Abstract
Biomedical applications of ZnFe2O4 nanoparticle are preferable among all kinds of ferrites due to the compatibility of Zn2+ ions for human bodies. We have followed the soft chemical route to synthesize chitosan and PEG coated ZnFe2O4 nanoparticles and also the chitosan-coated-nanoparticles encapsulated with liposome. X-ray diffraction studies by the Mo Kα target, showed the formation of single phase spinel structure. The lattice parameter turned out to be 8.48Å and grain size ~ 4.8 nm (± 0.1 nm). Similar particle size was observed by transmission electron microscope analysis. HRTEM studies showed the distinct lattice fringes thus confirming the good crystallinity of the synthesized nanoparticles. M-H curve at room temperature showed the prepared sample was superparamagnetic in nature, which is also confirmed by the doublets of Mössbauer spectroscopy. Relaxivity values (r2) of Chitosan and PEG coated ZnFe2O4 nanoparticles are 68 and 76 mM−1s−1 respectively. In order to achieve further biocompatibility the chitosan-coated-nanoparticles were encapsulated with liposome. The r2 relaxivity was found as 54mM−1s−1. MR images obtained from the in vitro experiments based on phantoms demonstrated good contrast enhancement. Induction heating of bare and coated particles was investigated to reveal the self heating temperature rising properties of ZnFe2O4 nanoparticles.
Keywords: Zinc Ferrite nanoparticle, Nuclear Magnetic Resonance, Hypethermia, Magnetization, Mossbauer spectroscopy
1. Introduction
Potential applications of ferrite nanoparticles as MRI contrast agent and hyperthermia led vibrant research activities in biomedical applications[1–9]. In recent time, ferrites containing divalent cations other than Zn such as Co, Ni, Fe, Mn, and Mg are under intense investigations[10] for its possible applications as MRI contrast agent as an attempt to find out newer contrast agents with optimum magnetic and structural properties[11,12]. Nuclear magnetic resonance is used to characterize the particles for its application in Magnetic Resonance Imaging with the variation of factors like size, shape, monodispersity and magnetization. NMR phenomenon is based on the fact that when nuclei of atoms are excited through an external pulse at Larmor frequency of particular nucleus (such as 1H) under static dc magnetic field the alignment of magnetic moment of the nucleus with external dc magnetic field is perturbed. The nucleus returns to its thermodynamic equilibrium state through processes of transverse relaxation (spin-spin) or R2 relaxation. Observed variation in signal in the presence of contrast agents is directly related to the above factors of the agents in different tissue.
Zinc ferrite nanoparticles earned a great deal of attention in nanomedicine due to the smaller toxicity of Zn2+. It is a long quest for biocompatible MRI contrast agents in the field of medical science because present contrast agents are toxic in nature. ZnFe2O4 nanoparticles has been known as good candidate for MRI contrast agents since its permissible RDI (Reference Daily Intake) doses for Fe and Zn are 18 and 15 mg/day, respectively, which is much higher than any other biocompatible material[9].
In this paper, we have synthesized ZnFe2O4 nanoparticles by the chemical co-precipitation method and coated with biocompatible chitosan and PEG. Chitosan coated nanoparticle was also encapsulated with liposome in order to investigate their possibility for biomedical applications. Possibility of obtaining good MR image was studied by using water phantoms. The extent of self-heating temperature rising properties was also studied by radio frequency field.
1. Materials and Methods
Analytical grade of Zn(NO3)2·6H2O and FeCl3 were mixed in the required molar ratio which is 1:2 under continuous stirring. As-synthesized ZnFe2O4 was coated with chitosan and PEG at room temperature. The chitosan-coated ferrites were encapsulated by liposome following the technique presented in [13]. The size of the encapsulated nanoparticles with liposome was <200nm which was achieved through extrusion. Extruder was preheated at 60–65°C with the heat plate. Samples were loaded into the gas-tight syringes and carefully placed into the end of the Mini-Extruder. After several passes through 3 pieces of 200 nm filter membrane, the sizes of nanoparticle loaded liposome were estimated to be less than 200 nm.
2. Results and discussion
2.1 Structural Properties
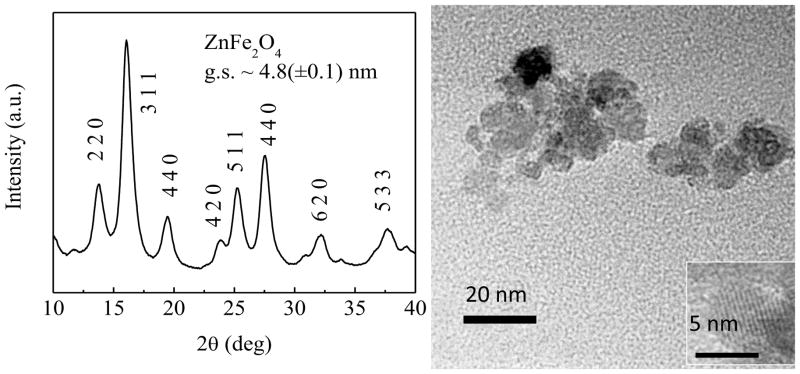
The XRD pattern for prepared ZnFe2O4 nanoparticles in the as prepared condition is shown in Fig 1(a). The prominent planes of the spinel structure (220), (311), (400), (422), (511) and (440) matched well [14]. The crystallite size was determined from the FWHM of (311) peak using Scherrer’s formula which is found ~5nm. The lattice parameter is found about ~ 8.48Å.
Fig 1.
(a)X-ray diffraction patterns of ZnFe2O4 in the as dried condition. (b) TEM and HRTEM (inset) images of the ZnFe2O4 nanoparticles in the as dried condition. TEM image shows the agglomeration of particles and HRTEM image shows the particle size is about 5nm.
In order to observe the physical size and shape of the ZnFe2O4 nanoparticle TEM bright field image and HRTEM image (inset) are presented in Fig. 1(b). From the Fig. 1(b) the physical size and shape of the nanoparticle is observed close to the value obtained from X-ray diffraction. From the HRTEM image, the lattice fringes clearly indicate the good crystallinity of the nanoparticles.
3.2 Magnetic Properties and Mössbauer analysis
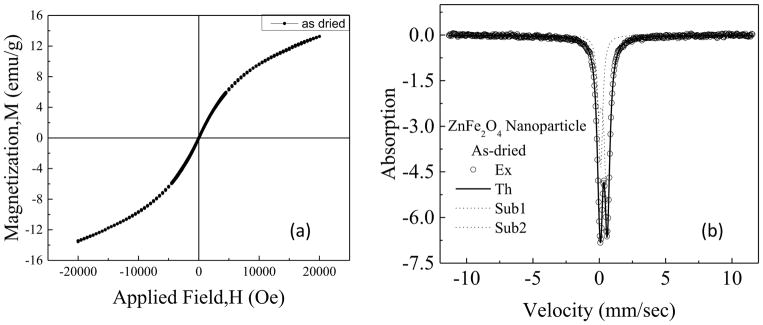
The M-H loops with Hmax of ±20kOe of as dried samples of ZnFe2O4 nanoparticles is presented in Fig 2(a). At the nano level there is a large change in the magnetic state due to the change in cation distribution and particle size which is reported in[15,16]. Introduction of large magnetization compared to paramagnetic bulk ZnFe2O4 is possible since at the nano dimension some of the Zn2+ ion is transferred to the octahedral position, which leads to the imbalance in spin configuration. Thus, zinc ferrite nanoparticles become a mixed spinel type due to change in cation distribution. It might be observed from Fig. 2 (a) that the nature of the hysteresis loop of ZnFe2O4 nanoparticles also indicates slightly S-shape rather than the straight line passing through the origin. Thus at the nano-dimension the synthesized ZnFe2O4 has attained superparamagnetic nature rather than paramagnetic. The maximum magnetization at 20kOe (Mmax) was found to be 13.4emu/g with negligible coercivity (Hc) and remanence.
Fig 2.
(a)Hysteresis loop of zinc ferrite nanoparticles in the as dried condition at room temperature (b) Mössbauer analysis of the ZnFe2O4 nanoparticles in the as dried condition at the room temperature
Mössbauer analysis at room temperature shows a doublet pattern which is shown in Fig 2 (b), which indicates faster relaxation. We found the chi^2 value as 0.828 which shows excellent fitting between experimental and theoretical curves. Required numbers of Fe3+ species were two which demonstrated 66% occupancy of Fe3+ on the octahedral site and 29.8% occupancy at the tetrahedral site. The isomer shift and the quadrupole splitting of the B site are 0.276 and 0.652 mm/s and the A site are 0.0633 and 0.13 mm/s respectively while Zeeman splitting are zero for both the sub-spectra.
3.3 NMR Analysis and MR images of Coated ZnFe2O4
The contrast enhancing efficacy of the synthesized ferrites as contrast agents may be expressed by the following equation[17]:
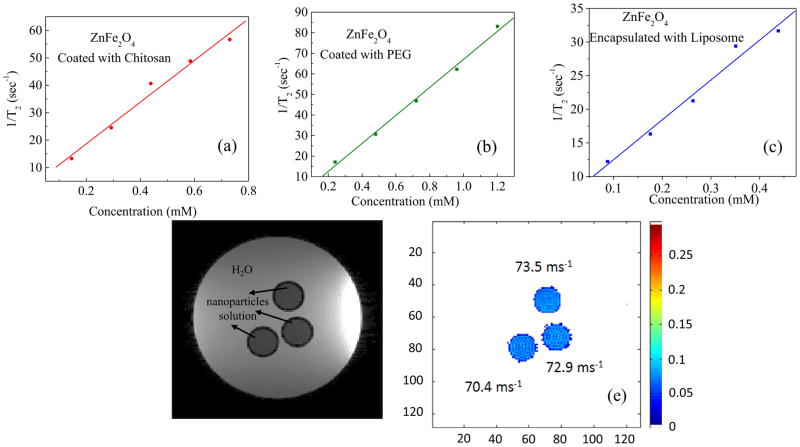
where, C is the concentration of the contrast agent, T2 is the observed relaxation time in the presence of contrast agent, and T2,0 is the relaxation time in the absence of contrast agent and r2 is the corresponding relaxivity. A contrast agent with a large relaxivity value r2 shortens relaxation time T2 drastically with a smaller concentration increment. It can be found in the literature that the value of r2 does not only depend on maximum magnetization but also depends on coating agents, particle size and shape[18]. In our present study, we have found a tangible difference between the r2 values of ZnFe2O4 nanoparticles coated with chitosan and PEG and with liposome encapsulation presented in Fig. 3(a), (b), and (c).The values of r2 for chitosan and PEG coated particles are about 76 (±6)mM−1s−1and 68 (±3)mM−1s−1 respectively which seems to be very close. In this case, surface coating didn’t have much effect on the r2 relaxivity. While the chitosan-coated ZnFe2O4 nanoparticles encapsulated with liposome exhibited slightly small value of about 54 (±10) mM−1s−1. The reason behind the lesser value of relaxivity might be associated with lesser particle-particle interaction due to encapsulation of the chitosan coated particles by the double layered wall of liposome.
Fig 3.
T2 Relaxivity by NMR spectroscopy of ZnFe2O4 nanoparticles with the coating of (a) Chitosan (b) PEG and (c) Encapsulated with Liposome. (d) MRI contrast of ZnFe2O4 coated with chitosan with respect to water (d) Respective relaxation measured from these images are quoted in the figure.
The MR images are presented in Fig 3(d). The image was obtained in-vitro using phantom which was designed by filling chitosan coated ZnFe2O4 nanoparticle solution of 0.66mM inside the small NMR tubes inserted inside the large Falcon tube of 50 ml containing water. In Fig. 3(d) the light background is water and the dark circles represent nanoparticle solution. The degree of darkening demonstrated that the chitosan coated ZnFe2O4 nanoparticle solution is suitable as T2 contrast agent. In Fig. 3(e) the value of relaxation time T2 or the (Relaxation) R2= 1/T2 has been determined by the MATLAB programme and presented in the figure with the scale bar. The average value of r2 has been obtained as 70ms−1 which is more or less close to the NMR results. The slight difference might be related to the difference in the value of Bo of 9.4 and 11.7 T respectively.
3. Induction heating of ZnFe2O4 nanoparticles
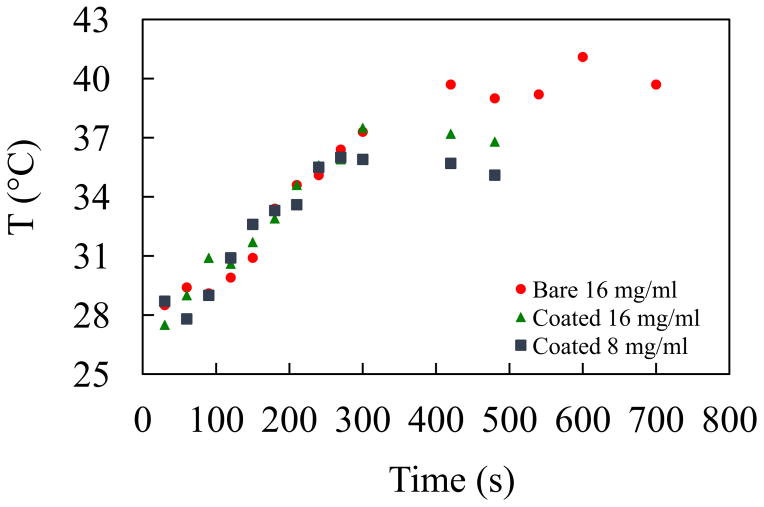
It is known that through hyperthermia therapy, cancer cells can be destroyed by rising the temperature of a lesion selectively to around 42–45°C while the healthy cells would remain unaffected. ZnFe2O4 ought to be inefficient for hyperthermia applications because of lower Mmax. Fig 4 (a), (b) and (c) represent time dependence of temperature profile for three conditions uncoated (a) coated with chitosan with the concentration of 16 mg/ml (b) and 8 mg/ml (c) respectively. From the figures it is observed that the maximum temperature rise of all the conditions the 16mg/ml solutions attains 41°C before saturation but it takes long exposure time of 10 minutes. This self-heating temperature rising properties might be used for various temperature sensitized applications.
Fig 4.
Hyperthermia effect of ZnFe2O4 nanoparticles at different concentrations (a) 16mg/mL (b) 16mg/mL with PVA and (c) 8mg/mL
Highlights.
Chitosan, PEG coated and liposome encapsulated ZnFe2O4 nanoparticles were developed.
T2 relaxivity of three solutions of ZnFe2O4 are 68, 76 and 59 mM−1sec1.
MR images of phantoms demonstrated contrast enhancement of the coated particles.
Particles were investigated for thermo therapeutic treatment of cancer.
Acknowledgments
Highest admiration for the NIH grant (R01 CA-140102, R01 EB-011968, P30 NS-052510). The authors also acknowledge greatly BAEC and Ministry of Science and Technology, GOB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmad T, Bae H, Iqbal Y, Rhee I, Hong S. Chitosan-coated nickel-ferrite nanoparticles as contrast agents in magnetic resonance imaging. J Magn Magn Mater. 2015;381:151–7. doi: 10.1016/j.jmmm.2014.12.077. [DOI] [Google Scholar]
- 2.Wan J, Jiang X, Li H, Chen K. Facile synthesis of zinc ferrite nanoparticles as non-lanthanide T1 MRI contrast agents. J Mater Chem. 2012;22:13500–5. doi: 10.1039/C2JM30684K. [DOI] [Google Scholar]
- 3.Lu J, Ma S, Sun J, Xia C, Liu C, Wang Z, et al. Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials. 2009;30:2919–28. doi: 10.1016/j.biomaterials.2009.02.001. http://dx.doi.org/10.1016/j.biomaterials.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Tong S, Hou S, Zheng Z, Zhou J, Bao G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10:4607–13. doi: 10.1021/nl102623x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng F-Y, Su C-H, Yang Y-S, Yeh C-S, Tsai C-Y, Wu C-L, et al. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26:729–38. doi: 10.1016/j.biomaterials.2004.03.016. http://dx.doi.org/10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Shultz MD, Calvin S, Fatouros PP, Morrison SA, Carpenter EE. Enhanced ferrite nanoparticles as MRI contrast agents. J Magn Magn Mater. 2007;311:464–8. http://dx.doi.org/10.1016/j.jmmm.2006.10.1188. [Google Scholar]
- 7.Na H, Bin Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009;21:2133–48. doi: 10.1002/adma.200802366. [DOI] [Google Scholar]
- 8.Bárcena C, Sra AK, Chaubey GS, Khemtong C, Liu JP, Gao J. Zinc ferrite nanoparticles as MRI contrast agents. Chem Commun (Camb) 2008:2224–6. doi: 10.1039/b801041b. [DOI] [PubMed] [Google Scholar]
- 9.Hoque SM, Srivastava C, Venkatesha N, Kumar PSA, Chattopadhyay K. Superparamagnetic behaviour and T1, T2 relaxivity of ZnFe2O4 nanoparticles for magnetic resonance imaging. Philos Mag. 2013;93:1771–83. [Google Scholar]
- 10.Mohapatra J, Mitra A, Bahadur D, Aslam M. Surface controlled synthesis of MFe 2 O 4 (M = Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic characteristics. CrystEngComm. 2013;15:524–32. doi: 10.1039/C2CE25957E. [DOI] [Google Scholar]
- 11.Zhou Z, Zhu X, Wu D, Chen Q, Huang D, Sun C, et al. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem Mater. 2015;27:3505–15. doi: 10.1021/acs.chemmater.5b00944. [DOI] [Google Scholar]
- 12.Mohapatra J, Mitra A, Tyagi H, Bahadur D, Aslam M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale. 2015;7:9174–84. doi: 10.1039/C5NR00055F. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan P, Giri J, Banerjee R, Bellage J, Bahadur D. Preparation and chracterization of manganes ferrite-besed magnetic liposomes for hyperthermia treatment of cancer. J Mag Mag Mater. 2007;311:208–15. [Google Scholar]
- 14.Tamhankar PMM, Kulkarni AC, Watawe S. Functionalization of Cobalt Ferrite Nanoparticles with Alginate Coating for Biocompatible Applications. Mater Sci Appl. 2011;02:1317–21. doi: 10.4236/msa.2011.29179. [DOI] [Google Scholar]
- 15.Jeyadevan B, Tohji K, Nakatsuka K. Structure analysis of coprecipitated ZnFe2O4 by extended x-ray-absorption fine structure. J Appl Phys. 1994;78:6325–7. [Google Scholar]
- 16.Choi EJ, Ahn Y, Hahn EJ. Size Dependence of the Magnetic Properties in Superparamagnetic Zinc-Ferrite Nanoparticles. J Korean Phys Soc. 2008;53:2090–4. [Google Scholar]
- 17.Hoque SM, Srivastava C, Srivastava N, Venkateshan N, Chattopadhyay K. Synthesis and characterization of Fe- and Co-based ferrite nanoparticles and study of the T 1 and T 2 relaxivity of chitosan-coated particles. J Mater Sci. 2013;48:812–8. doi: 10.1007/s10853-012-6800-9. [DOI] [Google Scholar]
- 18.Parkes LM, Hodgson R, Lu LT, Tung LD, Robinson I, Fernig DG, et al. Cobalt nanoparticles as a novel magnetic resonance contrast agent--relaxivities at 1. 5 and 3 Tesla. Contrast Media Mol Imaging. 2008;3:150–6. doi: 10.1002/cmmi.241. [DOI] [PubMed] [Google Scholar]