Abstract
P450 oxidoreductase (POR) transports electrons from NADPH to all microsomal cytochrome P450 enzymes, including steroidogenic P450c17, P450c21 and P450aro. Severe POR mutations A287P (in Europeans) and R457H (in Japanese) cause the Antley-Bixler skeletal malformation syndrome (ABS) plus impaired steroidogenesis (causing genital anomalies), but the basis of ABS is unclear. We have characterized the activities of ~40 POR variants, showing that assays based on P450c17 activities, but not cytochrome c assays, correlate with the clinical phenotype. The human POR gene is highly polymorphic: the A503V sequence variant, which decreases P450c17 activities to ~60%, is found on ~28% of human alleles. A promoter polymorphism (~8% of Asians and ~13% of Caucasians) at −152 reduces transcriptional activity by half. Screening of 35 POR variants showed that most mutants lacking activity with P450c17 or cytochrome c also lacked activity to support CYP1A2 and CYP2C19 metabolism of EOMCC (a fluorogenic non-drug substrate), although there were some remarkable differences: Q153R causes ABS and has ~30% of wild-type activity with P450c17 but had 144% of WT activity with CYP1A2 and 284% with CYP2C19. The effects of POR variants on CYP3A4, which metabolizes nearly 50% of clinically used drugs, was examined with multiple, clinically-relevant drug substrates, showing that A287P and R457H dramatically reduce drug metabolism, and that A503V variably impairs drug metabolism. The degree of activity can vary with the drug substrate assayed, as the drugs can influence the conformation of the P450. POR is probably an important contributor to genetic variation in both steroidogenesis and drug metabolism.
Keywords: cytochrome P450, P450 oxidoreductase, CYP3A4, CYP2D6, steroidogenesis, enzyme kinetics, drug metabolism, pharmacogenetics
P450 oxidoreductase
P450 oxidoreductase is the 78 kDa, 680 amino acid flavoprotein that transfers electrons from NADPH to all microsomal (type 2) cytochrome P450 enzymes, including steroidogenic P450c17 (17α-hydroxylase/17,20 lyase, encoded by the CYP17A1 gene), P450c21 (21-hydroxylase, encoded by CYP21A2) and P450aro (aromatase, encoded by CYP19A1), and the principal hepatic drug-metabolizing cytochrome P450 enzymes (Miller, 2005). POR is also a reductase for proteins that are not P450 enzymes, including squalene monoxygenases (Ono and Bloch, 1975), fatty acid elongase (Ilan et al., 1981), heme oxygenase (Wilks et al., 1995), and cytochrome b5 (Enoch and Strittmatter, 1979). POR receives two electrons from NADPH and transfers them one at a time to the P450 (Wang et al., 1997; Yamano et al., 1989). Crystallography of rat POR reveals two lobes, one binding FAD and the other binding FMN, connected by an α-helical domain and a disordered “hinge” of about 25 residues, permitting the FMN and FAD domains to move relative to each other (Wang et al., 1997). Nuclear magnetic resonance and X-ray scattering data show that POR undergoes dramatic conformational changes while receiving and discharging electrons (Ellis et al., 2009). After the FAD moiety receives a pair of electrons from NADPH, flexion of the hinge permits the FAD and FMN come into close apposition, permitting the electrons to flow from the FAD to the FMN. The POR molecule then reverts to its original, more open conformation, allowing the FMN domain to interact with the redox-partner binding site of the P450 by charge-charge interactions. The electron-donating surface of the FMN domain is dominated by acidic residues, whereas the redox-partner binding site of P450 enzymes is dominated by basic residues. Electrons travel from the FMN to the heme iron of the P450, where catalysis occurs (Fig. 1).
Fig. 1.
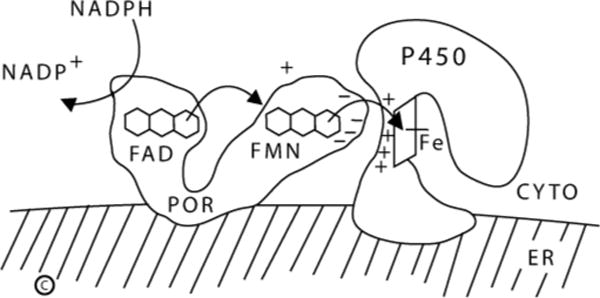
Role of POR in electron transfer to type 2 (microsomal) P450 enzymes. Copyright W.L. Miller.
POR Genetics
The human POR gene, located on chromosome 7, consists of 15 protein-coding exons and a recently discovered first untranslated exon that lies 38.8 kb upstream and initiates transcription (Scott et al., 2007). The human POR gene is highly polymorphic. We sequenced this gene in 842 normal persons from the San Francisco area who identified themselves as African-American (AA), Caucasian-American (CA), Mexican-American (MA) or Asian (Han Chinese) American (AS) on the basis of having all four grandparents claim membership in that ethnic group (Huang et al., 2008). We found a high degree of polymorphism, including 140 single nucleotide polymorphisms present in >1% of one of the four populations. By far the most common polymorphism changed the codon for amino acid 503 from alanine to valine (A503V); this variant was found on 19.1% of AA alleles, 26.4% of CA alleles, 31.0% of MA alleles and 36.7% of AS alleles, for an over-all incidence of 27.9% of all alleles (Huang et al., 2008). This sequence variant was in Hardy-Weinberg equilibrium, indicating no selective bias.
POR Deficiency Syndromes
The essential role of POR in so many reactions predicts that POR mutations should yield a very severe phenotype, and POR-knockout mice die during fetal development (Otto et al., 2003; Shen et al., 2002). When only the translational start site and N-terminal membrane-insertion domain are deleted, a soluble, cytoplasmic 66kDa protein is produced; transgenic mice expressing this truncated form of POR had two phenotypes: Type I embryos appeared normal until embryonic day 10.5 (E 10.5) but died by E 13.5; Type II embryos had generally retarded development by E 8.5 and died shortly thereafter (Shen et al., 2002). Knockout of the entire protein-coding region resulted in all embryos dying by E 9.5 (Otto et al., 2003). By contrast, liver-specific POR knockout mice were grossly normal, had a normal life span and had normal reproductive capacity, indicating normal gonadal P450 function (Gu et al., 2003; Henderson et al., 2003). These mice had hepatomegaly, hepatic steatosis and a 99% decrease in bile acids, a 65% decrease in serum cholesterol and a 50% decrease in serum triglycerides. These mice also had dramatically decreased capacities to metabolize drugs, despite having a 5-fold increase in total hepatic P450 content.
Despite its embryonic lethality in mice, we found that POR deficiency is compatible with human life, causing a severe skeletal malformation syndrome and a form of congenital adrenal hyperplasia (CAH) characterized by partial deficiencies in the activities of P450c17, P450c21 and P450aro (Fluck et al., 2004). The first report described three infants with the Antley-Bixler skeletal malformation syndrome (ABS), genital ambiguity, and hormonal findings suggesting partial deficiencies of 17α-hydroxylase and 21-hydroxylase; we also described a phenotypically normal adult woman with infertility and a hormonal profile suggesting partially impaired 17α-hydroxylase, 17,20 lyase and 21-hydroxylase activities (Fluck et al., 2004). All four patients had recessive, loss-of-function amino acid replacement mutations in POR. One infant was born to a woman who had become virilized during the pregnancy, suggesting partial feto-placental aromatase deficiency in this patient.
Over 50 human POR mutations have now been described, affecting various P450 enzymes to differing degrees, apparently explaining the great variability in the clinical and hormonal findings in POR deficiency (Adachi et al., 2004; Arlt et al., 2004; Fluck et al., 2004; Fukami et al., 2005; Hershkovitz et al., 2008; Huang et al., 2005; Sahakitrungruang et al., 2009; Scott and Miller, 2008). When these mutations are re-created and studied in vitro, the mutations found in the infants with ABS had severely impaired, but not totally absent, activities, whereas the mutations found in the phenotypically normal adult were less severe (Fluck et al., 2004). Careful examination of multiple biochemical assays of the recombinant human POR mutant proteins showed that the classical POR assay based on reduction of cytochrome c (a non-physiologic substrate) did not correlate with the severity of the patients’ phenotypes, whereas assays based on the 17,20 lyase activity of human P450c17 accurately predicted the phenotype (Fluck et al., 2004; Hershkovitz et al., 2008; Huang et al., 2005; Sahakitrungruang et al., 2009).
ABS is characterized by craniosynostosis, brachycephaly, radio-ulnar or radio-humeral synostosis, bowed femora, arachnodactyly, midface hypoplasia, proptosis, and choanal stenosis. When ABS is seen in association with abnormal steroids and ambiguous genitalia in either sex, the cause is an autosomal recessive mutation in POR (Adachi et al., 2004; Arlt et al., 2004; Fluck et al., 2004; Fukami et al., 2005; Hershkovitz et al., 2008; Huang et al., 2005; Sahakitrungruang et al., 2009; Scott and Miller, 2008); by contrast, when ABS is seen without disordered steroidogenesis or genital development, the cause is an autosomal dominant, gain-of-function mutation in fibroblast growth factor receptor 2; mutations in the POR and FGFR2 genes segregate completely (Huang et al., 2005). The ABS phenotypes resulting from POR or FGFR2 mutations are indistinguishable (Huang et al., 2005). The incidence of POR deficiency is unknown; the description of large numbers of patients within a few years of the first description of POR deficiency and the potentially very subtle clinical manifestations in individuals carrying mutations with partial activity (Fluck et al., 2004; Hershkovitz et al., 2008; Huang et al., 2005; Sahakitrungruang et al., 2009) suggest that POR deficiency may be fairly common.
Genital Ambiguity in POR Deficiency
Severe POR deficiency (associated with ABS) causes genital ambiguity in both sexes; females may be virilized and males may be under-developed. The 17,20 lyase activity of P450c17 is especially sensitive to perturbations in electron transport (Geller et al., 1997; Geller et al., 1999; Miller, 2005), thus affected males are hypo-androgenic. Consequently, severely affected males have under-developed external genitalia, and mildly affected males may have infertility (Hershkovitz et al., 2008; Huang et al., 2005; Sahakitrungruang et al., 2009). The partial virilization of female ABS patients is from two causes. First, there is partial deficiency placental aromatase (P450aro) activity in some, but not all, pregnancies (Fluck et al., 2004; Huang et al., 2005), resulting in fetal virilization similar to that seen with P450aro deficiency. This outcome is typical among infants carrying the R457H mutation prevalent in Japan, but not with the A287P mutation prevalent in Europe (Adachi et al., 2004; Arlt et al., 2004; Fluck et al., 2004; Fukami et al., 2005; Huang et al., 2005). As expected, women carrying such fetuses have low estriol levels (Fukami et al., 2006; Shackleton et al., 2004), and the R457H mutant does not support P450aro activity in vitro, whereas the A287P mutant retains full activity with P450aro (Pandey et al., 2007). Second, analysis of urinary steroids from European (Arlt et al., 2004; Shackleton et al., 2004) and Japanese (Fukami et al., 2006; Homma et al., 2006) patients with POR deficiency suggests that the alternative “backdoor” pathway of androgen biosynthesis (Auchus, 2004) converts fetal 17OHP to androgens (Arlt et al., 2004; Fukami et al., 2006; Shackleton et al., 2004). The relative importance of these two distinct mechanisms for virilizing the fetus with POR deficiency probably varies with the specific POR mutation involved.
Congenital Malformations in POR Deficiency
The cellular mechanisms by which POR deficiency causes skeletal malformations remain unclear, and there are no established roles for cytochrome P450 enzymes in the cardiac, neural tube, limb and eye systems that are disrupted in POR knockout mice. Nevertheless, four lines of evidence suggest a role for POR-supported cholesterol biosynthesis in bone formation, and hence in the ABS phenotype (Fluck and Miller, 2006). First, cholesterol biosynthesis requires two enzymes, squalene epoxidase (a non-P450 enzyme), and lanosterol 14α-demethylase (CYP51) which both require POR (Debeljak et al., 2003; Ono and Bloch, 1975). Lanosterol demethylase activity was reduced in fibroblasts from an infant with ABS and POR deficiency (Fluck et al., 2004; Kelley et al., 2002). Second, ABS has been associated with maternal ingestion of fluconazole (Aleck and Bartley, 1997; Pursley et al., 1996), an antifungal agent that acts by inhibiting CYP51 activity. Third, skeletal malformations are found in other disorders of cholesterol biosynthesis such as Smith-Lemli-Opitz syndrome. Finally, cholesterol derivitization of hedgehog proteins is required for signaling in bone formation (Cooper et al., 2003). Recent studies support this hypothesis. First, tissue-specific POR knockout in the limb bud mesenchyme of mice induces the expression of genes throughout the cholesterol biosynthetic pathway, suggesting that cholesterol deficiency could explain the skeletal phenotypes (Schmidt et al., 2009). Second, siRNA knockdown of POR decreased the proliferation and differentiation of rat chondrocytes, induced apoptosis, and reduced expression of Indian hedgehog (Aguilar et al., 2009), which is associated with the limb malformations seen in disorders of cholesterol biosynthesis (Gofflot et al., 2003). Thus cholesterol synthesis appears to be involved in the ABS phenotype of POR deficiency, but a mechanistic connection between the ABS of POR deficiency and of FGFR2 gain-of-function remains unknown. It has also been proposed that the ABS phenotype is attributable to POR mutations disrupting the metabolism of all-trans retinoic acid, as increased maternal ingestion of retinoic acid partially ameliorated the phenotype of the complete POR knockout mouse (Otto et al., 2003).
POR and Pharmacogenetics
Pharmacogenetics is the study of the genetic variations that account for most interindividual variation in drug responses (Evans and McLeod, 2003). Most studies of pharmacogenetics have addressed variations in genes encoding drug-metabolizing P450 enzymes and drug transporters. The human genome includes 22 genes and 16 pseudogenes in the CYP1, 2, and 3 families; the corresponding hepatic P450 enzymes metabolize about 70 to 80% of all clinically-used drugs (Ingelman-Sundberg, 2002; Weinshilboum, 2003). Allelic variations in these genes account for some, but not all of pharmacogenetic variation (Evans and Relling, 1999). We hypothesized that genetic variations in POR would account for some of the observed pharmacogenetic variation that is not attributable to variations in drug-metabolizing enzymes and drug transporters; therefore, we first studied the genetic variation in the POR gene in the normal population (Huang et al., 2008). Among the 140 SNPs found, only two were found in >2% of the population: these were the coding sequence variant A503V and a C to A change at −152 in the promoter.
By far the most common allelic variant was the coding sequence polymorphism A503V. We have measured the activity of POR A503V in numerous assays by measuring the parameter Vmax/Km for the activity of POR A503V compared to wild type (WT) control POR set to 100%. In the classic, non-physiologic assay based on cytochrome c, the ability of A503V to reduce cytochrome c was 67% of WT and its ability to oxidize NADPH was 56% of WT (Huang et al., 2008). Measurements of the activity of A503V to support catalysis by steroidogenic enzymes varied: A503V had 68% of WT ability to support the 17α-hydroxylase activity of human P450c17 and 58% of WT ability to support the 17,20 lyase activity of P450c17 (Huang et al., 2008; Huang et al., 2005). By contrast, A503V had 80% of WT activity to support the 21-hydroxylation of progesterone by human P450c21, and 95% of WT ability to support the 21-hydroxylation of 17OH-progesterone (Gomes et al., 2008). Thus, as has been observed in many studies, the ability of a POR sequence variant to support the activity of one P450 enzyme does not predict its ability to support the activity of other P450 enzymes. Therefore, we initiated a study of the activities of POR variants to support hepatic drug-metabolizing P450 enzymes.
Our first study of hepatic enzymes utilized commercially available, bacteriallyexpressed human CYP1A2 or CYP2C19; both enzymes can be assayed by measuring their conversion of the fluorogenic substrate EOMCC to its fluorescent product, permitting multiple measurements in a 96-well format assayed with a plate reader. We measured the Km and Vmax of this reaction with each P450 supported by 35 different mutants and sequence variants of POR (Agrawal et al., 2008). A503V supported 85% of WT activity with CYP1A2 and 113% of WT activity with CYP2C19, indicating that A503V was not an important variable for these two hepatic enzymes (Agrawal et al., 2008). However, CYP1A2 and CYP2C19 metabolize only about 15% of clinically-used drugs, and the assays based on EOMCC may not reflect activity with ‘real’ drugs. Therefore, we have studied the activities of A503V, the two common disease-causing mutants A287P and R457H, and a gain-of-function mutant, Q153R with human CYP3A4 and CYP2D6, which respectively metabolize ~45% and ~25% of clinically-used drugs.
POR and drug metabolism
CYP3A4 is responsible for nearly half of hepatic, P450-mediated drug disposal (Evans and Relling, 1999), metabolizing drugs with a broad array of sizes and shapes. Crystallography shows that human CYP3A4 has the same fold as other P450 enzymes, but that its substrate-binding pocket is highly distensible: it has a volume of ~520 Å3 in the absence of substrate or in association with metyropone (212 Da) or progesterone (318 Da) (Williams et al., 2004), but expands to ~2000 Å3 when binding erythromycin (734 Da) (Ekroos and Sjogren, 2006). Because it is not known whether such substrate-induced conformational changes in CYP3A4 affect electron donation from POR, we examined the ability of WT, Q153R, A287P, R457H and A503V POR to support catalysis by CYP3A4 using four substrates, testosterone, midazolam, quinidine and erythromycin, representing drugs of different sizes and chemical classes (Agrawal et al., 2010).
CYP3A4 metabolizes testosterone (288 Da) by 6β-hydroxylation. POR Q153R had 129% of WT activity, A287P had 17% of WT activity, R457H supported minimal activity, and A503V had 77% of WT activity in support of this activity of CYP3A4. CYP3A4 metabolizes the 326 Da benzodiazepine anesthetic midazolam to its 1-hydroxy and 4-hydroxy derivatives in about a 5:1 ratio. Others have reported increased metabolism of midazolam among people homozygous for A503V (Oneda et al., 2009); however, these subjects were receiving other CYP3A4/5 substrates that might induce CYP3A4, hence the data are not comparable. POR Q153R had 94% of WT activity for 1-hydroxylation and 92% for 4-hydroxylation; A287P had 17% and 14% of WT activity for 1-hydroxylation and 4-hydroxylation; R457H had minimal activity, so that kinetic parameters could not be calculated, and the activity of A503V was 61% for 1-hydroxylation and 74% for 4-hydroxylation. Quinidine, a 324 Da antimalarial compound with two fused rings, is 3-hydroxylated by CYP3A4. CYP3A4 metabolism of quinidine with the four POR variants was similar to the activity with midazolam. POR Q153R had 150% of WT activity, A287P and R457H had barely detectable activity, and A503V had of 89% of WT activity. CYP3A4 catalyzes N-demethylation of erythromycin, a 734 Da macrolide antibiotic containing a 14-member lactone ring, directly testing whether substrate-induced conformational changes in CYP3A4 will affect the efficiency of electron transfer from POR. Q153R, which had WT activity with midazolam and slightly increased activity with testosterone and quinidine had only 76% of WT activity with erythromycin. A287P and R457H supported minimal activity. A503V, which modestly impaired the metabolism of testosterone and midazolam, had 97% of WT activity with erythromycin. Thus the ability of different POR sequence variants to support the catalytic activity of CYP3A4 varied with the test substrate. Both the size of the substrate, as evidenced by erythromycin, and its chemical structure, as evidenced by midazolam, appear to be important (Agrawal et al., 2010). Other studies of CYP3A4 using fluorogenic non-drug substrates found no activity for POR Y181D, R457H, Y459H, V492E and R616X, 15–40% of WT activity with A287P, C569Y and V608F, and normal activity with Q153R, but these studies did not examine A503V (Fluck et al., 2010; Nicolo et al., 2010). We have done similar studies examining the ability of the same POR variants to support CYP2D6-mediated activation of EOMCC, its O-demethylation of dextromethorphan and its 1-hydroxylation of bufuralol, again showing decreased activity of A503V (Sandee et al., 2010). Thus A503V shows reduced activity to ~60% of wild type in 7 of 12 assays used, underscoring the need to test each reaction of interest (Table 1).
Table 1.
Activities of POR variants in various assays. The indicated substrates (second row) were incubated with the indicated CYP enzymes (top row) using the indicated POR sequence variants (left column) as electron donor. Enzymatic efficiencies was calculated as Vmax/Km and compared to the corresponding value obtained with wild type (WT) POR, arbitrarily set as 100%. The activities measured were: EOMCC, activation of fluorogenic vivid EOMCC [2H-1-benzopyran-3-carbonitrile,7-(ethoxy-methoxy)-2-oxo-(9Cl)]; O-dMe Dext, O-demethylation of dextromethorphan; 1OH Buf, 1-hydroxylation of bufuralol; 6OH T, 6β-hydroxylation of testosterone; 1OH M, 1-hydroxylation of midazolam; 4OH M, 4-hydroxylation of midazolam; 3OH Q, 3-hydroxylation of quinidine; N-dMe E, N-demethylation of erythromycin; 17OH, 17α-hydroxylation of progesterone; 17,20, conversion of 17α-hydroxypregnenolone to DHEA. (Data are from Agrawal et al., 2008; Agrawal et al., 2010; Huang et al., 2005; Huang et al., 2008; Sandee et al., 2010).
| CYP | 2D6 | 3A4 | 1A2 | 2C19 | 17 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate or Reaction | EOMCC | O-dMe Dext | 1OH Buf | 6OH T | 1OH M | 4OH M | 3OH Q | N-dMe E | EOMCC | EOMCC | 17-OH | 17, 20 |
| WT | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Q153R | 128 | 198 | 153 | 129 | 94 | 92 | 150 | 76 | 144 | 284 | 31 | 27 |
| A287P | Nil | 27 | 24 | 17 | 17 | 14 | 3 | Nil | Nil | Nil | 40 | 21 |
| R457H | Nil | Nil | Nil | Nil | Nil | Nil | 1 | Nil | Nil | Nil | 3 | Nil |
| A503V | 85 | 62 | 53 | 77 | 61 | 74 | 89 | 97 | 85 | 113 | 68 | 58 |
Transcriptional Regulation and Promoter Polymorphisms
Studies of POR regulation in intact rats and in transfected cells have shown that POR transcription requires thyroxine acting through a thyroid-response region having the sequence AGGTGAgctgAGGCCA at bases −564 to −536 (Li et al., 2001; Ram and Waxman, 1992). With our identification of the untranslated first exon of the human POR gene (Scott et al., 2007), it became possible to examine its transcriptional regulation. Computational searches of 10 kb of 5′ flanking DNA showed no evolutionarily conserved regions more than 2.5 kb upstream, and no apparent transcription factor binding sites more than 0.9 kb upstream from the transcriptional start site (Tee et al., 2010). Expression of deletional mutants containing up to 3.2 kb of the human POR promoter in human adrenal NCI-H295A and liver Hep-G2 cells indicated that most basal regulatory activity lies within 325 bp from the untranslated exon. Our sequencing of 274 bp of the human POR promoter in 701 normal persons (Huang et al., 2008) identified common polymorphisms at −208, −173 and −152 (Huang et al., 2008). When inserted into the −325 bp basal promoter, those at −208 and −173 had little influence on transcription, but the polymorphism at −152 reduced transcription by 40% in Hep-G2 cells and by 65% in NCI-H295A cells. Extensive analysis by electrophoretic mobility shift assays identified binding of Smad3/Smad4 between −249 and −261 and binding of thyroid hormone receptor-β (TRβ) at −240/−245, but did not detect proteins binding to either the WT or polymorphic sequence at −152. Chromatin immunoprecipitation showed that Smad3, Smad4, TRα, TRβ, and estrogen receptor-α (ERα) were bound between −374 and −149. Co-transfection of vectors for these transcription factors and POR promoter-reporter constructs into both cell types followed by treatment with estradiol or triiodothyronine showed that triiodothyronine exerts major tropic effects via TRβ, with TRα, ERα, Smad3 and Smad4 exerting lesser, modulatory effects (Tee et al., 2010). These data establish roles for TRβ and Smad3/4 in human POR expression and suggest that the common polymorphism at −152 may play a role in genetic variation in steroid biosynthesis and drug metabolism.
Conclusions
POR deficiency is a newly-described form of congenital adrenal hyperplasia; in its severe form it is characterized by ABS and genital ambiguity in both sexes, and hence is easily recognized, but mild forms may present only with infertility and subtle disorders of steroidogenesis. POR is required by all drug-metabolizing P450 enzymes. Extensive biochemical studies in vitro indicate that the common polymorphism A503V reduces catalysis by the principal drug-metabolizing P450 enzymes. In vivo studies of drug metabolism in carefully genotyped normal persons are needed to establish the role of POR A503V in human pharmacogenetics.
Acknowledgments
This study was supported by NIH grant R01 GM073020 to WLM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Tachibana K, Asakura Y, Yamamoto T, Hanaki K, Oka A. Compound heterozygous mutations of cytochrome P450 oxidoreductase gene (POR) in two patients with Antley-Bixler syndrome. Am J Med Genet A. 2004;128A:333–339. doi: 10.1002/ajmg.a.30169. [DOI] [PubMed] [Google Scholar]
- Agrawal V, Choi JH, Giacomini KM, Miller WL. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharmacogenet Genomics. 2010;20:611–618. doi: 10.1097/FPC.0b013e32833e0cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal V, Huang N, Miller WL. Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics. 2008;18:569–576. doi: 10.1097/FPC.0b013e32830054ac. [DOI] [PubMed] [Google Scholar]
- Aguilar A, Wu S, De Luca F. P450 oxidoreductase (POR) expressed in rat chondrocytes modulates chondrogenesis via cholesterol- and Indian hedgehog (Ihh)-dependent mechanisms. Endocrinology. 2009;150:2732–2739. doi: 10.1210/en.2009-0043. [DOI] [PubMed] [Google Scholar]
- Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253–256. [PubMed] [Google Scholar]
- Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. The Lancet. 2004;363:2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- Debeljak N, Fink M, Rozman D. Many facets of mammalian lanosterol 14alphademethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch Biochem Biophys. 2003;409:159–171. doi: 10.1016/s0003-9861(02)00418-6. [DOI] [PubMed] [Google Scholar]
- Ekroos M, Sjogren T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc Natl Acad Sci U S A. 2006;103:13682–13687. doi: 10.1073/pnas.0603236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Gutierrez A, Barsukov IL, Huang WC, Grossmann JG, Roberts GC. Domain motion in cytochrome P450 reductase: conformational equilibria revealed by NMR and small-angle x-ray scattering. J Biol Chem. 2009;284:36628–36637. doi: 10.1074/jbc.M109.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch HG, Strittmatter P. Cytochrome b5 reduction by NADPH-cytochrome P-450 reductase. J Biol Chem. 1979;254:8976–8981. [PubMed] [Google Scholar]
- Evans WE, McLeod HL. Pharmacogenomics–drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- Fluck CE, Miller WL. P450 oxidoreductase deficiency: a new form of congenital adrenal hyperplasia. Current Opinion In Pediatrics. 2006;18:435–441. doi: 10.1097/01.mop.0000236395.71956.5c. [DOI] [PubMed] [Google Scholar]
- Fluck CE, Mullis PE, Pandey AV. Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism. Biochem Biophys Res Commun. 2010;401:149–153. doi: 10.1016/j.bbrc.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Fluck CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T. Cytochrome P450 Oxidoreductase Deficiency in Three Patients Initially Regarded as Having 21-Hydroxylase Deficiency and/or Aromatase Deficiency: Diagnostic Value of Urine Steroid Hormone Analysis. Pediatr Res. 2006;59:276–280. doi: 10.1203/01.pdr.0000195825.31504.28. [DOI] [PubMed] [Google Scholar]
- Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, et al. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab. 2005;90:414–426. doi: 10.1210/jc.2004-0810. [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Mendonca BB, Miller WL. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet. 1997;17:201–205. doi: 10.1038/ng1097-201. [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Miller WL. P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- Gofflot F, Hars C, Illien F, Chevy F, Wolf C, Picard JJ, Roux C. Molecular mechanisms underlying limb anomalies associated with cholesterol deficiency during gestation: implications of Hedgehog signaling. Hum Mol Genet. 2003;12:1187–1198. doi: 10.1093/hmg/ddg129. [DOI] [PubMed] [Google Scholar]
- Gomes LG, Huang N, Agrawal V, Mendonca BB, Bachega TA, Miller WL. The Common P450 Oxidoreductase Variant A503V Is Not a Modifier Gene for 21-Hydroxylase Deficiency. J Clin Endocrinol Metab. 2008;93:2913–2916. doi: 10.1210/jc.2008-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Weng Y, Zhang QY, Cui H, Behr M, Wu L, Yang W, et al. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem. 2003;278:25895–25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Otto DM, Carrie D, Magnuson MA, McLaren AW, Rosewell I, Wolf CR. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem. 2003;278:13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab. 2008;93:3584–3588. doi: 10.1210/jc.2008-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, et al. Urine Steroid Hormone Profile Analysis in Cytochrome P450 Oxidoreductase Deficiency: Implication for the Backdoor Pathway to Dihydrotestosterone. J Clin Endocrinol Metab. 2006;91:2643–2649. doi: 10.1210/jc.2005-2460. [DOI] [PubMed] [Google Scholar]
- Huang N, Agrawal V, Giacomini KM, Miller WL. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A. 2008;105:1733–1738. doi: 10.1073/pnas.0711621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Z, Ilan R, Cinti DL. Evidence for a new physiological role of hepatic NADPH:ferricytochrome (P-450) oxidoreductase. Direct electron input to the microsomal fatty acid chain elongation system. J Biol Chem. 1981;256:10066–10072. [PubMed] [Google Scholar]
- Ingelman-Sundberg M. Polymorphism of cytochrome P450 and xenobiotic toxicity. Toxicology. 2002:181–182. doi: 10.1016/s0300-483x(02)00492-4. [DOI] [PubMed] [Google Scholar]
- Kelley RI, Kratz LE, Glaser RL, Netzloff ML, Wolf LM, Jabs EW. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet. 2002;110:95–102. doi: 10.1002/ajmg.10510. [DOI] [PubMed] [Google Scholar]
- Li HC, Liu D, Waxman DJ. Transcriptional induction of hepatic NADPH: cytochrome P450 oxidoreductase by thyroid hormone. Mol Pharmacol. 2001;59:987–995. doi: 10.1124/mol.59.5.987. [DOI] [PubMed] [Google Scholar]
- Miller WL. Minireview: Regulation of steroidogenesis by electron transfer. Endocrinology. 2005;146:2544–2550. doi: 10.1210/en.2005-0096. [DOI] [PubMed] [Google Scholar]
- Nicolo C, Fluck CE, Mullis PE, Pandey AV. Restoration of mutant cytochrome P450 reductase activity by external flavin. Mol Cell Endocrinol. 2010;321:245–252. doi: 10.1016/j.mce.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Oneda B, Crettol S, Sirot EJ, Bochud M, Ansermot N, Eap CB. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharmacogenet Genomics. 2009;19:877–883. doi: 10.1097/FPC.0b013e32833225e7. [DOI] [PubMed] [Google Scholar]
- Ono T, Bloch K. Solubilization and partial characterization of rat liver squalene epoxidase. J Biol Chem. 1975;250:1571–1579. [PubMed] [Google Scholar]
- Otto DM, Henderson CJ, Carrie D, Davey M, Gundersen TE, Blomhoff R, Adams RH, et al. Identification of novel roles of the cytochrome p450 system in early embryogenesis: effects on vasculogenesis and retinoic Acid homeostasis. Mol Cell Biol. 2003;23:6103–6116. doi: 10.1128/MCB.23.17.6103-6116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AV, Kempna P, Hofer G, Mullis PE, Fluck CE. Modulation of human CYP19A1 activity by mutant NADPH P450 oxidoreductase. Molecular Endocrinology. 2007;21:2579–2595. doi: 10.1210/me.2007-0245. [DOI] [PubMed] [Google Scholar]
- Pursley TJ, Blomquist IK, Abraham J, Andersen HF, Bartley JA. Fluconazoleinduced congenital anomalies in three infants. Clin Infect Dis. 1996;22:336–340. doi: 10.1093/clinids/22.2.336. [DOI] [PubMed] [Google Scholar]
- Ram PA, Waxman DJ. Thyroid hormone stimulation of NADPH P450 reductase expression in liver and extrahepatic tissues. Regulation by multiple mechanisms. J Biol Chem. 1992;267:3294–3301. [PubMed] [Google Scholar]
- Sahakitrungruang T, Huang N, Tee MK, Agrawal V, Russell WE, Crock P, Murphy N, et al. Clinical, Genetic, and Enzymatic Characterization of P450 Oxidoreductase Deficiency in Four Patients. J Clin Endocrinol Metab. 2009;94:4992–5000. doi: 10.1210/jc.2009-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, et al. Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenetics and Genomics. 2010;20:677–686. doi: 10.1097/FPC.0b013e32833f4f9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Hughes C, Chudek JA, Goodyear SR, Aspden RM, Talbot R, Gundersen TE, et al. Cholesterol metabolism: The main pathway acting downstream of cytochrome P450 oxidoreductase in skeletal development of the limb. Mol Cell Biol. 2009;29:2716–2729. doi: 10.1128/MCB.01638-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RR, Gomes LG, Huang NW, Van Vliet G, Miller WL. Apparent manifesting heterozygosity in P450 oxidoreductase deficiency and its effect on coexisting 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:2318–2322. doi: 10.1210/jc.2006-2345. [DOI] [PubMed] [Google Scholar]
- Scott RR, Miller WL. Genetic and clinical features of P450 oxidoreductase deficiency. Hormone Research. 2008;69:266–275. doi: 10.1159/000114857. [DOI] [PubMed] [Google Scholar]
- Shackleton C, Marcos J, Arlt W, Hauffa BP. Prenatal diagnosis of P450 oxidoreductase deficiency (ORD): a disorder causing low pregnancy estriol, maternal and fetal virilization, and the Antley-Bixler syndrome phenotype. Am J Med Genet A. 2004;129A:105–112. doi: 10.1002/ajmg.a.30171. [DOI] [PubMed] [Google Scholar]
- Shen AL, O’Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem. 2002;277:6536–6541. doi: 10.1074/jbc.M111408200. [DOI] [PubMed] [Google Scholar]
- Tee MK, Huang N, Damm I, Miller WL. Transcriptional regulation of the human P450 oxidoreductase gene: Hormonal regulation and influence of promoter polymorphisms. (submitted) 2010 doi: 10.1210/me.2010-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ. Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes. Proc Natl Acad Sci U S A. 1997;94:8411–8416. doi: 10.1073/pnas.94.16.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R. Inheritance and drug response. N Engl J Med. 2003;348:529–537. doi: 10.1056/NEJMra020021. [DOI] [PubMed] [Google Scholar]
- Wilks A, Black SM, Miller WL, Ortiz de Montellano PR. Expression and characterization of truncated human heme oxygenase (hHO-1) and a fusion protein of hHO-1 with human cytochrome P450 reductase. Biochemistry. 1995;34:4421–4427. doi: 10.1021/bi00013a034. [DOI] [PubMed] [Google Scholar]
- Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, et al. Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science. 2004;305:683–686. doi: 10.1126/science.1099736. [DOI] [PubMed] [Google Scholar]
- Yamano S, Aoyama T, McBride OW, Hardwick JP, Gelboin HV, Gonzalez FJ. Human NADPH-P450 oxidoreductase: complementary DNA cloning, sequence and vaccinia virus-mediated expression and localization of the CYPOR gene to chromosome 7. Mol Pharmacol. 1989;36:83–88. [PubMed] [Google Scholar]


