Summary
We have identified a phosphate transporter (TcPho91) localized to the bladder of the contractile vacuole complex (CVC) of Trypanosoma cruzi, the etiologic agent of Chagas disease. TcPho91 has 12 transmembrane domains, an N-terminal regulatory SPX domain and an anion permease domain. Functional expression in Xenopus laevis oocytes followed by two-electrode voltage clamp showed that TcPho91 is a low affinity transporter with a Km for Pi in the millimolar range, and sodium-dependency. Epimastigotes overexpressing TcPho91-GFP have significantly higher levels of pyrophosphate (PPi) and short chain polyphosphate (polyP), suggesting accumulation of Pi in these cells. Moreover, when overexpressing parasites were maintained in a medium with low Pi, they grew at higher rates than control parasites. Only one allele of TcPho91 in the CL strain encodes for the complete open reading frame, while the other one is truncated encoding for only the N-terminal domain. Taking advantage of this characteristic, knockdown experiments were performed resulting in cells with reduced growth rate as well as a reduction in PPi and short-chain polyP levels. Our results indicate that TcPho91 is a phosphate sodium symporter involved in Pi homeostasis in T. cruzi.
Introduction
Phosphate (Pi) is an essential macronutrient required for biosynthesis of cellular components. It also represents a source of energy, modulates protein activity and gene expression and it can be polymerized and accumulated as inorganic polyphosphate (polyP). Pi is negatively charged and needs to be transported into cells or organelles via an active transport process. Pi transporters are present in all cells and use either the transmembrane Na+ (animals, fungi) or H+ (plants, bacteria) gradients to drive Pi transport into the cells (Ravera et al., 2007). In addition, some Pi transporters occur in organelles, such as the mitochondrial proton/phosphate symporter (Pi carrier), an inner membrane-embedded protein which translocates Pi from the cytosol into the mitochondrial matrix (Ferreira and Pedersen, 1993), and Pho91p, a low affinity transporter from Saccharomyces cerevisiae that localizes to the vacuolar membrane and was postulated to export Pi from the vacuolar lumen to the cytosol (Hurlimann et al., 2007).
In Trypanosoma cruzi, the etiologic agent of Chagas disease, Pi and polyP play an important role in osmoregulation, key process for the parasite survival throughout its life cycle (Docampo et al., 2011; Li et al., 2011; Ruiz et al., 2001). Resistance to osmotic stress is essential for digenetic trypanosomatids as they encounter marked osmotic changes in both the insect vectors and vertebrate hosts (Go et al., 2004; Kollien et al., 2001; Lang et al., 2007). Large amount of Pi, pyrophosphate (PPi) and polyP are accumulated in acidocalcisomes, which are lysosome-related organelles (Docampo and Moreno, 2011). PolyP hydrolysis occurs during hyposmotic stress of trypanosomes (Ruiz et al., 2001), increasing the osmotic pressure of the acidocalcisomes and facilitating water movement. Acidocalcisomes then fuse with the contractile vacuole complex (CVC) and could transfer Pi to the bladder where it could act as an osmolyte, attracting water that is expelled through the flagellar pocket (Niyogi et al., 2015; Rohloff et al., 2004). In this process, Pi is not released to the media, suggesting the presence of mechanisms that facilitate Pi recovery from the lumen of the CVC into the cytosol.
In a previous proteomic study of the contractile vacuole of T. cruzi we identified a Pi transporter (previously annotated as a sulfate/sodium symporter) with similarity to Pho91p, localized it to the CVC by expressing the green fluorescent protein (GFP)-tagged protein, and suggested that this transporter, TcPho1, could be involved in returning Pi to the cytosol once water is discharged by the CVC upon hyposmotic stress (Ulrich et al., 2011). In this study, we have characterized this Pi transporter (now renamed as TcPho91) at the molecular and electrophysiological levels and report its relevance in Pi and polyP homeostasis and growth in trypanosomes.
Results
Characteristics and localization of TcPho91
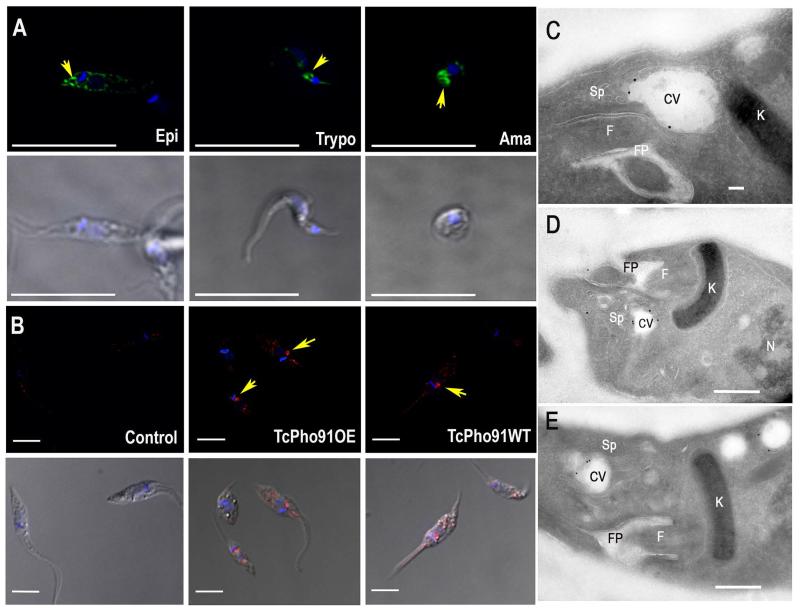
One gene (TcCLB.508831.60), annotated as sulfate/sodium symporter, and encoding for a putative ScPho91 orthologue was found in the T. cruzi genome (http://tritrypdb.org/tritrypdb/), and named TcPho91. The full-length gDNA of TcPho91 was cloned by PCR amplification from the CL strain of T. cruzi, confirmed by sequencing and shown to be identical to the gene in the database. The orthologs identified in T. brucei (Tb927.11.11160) (Huang et al., 2014) and Leishmania major (LmjF.28.2930) shared 65% and 59% amino acid identity, respectively, to TcPho91 (Fig. S1A). The ORF predicts a 727 amino acid protein with an apparent molecular weight of 80 kDa, 12 transmembrane domains (Fig. S1B), an N-terminal regulatory SPX domain and an anion-permease domain also present in other anion transporters (Fig. S1C). Interestingly, only one of the alleles (30s) encodes for the complete protein, while the other one (30p) seems to be truncated and only contains the sequence corresponding to the SPX domain (Fig. S1C). In previous work we reported the C-terminal tagging of TcPho91 with the green fluorescent protein (GFP) gene, and the localization of TcPho91-GFP predominantly to the bladder of the CVC of epimastigotes of T. cruzi and also to other intracellular membranes (Ulrich et al., 2011). Fig. 1A shows that TcPho91-GFP localizes mainly to the bladder of the CVC of epimastigotes, trypomastigotes, and amastigotes, as revealed by its circular staining pattern (Fig. 1A, arrows). CVC localization of the protein was also detected using an affinity-purified antibody against TcPho91 (Fig. 1B). This antibody labels TcPho91 in the CVC of both wild type and TcPho91-overexpressing epimastigotes. The antibody predominantly reacts with a protein of about 87 kDa in wild type parasites, while in cells overexpressing TcPho91 the antibody recognizes a band of around 100 kDa, as expected for TcPho91-GFP (Fig. S1D). No native protein was detected in the overexpressing parasites, probably due to a suppressive effect of the episomal expression. Cryo-immunogold electron microscopy confirmed the predominant localization of TcPho91-GFP in the bladder of the CV (Fig. 1C-E). No labeling was observed in control cells. Co-labeling with endoplasmic reticulum (ER)-Golgi (BODIPY-ceramide) and mitochondrial (MitoTracker red) probes show partial co-localization with BODIPY-ceramide in parasites overexpressing TcPho91-GFP (Fig. S2A), indicating that the protein could be also localized in the ER. No significant co-localization was detected when parasites were labeled with MitoTracker red (Fig. S2B).
Fig. 1.
Localization of TcPho91 in different trypanosome stages. A. TcPho1-GFP localizes to the CVC (arrows) and some intracellular membranes of epimastigotes (Epi), trypomastigotes (Trypo) and amastigotes (Ama). TcPho91 was detected with anti-GFP antibodies in trypanosomes expressing TcPho91-GFP. Lower panels are the same cells using differential interference microscopy (DIC). B. Localization of TcPho91 in wild type (WT) and TcPho91-GFP-overexpressing (Pho91-OE) epimastigotes, as detected with specific antibodies. Control was done using the same amount of preimmune serum. Nuclei were DAPI stained. Bars; A = 10 μm; B = 5 μm. C-E. Cryo-immunogold electron microscopy of epimastigotes expressing TcPho91-GFP, fixed, and incubated with rabbi anti-GFP antibodies (1:50) at room temperature for 1 h, and then treated with secondary antibodies (goat anti-rabbit conjugated to 18 nm colloidal gold) for 1 h at room temperature, showing localization in the contractile vacuole (CV) bladder and small vesicles of the spongiome (D) or larger empty vacuoles (E). F, flagellum; FP, flagellar pocket; K, kinetoplast; N, nucleus; Sp, spongiome. Scale bars = 100 nm (C) and 500 nm (D, E).
Functional expression in Xenopus laevis and electrophysiological characterization
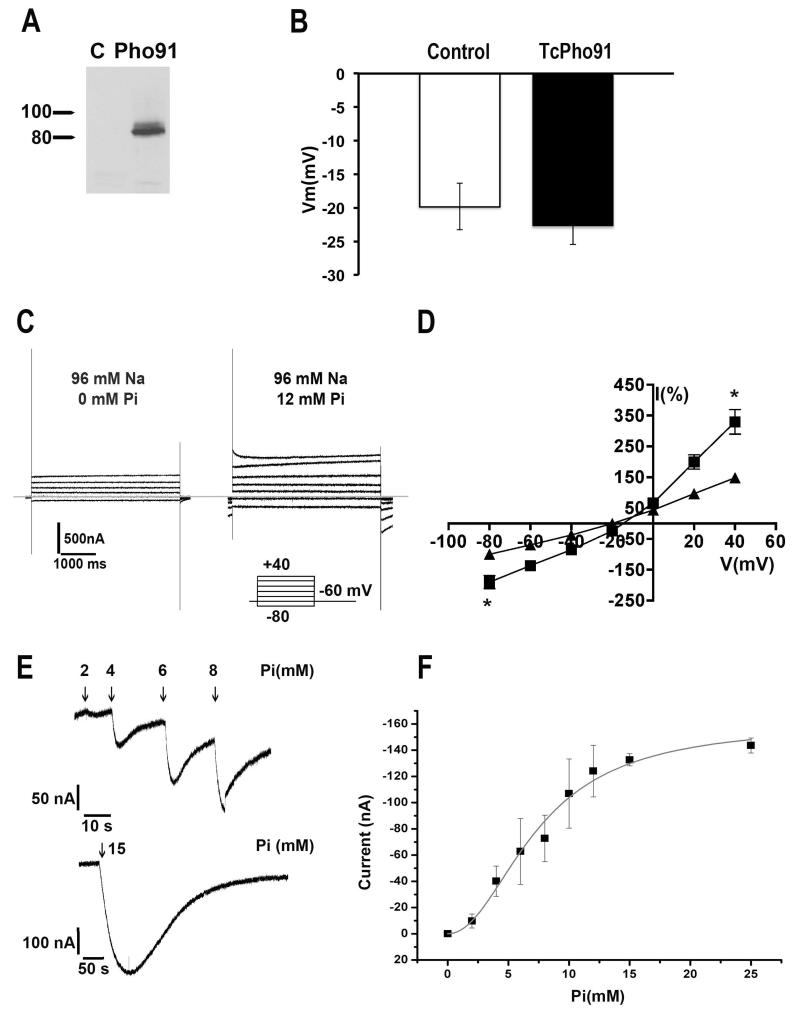
Heterologous expression of TcPho91-GFP was evaluated by western-blot analysis in oocytes injected with water (C, control) or cRNA encoding for the transporter (Fig. 2A). No significant difference was observed in the resting membrane potential (Vm) of oocytes injected with TcPho91 cRNA or the same volume of water (control = −19.8 ± 3.5 mV, n = 20; TcPho91 = −22.7 ± 2.7 mV, n = 28), indicating that expression of the gene was not toxic to the cells (Fig. 2B). Fig. 2C show representative currents recorded in response to the voltage step protocol indicated, in oocytes expressing TcPho91 when perfused in ND96 solution in the absence (left) or presence (right) of 12 mM Pi. Quantification of normalized currents of oocytes expressing TcPho91 (Fig. 2D) shows a significant increase in the current at positive and negative potential when comparing the control conditions in ND96 without Pi (black triangles) after addition of 12 mM Pi (black squares). The magnitude of the current in the presence of Pi is 329% the value of the control at +40 mV and 189% at −80 mV (*p < 0.05, n = 25). Fig. 2E shows the currents elicited at holding potential (−60 mV) by increasing concentrations of Pi in oocytes expressing TcPho91 with bath solution ND96. In the presence of Na+, 15 mM Pi induces an inward current of −237 ± 12 nA at a holding potential of −60 mV (n = 6). Water injected oocytes showed no significant changes in current (data not shown). Fig. 2F shows steady state kinetic parameters obtained by fitting the peak currents recorded in the presence of increasing concentrations of Pi according with the Hill equation (IPi= Ipimax * SnH/(SnH + KmnH) where IPi is the peak current in presence of Pi, IPimax is the maximum current elicited by Pi, S in the concentration of Pi, nH represents the Hill coefficient and Km the apparent affinity constant. The calculated Km for Pi is 7.43 ± 0.28 mM and demonstrates that TcPho91 is a low affinity transporter. Values are mean ± SD of six independent experiments.
Fig. 2.
Electrophysiological characterization of TcPho91. A. Western blot analysis of Xenopus oocytes lysates control (C) or transfected with TcPho91-GFP cRNA using anti-GFP antibody. Markers are shown on the left (in kDa). B. Resting membrane potential (Vm) of oocytes injected with TcPho91-GFP cRNA or same volume of water (control). No significant differences were observed in both groups indicating that the expression is not toxic for the cells. Values are means ± SEM of n = 20 control and n = 28 TcPho91-GFP-injected oocytes. C. Representative currents recorded in response to a voltage step protocol between −80 and +40 mV, in oocytes expressing TcPho91-GFP when superfused in ND96 solution in absence (left) or presence (right) of 12 mM Pi. D. I/V curve of the normalized currents obtained form the voltage protocols showed in (C), in the absence (triangles) or presence (squares) of 12 mM Pi. Values are means ± SEM (* p <0.05, n = 25). E. Inward currents elicited by Pi in the presence increasing concentrations of Pi. F. TcPho91 is a low affinity transporter. Steady-state kinetic parameters were obtained by fitting the peak currents recorded in the presence of increasing concentrations of Pi according with the Hill equation. Values are means ± SEM of 6 independent experiments.
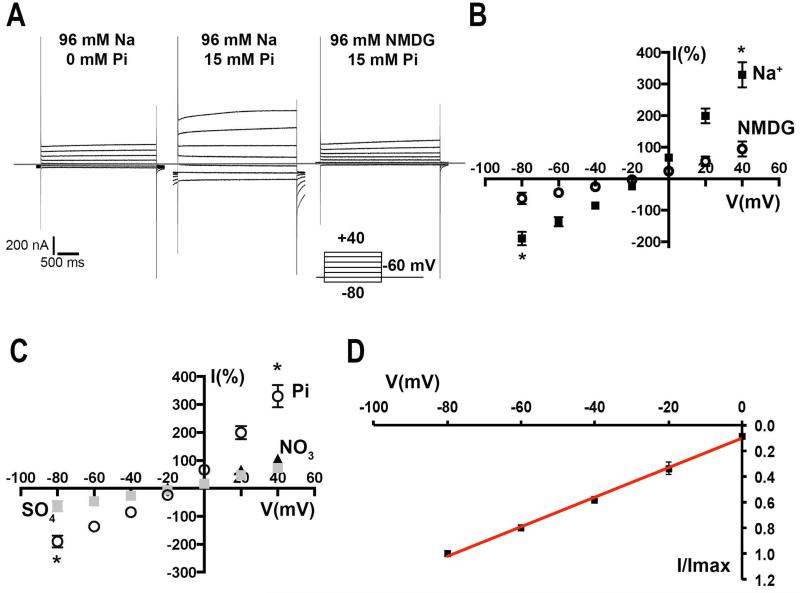
Sodium dependency was evaluated by replacement with the non-permeable cation N-methyl-D-Glucamine (NMDG). Fig. 3A shows a family of currents in response to the pulse protocol indicated in the figure. Control currents in injected oocytes without Pi are shown in the left panel, compared with the responses in the presence of Pi (middle) or when Na+ is replaced with equimolar concentration of NMDG (right). In NMDG (Fig. 3B, open circles), Pi elicited currents 3 times smaller than in presence of Na+ (black squares) at −80 mV and only 61% of the control current when Pi is not present. Differences were even bigger at +40 mV with Pi/Na+ currents 350% of the values of Pi/NMDG recordings (*p < 0.05, n = 5). No significant increase in current was observed when Na+ was replaced by K+ (data not shown). Anion selectivity was demonstrated by applying voltage pulses as described before (Fig. 3A), in the presence of either 15 mM Pi (Fig. 3C, open circles), sulfate (gray squares) or nitrate (black triangles). With both anions, no significant increase in the recorded currents was observed when compared with the control in absence of Pi (at −80 mV: sulfate= 63.5 ± 9; nitrate= 62.4 ± 3 and at +40 mV: sulfate 72.2 ± 6; nitrate 106 ± 7. Values are I (%), normalized against the control current at the same voltage without Pi (n = 5). When Pi was added to the same oocyte, an increase of 300% of the depolarization current and 200% of the inward current was observed (*p < 0.01) (Fig. 3C).
Fig. 3.
A. Sodium-dependency of TcPho91 activity. Currents elicited by Pi in the presence or absence of Na+ when a voltage pulse protocol was applied. When Na+ is replaced by N-methyl-D-glucamine (NMDG) the steady-state and inactivation currents are smaller. B. Quantification of the normalized currents in the presence of 15 mM Pi in ND96 (black squares) or NMDG (open circles). Values are means ± SEM of 5 independent experiments (* p < 0.05 at −80 and +40 mV). C. Anion selectivity analysis of the currents recorded under a voltage step protocol in the presence of 15 mM Pi (open circles), sulfate (gray squares) or nitrate (black triangles). Values are means ± SEM of 5 independent experiments (* p < 0.05 at −80 and +40 mV). D. Voltage dependency analysis of the Pi elicited currents. I/Imax correspond to the normalized values respect to the maximum current at −80 mV. Red line represents the fitting of the data to the linear equation. Values are means ± SEM of 14 independent experiments.
Voltage dependency of the transporter was analyzed in the steady-state currents in the presence of 96 mM Na+/12 mM Pi (Fig. 3D). The currents normalized relative to the value at −80 mV have a linear behavior respect to the holding potential. This is a characteristic often described in type II Na+/Pi co-transporters (Forster et al., 1997). In summary, our functional studies indicate that TcPho91 is a low affinity, Na+-dependent Pi transporter.
Generation of TcPho91 knockdown strains
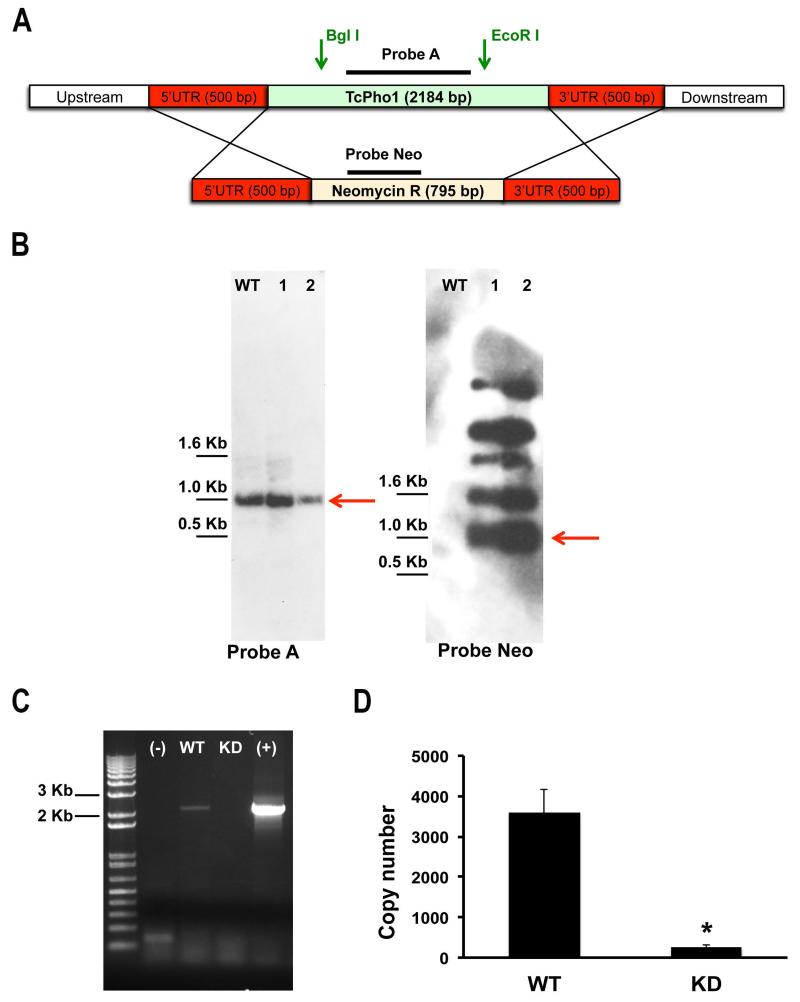
Only one allele of TcPho91 in the CL strain encodes for the complete open reading frame of this transporter, while the other one is truncated encoding for only the N-terminal domain (Fig. S1C). We took advantage of this characteristic to try to knockout the expression of TcPho91 by replacing the complete allele with a neomycin selectable marker (see Experimental procedures) (Fig. 4A). Although attempts to generate a complete TcPho91 knockout strain failed, we obtained strains in which TcPho91 expression was down regulated. The genotype of two mutant cell lines was verified by Southern blot analysis (Fig. 4B). One of the clones (lane 2) showed slightly decreased labeling with probe A, which detects TcPho91 (Fig. 4B, left panel) while the neomycin-resistant gene was present at different loci either episomally or integrated into other regions (Fig. 4B, right panel, red arrow indicates the expected size). RT-PCR (Fig. 4C) and qRT-PCR (Fig. 4D) of TcPho91 from mutant and control cells confirmed that TcPho1 RNA levels (normalized to tubulin transcript abundance) were knocked down > 95% (Fig. 4D). Furthermore, immunofluorescence analysis with anti-TcPho91 antibody shows that the transporter is localized in the CVC of wild-type and overexpressing parasites but absent in knockdown parasites (Fig. S3). It is possible that overexpression of the neomycin-resistant cassette had a dominant negative effect on the translation of the mRNA, as we observed previously in the case of an aquaporin (TcAQP1) (Li et al., 2011), and this phenomenon is currently being investigated.
Fig. 4.
Generation of TcPho91 knockdown cell line. A Schematic representation of the strategy used for the generation of a TcPho91 knockout mutant in T. cruzi epimastigotes. One allele of TcPho91 would be replaced with the neomycin-resistance gene by homologous recombination, generating a TcPho91 SKO cell line. B. Southern blot analysis indicates that two neomycin resistant cells lines still conserve the TcPho91 gene (left panel). A probe against the resistant marker showed hybridization signals specific to Neo gene in multiple loci either episomally or integrated into other regions (right panel). C. RT-PCR analysis of wild type (WT) and KD mutants shows considerable decrease in the signal corresponding to TcPho91 mRNA indicating knockdown of TcPho91 expression. Negative (−) and positive (+) controls are also shown. D. Quantification of the results in (C) * p < 0.05.
Phenotypic changes in overexpressed and knocked down TcPho91
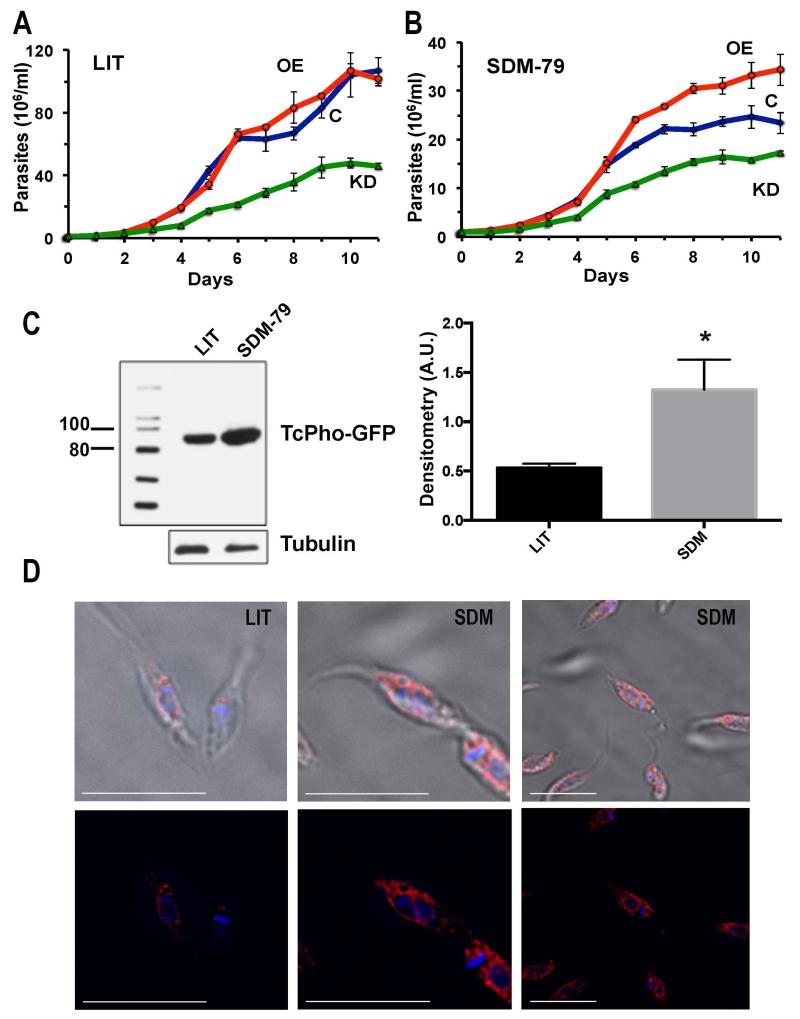
The growth in LIT medium of epimastigotes in which the expression was knocked down was considerably decreased as compared to wild type cells, while growth of cells overexpressing TcPho91-GFP (TcPho91-OE) was not different from that of wild type cells (Fig. 5A). It has been reported that proliferation of epimastigotes under limiting Pi conditions is slower (Dick et al., 2013) and we therefore investigated whether knockdown and TcPho91-GFP-OE parasites were more affected under those conditions. We compared growth of mutant and wild type parasites in SDM-79 supplemented with putrescine, a medium used before for growing T. cruzi (Hasne et al., 2010) that contains 5 mM Pi instead of 55 mM Pi present in LIT medium. Overexpression of TcPho91 was higher under Pi restricted conditions, as detected by western blot analysis (Fig. 5C), and cells overexpressing TcPho91 grew better than wild type cells and TcPho91 knocked down cells (Fig. 5B). Interestingly, epimastigotes overexpressing TcPho91 showed strong plasma membrane and perinuclear localization of the protein, as detected by IFA using anti-GFP antibodies (Fig. 5D). The perinuclear labeling is typical of the endoplasmic reticulum of trypanosomes (Bangs et al., 1993) and is frequently detected after overexpression of proteins (Fang et al., 2007a). As indicated above the partial localization of TcPho91-GFP in the ER was also detected by labeling with BOPIDY-ceramide in parasites maintained in LIT or SDM 79 (Fig. S2A).
Fig. 5.
Effect of downregulation and overexpression of TcPho91 on cell growth and TcPho91 localization. In vitro growth of wild type (C, open rombs, blue), TcPho91 knockdown parasites (KD, open triangles, green), and TcPho1-OE parasites (OE, open circles, red) in LIT (A) or SDM-79 (B) medium. C. Western blot analysis (left panel) of lysates from TcPho91-OE mutant grown in LIT of SDM-79 medium probed with an anti-GFP antibody. Markers are shown on the left (in kDa). Anti-tubulin antibodies were used as loading controls and for densitometry analysis (right panel). D. Plasma membrane and ER localization of TcPho91 in TcPho91-OE mutants grown in LIT or SDM-79 medium. Bars = 10 μm.
PPi and polyP levels in TcPho91-OE and knocked down epimastigotes
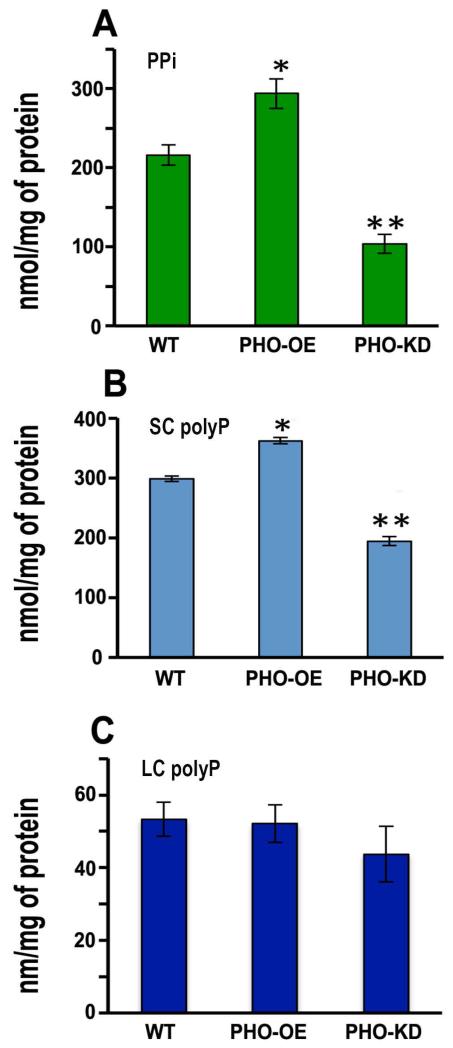
Overexpression of TcPho91 in epimastigotes led to a significant increase in the levels of PPi (Fig. 6A), and short-chain (SC) polyP (Fig. 6B), and no significant changes in intracellular long-chain (LC) polyP levels (Fig. 6C), while down regulation of TcPho91 expression led to a significant decrease in PPi (Fig. 6A) and short-chain polyP (Fig. 6B) without affecting long-chain polyP levels (Fig. 6C).
Fig. 6.
Effect of downregulation and overexpression of TcPho91 on PPi, short and long-chain polyP levels. Extracts from TcPho91-OE (OE), or TcPho91 knockdown (KD) mutants showed increase or reduction in PPi (A), and short chain polyP (B) content, respectively, with no significant changes in long chain polyP content (C), as compared to the parental cell line (WT). Values are means ± SD of three different experiments. *Differences are statistically significant as compared to respective controls, p < 0.05 (Student’s t test).
TcPho91-KD mutant parasites display an osmoregulatory defect
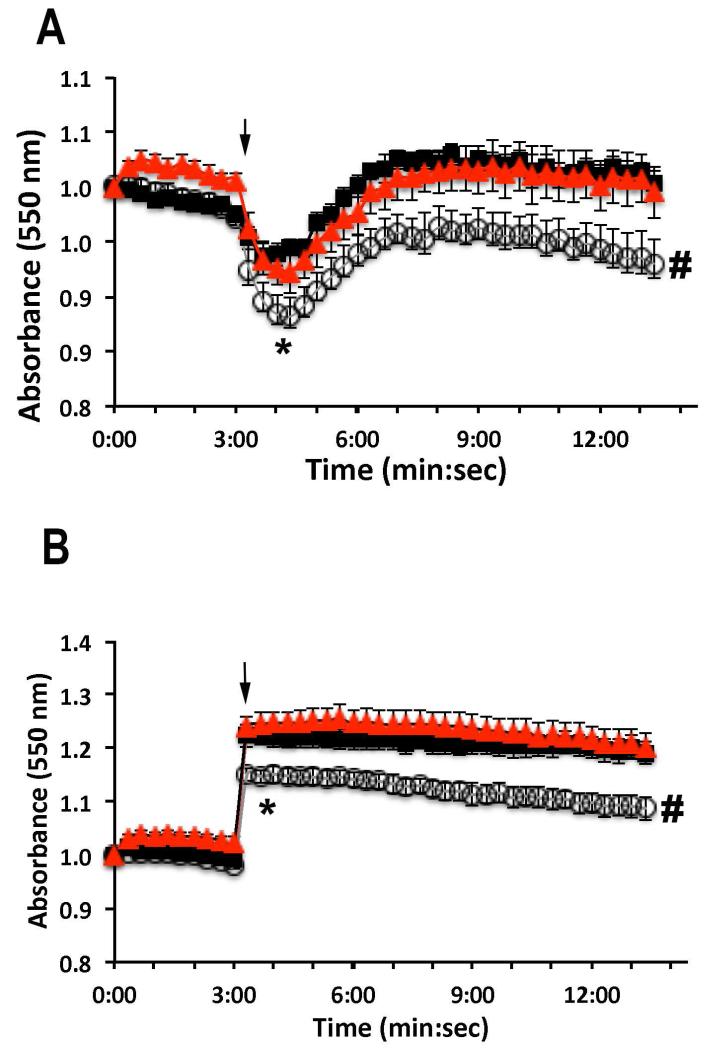
To investigate the role of TcPho91 in osmoregulation, we exposed TcPho91-deficient or TcPho91-OE epimastigotes to hyposmotic and hyperosmotic conditions and evaluated changes of cell volume with time. Under hyposmotic conditions (Fig. 7A) TcPho91-KD epimastigotes (open circles) have a higher increase in volume at 4 min (* p < 0.05) than wild type (black squares) and TcPho91-OE parasites (red triangles). These parasites also showed a significant defect in the ability to recover cell volume (a process known as Regulatory Volume Decrease or RVD during hyposmotic stress when compared with wild type cell lines (Fig. 7A, # p < 0.05). Shrinkage under hyperosmotic conditions (Fig. 7B) was reduced in TcPho91-KD (open circles) at 4 and 12 min post-stress (*, # p < 0.05), indicating a reduced capacity of TcPho91 mutant parasites to respond to osmotic stress.
Fig. 7.
Effect of downregulation and overexpression of TcPho91 on the response of epimastigotes to hyposmotic and hyperosmotic stresses. The same amount of wild type (WT, black), TcPho91 knockdown (KD, white), and TcPho91 overexpressing parasites (OE, red) were suspended in isotonic buffer. The cells were then treated as described under Experimental procedures and relative changes in cell volume were followed by monitoring the absorbance at 550 nm. A. Changes in cell volume after hyposmotic stress (117 mOsm). B. Changes in cell volume after hyperosmotic stress (650 mOsm). Arrows indicate the time point (3 min) when osmotic stress was induced. A decrease in absorbance corresponds to an increase in cell volume and vice versa. Values are means ± SEM of three independent experiments each one in triplicate. Differences between different treatments were significant at 4 min (*) and 12 min (#) (p < 0.05, Students t-test).
Discussion
We report the biochemical and electrophysiological characterization of a Pi transporter of T. cruzi. This transporter localizes to the bladder of the CVC and to intracellular membranes of different stages of T. cruzi. Expression of TcPho91 in Xenopus oocytes followed by two-electrode voltage clamp studies showed that TcPho91 is sodium-dependent and is a low affinity transporter with a Km for Pi in the millimolar range. Overexpression of this transporter resulted in plasma membrane localization, improved growth under Pi-deficient conditions, and accumulation of PPi and short chain polyP. Conversely, downregulation of TcPho91 expression resulted in lower growth rate and reduced levels or PPi and polyP. Long chain polyP levels were not affected in either case. These mutant parasites displayed osmoregulatory defects when submitted to hyposmotic or hyperosmotic stress.
Few Pi transporters from early eukaryotes have been characterized. Saccharomyces cerevisiae possesses four plasma membrane localized Pi transporters, two low affinity transporters named Pho87p and Pho90p, and two high affinity transporters named Pho84p and Pho89p (Secco et al., 2012). Another Pi transporter, Pho91p, is localized to the vacuole and proposed to be involved in exporting Pi from the vacuole to the cytosol (Hurlimann et al., 2007). While Pho84p is a H+/Pi symporter, all the others are Na+/Pi symporters. A Na+/Pi symporter has also been characterized in the malaria parasite, Plasmodium falciparum (Saliba et al., 2006). Pi transport has been described in T. cruzi (De Boiso and Stoppani, 1972; Dick et al., 2013), and several sequences with characteristics of Pi transporters are present in its genome. Phosphate transport and partial sequences corresponding to phosphate transporters of Trypanosoma rangeli (Dick et al., 2012) and Leishmania infantum (Russo-Abrahao et al., 2013) have also been reported. However, this is the first molecular and electrophysiological characterization of a Pi transporter in T. cruzi or in any trypanosome.
The CVC localization of TcPho91 could be important for Pi return to the cytosol after the cells are submitted to hyposmotic stress. Acidocalcisomes have been shown to fuse with the CVC upon hyposmotic stress and transfer aquaporin (TcAQP1) to the CV membrane (Rohloff et al., 2004). When fusion occurs, Pi resulting from polyP hydrolysis (Ruiz et al., 2001) and cations released from their binding to the polymer, were proposed to enter the CV and increase its osmotic pressure facilitating water entry (Docampo et al., 2013). After water is eliminated through the flagellar pocket, cations and Pi should return to the cytosol as no significant release of these compounds has been detected (Rohloff et al., 2003). The presence of a Na+/Pi symporter would serve to recover both Na+ and Pi to the cytosol favored by their concentration gradient. Interestingly, the orthologue of TcPho91 in T. brucei (TbPho91), an organism that apparently lacks a CVC, localizes to acidocalcisomes, where it has been proposed to be involved in Pi release (Huang et al., 2014).
Overexpression of TcPho91 resulted in transfer of the protein to the plasma membrane and increased levels of PPi and short chain polyP, which would be compatible with an enhanced Pi transport. The plasma membrane localization of the transporter was more evident when cells were grown in medium deficient in Pi (SDM-79). Under those conditions, epimastigotes overexpressing TcPho91 had a clear growth advantage over wild type cells. Interestingly, a similar translocation of a polyamine transporter (TcPOT1.1) from the CVC to the plasma membrane was described in T. cruzi when cultivated in medium deficient in polyamines (Hasne et al., 2010). In this regard, a role of the CVC in traffic of proteins to the plasma membrane is well established in both Dictyostelium discoideum and T. cruzi (Docampo et al., 2013; Niyogi et al., 2014).
Few genetic tools are available to work with T. cruzi (Docampo, 2011). This parasite is predominantly diploid and thus inactivation of most genes requires two rounds of gene replacement. However, attempts to generate null mutants by homologous replacement sometimes yields parasites bearing the two planned replacements but also bearing extra copies of the genes through aneuploidy. In addition, RNAi, which is present in T. brucei, is absent in T. cruzi (Docampo, 2011). In the case of TcPho91, only one allele coded for a complete ORF while the other was truncated. Taking advantage of this characteristic and using a method that was previously successful in knocking down the expression of TcAQP1 (Li et al., 2011), we were able to downregulate the expression of TcPho91. These parasites showed a significant growth defect under both deficient and normal medium Pi concentration and had lower levels of PPi and short chain polyP, suggesting that TcPho91 is essential for optimal growth of epimastigotes.
PolyP synthesis results from the polymerization from 3 to thousands of Pi residues (Moreno and Docampo, 2013) while PPi is mainly a byproduct of many biosynthetic reactions, such as the synthesis of nucleic acids, coenzymes, proteins, and isoprenoids, and the activation of fatty acids. Both PPi and polyP accumulate in acidocalcisomes (Moreno et al., 2000). Several classes of polyP are present in trypanosomes: very short chain polyP, such as polyP3, polyP4, and polyP5, which are detected by 31P-NMR analysis (Moreno et al., 2000), and short and long chain polyP, containing ~100-300, or ~1,000 Pi residues, respectively (Ruiz et al., 2001). Synthesis of short chain polyP (~100-300 mer) in T. brucei (Lander et al., 2013) and T. cruzi (Ulrich et al., 2014) is catalyzed by a vacuolar transporter chaperone 4 (TbVtc4 and TcVtc4, respectively), which synthesizes and translocates polyP to the acidocalcisomes. However, the enzyme(s) involved in the synthesis of very short and long chain polyP are unknown. Interestingly, osmoregulatory defects have been described before when short chain polyP levels are altered in trypanosomes by either knockdown or knockout (Fang et al., 2007a; Lander et al., 2013) of enzymes involved in their synthesis or overexpression of enzymes involved in their metabolism (Fang et al., 2007b) and our results are consistent with a role of short chain polyP in osmoregulation. In conclusion, we have characterized for the first time a Pi/Na+ symporter involved in Pi metabolism in T. cruzi.
Experimental procedures
Culture methods
T. cruzi epimastigotes (Y and CL strains) were grown at 28°C in liver infusion tryptose medium (LIT) (Bone and Steinert, 1956) supplemented with 10% heat-inactivated fetal bovine serum. The epimastigotes transformed with pTREX constructs were maintained in LIT medium supplemented with 10% heat-inactivated fetal bovine serum and 0.25 mg/ml geneticin (G418) (Montalvetti et al., 2004). When indicated, epimastigotes were also cultured in medium SDM-79 (Cunningham, 1977) supplemented with 200 μM putrescine and hemin (Hasne et al., 2010). Tissue culture cell-derived trypomastigotes were obtained from L6E9 myoblasts infected with metacyclic trypomastigotes from stationary cultures of TcPho91-GFP parasites. T. cruzi amastigote and trypomastigote forms were collected from the culture medium of infected host cells, using a modification of the method of Schmatz and Murray (Schmatz and Murray, 1982) as described previously (Moreno et al., 1994).
Growth curves in LIT and SDM-79
Wild type, overexpressing or knock down epimastigotes were diluted to 1×106 cells ml−1 in LIT or SDM-79 supplemented as described above. Aliquots of parasites were taken daily, diluted in Isoton solution (Beckman) and cell density was evaluated in duplicate using an automated cell counter (Z2 Beckman Coulter). Results correspond to mean ± SD of three independent experiments each one in triplicate.
Chemicals and reagents
Fetal bovine serum was from Atlanta Biologicals. Dulbecco’s phosphate-buffered saline (PBS), protease inhibitor cocktails (P8849 and P8340), paraformaldehyde, glutaraldehyde, bovine serum albumin, malachite green, ATP, PPi, polyphosphate glass (polyP75), and monoclonal antibody against tubulin were purchased from Sigma (St. Louis, MO). Restriction enzymes were from New England BioLabs (Ipswich, MA), T4 DNA ligase, Taq polymerase, DNA ladder and goat serum were from Gibco BRL, Life Technologies, (Gaithersburg, MD). The pET28a+ expression system, nickel nitriloacetic acid His-Bind resin, and benzonase nuclease were from Novagen (Madison, WI). pCR2.1-TOPO BLUNT cloning kit was from Invitrogen (Carlsbad, CA). Hybond-N nylon membrane, and ECLTM chemiluminescence kit were obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). [α-32P]-dCTP (3.0 Ci/mol) was from Perkin Elmer. Coomassie Brilliant Blue protein assay reagent was from Bio-Rad. Antibodies against TbVP1 were a gift from Norbert Bakalara (University of Montpellier, France). Antibodies against GFP were from Invitrogen (Life Technologies, Grand Island, NY). All other reagents were analytical grade.
Construction of TcPho91 knockout cassettes
A homologous recombination PCR-based approach was used to generate the knockouts. The 5’- and 3’-flanking sequences of TcPho91 gene were amplified by PCR. The open reading frame (ORF) of the neomycin-resistant gene was also amplified by PCR using primers containing ~20 extra nucleotides at the 5’ end overlapping with the 5’- and 3’-flanking sequences of TcPho91, respectively, The three PCR fragments (5’-TcPho91 flanking sequence, neomycin-resistant gene, and the 3’-TcPho91 flanking sequence) were linked together by sequential PCR. The drug resistant gene was therefore flanked with the exact 5’ and 3’-UTR of TcPho91. The final PCR products were cloned into pCR 2.1 TOPO BLUNT vector. Several clones were sequenced to confirm that the drug resistant gene ORF was correct. The linearized plasmid with the drug-resistance cassette was then used to transfect CL strain epimastigotes. After drug selection of resistant parasites, the genomic DNA was extracted for PCR and Southern blot analysis.
Cell transfection
T. cruzi epimastigotes were grown to a density of 2 × 107/ml, washed once with Buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM Hepes, pH 7.2) with glucose (5.5 mM) at room temperature and resuspended in cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4, 2 mM EDTA, 5 mM MgCl2, pH 7.6) at a density of 108 cells/ml in electroporation buffer. Transfections were carried out in a 4 mm gap cuvette with 50 μg recombinant plasmid DNA using a Bio-Rad Gene Pulser II set at 1.5 kV and 50 microfarads with two pulses, and then incubated on ice for 5 min. Parasites were recovered in 5 ml of LIT supplemented with 10% fetal bovine serum at 28°C, and after 24 h in culture, geneticin (G418) was added to a final concentration of 250 μg/ml to select stably growing transformants.
qRT-PCR
Total RNA was isolated from wild-type and knock down epimastigotes using the TRI® reagent (Sigma) by following the manufacturer’s instructions. The total RNA was treated with RQ1 RNase-free DNase I to remove genomic DNA contamination. cDNA was synthesized from 1 μg total RNA with Superscript III reverse transcriptase (Life Technologies), using both oligodT and TcPho91 gene specific primers. Heat-inactivated cDNA reaction mixtures were treated with RNase H at 37°C for 45 min. Quantification of TcPho91 was done by real-time PCR with primers listed in Table S1 and standardized against tubulin as a control gene. The copy number was calculated based on standard curves of the control gene and serial dilutions of TcPho91 cloned gene. The average ratios were calculated from 3 independent experiments each performed in triplicate.
Heterologous expression of TcPho91-GFP in Xenopus laevis oocytes
TcPho91 ORF was amplified with the primers indicated in Table S1 flanked by EcoRI and HindIII restriction sites, cloned into TOPO BLUNT vector. TcPho91 was subcloned into pTREX-GFP vector and sequenced to confirm the correct frame. pTREX-TcPho91GFP was purified and linearized with XmnI. cRNA was obtained by in vitro transcription with mMESSAGE mMACHINE kit (Ambion) according with the manufacturer’s protocol. Stage IV or V oocytes were surgically collected, treated with collagenase (1 mg/ml) at room temperature for 1 h and manually de-folliculated. 20 ng cRNA (25-50 nL) per oocyte were injected and the cells were incubated for 72 h at 18°C in Barth’s solution (88 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, 0.33 mM Ca(NO3)2 and 10 mM Hepes, pH 7.4) with antibiotics. Control oocytes were injected with same volume of DEPC water. Prior to recording by two-electrode voltage clamp, cell with appropriate morphology and poles clearly delineated were selected and the oocytes were placed in normal ND96 buffer (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes-Tris, pH 7.4).
Electrophysiology
The standard two-electrode voltage-clamp technique was used, as previously described (Diaz et al., 2008). Briefly, oocytes were placed in a recording chamber and superfused with ND96 solution at room temperature. TcPho91 activity was evaluated by whole cell voltage clamp in an Oocyte Clamp OC-725 amplifier (Warner Instruments, USA). Recordings were filtered at 500 Hz, digitized at 5 kHz using a Digidata 1440 (Axon Instruments, USA), and analyzed using PClamp 10 software (Axon Instruments, USA). Intracellular electrodes were pulled to a resistance of 1 – 4 mega ohms and filled with a 3 M KCl solution. Steady state membrane currents were recorded in response to voltage step or ramp protocols from −80 to +40 mV. The holding potential in all the experiments was −60 mV and responses to Pi and other anions were always examined at holding potential in the presence or absence of sodium. All experiments were performed on oocytes from at least 6 donor frogs to confirm reproducibility. Data presented correspond to a total of 28 TcPho91 expressing oocytes and 20 control oocytes obtained from 8 independent frogs. Currents were normalized against the control current at −80 mV in the absence of Pi, unless indicated otherwise. Statistic analysis was performed in Origin 7 and Prism 6 software. Significant differences between treatments were compared by Student’s t-test.
Electrophysiology solutions
All recording were conducted at room temperature in ND96 normal buffer described above. Sodium free solutions (NDX) were prepared by equimolar substitution of sodium chloride with either potassium or N-methyl-D-glucamine (NMDG) chloride. Effect of anions (phosphate, sulfate and nitrate) was evaluated by addition of the ions to ND96 (unless indicated otherwise) at increasing concentrations. pH was readjusted to 7.4 prior to use to avoid acidification effects.
SDS-PAGE and western blot analyses
Electrophoresis was performed as described by Laemmli (Laemmli, 1970) under reducing conditions. Electrophoresed proteins were transferred to nitrocellulose membranes using a Bio-Rad transblot apparatus. Following transfer, the membrane blots were blocked with 5% non-fat dry milk in PBS containing 0.1% Tween-20 (PBS-T) at 4°C overnight. Blots were probed with affinity-purified anti-TcPho91 polyclonal antibody (1:5,000 dilution), anti-GFP monoclonal antibody (1:10,000 dilution), or anti-α-tubulin monoclonal antibody (1:40,000) for 1 h. After washing five times with PBS-T, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:20,000), or goat anti-mouse antibody (1:20 000). Immunoblots were visualized on blue-sensitive X-ray film (Midwest Scientific, St. Louis, MO) using the ECL chemiluminescence detection kit according to the instructions of the manufacturer.
Expression of TcPho91-GFP in oocytes was evaluated by western blot analysis. Pools of 10 control and injected oocytes were washed in solution A (83 mM NaCl, 1 mM MgCl2 and 10 mM Hepes-Na pH 7.5) and resuspended in the same buffer. After homogenization, the yolk was eliminated by centrifugation at 1,000 × g for 10 min and the supernatant containing the membrane fraction was sedimented at 50,000 × g for 45 min at 4°C. The pellet was resuspended in modified RIPA buffer (150 mM NaCl, 20 mM Tris-Cl pH 7.5, 1 mM EDTA, 1% SDS and 0.1% Triton X-100) and TcPho91-GFP expression was evaluated by SDS-PAGE and western-blot analysis with anti-GFP polyclonal antibody (1:10,000 dilution) and anti-rabbit HRP antibody (1:20,000).
Antibody production
Polyclonal antibodies against TcPho91 were produced in mice using as antigen a synthetic peptide TALYRLTAHRPPFYLLGVML corresponding to TcPho91 amino acids 366-385, conjugated with KLH (Keyhole Limpet Hemocyanin). Final bleeds from five inoculated mice were affinity purified by immunoadsorption to the peptide. The adsorbed antibodies were eluted with 0.1 M glycine, pH 2.5, and neutral pH was restored immediately by adding 1 M Tris-HCl buffer, pH 8.0. Mice and X. laevis experiments were approved by the Institutional Animal Care and Use Committee (IACUC).
Fluorescence microscopy
We directly observed subcellular localization of GFP-fusion proteins in epimastigotes under isosmotic (280 mOsm) and hyposmotic conditions (117 mOsm). We prepared cells for observation under hyposmotic stress by washing twice in Buffer A-glucose (pH 7.4) and resuspending them in isosmotic (64 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 0.53 mM MgCl2, 5.5 mM glucose, 150 mM D-mannitol, 5 mM Hepes-Na, pH 7.4, 282 mosmol/L), hyposmotic (64 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 0.53 mM MgCl2, 5.5 mM glucose, 5 mM Hepes-Na, pH 7.4, 117 mosmol/L) or hyperosmotic buffer (64 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 0.53 mM MgCl2, 5.5 mM glucose, 500 mM D-mannitol, 5 mM Hepes-Na, pH 7.4, 650 mosmol/L). For immunofluorescence microscopy, cells were fixed in PBS (pH 7.4) with 4% paraformaldehyde, adhered to poly-lysine coverslips, and permeabilized for 5 min with PBS (pH 7.4) containing 0.3% Triton X-100. Permeabilized cells were treated with 50 mM NH4Cl for 30 min and blocked overnight in PBS (pH 8) containing 3% bovine serum albumin. GFP was labeled with a monoclonal anti-GFP antibody (3E6, 1:300 dilution, Invitrogen) or polyclonal anti-GFP antibody (1:1,000 dilution, Invitrogen) and the corresponding goat anti-mouse or anti-rabbit Alexa conjugated secondary antibody (1:2,000 dilution, Invitrogen). For TcPho91-GFP cell lines, the parasites were fixed and permeabilized as described. After blocking overnight at 4°C in 3% bovine serum albumin (PBS, pH 8), the cells were incubated with rabbit polyclonal anti-GFP antibody (1:2,000 dilution, Invitrogen) and goat anti-rabbit Alexa conjugated secondary antibody (1:2,000 dilution, Invitrogen). Specimens were imaged using a Delta Vision Elite deconvolution microscope (Applied Precision).
Cryo-immunogold electron microscopy
T. cruzi epimastigotes expressing TcPho91-GFP were washed twice in 0.1 M sodium cacodylate buffer, pH 7.4, and fixed for 1 h on ice with 0.1% glutaraldehyde, 4% paraformaldehyde and 0.1 M sodium cacodylate buffer, pH 7.4. Samples were processed for cryo-immunoelectron microscopy at the Molecular Microbiology Imaging Facility, Washington University School of Medicine. GFP-fusion protein localization was detected with a polyclonal antibody against GFP (Abcam) at a 1:50 dilution and anti-rabbit conjugated to 18 nm colloidal gold as a secondary antibody.
BODIPY-ceramide labeling and live cell imaging
Epimastigotes overexpressing TcPho91-GFP and maintained either in LIT or SDM-79 media were collected by centrifugation in the exponential phase of growth and resuspended at a density of 1×107 parasites/ml in LIT or SDM79 media containing 5 μM of BODIPY-TR C5 ceramide (Invitrogen). After 1 h of incubation at 28°C, the cells were collected by centrifugation at 1,600 × g, and washed twice in PBS, pH 7.4. Labeled epimastigotes were then placed in bottom-glass petri dishes previously treated with poly-lysine. Cells were allowed to settle in the dish for 2 min and loose parasites were washed away with isosmotic buffer. Live cell imaging was done in isosmotic conditions in an Olympus IX-83 inverted fluorescence microscope and processed with CellSense Dimensions software.
MitoTracker labeling and immunofluorescence
Epimastigotes overexpressing TcPho91-GFP and maintained in LIT or SDM-79 media were collected by centrifugation in the exponential phase of growth and resuspended in LIT or SDM79 media containing 1 μM of MitoTracker Red CMXRos (Life Technologies). After 1 h of incubation at 28°C, the cells were collected by centrifugation at 1,600 × g, washed twice in PBS, pH 7.4 and fixed in 4% para-formaldehyde. Indirect immunofluorescence to detect TcPho91-GFP was performed as described above using a polyclonal anti-GFP antibody (1:1,000 dilution, Invitrogen) and the corresponding goat anti-mouse or anti-rabbit Alexa conjugated secondary antibody (1:2,000 dilution, Invitrogen). Images were taken with CellSense Dimensions software in an Olympus IX-83 inverted fluorescence microscope.
Extraction and determination of PPi, short chain and long chain polyP levels in epimastigotes
Cells in log phase were harvested and washed twice with Buffer A. The PPi, and short-chain polyP were extracted using 0.5 M perchloric acid (HClO4) (Ruiz et al., 2001), and the long-chain polyP was extracted using glass milk (Molecular Probes) as described by Ault-Riche et al. (Ault-Riche et al., 1998). PPi was determined by measuring the amount of Pi released upon treatment with S. cerevisiae (Sigma) or Thermoccocus litoralis (New England Biolabs) inorganic pyrophosphatase. Extracts were incubated with 5 units/ml of yeast inorganic pyrophosphatase in 50 mM Tris-HCl, pH 7.5, and 6 mM MgCl2, at 30°C for 30 min, and the released Pi was measured using malachite green as described before (Ruiz et al., 2001). PolyP was determined by the amount of Pi released upon treatment with an excess of S. cerevisiae exopolyphosphatase 1 (ScPPX1), freshly purified in our laboratory (Ruiz et al., 2001). The concentration of PPi, and polyP in T. cruzi epimastigotes was expressed per mg of protein used to perform the extractions. Protein concentration was measured by BCA method following the manufacturer protocol (Pierce). Results correspond to 6 independent experiments.
Regulatory volume changes under osmotic stress conditions
For osmotic stress under constant ionic strength, we used the buffers described previously (de Jesus et al., 2010; Jimenez and Docampo, 2012). Briefly, the cells were washed twice in PBS and resuspended at a density of 4 × 108 cells/ml in isosmotic buffer. Aliquots of 4 × 107 parasites (100 μl) were distributed in 96 well plates and osmotic stress was induced after 3 min of reading by adding 200 μl of hyposmotic or hyperosmotic buffer was added to reach a final osmolality of 115 and 650 mosmol/L, respectively. Absorbance at 550 nm was monitored every 10 sec for 12 min. The results were normalized respect to the average of a 3 min pre-reading under isosmotic conditions. Cell viability was verified in the microscope after 10 min under osmotic stress.
Supplementary Material
Acknowledgments
We thank Wandy L. Beatty for help with the cryo-immunogold electron microscopy. This work was funded by the U.S. National Institutes of Health (grants AI101167 to V.J., and AI107663 to R.D.)
References
- Ault-Riche D, Fraley CD, Tzeng CM, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci. 1993;105:1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- Bone GJ, Steinert M. Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature. 1956;178:308–309. doi: 10.1038/178308a0. [DOI] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977;24:325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- De Boiso JF, Stoppani AO. Phosphate transport in Trypanosoma cruzi. Experientia. 1972;28:1162–1164. doi: 10.1007/BF01946144. [DOI] [PubMed] [Google Scholar]
- de Jesus TC, Tonelli RR, Nardelli SC, da Silva Augusto L, Motta MC, Girard-Dias W, et al. Target of rapamycin (TOR)-like 1 kinase is involved in the control of polyphosphate levels and acidocalcisome maintenance in Trypanosoma brucei. J Biol Chem. 2010;285:24131–24140. doi: 10.1074/jbc.M110.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P, Vallejos C, Guerrero I, Riquelme G. Barium, TEA and sodium sensitive potassium channels are present in the human placental syncytiotrophoblast apical membrane. Placenta. 2008;29:883–891. doi: 10.1016/j.placenta.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Dick CF, Dos-Santos AL, Majerowicz D, Gondim KC, Caruso-Neves C, Silva IV, et al. Na+-dependent and Na+-independent mechanisms for inorganic phosphate uptake in Trypanosoma rangeli. Biochim Biophys Acta. 2012;1820:1001–1008. doi: 10.1016/j.bbagen.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Dick CF, Dos-Santos AL, Majerowicz D, Paes LS, Giarola NL, Gondim KC, et al. Inorganic phosphate uptake in Trypanosoma cruzi is coupled to K+ cycling and to active Na+ extrusion. Biochim Biophys Acta. 2013;1830:4265–4273. doi: 10.1016/j.bbagen.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Docampo R. Molecular parasitology in the 21st century. Essays in biochemistry. 2011;51:1–13. doi: 10.1042/bse0510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Jimenez V, King-Keller S, Li ZH, Moreno SN. The role of acidocalcisomes in the stress response of Trypanosoma cruzi. Adv Parasitol. 2011;75:307–324. doi: 10.1016/B978-0-12-385863-4.00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int Rev Cell Mol Biol. 2013;305:69–113. doi: 10.1016/B978-0-12-407695-2.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rohloff P, Miranda K, Docampo R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007a;407:161–170. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Ruiz FA, Docampo M, Luo S, Rodrigues JC, Motta LS, et al. Overexpression of a Zn2+-sensitive soluble exopolyphosphatase from Trypanosoma cruzi depletes polyphosphate and affects osmoregulation. J Biol Chem. 2007b;282:32501–32510. doi: 10.1074/jbc.M704841200. [DOI] [PubMed] [Google Scholar]
- Ferreira GC, Pedersen PL. Phosphate transport in mitochondria: past accomplishments, present problems, and future challenges. J Bioenerg Biomembr. 1993;25:483–492. doi: 10.1007/BF01108405. [DOI] [PubMed] [Google Scholar]
- Forster IC, Wagner CA, Busch AE, Lang F, Biber J, Hernando N, et al. Electrophysiological characterization of the flounder type II Na+/Pi cotransporter (NaPi-5) expressed in Xenopus laevis oocytes. J Membr Biol. 1997;160:9–25. doi: 10.1007/s002329900291. [DOI] [PubMed] [Google Scholar]
- Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasne MP, Coppens I, Soysa R, Ullman B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol Microbiol. 2010;76:78–91. doi: 10.1111/j.1365-2958.2010.07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Ulrich PN, Storey M, Johnson D, Tischer J, Tovar JA, et al. Proteomic analysis of the acidocalcisome, an organelle conserved from bacteria to human cells. PLoS Pathog. 2014;10:e1004555. doi: 10.1371/journal.ppat.1004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlimann HC, Stadler-Waibel M, Werner TP, Freimoser FM. Pho91 Is a vacuolar phosphate transporter that regulates phosphate and polyphosphate metabolism in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:4438–4445. doi: 10.1091/mbc.E07-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez V, Docampo R. Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi. PLoS Pathog. 2012;8:e1002750. doi: 10.1371/journal.ppat.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J Insect Physiol. 2001;47:739–747. doi: 10.1016/s0022-1910(00)00170-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lander N, Ulrich PN, Docampo R. Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J Biol Chem. 2013;288:34205–34216. doi: 10.1074/jbc.M113.518993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Foller M, Lang K, Lang P, Ritter M, Vereninov A, et al. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–225. doi: 10.1016/S0076-6879(07)28011-5. [DOI] [PubMed] [Google Scholar]
- Li ZH, Alvarez VE, De Gaudenzi JG, Sant’Anna C, Frasch AC, Cazzulo JJ, Docampo R. Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi. J Biol Chem. 2011;286:43959–43971. doi: 10.1074/jbc.M111.311530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- Moreno B, Urbina JA, Oldfield E, Bailey BN, Rodrigues CO, Docampo R. 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates. J Biol Chem. 2000;275:28356–28362. doi: 10.1074/jbc.M003893200. [DOI] [PubMed] [Google Scholar]
- Moreno SN, Docampo R. Polyphosphate and its diverse functions in host cells and pathogens. PLoS Pathog. 2013;9:e1003230. doi: 10.1371/journal.ppat.1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SN, Silva J, Vercesi AE, Docampo R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med. 1994;180:1535–1540. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi S, Jimenez V, Girard-Dias W, de Souza W, Miranda K, Docampo R. Rab32 is essential for maintaining functional acidocalcisomes and for growth and infectivity of Trypanosoma cruzi. J Cell Sci. 2015 doi: 10.1242/jcs.169466. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi S, Mucci J, Campetella O, Docampo R. Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi. PLoS Pathog. 2014;10:e1004224. doi: 10.1371/journal.ppat.1004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravera S, Virkki LV, Murer H, Forster IC. Deciphering PiT transport kinetics and substrate specificity using electrophysiology and flux measurements. Am J Physiol Cell Physiol. 2007;293:C606–620. doi: 10.1152/ajpcell.00064.2007. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J Biol Chem. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem. 2001;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- Russo-Abrahao T, Alves-Bezerra M, Majerowicz D, Freitas-Mesquita AL, Dick CF, Gondim KC, Meyer-Fernandes JR. Transport of inorganic phosphate in Leishmania infantum and compensatory regulation at low inorganic phosphate concentration. Biochim Biophys Acta. 2013;1830:2683–2689. doi: 10.1016/j.bbagen.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Saliba KJ, Martin RE, Broer A, Henry RI, McCarthy CS, Downie MJ, et al. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature. 2006;443:582–585. doi: 10.1038/nature05149. [DOI] [PubMed] [Google Scholar]
- Schmatz DM, Murray PK. Cultivation of Trypanosoma cruzi in irradiated muscle cells: improved synchronization and enhanced trypomastigote production. Parasitology. 1982;85:115–125. doi: 10.1017/s0031182000054202. [DOI] [PubMed] [Google Scholar]
- Secco D, Wang C, Shou H, Whelan J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS letters. 2012;586:289–295. doi: 10.1016/j.febslet.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Ulrich PN, Jimenez V, Park M, Martins VP, Atwood J, 3rd, Moles K, et al. Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS One. 2011;6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich PN, Lander N, Kurup SP, Reiss L, Brewer J, Soares Medeiros LC, et al. The acidocalcisome vacuolar transporter chaperone 4 catalyzes the synthesis of polyphosphate in insect-stages of Trypanosoma brucei and T. cruzi. J Eukaryot Microbiol. 2014;61:155–165. doi: 10.1111/jeu.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.