in normoxia and transforms the noncorrected negative (a-ET)Pco2 during maximal exercise in hypoxia to positive.
Negative (a-ET)Pco2 values: fact or artifact?
In agreement with the previous investigators (Jones et al. 1979; Robbins et al. 1990; Liu et al. 1995; St Croix et al. 1995; Williams and Babb 1997), we have also observed negative (a-ET)Pco2 values during exercise, which increased with exercise intensity, as previously reported (Wasserman et al. 1967; Whipp and Wasserman 1969). It has been the subject of controversy whether negative (a-ET)Pco2 values really exist or if they result from multiple inaccuracies, including the use of different procedures to measure respiratory and blood gases (Forster 1977; Gurtner 1977; Scheid and Piiper 1980; Piiper 1986). In theory (a-ET)Pco2 negative values may be caused by several mechanisms acting conjointly or separately (for review see [Scheid and Piiper 1980; Stickland et al. 2013]).
In well-ventilated and perfused alveoli, the PETco2 represents the Pco2 during the phase of the respiratory cycle at which the PAco2 becomes closer to the mixed venous Po2. Thus, the PETco2 will always overestimate the actual PAco2 in well-ventilated and perfused alveoli. Underperfused alveoli have a rather low PAco2, which is even lower in areas that do not participate in gas exchange (dead space). Consequently, dead space ventilation may contribute to reduce PETco2 below mean PAco2, as observed at rest (Dubois et al. 1952). The increase in Vt, VCO2, and mixed venous CO2 with exercise causes greater within-breath fluctuations of alveolar gas composition (Dubois et al. 1952) such that during expiration, PAco2 increases toward mixed venous Pco2 (Pvco2) more rapidly as the increased CO2 production of exercise is evolved into a lung volume becoming smaller as expiration continues (Jones et al. 1979). This may result in PETco2 actually being higher than mean Paco2 during exercise (Jones et al. 1966). According to this description, we must have seen increasingly negative (a-ET)Pco2 with the increase of exercise intensity because the difference between Pvco2 and Paco2 increases with exercise intensity. We actually observed the opposite, that is, (a-ET)Pco2 becomes less negative with the increase of exercise intensity. Our findings can be explained by several mechanisms. First, lack of Paco2 correction for arterial blood temperature as shown in this study.
Second, the increase in PETco2 with the exercise-induced widening of the intra-breath fluctuation in PAco2 is expected to be lower in severe hypoxia than in normoxia because the mixed venous Pco2 is lower while the inspiratory CO2 is similar to normoxia. Consequently, the magnitude of the mean PETco2 is lower in hypoxia and remains closer to the mean PAco2. Thus, the second mechanism agrees with a greater (less negative or more positive) (a-ET)Pco2 during exercise in severe hypoxia, as observed in the present study.
Third, lack or a very small right-to-left shunt may cause an elevation of (a-ET)Pco2 as Paco2 is expected to increase in proportion to the magnitude of the venous admixture (Whyte et al. 1993). Using the data generated in this study, we have estimated that during maximal exercise in normoxia, a 2% and 10% right-to-left shunt would increase Paco2 by 5 and 15 mmHg, respectively, even after accounting for the Haldane effect. In severe acute hypoxia, a 2% and 10% shunt will cause a 4 and 11 mmHg increase of Paco2, respectively. However, experiments using the multiple inert gas elimination technique have found no evidence of shunt during exercise (Hammond et al. 1986; Wagner et al. 1986; Hopkins et al. 1994, 1998). Although some passage of blood through arterial-venous anastomosis has been demonstrated in humans (Lovering et al. 2008, 2009), its magnitude is likely low. The fact that the (a-ET)Pco2 difference was negative or close to 0 mmHg concurs with a small or inexistent shunt in our experimental conditions. Moreover, shunt at maximal exercise has a greater impact on Paco2 than on Pao2 because the mixed venous CO2 content during exercise increases proportionally more than mixed venous O2 is reduced. Thus, a good correlation between the A-aDO2 and the A-aDCO2 is not expected with a high contribution of shunt to the impairment of pulmonary gas exchange during exercise because the shunt affects differently the A-aDO2 and the (a-ET)Pco2.
In summary, our results suggest that the negative (a-ET)Pco2 values observed in previous studies are likely due to lack of correction of Paco2 for blood temperature. The (a-ET)Pco2 difference is less negative during exercise in hypoxia than normoxia. At peak exercise, the mean Paco2 and the mean PETco2 are similar, suggesting that PETco2 is a useful surrogate for Paco2. The mean (a-ET)Pco2 difference increases with exercise intensity and is closely related to the mean A-aDO2 difference. This is expected if similar mechanisms perturb the lung gas exchanges of O2 and CO2.
Acknowledgments
Our special thanks to José Navarro de Tuero for his excellent technical assistance. We express our gratitude to Prof. Robert Boushel for his insightful comments.
Conflict of Interest
None declared.
References
- Andersen P. Saltin B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA. Boushel R. Assessment of cardiac output with transpulmonary thermodilution during exercise in humans. J. Appl. Physiol. 2015;118:1–10. doi: 10.1152/japplphysiol.00686.2014. [DOI] [PubMed] [Google Scholar]
- Calbet JA. Lundby C. Air to muscle O2 delivery during exercise at altitude. High Alt. Med. Biol. 2009;10:123–134. doi: 10.1089/ham.2008.1099. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B. Boushel R. Effects of ATP-induced leg vasodilation on VO2peak and leg O2 extraction during maximal exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R447–R453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Robach P, Lundby C. Boushel R. Is pulmonary gas exchange during exercise in hypoxia impaired with the increase of cardiac output? Appl. Physiol. Nutr. Metab. 2008;33:593–600. doi: 10.1139/H08-010. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Losa-Reyna J, Torres-Peralta R, Rasmussen P, Ponce-Gonzalez JG, Sheel AW, et al. Limitations to oxygen transport and utilisation during sprint exercise in humans: evidence for a functional reserve in muscle O2 diffusing capacity. J. Physiol. 2015 doi: 10.1113/JP270408. doi: 10.1113/JP270408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretelli P. Di Prampero PE. Gas exchange in exercise. In: Fahri LE, Tenney SM, editors; Handbook of physiology - The respiratory system IV. Bethesda: The American Physiological Society; 1987. pp. 297–339. [Google Scholar]
- Dempsey JA, Hanson PG. Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J. Physiol. 1984;355:161–175. doi: 10.1113/jphysiol.1984.sp015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois AB, Britt AG. Fenn WO. Alveolar CO2 during the respiratory cycle. J. Appl. Physiol. 1952;4:535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- Forster RE. Can alveolar pCO2 exceed pulmonary end-capillary CO2? No. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977;42:326–328. doi: 10.1152/jappl.1977.42.3.326. [DOI] [PubMed] [Google Scholar]
- González-Henriquez JJ, Losa-Reyna J, Torres-Peralta R, Göran R, Koskolou M. Calbet JAL. 2015. A new equation to estimate temperature-corrected PaCO2 from PETCO2 during exercise in normoxia and hypoxia. Scand. J. Med. Sci. Sports. (in press) [DOI] [PubMed] [Google Scholar]
- Gurtner GH. Can alveolar pCO2 exceed pulmonary end-capillary CO2? Yes. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977;42:323–326. doi: 10.1152/jappl.1977.42.3.323. [DOI] [PubMed] [Google Scholar]
- Hammond MD, Gale GE, Kapitan KS, Ries A. Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J. Appl. Physiol. 1986;61:1749–1757. doi: 10.1152/jappl.1986.61.5.1749. [DOI] [PubMed] [Google Scholar]
- Hlastala MP. A model of fluctuating alveolar gas exchange during the respiratory cycle. Respir. Physiol. 1972;15:214–232. doi: 10.1016/0034-5687(72)90099-0. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Linderholm H. Oxygen and carbon dioxide tensions of arterial blood during heavy and exhaustive exercise. Acta Physiol. Scand. 1958;44:203–215. doi: 10.1111/j.1748-1716.1958.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, McKenzie DC, Schoene RB, Glenny RW. Robertson HT. Pulmonary gas exchange during exercise in athletes. I. Ventilation-perfusion mismatch and diffusion limitation. J. Appl. Physiol. 1994;77:912–917. doi: 10.1152/jappl.1994.77.2.912. [DOI] [PubMed] [Google Scholar]
- Hopkins SR, Gavin TP, Siafakas NM, Haseler LJ, Olfert IM, Wagner H, et al. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J. Appl. Physiol. 1998;85:1523–1532. doi: 10.1152/jappl.1998.85.4.1523. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Heigenhauser GJF, Hsia CW, Jones NL. Wagner PD. Vol. 29. 2011. Determinants of gas exchange and acid–base balance during exercise. Handbook of physiology, exercise: regulation and integration of multiple systems; pp. 515–584. Compr. Physiol. Suppl. doi: 10.1002/cphy.cp120112. [Google Scholar]
- Jones NL, McHardy GJ, Naimark A. Campbell EJ. Physiological dead space and alveolar-arterial gas pressure differences during exercise. Clin. Sci. 1966;31:19–29. [PubMed] [Google Scholar]
- Jones NL, Robertson DG. Kane JW. Difference between end-tidal and arterial PCO2 in exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979;47:954–960. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- Liu Z, Vargas F, Stansbury D, Sasse SA. Light RW. Comparison of the end-tidal arterial PCO2 gradient during exercise in normal subjects and in patients with severe COPD. Chest. 1995;107:1218–1224. doi: 10.1378/chest.107.5.1218. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS. Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J. Appl. Physiol. 2008;104:1418–1425. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Haverkamp HC, Romer LM, Hokanson JS. Eldridge MW. Transpulmonary passage of 99mTc macroaggregated albumin in healthy humans at rest and during maximal exercise. J. Appl. Physiol. 2009;106:1986–1992. doi: 10.1152/japplphysiol.01357.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft UC, Loeppky JA. Mostyn EM. Mean alveolar gases and alveolar-arterial gradients in pulmonary patients. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979;46:534–540. doi: 10.1152/jappl.1979.46.3.534. [DOI] [PubMed] [Google Scholar]
- Lundby C, Calbet JA, van Hall G, Saltin B. Sander M. Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4,100 m and in high-altitude Aymara natives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1202–R1208. doi: 10.1152/ajpregu.00725.2003. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Matthay RA, Snyder PE, Neff RK. Loke J. Determination of cardiac output at rest and during exercise by carbon dioxide rebreathing method in obstructive airway disease. Am. Rev. Respir. Dis. 1985;131:73–78. doi: 10.1164/arrd.1985.131.1.73. [DOI] [PubMed] [Google Scholar]
- Morales-Alamo D, Losa-Reyna J, Torres-Peralta R, Martin-Rincon M, Perez-Valera M, Curtelin D, et al. What limits performance during whole body incremental exercise to exhaustion in humans? J Physiol. 2015 doi: 10.1113/JP270487. doi: 10.1113/JP270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S. Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013;4:133–142. [Google Scholar]
- Paterson DJ, Wood GA, Marshall RN, Morton AR. Harrison AB. Entrainment of respiratory frequency to exercise rhythm during hypoxia. J. Appl. Physiol. 1987;62:1767–1771. doi: 10.1152/jappl.1987.62.5.1767. [DOI] [PubMed] [Google Scholar]
- Piiper J. Blood-gas equilibrium of carbon dioxide in lungs: a continuing controversy. J. Appl. Physiol. 1986;60:1–8. doi: 10.1152/jappl.1986.60.1.1. [DOI] [PubMed] [Google Scholar]
- Robbins PA, Conway J, Cunningham DA, Khamnei S. Paterson DJ. A comparison of indirect methods for continuous estimation of arterial PCO2 in men. J. Appl. Physiol. 1990;68:1727–1731. doi: 10.1152/jappl.1990.68.4.1727. [DOI] [PubMed] [Google Scholar]
- Schaffartzik W, Poole DC, Derion T, Tsukimoto K, Hogan MC, Arcos JP, et al. VA/Q distribution during heavy exercise and recovery in humans: implications for pulmonary edema. J. Appl. Physiol. 1992;72:1657–1667. doi: 10.1152/jappl.1992.72.5.1657. [DOI] [PubMed] [Google Scholar]
- Scheid P. Piiper J. Blood/gas equilibrium of carbon dioxide in lungs. A Critical Review. Respir. Physiol. 1980;39:1–31. doi: 10.1016/0034-5687(80)90011-0. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J. Appl. Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. The acid-base status of blood. Copenhagen: Munksgaard; 1974. [Google Scholar]
- St Croix CM, Cunningham DA, Kowalchuk JM, McConnell AK, Kirby AS, Scheuermann BW, et al. Estimation of arterial PCO2 in the elderly. J. Appl. Physiol. 1995;79:2086–2093. doi: 10.1152/jappl.1995.79.6.2086. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Lindinger MI, Olfert IM, Heigenhauser GJ. Hopkins SR. Pulmonary gas exchange and acid-base balance during exercise. Compr. Physiol. 2013;3:693–739. doi: 10.1002/cphy.c110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE. Moon RE. Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J. Appl. Physiol. 1985;58:989–995. doi: 10.1152/jappl.1985.58.3.989. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Ventilation-perfusion matching during exercise. Chest. 1992;101:192S–198S. doi: 10.1378/chest.101.5_supplement.192s. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW. Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J. Appl. Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Van Kessel AL. Burton GG. Interaction of physiological mechanisms during exercise. J. Appl. Physiol. 1967;22:71–85. doi: 10.1152/jappl.1967.22.1.71. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. Wasserman K. Alveolar-arterial gas tension differences during graded exercise. J. Appl. Physiol. 1969;27:361–365. doi: 10.1152/jappl.1969.27.3.361. [DOI] [PubMed] [Google Scholar]
- Whyte MK, Hughes JM, Jackson JE, Peters AM, Hempleman SC, Moore DP, et al. Cardiopulmonary response to exercise in patients with intrapulmonary vascular shunts. J. Appl. Physiol. 1993;75:321–328. doi: 10.1152/jappl.1993.75.1.321. [DOI] [PubMed] [Google Scholar]
- Williams JS. Babb TG. Differences between estimates and measured PaCO2 during rest and exercise in older subjects. J. Appl. Physiol. 1997;83:312–316. doi: 10.1152/jappl.1997.83.1.312. [DOI] [PubMed] [Google Scholar]