Abstract
Management of patients with pancreatic cancer is a multidisciplinary approach that presents enormous challenges to the clinician. Overall 5-year survival for all patients remains <3%. Symptoms of early pancreas cancer are nonspecific. As such, only a fraction of patients are candidates for surgery. While surgical resection provides the only curative option, most patients will develop tumor recurrence and die of their disease. To date, the clinical benefits of chemotherapy and radiation therapy have been important but have led to modest improvements. Tumor vaccines have the potential to specifically target the needle of pancreas cancer cells amidst the haystack of normal tissue. The discovery of pancreas tumor-specific antigens and the subsequent ability to harness this technology has become an area of intense interest for tumor immunologists and clinicians alike. Without knowledge of specific antigen targets, the whole tumor cell represents the best source of immunizing antigens. This chapter will focus on the development of whole tumor cell vaccine strategies for pancreas cancer.
Keywords: Pancreas cancer, Immunotherapy, Tumor antigens, Genetically modified whole cell allogeneic vaccine
1. Introduction
Surgical resection provides the only curative option for patients with pancreatic cancer. Unfortunately, only 10–20% of patients are candidates for surgical resection. Even in this selected population, the 5-year survival is approximately 20% for resectable disease with a median survival of 20–24 months (1–4). To date, the clinical benefits of chemotherapy and/or radiation therapy have been relatively modest (5–8). More effective therapies for all stages of pancreatic adenocarcinoma are needed.
Immunotherapy has the potential to provide a non-cross resistant mechanism of antitumor activity that can be integrated with surgery, radiation, and chemotherapy. A major advantage of immune-based therapies is their ability to specifically target a tumor cell relative to the normal cell of origin, thereby minimizing nonspecific toxicities. Antitumor immune responses can be diverse and consist of both antibody and cellular responses. In the case of solid tumors and, in particular, pancreatic cancer, specific targets of the immune response that serve as tumor rejection antigens have not been defined. However, a few candidate antigens have been identified against which cellular and antibody responses can be induced. Because the majority of these antigens are intracellular, vaccine strategies should be able to predominantly induce T cell rather than antibody responses.
Without knowledge of specific antigen targets, the whole tumor cell represents the best source of immunizing antigens. Therefore, this chapter will focus on the development of whole tumor cell vaccine strategies. However, with the sequencing of the human genome (9), the sequencing of the pancreas cancer genome (10), and the development of rapid methods for identifying genes that are differentially expressed by tumor cells, many more candidate immune targets are expected to be uncovered that may serve as immunogens for the development of vaccine strategies that aim at both the treatment and the prevention of pancreatic cancer (11–18). This chapter will: (1) review the important features of an effective antitumor immune response; (2) discuss the results of some of the more promising strategies that are currently under clinical consideration with a focus on a cytokine-modified allogeneic whole cell vaccine approach; and (3) provide a detailed description of the materials and methods employed for the development of a GM-CSF-modified allogeneic whole cell pancreatic cancer vaccine. It is important to point out that these methods can be modified to develop whole cell vaccines that express other cytokines and co-stimulatory molecules that are known to be important for activating tumor-specific immune responses.
1.1. Features of the Immune System That Are Advantageous for Cancer Immunotherapy
The immune system comprises a number of cell types which, when activated, are extremely efficient at recognizing and killing their target. In particular, B cells and T cells each possess vast arrays of clonally distributed antigen receptors that enable them to recognize foreign antigens and to discriminate self from non-self. It has been estimated that both T and B cells can express more than a million different antigen-specific receptors through recombination of the genes encoding for their receptor at the time of maturation in the thymus and bone marrow, respectively. Therefore, the immune system should have an unlimited capability to recognize antigenic differences between normal and malignant cells, whether they are in the form of the product of a new genetic alteration, a reactivated embryonic gene, or an over-expressed gene.
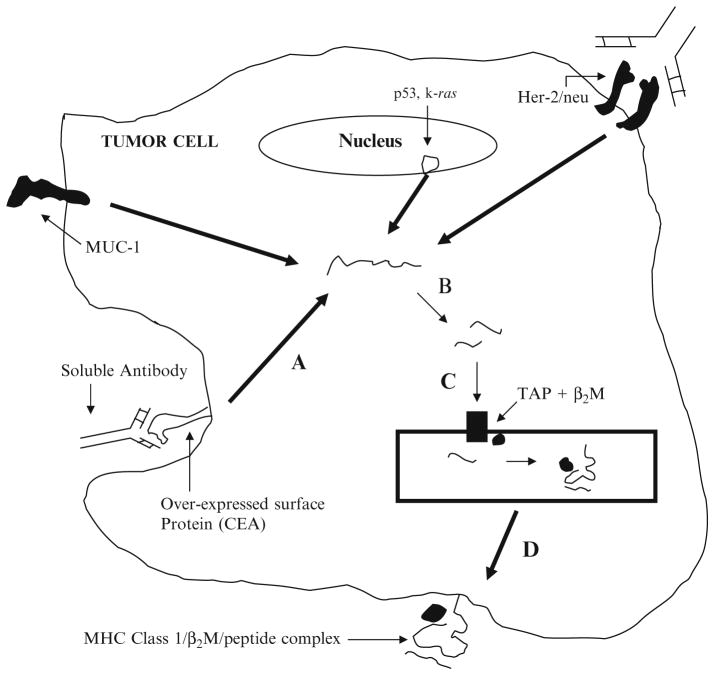
The B cell receptor, which is a surface immunoglobulin with the ability to bind antigens on soluble molecules, recognizes free antigenic determinants (Fig. 1). Therefore, special antigen processing is not required for B cell receptor or soluble antibody binding to its antigen. In contrast, the T cell receptor recognizes fragments of the antigenic protein bound to HLA class I and II molecules on another cell. This peptide–HLA complex is formed as a result of fragmentation of proteins within specialized cellular compartments and subsequent association with a binding site on the HLA molecule (Fig. 1).
Fig. 1.
Tumor antigen processing and presentation: This model demonstrates that there are many types of proteins or glycoproteins expressed by tumor cells that can be recognized by T cells of the immune system. The tertiary structure of the surface mucins or surface glycoproteins that are the product of reactivated embryonic genes, or the tertiary structure of over-expressed growth factor receptors such as HER-2/neu, can be recognized by soluble antibodies secreted by B cells. However, these same proteins or glycoproteins can be internalized into endocytic vacuoles, and then traf ficked either into the lysosomal antigen processing pathway to generate MHC class II bound tumor antigens via the exogenous pathway or into the cytoplasm where they will be degraded into MHC class I bound tumor antigens via the endogenous pathway. Tumor cells do not usually express MHC class II. Therefore, most tumor antigens will not be presented for MHC class II presentation to CD4+ helper T cells. However, the surface tumor antigens that will enter the cytoplasm will be degraded into small peptide fragments by the proteasomes (a, b). The small peptide fragments will then be transported into the endoplasmic reticulum (ER) where they will bind to MHC class I (c ) and subsequently be presented on the tumor’s surface bound to MHC class I molecules, and recognized by the cytotoxic T cell (d) tumor’s surface bound to MHC class I molecules, and recognized by the cytotoxic T cell (d). Any protein within a tumor cell, whether it is presented on the cell surface, expressed in the nucleus, cytoplasm, or endoplasmic reticulum, can gain access to the cytoplasm for processing and presentation on MHC class I molecules and be recognized by cytotoxic T cells. B2M beta-2 microglobulin, TAP transporter associated with antigen processing.
Two forms of T cell antigen processing exist (19–24). Professional antigen presenting cells (macrophages, B cells, and dendritic cells) have the ability to capture extracellular proteins that are released by the tumor through secretion, shedding, or tumor lysis. These proteins are subsequently internalized via endocytosis and processed through the exogenous pathway. These proteins are taken up into low pH vesicles (the lysosomal compartment) where they undergo fragmentation. Peptide fragments (10–25 amino acids in length) then bind to the HLA class II protein, prior to expression of the complex on the cell surface. This complex is recognized exclusively by CD4+ helper T cells in the context of a second co-stimulatory molecule such as B7 (25, 26). In the presence of both of these signals, activated CD4+ T cells can amplify the CD8+ T cell response. In addition, memory CD4+ T cells are generated and play the key role in the maintenance of protective immunity. Presentation of antigen on HLA class II and the ability to express co-stimulatory molecules are the specialized function of these “professional” antigen presenting cells that derive from hematopoietic precursors in the bone marrow.
In contrast to professional antigen presenting cells, pancreatic and most solid tumors derive from epithelial cells rather than hematopoietic cells. Therefore, pancreatic cancer cells cannot process and present antigen through the exogenous pathway. However, all cells including tumor cells have the ability to process and present antigens that derive from cellular proteins through the endogenous pathway (Fig. 1) (27, 28). Any protein within a tumor cell can gain access to the cytosol and undergo enzymatic degradation into 8–10 amino acid fragments by specialized machinery (the proteasome). The peptide fragments are subsequently transported into the endoplasmic reticulum via TAP (transporter associated with antigen processing) where they bind to HLA class I molecules and are transported to the cell surface for recognition by CD8+ T cells. CD8+ T cells exclusively recognize antigen in this way. In general, CD4+ T cells provide helper or regulatory function while CD8+ T cells carry out direct tumor lysis. A few candidate pancreatic antigens recognized by B and T cells have already been identified and are listed in Table 1.
Table 1.
Candidate B and T cell pancreatic targets
| Category of antigena | T cell antigens | B cell antigens |
|---|---|---|
| Reactivated embryonic genes | CEA | CEA |
| Mutated oncogene/suppressor genes | k-ras, p53 | p53 |
| Tissue-specific antigens | HER-2/neu | HER-2/neu |
| Glycoproteins | MUC-1 | MUC-1 |
Categories are defined based on already defined categories of human melanoma antigens (77)
1.2. Immunotherapy in Practice
The goal of immune-based therapies is to either: recruit and activate tumor-specific T cells that have the ability to directly lyse a tumor cell; or employ monoclonal antibodies that can target tumor-specific antigens and either directly lyse the tumor or lyse the tumor via delivery of a cytotoxic agent. Both approaches are attractive for several reasons. (1) The activation of tumor-specific T cells or the use of monoclonal antibodies act via a mechanism that is distinct from chemotherapy or radiation therapy and would represent a non-cross resistant treatment with an entirely different spectrum of toxicities. (2) The immune system is capable of recognizing a broad diversity of potential antigens while orchestrating selective as well as specific cytotoxic responses. This feature may be essential in recognizing and eliminating a heterogeneous tumor population while avoiding normal tissue toxicity. (3) Preclinical animal models using both forms of immunotherapy have been able to eliminate small burdens of established tumors, a situation that corresponds to the state of minimal residual disease commonly found after resection of human tumors (29, 30).
Immunotherapy can be broadly divided into passive and active therapeutic approaches (Table 2). Passive immunotherapy mainly involves the use of unlabeled or labeled monoclonal antibodies that are specifically raised against tumor antigens. Antibodies have so far been the most successful form of immunotherapy clinically. They are being employed as diagnostic tools, prognostic indicators, and for the treatment of cancer. Advantages include specific targeting of tumor cells while sparing normal tissue, relative ease of administration, and low toxicity profile. The major disadvantages include the absence of T cell activation which therefore precludes T-cell-mediated cytotoxic killing and the generation of memory immune responses. In addition, a potential limiting factor in its use involves tumor heterogeneity. Specifically, all tumor cells within a proliferating mass may not express the antigen being targeted by the antibody. A number of monoclonal antibodies have been used for solid tumors including advanced pancreatic cancer (31–34). However, these results provide additional rationale for developing vaccine approaches that can induce natural tumor-specific antibody responses in the patient.
Table 2.
Different categories of immunotherapy
Passive immunotherapy
|
Active nonspecific immunotherapy
|
Active specific immunotherapy (vaccines)
|
Active immunotherapy is typically divided into nonspecific and specific processes. Nonspecific therapy attempts to augment an immune response without actually targeting a specific tumor antigen. Examples include the use of BCG, IL-2, and levamisole. In contrast, active specific or vaccine therapy targets specific tumor antigens as a result of the induction of antigen-specific B-cell- or T-cell-mediated immune responses. Active specific therapy can also generate antigen-specific memory T cell responses that are capable of being reactivated if tumor cells expressing the same antigen profile recur. Furthermore, the induction of cellular immune responses has the added benefit of allowing natural access to the microenvironment of the tumor. Preclinical studies have already shown that T-cell-mediated vaccine therapy can induce antitumor immune responses that are potent enough to eradicate colorectal tumors (35, 36). Translation of these vaccine approaches into therapies for patients with pancreatic and colorectal tumors is in early phases of clinical development. Examples of the different vaccine approaches that are currently undergoing clinical testing will be discussed below.
1.2.1. Peptide- and Protein-Based Vaccines
Although significant progress has been made in understanding the biology of pancreatic cancer at the genetic level, specific pancreatic tumor antigens that can serve as rejection targets have not yet been identified. However, point mutations in a variety of oncogenes (k-ras) or tumor suppressor genes (p53, p16, DPC4, BRCA2, Her-2/neu) have been associated with different histologically defined precursor lesions (15–18, 37–40) and are being studied as candidate immune targets. Mutated k-ras is a particularly attractive immune target because it is mutated in >90% of pancreatic adenocarcinomas (41–44). The ras p21 protooncogenes including K-ras, N-ras, and H-ras encode proteins that are important for regulating cellular events including growth and differentiation. Point mutations at codons 12, 13, and 61 have been identified in many cancers including pancreatic adenocarcinoma (43, 44). These mutations encode distinct proteins that are potential immunogens. The major advantage of a protein- or peptide-based vaccine is the ability to deliver high doses of the potential immunogen safely and at a relatively modest cost. However, there are also several limitations to vaccine approaches that employ peptides and proteins. First, the vaccine approaches that will be most successful at optimally priming with the peptide and/or protein have not yet been determined. Second, proteins that are identified as a candidate immunogen based on the criteria that they are over-expressed in pancreatic adenocarcinoma may turn out not to be the most relevant target of the immune response.
Mutated k-ras-based vaccines have been the most extensively studied peptide-/protein-based vaccine approach in patients with pancreatic adenocarcinoma. In preclinical models, vaccination with mutant k-ras peptides induces both major histocompatibility complex (MHC) class I and II restricted T cell responses. K-ras-specific T cell responses have also been elicited in patients treated with vaccines that consist of k-ras peptides that contain a point mutation at codon 12 (45–49).
Heat shock proteins (HSPs) are ubiquitous and highly conserved cellular proteins that are upregulated during cell stress. They are thought to bind to cellular proteins that become damaged when a cell experiences stress, thereby facilitating the protein’s re-folding to an active conformation. In the non-stressed environment, HSPs are thought to have multiple functions including helping newly synthesized polypeptides fold, assisting in protein transport, and associating with peptides generated during protein degradation. They are also thought to stimulate macrophage and dendritic cell activation and assist in re-presentation of peptides. HSPs as vaccine have already been used in clinical trials (50, 51).
1.2.2. Glycoproteins as Antigens
There are other antigen targeted immunologic approaches that have been tested in patients with pancreatic adenocarcinoma. Mucin-1 (MUC-1) is a glycosylated transmembrane protein that is uniquely characterized by an extracellular domain that consists of a variable number of tandem repeats of 20 amino acids rich in pro-line, serine, and threonine residues (52). While normally present lining ductal epithelial surfaces including the gastrointestinal tract, MUC-1 is over-expressed on the cell surface of many cancers including pancreatic adenocarcinoma (52, 53). There is also evidence to suggest that MUC-1 protein expression is associated with an increased risk for metastasis and poor prognosis (54). As a glycoprotein with altered expression in pancreatic cancers, MUC-1 is considered another attractive candidate target for immunologic manipulation (52, 53, 55). There is also evidence to suggest that the alterations in the glycosylation of mucin may initiate the events that generate the antitumor immune response. Presumably in normal mucin secreting cells, the bulky carbohydrate side chains block the presentation of potential peptide to T cells. As a consequence of under glycosylation of mucin thought secondary to decreased glycosyl transferase activity in malignant cells, new peptide epitopes are exposed. Data from animal and phase I clinical studies have demonstrated that HLA-unrestricted T cells isolated from patients with MUC-1 expressing tumors can recognize these exposed epitopes and can induce MUC-1-specific DTH responses (55). More recently, immunization with MUC-1 peptide has been shown to induce an HLA-A2-restricted T cell response (56). Another approach has targeted murine alpha-1,3 galactosyl transferase gene product (alpha-GT). Murine Alpha-GT epitopes are not present in human cells. When transfected into human pancreas tumor cells, murine Alpha-GT epitopes are thought to induce an immune response in an antibody-dependent, cell-mediated cytotoxicity manner. A small phase II study has been completed integrated with gemcitabine and 5-FU-based radiation therapy in patients with resected pancreas cancer with encouraging disease-free survival (56). A phase III study is currently open testing this vaccine approach plus chemotherapy or chemoradiotherapy versus chemotherapy or chemoradiotherapy alone with planned completion date of 2014 (Clinical Trials.gov identifier NCT01072981).
Carcinoembryonic antigen (CEA) is another glycoprotein that is over-expressed in pancreatic cancers. Although it is known to be a member of the immunoglobulin supergene family, the exact function of CEA is unclear (57). A CEA vaccine approach has also already been tested in a clinical trial (58). Additional potential glycoprotein targets have recently been identified by gene expression analysis (15–18). These candidate targets will likely be tested as immunogens in clinical trials in the near future.
1.2.3. New Immunotherapy Targets
It is now clear that both local characteristics of the tumor microenvironment and systemic factors are important for the immune evasion of tumors (59, 60). Among these immune inhibitory interactions involves CTLA4 expressed on T cells and B7 expressed on antigen presenting cells. The CTLA-4 monoclonal antibody Ipilimumab (Bristol Myers Squibb, Princeton, NJ) has been recently approved in the management of metastatic malignant melanoma. A recently completed small phase II study in chemotherapy refractory advanced pancreas cancer of Ipilimumab versus Ipilimumab and a pancreas cancer whole cell allogeneic vaccine demonstrated that this treatment alone or in combination was safe with improved survival correlating to enhanced T cell response to the pancreas cancer specific antigens mesothelin and galactin-3 (61). There is also a better understanding about the immune activating interactions between specific T cell receptors and antigen presenting cell ligands such as CD40 and the CD40 ligand CD154 (62). A CD40 agonist was recently examined in the K-Ras, P53 mutant (KPC) pancreas cancer mouse model and subsequently tested in a phase I clinical trial of gemcitabine and the CD40 agonist CP-870,893 (Pfizer) in patients with meta-static pancreas cancer (63, 64).
1.2.4. Whole Tumor Cell Vaccines
Currently, one major limitation of defined antigen-based vaccines is the lack of identified pancreatic tumor antigens that are the known targets of the immune response. Until pancreatic tumor-specific antigens are validated, the whole tumor cell represents the best source of immunogens. In addition, at this time it is not clear which if any of the currently studied antigen-based vaccine approaches are most effective at delivering these immunogens for activation of antitumor immunity.
A whole tumor cell vaccine approach involves the use of autologous or allogeneic tumor cells to stimulate an immune response. The whole tumor cell can provide multiple antigens for immunization and therefore leaves it up to the immune system to select which antigens are relevant. However, studies aimed at dissecting antitumor immune responses have confirmed that most tumors are not naturally immunogenic (65). Evidence from preclinical models suggests that the failure of the immune system to reject spontaneously arising tumors is unrelated to the absence of sufficiently immunogenic tumor antigens. Instead, the problem is derived from the immune system’s inability to appropriately respond to these antigens (66, 67). The importance of the local release of stimulatory cytokines to provide an immunological boost and to attract other immune cells has been extensively examined. These findings have lead to the concept that a tumor cell can become more immunogenic if engineered to secrete immune activating cytokines.
Tumor cells genetically modified to secrete immune activating cytokines have been extensively studied for their ability to induce systemic antitumor immune responses (68). Preclinical studies have shown that these vaccines can induce immune responses potent enough to cure mice of pre-established tumor (29, 68). In one comparison study of ten cytokines, GM-CSF was most potent, generating systemic immunity dependent on both CD4+ and CD8+ T cells (69). GM-CSF is known to be involved in the recruitment and differentiation of bone marrow-derived dendritic cells and dendritic cells are known to be the most efficient “professional” APCs at activating T cells (70, 71). In addition, GM-CSF is produced by activated CD4+ T helper cells further supporting the concept that this cytokine may function by priming immune effector cells (66, 71, 72). Studies aimed at optimizing this cytokine-secreting tumor vaccine approach confirmed that GM-CSF secretion must be at the site of relevant tumor antigen. Simple injection of soluble GM-CSF along with the appropriate tumor cells does not provide sustained local levels required to provide a sufficient immunologic boost (73). This information suggested that the mere presence of GM-CSF was not sufficient. Rather, the sustained release and duration of GM-CSF secretion appeared to be critical for priming the immune response. Furthermore, high levels must be sustained for several days. In the preclinical data, it appeared that a minimum of 35 ng/106 cells/24 h is necessary to generate effective antitumor immunity (73, 74).
A GM-CSF secreting tumor vaccine was first tested in patients with renal cell carcinoma. Lethally irradiated autologous renal cell carcinoma cells transduced with the GM-CSF gene were prepared and tested in a Phase I trial of patients with metastatic renal cell carcinoma. Although the maximally tolerated dose (MTD) as well as the dose of maximal bioactivity could not be determined secondary to technical difficulty of expanding each patient’s tumor cells beyond 4 × 107 cells, a dose of 4 × 107 cells resulted in post-vaccination DTH responses against autologous tumor cells that were similar to those measured in mouse studies testing this vaccine approach. Other immune parameters including histologic evaluation of the vaccine biopsy and DTH sites revealed similar immune infiltrates when compared with preclinical models. The vaccine was well tolerated at all vaccine doses tested. The most common side effects were local induration and erythema at the vaccine site (75). A similar spectrum of toxicities was subsequently observed in autologous prostate and melanoma studies and in allogeneic prostate vaccine studies (76, 77).
While the use of autologous tumor cells may preserve unique antigens expressed by each patient’s cancer, the development of an autologous vaccine requires extensive processing, in vitro expansion, and regulatory testing be performed for each individual patient vaccine. In the case of metastatic disease, the development of autologous tumor vaccine would also require the ability to obtain adequate tissue. These limitations preclude the use of autologous cellular vaccines for most cancers including pancreatic adenocarcinoma. Recent data support the immunologic rationale for using allogeneic cells as the source of immunogen. First, studies evaluating human melanoma antigens have demonstrated that most antigens identified so far are shared among at least 50% of other patient melanomas, regardless of whether or not they share the same HLA type (75–79). In addition, there is data to support that the professional APCs that present immunogen to specific T cells in the context of HLA are host derived. Therefore, the vaccine cells do not need to be HLA compatible with the host’s immune system as long as they can release cellular proteins (the tumor antigens) for uptake by the professional APCs (macrophages and dendritic cells) that are attracted to the vaccine site by GM-CSF. Tumor antigens are taken up by the APC’s exogenous processing pathway. However, these antigens have been shown to also gain access to the cytosol for processing onto HLA class I through the recently defined cross-priming mechanism (66, 67). Taken together, the data suggest that relevant tumor antigens can be delivered by an allogeneic tumor and still sufficiently mount an effective CD4+ and CD8+ T cell response against a tumor.
The results of a phase I study using irradiated allogeneic pancreatic tumor cell lines transfected with GM-CSF as adjuvant treatment administered in sequence with adjuvant chemoradiation in patients with resected adenocarcinoma of the pancreas were recently reported (81). This was the first GM-CSF secreting vaccine study to escalate the vaccine dose to 5 × 108 GM-CSF secreting cells. However, toxicities remained mostly limited to grade I/II local reactions at the vaccine site. In addition, there were self-limited systemic rashes, including one documented case of Grover’s syndrome (82). Systemic GM-CSF levels were evaluated as an indirect measure of the longevity of vaccine cells at the immunizing site. This pancreatic vaccine study is the first GM-CSF vaccine clinical trial to measure low but detectable serum GM-CSF levels in patients. As was observed in preclinical studies (69, 70), GM-CSF levels peaked at 48 h following vaccination. In addition, serum GM-CSF levels could be detected for up to 96 h following vaccination. Subsequently, a phase II study of 60 patients with resected pancreatic adenocarcinoma was performed. Each immunotherapy treatment consisted of a total of 5 × 108 GM-CSF-secreting cells distributed equally among three lymph node regions. The first immunotherapy treatment was administered 8–10 weeks following surgical resection. Patients received 5-FU-based chemoradiation. Patients who remained disease free after completion of chemoradiotherapy received treatments 2–4, each 1 month apart. A fifth and final booster was administered 6 months after the fourth immunotherapy. The primary endpoint was disease-free survival and secondary endpoints were overall survival and toxicity, and the induction of mesothelin-specific T cell responses.
The median disease-free survival is 17.3 months with median survival of 24.8 months. The administration of immunotherapy was well tolerated. In addition, the post-immunotherapy induction of mesothelin-specific CD8+ T cells in HLA-A1+ and HLA-A2+ patients correlates with disease-free survival. These data, together with data from preclinical models, would suggest that serum GM-CSF levels may serve as a bio-marker of immune response. Follow-up studies are ongoing to determine if these promising effects on immune activation will translate into a true clinical benefit for patients with pancreatic cancer (83).
1.3. Features of Gene Transfer Systems
1.3.1. Rationale for Choosing a Retroviral Vector for Gene Transfer into Tumor Cells
Gene transfer into tumor cells can be accomplished by a variety of methods involving either naked DNA or the use of viral vectors. There are many methods for transferring naked DNA into cells, including: (1) co-precipitation with calcium phosphate (84); (2) the use of electroporation which exposes cells to rapid pulses of high voltage current thereby providing a physically induced opening in the cell membrane for the entry of DNA (85); (3) direct introduction of DNA into cells by microinjection (86); and (4) encapsidation of DNA into liposomes (87). Use of any of these methods will often result in the introduction of multiple copies of the cytokine gene randomly into the host cell’s genome. Several of these methods can result in transient expression of the gene (for 24–72 h) by as many as 50% of the cells in the transfected population, because the transfected DNA can exist free in the cell nucleus for a short time (88). However, stable gene expression, which requires integration of the transfected DNA into the host’s genome, usually occurs in much less than 1% of the cells within the population undergoing transfection. To achieve stable integration and expression of the DNA into a high proportion of the cell population, it is often necessary to select for the minority of cells in the transfected population that have successfully retained the foreign DNA. This can be accomplished by co-transfecting DNA that encodes for a selectable marker that will allow cells expressing its product to survive in growth media that contain a substrate for the gene’s product that is normally toxic to most mammalian cells. In this way, in vitro selection of those cells that have successfully incorporated the transferred DNA can be accomplished. However, although in vitro selection will enhance the number of gene-transduced tumor cells in the cell population to nearly 100%, it is at the theoretical expense of antigen expression loss among that tumor cell population. In theory, loss of particular antigenic populations of tumor cells will decrease the effectiveness of the vaccine whether it be an allogeneic or autologous vaccine approach.
Currently, the most efficient method of stable gene delivery into mammalian cells is through the use of viral vectors, which infect their target cell by binding specific cell surface receptors. Most viral vectors are constructed so that they contain the sequences encoding for the expression of the gene and all of the genetic signals including the promoters, enhancers, splicing signals, and signals for polyadenylation of RNA transcripts, all of which are necessary for the transcription and ultimate translation of the inserted cytokine gene sequences. Often, the vectors will also contain selectable markers (89). Potential adverse consequences following viral infection can include: (1) damage or death to the host cell; (2) the activation of other latent viruses integrated into the host’s genome; (3) the activation of silent host genes such as protooncogenes; or (4) transformation of the defective viral vector from replication incompetent to replication competent by recombination with host gene sequences (which results in the production of helper virus). All of these possibilities should be considered in choosing a viral vector system for gene transfer.
Retroviruses have been the most commonly employed vectors for the preparation of cytokine-secreting tumor vaccines, for study in murine models, and in human vaccine therapy trials. There are at least two reasons for this. First, as mentioned above, retroviruses usually do not enter into a lytic cycle of viral replication and therefore, do not kill their host cell soon after viral infection. Second, retroviruses can infect most mammalian cells and integrate into the host genome, which is a critical requirement for efficient gene transfer and expression in a stable and heritable fashion. Most of the retroviral vectors employed in cytokine-secreting tumor vaccine studies have been developed from either avian or murine retroviruses. A key feature of these retroviral vectors is their incompetence to replicate following transduction into the host cell. Details of the mechanisms of infection, replication, integration, and gene expression of these viral vectors have already been described in significant detail in the literature (90–95). However, it is important to note that host cell replication and DNA synthesis are required for provirus integration, and thus, efficient gene transfer is restricted to replicating cells.
This chapter will focus on the retrovirus gene transfer system for several reasons: (1) it is still one of the most efficient gene transfer systems for replicating tumor cells; (2) these viruses have already been used clinically for this purpose and have not been demonstrated to cause clinically significant side effects. However, the Lentiviruses are a newer gene transfer system that appears to have equivalent gene transfer efficiency but to a wider range of cells (96, 97). In particular, Lentiviruses do not require a cell be replicating for successful gene transfer. In addition, Lentiviruses infect bone marrow-derived cells more efficiently than other viral vector systems. However, these viruses have not been tested in the clinics as of yet. There is still ongoing discussion over the increased safety concerns associated with the gene transfer system. For gene transfer to pancreatic tumor cells, retroviruses should be sufficient.
1.3.2. Structure of the Retroviral Vector
The transduction procedures that will be described employ the MFG retroviral vector system which we have extensive experience using and which has been tested in the clinics. However, other similar and equally effective retroviral vectors are also available for this use (98). The structure of MFG has recently been described (95). Briefly, in this vector, Moloney murine leukemia virus (Mo-MuLV) long terminal repeat (LTR) sequences are used to generate both a full-length viral RNA (for encapsidation into virus particles) and a subgenomic mRNA (analogous to the Mo-MuLV env mRNA) which is responsible for the expression of inserted sequences. The vector retains both sequences in the viral gag region shown to improve the encapsidation of viral RNA and the normal 5′ and 3′ splice sites necessary for the generation of the env mRNA. Protein coding sequences are inserted between the NcoI and BamHI sites in such a way that the cDNA sequences encoding the gene of interest are cloned into the downstream site such that the cDNA inserts AUG at the exact position relative to the 3′ splice acceptor site where the env gene starts in the original virus. It is therefore expressed as a subgenomic transcript off of the 5′ LTR. No selectable marker exists in the vector. This feature, together with deletion of sequences in the 5′ portion of the gag-region intron, results in high titer production of viral particles by the packaging line as well as uniformly high levels of expression regardless of the particular gene cloned into the vector.
1.3.3. Construction of the Packaging and the Producer Lines
The other critical component of the retroviral vector system is a cell line that produces the viral proteins that are required for encapsidation of the viral RNA. This cell line, referred to as the retrovirus packaging line, is produced by transfection of proviral DNA containing the retroviral genes necessary for the synthesis of the viral proteins into a fibroblast cell line. The most commonly employed fibroblast cell line is the murine NIH 3T3 cell line. After transfection of the proviral DNA vector into the packaging cell line, this line is then referred to as the retrovirus producer cell line. The first generation of packaging lines contained the stable introduction of a mutant Moloney murine leukemia virus proviral genome that contained a deletion of the encapsidation sequence (psi sequence) (92–94, 99–102). Most of the producer cell line clones derived from these packaging cell lines have been shown to produce retroviral titers in the range of 106 cfu/mL, levels that allow for high efficiency gene transfer (101). These titers are comparable to low normal titers obtained for replication-competent retroviruses.
1.4. Choice of Pancreatic Tumor Cells for Vaccine Production
As discussed earlier, autologous or allogeneic tumor lines can theoretically be used for this purpose. The major benefit of autologous tumor cells has to do with the ability to present antigens that are unique to the patient as well as common among the majority of pancreatic tumors. Unfortunately, it is technically difficult and expensive to isolate tumor cells from every pancreatic cancer patient. In addition, 20% or less of patients presenting with pancreatic cancer are surgical candidates. For these two reasons, we have chosen to use allogeneic tumor cells as the source of immunogen. Based on data from human melanoma, it appears that the majority of T cell specific tumor antigens identified for that disease are shared by at least 50% of other patients’ tumors. Therefore, we have taken the approach of mixing pancreatic tumor lines that derived from two different patients (80). A significant number of pancreatic tumor lines that are derived from either primary pancreatic tumors or from metastases are available from the American Tissue Culture Collection (ATCC, www.atcc.org). These lines have been characterized and could be a good source of tumor lines for this purpose. In choosing a line several parameters should be considered: (1) Rate of growth since these cells will have to be expanded to large numbers for a vaccine trial; (2) Number of known pancreatic cancer specific antigens. This second parameter is important for several reasons. First, expression of at least one pancreatic cancer specific antigen will provide a marker for stability testing following gene transfer and cell expansion. Second, these expressed pancreatic antigens can serve as surrogate targets for immune monitoring studies as part of evaluating efficacy. Alternatively, we have described methods for establishing new pancreatic cancer cell lines from primary tumor specimens (103).
2. Materials
2.1. Retroviral Vector Producer Lines
The MFG retroviral producer cell lines were originally obtained from R. C. Mulligan (Whitehead Institute for Biomedical Research, Cambridge, MA). Both the amphotropic and ecotropic retroviral producer cell lines, CRIP and CRE, respectively, are grown in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose (4,500 g/L), supplemented with 10% bovine calf serum, penicillin (100 U/mL final concentration), streptomycin (100 μg/mL final concentration), L-glutamine (2 mM final concentration), and gentamycin (50 μg/mL final concentration), at 37°C, and 10% CO2. Trypsin (0.25%)–EDTA (0.1%) for passaging the cell lines.
2.2. Tumor Cell Lines
All tumor cell lines to be transduced should be maintained in their optimal growth media before and after performing the transduction procedure to enhance the proliferation capacity of the cell population.
2.3. Retroviral Gene Transfer to Tumor Cells
Tumor cells and retroviral supernatant has been prepared as described in the methods below. In addition, DEAE-dextran 10 mg/mL stock solution prepared by dissolving 1 g into 100 mL of the producer line growth media (DMEM + 10% calf serum) and filtered through an 0.45 μm filter. Store in sterile 5 mL aliquots at 4°C for up to 6 months. Sterile tumor growth media and sterile 1× PBS.
3. Methods
3.1. Maintenance of Retroviral Vector Producer Lines
Grow the retroviral producer cell line in culture to confluency in large flasks (162 cm2 or greater). These cells grow in an adherent monolayer (see Note 1).
When the cells have reached confluency, remove the supernatant, and incubate the cells with enough trypsin–EDTA to cover the bottom of the flask (usually 2–3 mL), at room temperature until the cells become non-adherent (usually 1–2 min) (see Note 2).
Quench the trypsin with at least 3–4 volumes of the growth media containing the calf serum.
Centrifuge for 10 min at 400 × g, at 4°C (see Note 3).
Remove the supernatant and count the cells.
Replate the cells at about a 1:10 dilution of the number of cells in the confluent flask (about 2 × 106 cells per 162 cm2 flask).
Split the cell lines 1:10 every 3–4 days or when each flask reaches confluency (see Notes 4 and 5).
3.2. Preparation of Retroviral Supernatants
Two days prior to transduction, trypsinize the producer cells, wash them once, and replate them at a density of 2 × 106 cells per 100 mm culture dish (see Note 6).
One day prior to transduction, remove the media, and add 10 cm3 of fresh media to the cells.
On the day of transduction, collect a 24 h retroviral supernatant and filter through an 0.45 micrometer (μm) filter to remove contaminating retroviral producer cells (see Notes 7–11).
3.3. Maintenance of Tumor Cell Lines
3.4. Performing Retroviral Gene Transfer to Tumor Cells
Incubate the freshly collected retroviral supernatant with the 10 μg/mL final concentration of the transduction enhancer DEAE-dextran for approximately 10 min at room temperature, so that the retrovirus will bind to the enhancer prior to exposure to the tumor cells (see Note 14).
Remove the growth media from the tumor cells and replace it with 10 cm3 of retroviral supernatant containing the enhancer.
Following incubation of the tumor cells with the retroviral supernatant, remove the supernatant, and wash the cells twice with sterile PBS to rinse away residual retroviral supernatant (see Notes 17–19).
Add 10 cm3 of tumor growth media and allow the cells to grow for 48 h (see Notes 20 and 21).
3.5. Testing for Cytokine Gene Product
At 48 h following transduction, remove the growth media and add 10 cm3 of fresh tumor growth media.
Collect a 24 h supernatant for evaluation of cytokine secretion. To do this, remove the supernatant, centrifuge or filter through an 0.45 μm filter to remove the cells, and aliquot the supernatant into three, 1 mL sterile aliquots that can be stored frozen at −70°C until the time of testing for cytokine secretion. Three separate aliquots should be stored so that repeat testing can be performed without multiple freeze/thaws (which might reduce the concentration of the gene product).
Following collection of the cell supernatant, take up the cells, and count them. Record the total number of cells that contributed to the production of the cytokine over the 24 h. This number will be used to calculate the concentration of cytokine secretion per given number of tumor cells after the concentration of cytokine in the supernatant is determined (see Notes 22–31).
4. Notes
Optimizing Growth Conditions of Retroviral Vector Producer Lines
Producer lines derived from the NIH 3T3 fibroblast cell line grow in an adherent monolayer. They do best when they are plated at a threshold density of about 1/10th the flask’s total cell capacity. Plating the cells at a lower density may result in loss of the cell line.
Exposure to trypsin results in the rapid release of the producer cells from the tissue culture flask. Be aware that overexposure to trypsin will result in significant cell death.
The MFG producer line usually grows in media supplemented with bovine calf serum. Substitution of fetal bovine serum may result in a change in growth kinetics and viral particle production. The growth requirements recommended by the laboratory in which the producer line originated should always be used to culture and cryopreserve the producer line being employed.
Considerations for Storage of Producer Cell Lines
It is advantage to initially expand enough of the producer cells to allow for freezing down of a large stock of aliquots for two reasons. First, prolonged passage in culture may increase the possibility of recombination events within the producer cells which may result in the production of helper virus. Second, there is the theoretical concern that prolonged passage in culture may result in a decrease in the population of producer cells capable of efficiently producing the viral particles.
The MFG producer cell lines freeze well in 90% calf serum + 10% DMSO. Recovery of viable producer cells will be severely compromised if these cells are frozen in other types of serum. Each producer line should be frozen in the same type of serum that is used for in vitro growth. These cells can be stored long term in liquid nitrogen.
Tittering Retroviral Vectors
Retroviruses are difficult to titer because they do not form plaques. It is therefore recommended that every retroviral producer line be tittered for transduction efficiency using an easily transducible cell line. NIH 3T3 cells are a good choice for comparison with other murine cell lines. For the transduction of human primary cultures, a human cell line may be a more appropriate cell line for comparison. Tittering can be accomplished by using the transduction procedure described above and by performing serial two- to fivefold dilutions of the retroviral supernatant prior to exposure of retrovirus to the cell line. Dilutions of the retroviral supernatant should be made with the cell line’s growth media for best results. Most supernatants are optimal either undiluted or between a 1:2 and 1:10 dilution.
Troubleshooting Low Titer Supernatant Collections
Before assuming that insufficient transduction rates are due to low titer supernatants as a result of a poor supernatant collection, it is important to first determine if the producer cells themselves are still capable of producing high quantities of retroviral particles. Because the producer cells themselves also express the gene encoded by the retroviral vector, an easy way to evaluate the producer line for production of the vector is to assay the cells for expression of the gene product. However, in vitro loss of high titer producer lines due to long-term culture can easily be avoided by routinely thawing a new aliquot of producer cells every 3–4 weeks.
If expression is at the expected level, then the problem is more likely to be due to a low titer retroviral supernatant resulting from suboptimal supernatant collection. There are two major causes of low titer retroviral supernatants: (1) Inadequate retroviral supernatant collection due to insufficient numbers of producer cells or overgrowth of producer cells; and (2) Suboptimal growth conditions for retroviral supernatant collection, in particular, a bad lot of calf serum, use of the wrong media and supplements, inadequate CO2 concentration during incubation, etc.
A recent study performed to evaluate the improvement of retroviral vector production observed that the growth of 21/22 producer cell lines at 32°C for up to 2 weeks after the cells reached 100% confluence increased vector titers (105). Growth of the producer lines at 32°C is thought to increase the stability of the viral particles. In addition, improved vector production may be due to the decreased metabolism of the producer cells at this lower temperature.
Short-Term and Long-Term Storage Considerations
For optimal transduction efficiencies, freshly collected retroviral supernatants should be used. Although it is possible to store the supernatants at 4°C for several days, and to freeze these supernatants at −70°C for several weeks, the efficiency of transduction may decrease by as much as 50% following thaw of the supernatant.
Producer lines must be frozen in the same type of serum used for in vitro growth unless otherwise advised. The substitution of other serum may not support the growth of these cells well and may result in significant cell death during freezing and storage.
Optimizing Growth Conditions to Enhance Cell Proliferation Rate
Most proliferating cell lines can be transduced with a retroviral vector. However, the efficiency of transduction will depend on the percentage of cells that are actively proliferating at the time of exposure to the retrovirus, since integration into the host genome is required for stable expression of the transferred gene. Therefore, the growth conditions for each cell line being transduced should be optimized before attempting this transduction procedure. Most long-term cell lines already have defined growth conditions that support optimal growth. However, it is now possible to transduce many primary, short-term cancer cell lines, and conditions for optimizing their growth may already have been described.
The actual density of cells in the flask should be optimized for every tumor cell type, keeping in mind that the cells will need to be able to proliferate maximally for at least 48 h following transduction to allow for optimal integration and expression of the gene. For cells with a 48–72 h doubling time, adequate transduction can be achieved by plating the cells at a density that will result in approximately one-third confluency of the flask on the day of transduction.
Consideration of Control Groups for the Transduction Procedure
Both a negative and a positive control group should be included in each transduction experiment to confirm that gene expression is the result of gene transfer. A good negative control group is to incubate a flask of each cell type to be transduced with retroviral producer cell growth media containing the enhancing polymer alone. An adequate positive control group would include the transduction of any easily transducible cell line with the same lot of retroviral supernatant used to transduce the test tumor cells.
Suggestions for Optimizing the Gene Transfer Method
Transduction efficiency can be enhanced by the addition of polymers to the retroviral supernatant just prior to exposure of the target cells to the retroviral vector. Enhanced gene transfer is thought to occur via a charge-mediated mechanism that affects virus binding to or penetration of the target cell. The polycations protamine, polybrene, and DEAE-dextran are routinely used for this purpose (104–106). In addition, liposome-forming compounds such as DOTAP (Boehringer Mannheim, Indianapolis, IN) have also been successfully used to enhance retroviral gene transfer and may be less toxic to the host cell than other enhancers. Liposome-forming agents probably enhance gene transfer into the host cell by first forming stable interactions with the virus, then adhering to the cell surface, followed by fusing with the cell membrane and releasing the virus into the cell cytoplasm (107). Because most enhancers are toxic to the cell lines at high concentrations, yet higher concentrations of polymers may be required for enhanced transduction efficiency to some cell lines, it is recommended that a titer of the enhancer be performed on each new batch of enhancers used, to determine the least toxic, most enhancing concentration of the polycation or lipid compound. Table 3 illustrates recommended ranges of polycation and lipid reagent concentrations for the commonly employed transduction enhancers.
Longer incubation times will increase the number of proliferating cells that are exposed to the retroviral vector, and therefore, may increase the efficiency of transduction. Hardy tumor cell lines may tolerate the retroviral supernatant containing low concentrations of enhancer for 24–48 h without significant cell death. However, primary human tumor cultures may not tolerate a change in the growth media for more than several hours. Therefore, it is best to perform a pilot study evaluating the rate of tumor cell death over time when exposed to the retroviral supernatant containing the enhancer, to optimize the transduction procedure.
There is also evidence to suggest that the efficiency of retroviral transduction can be improved by a 90 min centrifugation at 2,500 rpm at 32°C, prior to an overnight incubation (at 32°C) of the tumor cells with the retroviral supernatant (105). However, some tumor cells may not tolerate an overnight incubation at 32°C.
It is often useful to perform the initial transduction studies on new tumor cell lines using the retroviral vector containing a marker gene (for example, the lacz gene that expresses the cytoplasmic enzyme beta-galactosidase, which will turn the transduced cell’s cytoplasm blue when exposed to the substrate bluogal or Xgal). Marker genes can be used quantitatively to determine the number of tumor cells in the transduced population that are capable of expressing the transferred gene (the transduction efficiency of the vector for a particular tumor cell line).
It is not uncommon to have a high titer retroviral supernatant. If this is the case, the supernatant can be diluted 1:5 or 1:10 (depending on titer) with target cell growth media, prior to the transduction procedure, to decrease target cell toxicity from the retroviral supernatant. In fact, be aware that a dilution of a high titer retroviral supernatant may be necessary because higher titer supernatants may contain inhibitors against successful retroviral transduction.
Storage Considerations of Transduced Tumor Cells
Freezing of large stocks of the transduced tumor cells is recommended to prevent loss of gene expression as well as to prevent in vitro selection with loss of antigen expression. Transduced tumor cell lines can be frozen down and stored in liquid nitrogen long term without loss of gene expression. Controlled-rate freezing is recommended to prevent a significant decrease in viability following thawing. A cheap and efficient way of control-rate freezing is to immerse the freezing vial of cells in a propanol bath (Nalgene Cryo 1°C Freezing Container) and to place the apparatus into a −70°C freezer overnight. This will freeze the cells at approximately 1°C/min. The cells can then be placed into liquid nitrogen for long-term storage. However, more controlled and regulated freezing methods should be used for vaccine intended for clinical use.
Special Considerations for Human Tumor Cells
Primary human tumor lines are more difficult to transduce than long-term established lines. However, with the increasing applications of gene therapy to the clinics, there is an increasing need for improved methods of gene transfer to these cells. The most important criteria for efficient gene transfer to primary human tumor cultures are to optimize the growth conditions for maximally proliferation capacity. In addition, increasing the concentration of transduction enhancing polymer may result in improved transduction efficiency. It is often beneficial to initially screen the different enhancing polymers for the upper limits of concentration of polymer, and incubation time, that each primary tumor cell line can tolerate, before significant cell death is observed.
In Vitro Testing of Cytokine-Secreting Tumor Vaccines
Following transduction of tumor cells with the cytokine gene, the transduced cells should be evaluated for the total quantity of cytokine produced and for the quantity of cytokine that is bioactive. The total quantity of cytokine produced is best determined by ELISA. ELISA kits are now commercially available for quantitation of most murine and human cytokines (Genzyme, Endogen, R&D systems). Although these kits are expensive, they usually have a sensitivity of 1–4 pg/mL and are specific for the cytokine being tested. Bioassays are also available for many murine and human cytokines. Although they are often not as sensitive or specific as ELISA, they provide important information concerning the function of the cytokine being secreted by the tumor cells. Cell lines for bio-assay of common murine and human cytokines are listed in Table 3. For all of these assays, serial dilutions are made of the tumor cell supernatants collected as described above. Most of the bioassays rely on cell lines that are growth factor dependent. In these assays, the degree of proliferation of the cell lines in the presence of the serially diluted cytokines is determined by 3H-thymidine incorporation. A recombinant standard is also run along with the test samples to accurately quantitate the cytokine in the test samples. Because several cytokines may stimulate the same cell line, duplicate curves are often run for each sample, one curve in the presence of cytokine blocking antibody, to evaluate the percent of proliferation that is specifically the result of that cytokine. The exceptions are: the TNF-alpha assay, which is a cytotoxic assay; and the interferon-gamma assay, which is an anti-viral assay. The exact procedures for performing these assays can be found in the references listed in Table 4.
Testing for the Expression of Gene Products Other Than Cytokines
Genes encoding cytokines are currently one of the most commonly employed genetically altered tumor vaccine strategies, in preclinical models, and in clinical trials. However, other gene-modified vaccine strategies, including tumor cell surface expression of MHC class I and II molecules, and co-stimulatory cell surface molecules (for example, B7), are also under investigation. Successful gene transfer of these gene products can be assayed using cell surface staining with monoclonal antibodies specific for the gene product and analyzed by standard flow cytometric methods.
Troubleshooting an Inefficient Gene Transfer Result
Evaluation of vector copy number should be considered, particularly in cases of suboptimal gene product expression, to determine if the problem is at the level of transcription or due to inadequate transduction. Vector copy number can be evaluated by southern blot hybridization using standard procedures. This procedure is also important to do for characterizing cells that will be used for clinical use.
If the problem is due to inadequate transduction and all of the transduction conditions have been optimized, it is possible to significantly improve on the transduction efficiency by subjecting the transduced cells to one or two more rounds of transduction.
Sometimes alternative gene transfer vectors are required.
Production of Vaccine Cells Using Good Laboratory Practice Guidelines
Although beyond the scope of this review, there are some general principles to consider when developing vaccine cells for clinical use. It is always a good idea to be familiar with FDA recommendations and to consider a pre-IND meeting to discuss the process to be used. Some general principles to consider include:
All procedures should be done under the sterile conditions.
Well-characterized vector systems should be used to produce transduced cells. There are specific requirements for each vector system. However, all vectors require complete sequencing, a well-documented construction history, sterility testing, and a BLAST search to confirm that it does not contain sequences with potential oncogene activity. Repeat sequencing of the vector should be performed after insertion into the cells to confirm that it was inserted without new mutations or new sequences that could be potentially oncogenic. In addition, copy number should be determined.
Antibiotic selection markers within vectors are of potential concern due to the potential for antibiotic resistance that they can confer long term.
Antibiotics used for selection of genetically modified cells will probably need to be completely eliminated from the final vaccine formulation.
Specific parameters should be identified for demonstrating stability of the genetically modified tumor cells. Examples include: expression of genes or surface antigens, HLA typing, levels of cytokine production, etc.
Table 3.
Commonly employed transduction enhancing reagents
| Enhancer | Target cell | Concentration range a (final conc. in retroviral supernatant) (μg/mL) |
|---|---|---|
| DEAE-dextran (Sigma, St. Louis, MO) | Murine tumor cell lines Human tumor cell lines |
5–10 10–100 |
| Polybrene (Sigma, St. Louis, MO or Aldrich, Milwaukee, WI) | Murine tumor cell lines Human tumor cell lines |
5–10 10–100 |
| Protamine sulfate (Lilly, Indianapolis, IN) | Murine tumor cell lines Human tumor cell lines |
5–10 5–100 |
| DOTAP (Boehringer Mannheim, Indianapolis, IN) | Murine tumor cell lines Human tumor cell lines |
5–10 10–100 |
Final conc. in retroviral supernatant
Table 4.
Common bioassays used to quantitate cytokine production
| Cytokine | Bioassay |
|---|---|
| Human IL-2 | CTLL-2 |
| Murine IL-2 | CTLL |
| Human IL-3 | TF-1 cells |
| Murine IL-3 | NFS 60 |
| Human IL-4 | PHA-activated peripheral blood mononuclear cells |
| Murine IL-4 | CT4S or HT-2 cells |
| Human IL-5 | TF-1 cells |
| Murine IL-5 | Same |
| Human IL-6 | T 1165.85.2.1 cells |
| Murine IL-6 | Same |
| Human IL-7 | PHA-activated peripheral blood mononuclear cells |
| Murine IL-7 | Same |
| Human GM-CSF | TF-1 cells |
| Murine GM-CSF | NFS-60 cells |
| Human interferon-gamma | Anti-viral assay |
| Murine interferon-gamma | Anti-viral assay |
| Human TNF-alpha | Cytotoxic assay |
| Murine TNF-alpha | Cytotoxic assay |
References
- 1.Evans DB, Abbruzzese JL, Rich TA. Cancer of the pancreas. In: DeVita VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 5. J.B. Lippincott Co; Philadelphia: 1997. pp. 1054–1087. [Google Scholar]
- 2.Conlon KC, Klimstra DS, Brennan MF. Long term survival after curative resection for pancreatic ductal adenocarcinoma. Ann Surg. 1996;223(3):273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduo-denectomies in the 1990s: pathology, complications and outcomes. Ann Surg. 1997;226(3):248–260. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL, Laheru DA, Farnell MB, Swartz MJ, Gunderson LL, Miller RC. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17(4):981–990. doi: 10.1245/s10434-009-0743-7. Epub 2010 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S, Vauthey JN, Abdalla EK, Lee JE, Pisters PW, Evans DB. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16(4):836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 9.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 10.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. Epub 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW. Genome expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 13.Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RE, Marra MA, Prange C, Morin PJ, Polyak K, Papadopoulos N, Vogelstein B, Kinzler KW, Strausberg RL, Riggins GJ. A public database for gene expression in human cancers. Cancer Res. 1999;59:5403–5407. [PubMed] [Google Scholar]
- 14.Lash AE, Tolstoshev CM, Wagner L, Schuler GD, Strausberg RL, Riggins GJ, Altschul SF. SAGEmap: a public gene expression resource. Genome Res. 2000;10:1051–1060. doi: 10.1101/gr.10.7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Maitra A, Shen-Ong GL, et al. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160(4):1239–1249. doi: 10.1016/S0002-9440(10)62551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinoma of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 17.Ryu B, Jones J, Blades NJ, et al. Relationships and differentially expressed genes among pancreatic cancers examined by large scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- 18.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 19.Germain RN. Immunology: the ins and outs of antigen processing and presentation. Nature. 1986;322:687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- 20.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 21.Pieters J. MHC class II restricted antigen processing and presentation. Adv Immunol. 2000;75:159–208. doi: 10.1016/s0065-2776(00)75004-8. [DOI] [PubMed] [Google Scholar]
- 22.Solheim JC. Class I MHC molecules: assembly and antigen presentation. Immunol Rev. 1999;172:11–19. doi: 10.1111/j.1600-065x.1999.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 23.Hammerling GJ, Vogt AB, Kropshofer H. Antigen processing and presentation-towards the millennium. Immunol Rev. 1999;172:5–9. doi: 10.1111/j.1600-065x.1999.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 24.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Ashe S, Brady WA, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz RH. Costimulation of T lymphocytes, the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 27.Lechler R, Aichinger G, Lightstone L. The endogenous pathway of MHC class II antigen presentation. Immunol Rev. 1996;151:51–79. doi: 10.1111/j.1600-065x.1996.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 28.Ostrand-Rosenberg S. Tumor immunotherapy: the tumor cell as an antigen presenting cell. Curr Opin Immunol. 1994;6(5):722–727. doi: 10.1016/0952-7915(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 29.Golumbek P, Lazenby A, Levitsky HI, et al. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 30.Dranoff G, Jaffee EM, Golumbek P, et al. Vaccination with irradiated tumor cells engineered to secrete murine GM-CSF stimulates potent, specific and long lasting anti tumor immunity. Proc Natl Acad Sci. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishihara T, Sawada T, Yamamoto A, et al. Antibody-dependent cytotoxicity mediated by chimeric monoclonal antibody Nd2 and experimental immunotherapy for pancreatic cancer. Jpn J Cancer Res. 2000;91(8):817–824. doi: 10.1111/j.1349-7006.2000.tb01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruns CJ, Harbison MT, Davis DW, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6(5):1936–1948. [PubMed] [Google Scholar]
- 33.Green MC, Murray JL, Hortobagyi GN. Monoclonal antibody therapy for solid tumors. Cancer Treat Rev. 2000;26(4):269–286. doi: 10.1053/ctrv.2000.0176. [DOI] [PubMed] [Google Scholar]
- 34.Tempero M. Biologic therapy of gastrointestinal cancer. Cancer Treat Res. 1998;98:227–237. doi: 10.1007/978-1-4615-4977-2_9. [DOI] [PubMed] [Google Scholar]
- 35.Foon KA, Yannelli J, Bhattacharya-Chatterjee M. Colorectal cancer as a model for immunotherapy. Clin Cancer Res. 1999;5(2):225–236. [PubMed] [Google Scholar]
- 36.Offringa R, Vierboom MP, van der Burg SH, Erdile L, Melief CJ. p53: a potential target antigen for immunotherapy of cancer. Ann N Y Acad Sci. 2000;910:223–233. doi: 10.1111/j.1749-6632.2000.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 37.Abbruzzese JL. Molecular diagnosis of pancreatic and biliary cancer: ready for broad implementation? Cancer J. 2000;6(5):282–284. [PubMed] [Google Scholar]
- 38.Saforafas GH, Tsiotou AG, Tsiotos GG. Molecular biology of pancreatic cancer; oncogenes, tumor suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev. 2000;26(1):29–52. doi: 10.1053/ctrv.1999.0144. [DOI] [PubMed] [Google Scholar]
- 39.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156(6):1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn SA, Kern SE. Molecular genetics of exocrine pancreatic neoplasms. Surg Clin North Am. 1995;75(5):857–869. doi: 10.1016/s0039-6109(16)46732-0. [DOI] [PubMed] [Google Scholar]
- 41.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 42.Flanders TY, Foulkes WD. Pancreatic adenocarcinoma: epidemiology and genetics. J Med Genet. 1996;33(11):889–898. doi: 10.1136/jmg.33.11.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hruban RH, Van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the pancreas. Am J Pathol. 1993;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- 44.Gjertsen MK, Bakka A, Breivik J, et al. Vaccination with mutant ras peptides and induction of T-cell responsiveness in pancreatic carcinoma patients carrying the corresponding ras mutation. Lancet. 1995;346:1399–1400. doi: 10.1016/s0140-6736(95)92408-6. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann-Leitner ES, Kantor JA, Shupert WL, Schlom J, Abrams SI. Identification of a human CD8+ T lymphocyte neo-epitope created by a ras codon 12 mutation which is restricted by the HLA-A2 allele. Cell Immunol. 1998;187:103–116. doi: 10.1006/cimm.1998.1325. [DOI] [PubMed] [Google Scholar]
- 46.Khleif SN, Abrams SI, Hamilton JM, et al. A Phase I vaccine trial with peptides reflecting Ras oncogene mutations of solid tumors. J Immunother. 1999;22(2):155–165. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Toubaji A, Achtar M, Provenzano M, et al. Pilot study of mutant ras peptide-based vaccine as an adjuvant treatment in pancreatic and colorectal cancers. Cancer Immunol Immunother. 2008;57(9):1413–1420. doi: 10.1007/s00262-008-0477-6. Epub 23 Feb 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gjertsen MK, Buanes T, Rosseland AR, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony stimulating factor as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer. 2001;92:441–450. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 49.Wang XY, Kaneko Y, Repasky E, Subjeck JR. Heat shock proteins and cancer immunotherapy. Immunol Invest. 2000;29(2):131–137. doi: 10.3109/08820130009062296. [DOI] [PubMed] [Google Scholar]
- 50.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor specific cytotoxic T lymphocytes and memory T cells by immunization with tumor derived heat shock protein gp96. J Immunother. 1998;21(4):269–276. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Maki RG, Livingston PO, Lewis JJ, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52(8):1964–1972. doi: 10.1007/s10620-006-9205-2. Epub 10 Apr 2007. [DOI] [PubMed] [Google Scholar]
- 52.Finn OJ, Jerome KR, Henderson RA, et al. MUC-1 epithelial tumor mucin-based immunity and vaccines. Immunol Rev. 1995:14561–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 53.Apostopopoulos V, McKenzie IF. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14(3/4):293–309. doi: 10.1615/critrevimmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 54.Mukherjee P, Ginardi AR, Madsen CS, et al. Mice with spontaneous pancreatic cancer naturally develop MUC-1 specific CTLs that eradicate tumors when adoptively transferred. J Immunol. 2000;165:3451–3460. doi: 10.4049/jimmunol.165.6.3451. [DOI] [PubMed] [Google Scholar]
- 55.Ramanathan RK, Lee K, Mckolanis J, et al. Phase I study of a MUC-1 synthetic vaccine admixed with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–264. doi: 10.1007/s00262-004-0581-1. Epub 2004 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J Gastrointest Surg. 2013;17(1):94–101. doi: 10.1007/s11605-012-2064-6. Epub 15 Nov 2012. [DOI] [PubMed] [Google Scholar]
- 57.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 58.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime and boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18(23):3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 59.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer—science driving clinical progress. Nat Rev Cancer. 2005;5(6):459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 60.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26(32):5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 61.Le D, Lutz E, Huang L, Onners B, Uram J, Solt S, Sugar E, Zheng L, Jaffee E, Laheru D. Phase Ib study of ipilimumab alone or in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene (vaccine) in pancreatic cancer. J Clin Oncol. 2012;30(suppl 4):abstr 211. [Google Scholar]
- 62.Fonsatti E, Maio M, Altomonte M, Hersey P. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37(5):517–523. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 64.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Restifo NP. Cancer vaccines: basic principles. In: Rosenberg SA, editor. The principles and practice of the biologic therapy of cancer. 3. Lippincott Williams and Wilkens; Philadelphia: 2000. pp. 571–584. [Google Scholar]
- 66.Pardoll DM, Jaffee EM. Cancer vaccines: clinical applications. In: Rosenberg SA, editor. The principles and practice of the biologic therapy of cancer. 3. Lippincott Williams and Wilkens; Philadelphia: 2000. pp. 647–662. [Google Scholar]
- 67.Greten TF, Jaffee EM. Cancer vaccines. J Clin Oncol. 1999;17(3):1047–1060. doi: 10.1200/JCO.1999.17.3.1047. [DOI] [PubMed] [Google Scholar]
- 68.Fearon ER, Itaya T, Hunt B, Vogelstein B, Frost P. Induction in a murine tumor of immunogenic tumor variants by transfection with a foreign gene. Cancer Res. 1988;48(11):2975–2980. [PubMed] [Google Scholar]
- 69.Dranoff G, Jaffee EM, Golumbek P, et al. Vaccination with irradiated tumor cells engineered to secrete murine GM-CSF stimulates potent, specific and long lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inaba K, Steinman R, Pack M, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang AY, Golumbek PT, Ahmadzadeh M, et al. Role of bone marrow derived cells in presenting MHC class I restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 72.Nakazaki Y, Tani K, Lin ZT, et al. Vaccine effect of granulocyte-macrophage colony stimulating factor or CD80 gene transduced murine hematopoietic tumor cells and their cooperative enhancement of anti-tumor immunity. Gene Ther. 1998;5(10):1355–1362. doi: 10.1038/sj.gt.3300726. [DOI] [PubMed] [Google Scholar]
- 73.Golumbek PT, Azhari R, Jaffee EM, et al. Controlled release biodegradable cytokine depots: a new approach to cancer vaccine design. Cancer Res. 1993;53:1–4. [PubMed] [Google Scholar]
- 74.Jaffee EM, Abrams RA, Cameron JL, et al. A phase I trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene for the treatment of pancreatic adenocarcinoma. Hum Gene Ther. 1998;9:1951–1971. doi: 10.1089/hum.1998.9.13-1951. [DOI] [PubMed] [Google Scholar]
- 75.Simons JW, Jaffee EM, Weber C, et al. Bioactivity of human GM-CSF gene transduced autologous renal vaccines. Cancer Res. 1997;57:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 76.Simons JW, Mikhak B, Chang JF, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 77.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95(22):13141–13146. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma specific human cytotoxic T cell lines. Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 79.Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaffee EM, Schutte M, Gossett J, et al. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am. 1998;4:194–203. [PubMed] [Google Scholar]
- 81.Jaffee EM, Hruban R, Biedzycki B, et al. A novel allogeneic GM-CSF secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19(1):145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 82.Davis MP, Dinneen AB, Landa N, et al. Grover’s disease: clinicopathologic review of 72 cases. Mayo Clin Proc. 1999;74(3):229–234. doi: 10.4065/74.3.229. [DOI] [PubMed] [Google Scholar]
- 83.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, Choi M, Sharma R, Illei PB, Hruban RH, Abrams RA, Le D, Jaffee E, Laheru D. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253(2):328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boon T, Van Den Eynde BJ. Cancer vaccines; cancer antigens. In: Rosenberg SA, editor. The principles and practice of the biologic therapy of cancer. 3. Lippincott Williams and Wilkens; Philadelphia: 2000. pp. 493–504. [Google Scholar]
- 85.Graham FL, Van Der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 86.Potter H, Weir L, Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci USA. 1984;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- 88.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banerji J, Rusconi S, Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981;27:299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- 90.Kingston RE. Introduction of DNA into mammalian cells. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. Vol. 1. Wiley; Hoboken: 1993. [Google Scholar]
- 91.Mulligan RC. Gene transfer and gene therapy. Principles, prospects, and perspective. In: Lindsten J, Pettersson U, editors. Etiology of human diseases at the DNA level. Raven Press, Ltd; New York: 1991. [Google Scholar]
- 92.Danos O, Mulligan RC. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 94.Miller DA, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Armentano D, Sheau-Fung Y, Kantoff P, von Ruden T, Anderson WF, Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987;61:1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lindemann D, Patriquin E, Feng S, Mulligan RC. Versatile retroviral vector systems for regulating gene expression in vitro and in vivo. Mol Med. 1997;3(7):466–476. [PMC free article] [PubMed] [Google Scholar]
- 97.Uberla K. Lentivirus vector based on simian immunodeficiency virus. Development and use. Methods Mol Med. 2002;69:351–360. [PubMed] [Google Scholar]
- 98.Srinivasakumar N. Packaging cell system for lentivirus vectors. Preparation and use. Methods Mol Med. 2002;69:275–302. doi: 10.1385/1-59259-141-8:275. [DOI] [PubMed] [Google Scholar]
- 99.Miller DA, Miller DG, Garcia VJ, Lynch CM. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 100.Miller AD, Law MF, Verma IM. Generation of helper-free amphotropic retroviruses that transduce a dominant acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985;5:431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mann R, Baltimore D. Varying the position of a retrovirus packaging sequence results in the encapsidation of both unspliced and spliced RNAs. J Virol. 1985;54:401–407. doi: 10.1128/jvi.54.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bosselman RA, Hsu RY, Bruszewski J, Hu F, Martin F, Nicholson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Muloney murine leukemia virus structural genes via the metallothionein promoter. Mol Cell Biol. 1987;7(5):1797–1806. doi: 10.1128/mcb.7.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jaffee EM, Schutte M, Gossett J, Morsberger L, Adler AJ, Thomas M, Greten TF, Hruban RH, Yeo CJ, Griffin GA. Development and characterization of a cytokine secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J Sci Am. 1998;4(3):194–203. [PubMed] [Google Scholar]
- 104.Small J, Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983;219:174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- 105.Kotani H, Newton PB, Zhang S, Chiang YL, Otto E, Weaver L, Balese MR, Anderson FW, McGarrity GJ. Improved methods of retroviral vector transduction and production for gene therapy. Hum Gene Ther. 1994;5:19–28. doi: 10.1089/hum.1994.5.1-19. [DOI] [PubMed] [Google Scholar]
- 106.Cornetta K, Anderson F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene transfer: implications for human gene therapy. J Virol Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- 107.Wilson JM, Jefferson DM, Chowdhury JR, Novikoff PM, Johnston DE, Mulligan RC. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci USA. 1988;85:3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]