Introduction
For many thousands of years, the sun was our only source of light, and human behavior followed a natural day-night cycle. This milieu began to change approximately 150 years ago with the invention of incandescent lighting. Electric lighting disrupted our behavioral dependence on the day-night cycles of the sun, and facilitated alterations in our circadian sleep-wake cycles. Recent research has begun to identify the physiologic consequences of unnatural light exposure and subsequently altered circadian rhythms.1 In this paper, we review the molecular basis of circadian rhythms and discuss the established connection between disrupted circadian rhythms and clinical disease. We also explore the concept of daylight as therapy to restore disrupted circadian rhythms and improve clinical outcomes.
Molecular mechanisms: daylight as an essential regulator of circadian rhythms
For nearly four billion years, life on Earth, outside of the poles and deep oceans, has evolved under a consistent pattern of alternating bright days and dark nights. As a result, most organisms on our planet synchronize “daily life” to a 24-hour cycle. This is called a circadian (from Latin circa, meaning “around”, and dies, meaning “day”) rhythm. It is in this same environment that modern humans evolved over the past 200,000 years.
Today we know that the circadian rhythm does not simply regulate sleep-wake cycles, but also influences the molecular biology of individual cells and organ systems. The molecular mechanism of the circadian rhythm itself was discovered around 1970 in the common fruit fly, Drosophila melanogaster, and shortly thereafter described in humans. Genes including Clock and Period were identified as important regulators of the circadian rhythm, through patterns of protein expression that oscillate approximately every 24 hours. Expression of these proteins reflects the circadian rhythm on a molecular level in all mammals (Figure 1A). The molecular mechanism of the circadian rhythm involves a very complex and autonomous transcriptional-translational feedback loop which consists of a core set of oscillating, ubiquitously expressed genes, including Clock, Bmal1, Period homologues 1 and 2 (Per1 and Per2), and Cryptochromes 1 and 2 (Cry1 and Cry2) (Figure 1A).2 This transcriptional-translational feedback loop takes approximately 24 hours to complete.2 In addition to this classical transcriptional and post-translational mechanism, many interacting pathways have been described, but the complete regulatory system is not yet fully understood.
Figure 1.
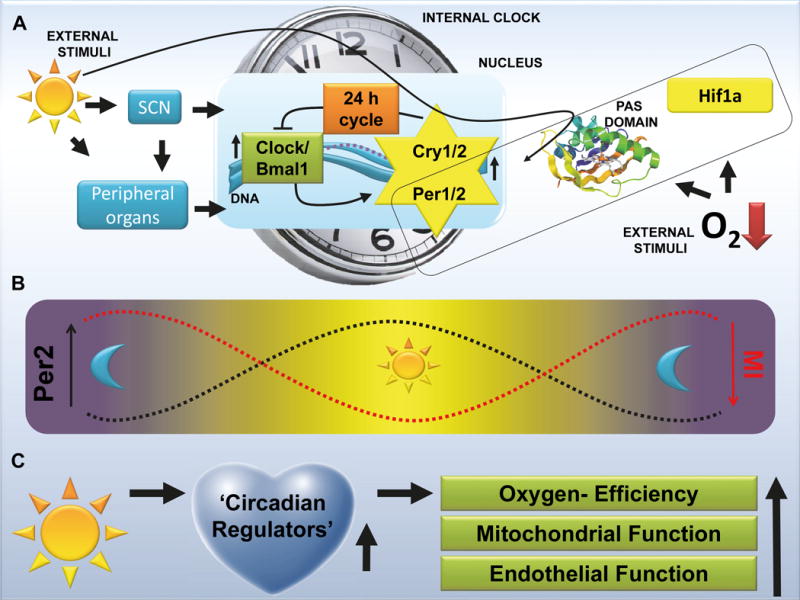
Mechanisms of circadian rhythms. A. Molecular mechanism: The circadian clock is composed of a primary negative-feedback loop involving the genes Clock, Bmal1, Period homologue 1 (Per1), Per2, Cryptochrome 1 (Cry1) and Cry2. This clockwork is composed of a set of proteins that are synchronized by daylight (Per1, Per2) in the central nervous system and peripheral organs. Periods (Per) are nuclear proteins that belong to the so-called PAS domain superfamily and are cofactors of transcription. PAS stands for Period, Arnt and Sim, three drosophila genes in which the PAS domain was discovered. Hypoxia Inducible Factor 1 Alpha (HIF1A) that plays an important role in hypoxic and ischemic disease states also belongs to this family of PAS domain positive proteins. The PAS domain has been described as a binding site to allow interactions between those proteins and to sense oxygen or light; SCN=Suprachisasmatic Nuclei; DNA=Deoxyribonucleic acid;
B. Correlation between infarct sizes (myocardial infarction, MI) and cardiac Per2 levels. Per2 levels are regulated by daylight. Recent studies recognized a relation between daylight elicited Per2 levels and the severity of myocardial ischemia. C. Proposed cardio-protective mechanisms mediated by circadian regulators via day daylight exposure.
Though circadian rhythms are theoretically endogenously self-sustained, powerful external stimuli influence and fine-tune the timing of the circadian system. Those external stimuli change the expression profile of our circadian proteins to synchronize our endogenous circadian rhythm to the environment. While in some species (e.g. Drosophila Melanogaster) circadian rhythms are synchronized by the temperature and other environmental factors, in humans the most powerful of these external stimuli has been shown to be daylight.3,4 Daylight stimulates melanopsin receptors in retinal ganglion cells and these cells transform the physical signal into an electrical signal (neurotransmission) that stimulates neurons in the suprachiasmatic nuclei (SCN). In these neurons, the electrical signal activates the circadian proteins in the SCN and subsequently all peripheral organs.2
Important players in daylight-elicited synchronization of the circadian rhythm are the Period proteins (Per1, Per2), as daylight exposure leads directly to the induction of Per1/Per2 transcript and protein (Figure 1A).5 Periods are expressed in a circadian manner not only in the SCN but throughout the body and are involved in the regulation of metabolism and sleep.6 Periods belong to the so-called PAS domain superfamily. On a cellular level, the PAS domain functions as a sensor of oxygen or light (Figure 1A).7 Interestingly, genes such as Hypoxia Inducible Factor 1 Alpha (HIF1A) that play an important role in hypoxic and ischemic disease states – such as sepsis or heart disease – also belong to the same family of PAS-domain-positive proteins. From an evolutionary standpoint, light and oxygen sensing represent important adaptive mechanisms to environmental stress and the advantage for cell survival of sensing oxygen or light has been widely recognized.7 An important development in the study of circadian proteins has been the recognition that light and oxygen sensing are fundamentally linked.
Circadian rhythms in the pathogenesis of cardiovascular disease
Myocardial infarction has a long established connection to the circadian rhythm. A landmark study published in 1985 documented a three-fold higher incidence of myocardial infarction in the morning as compared to the late evening hours.8 An ensuing meta-analysis with more than 60,000 patients confirmed this result, and many subsequent studies have identified circadian patterns across a broad range of cardiovascular events, including unstable angina and stent thrombosis, stroke, ventricular arrhythmia, and aortic dissection.8 The mechanisms underlying these circadian patterns are complex and incompletely understood. What is clear, however, is that there is convincing human data for involvement of circadian-regulated proteins and hormones that likely contribute to plaque rupture and modulate myocardial response to ischemia. Regulators include cortisol and other stress hormones through activation of the sympathetic nervous system and increases in platelet reactivity that contribute to thrombosis.8 Two recent clinical studies demonstrated that in addition to peak incidence of myocardial infarction, the heart is also more vulnerable to an ischemic event in the early morning hours leading to larger infarct sizes.9,10
This diurnal pattern of infarct size has also been reproduced in animal studies. We recently discovered a correlation between light intensity and cardiac Per2 protein levels. We exposed mice to daylight (>10,000 LUX intensity) and found a robust induction of the light-regulated circadian protein Per2 in the heart when compared to typical indoor light (200 LUX) exposure. Treatment with daylight exposure prior to induction of myocardial ischemia also reduced troponin levels and infarct sizes by more than 50%. Subsequent studies over a 24 hour time period revealed a reciprocal correlation between infarct sizes and cardiac Per2 protein levels (Figure 1B).5 Studies on cardiac metabolism during ischemia using a mouse model for myocardial ischemia, established that Per2 knockout mice were less metabolically oxygen-efficient (Figure 1C). A metabolic switch from an oxygen dependent “energy-efficient” metabolism to a more oxygen conserving or “oxygen-efficient” metabolism is pivotal to allow the myocardium to function under ischemic conditions.11 This means that the heart must replace conventional fatty acid beta-oxidation with anaerobic glycolysis. Interestingly, anaerobic glycolysis is under the control of HIF1A. As noted above, HIF1A is an important transcription factor known to allow tissues and organs to adapt to conditions of low oxygen availability.12 Mechanistically, recent research demonstrated that Per2 is an important co-factor for HIF1A and several other transcription factors that are well-known therapeutic targets for cardiovascular disease.5,13,14 In our own studies, we were able to demonstrate that daylight-elicited cardiac Per2 stabilization led to the transcriptional induction of metabolic pathways in hearts from wildtype but not Per2 deficient mice.5 These findings implicate daylight-elicited Per2 stabilization in the heart as an endogenous cardioprotective mechanism, which mediates a metabolic switch to enhance “oxygen-efficient” metabolism and thereby renders the heart more resistant to ischemia (Figure 1). Current research from our lab indicates that daylight exposure of human volunteers increases Per2 levels and glycolytic enzymes in human plasma samples (unpublished data). Future studies in cardiac patients are needed to determine whether light exposure can help to make the heart more resistant to an ischemic event.
To date, genetic studies on circadian rhythm proteins and their effect on human disease are rare. While there is strong evidence based on clinical and basic science observations that circadian rhythm proteins are involved in the pathogenesis of cardiovascular disease, clinical studies using circadian proteins as a therapeutic target or as diagnostic biomarkers are still needed. Next steps should include the sequential collection of tissue and blood samples from patients over a 24 hour circadian cycle to generate tissue banks for the analysis of circadian rhythm gene polymorphisms, mutations, and protein expression patterns.
Given the power of daylight to entrain and re-synchronize circadian rhythms, exposure to naturally-cycled daylight may prevent or treat circadian related illnesses such as myocardial ischemia (Figure 1). Standard indoor lighting is not an effective substitute for sunlight in entraining the circadian oscillator due to its significantly lower intensity.15
Though studies from our group demonstrate daylight exposure in animals increases the expression of Per2 in the heart, ultimately resulting in cardio-protection, it is well known from human studies that daylight treatment is of greatest benefit when timed with circadian rhythms.16 Moreover, previous animal research has shown that either constant darkness or constant light was worse in an animal model for sepsis when compared to natural day/night cycles.17 In addition, nighttime exposure to background light in humans disrupts circadian rhythms, and such disruptions have been linked to multiple pathophysiological changes, including increased body mass and waist circumference, elevated triglyceride levels, and poor cholesterol balance.1 These alterations are known risk factors for cardiovascular disease and thus help explain the increased prevalence of cardiovascular disease in evening-shift and overnight workers.18
Circadian rhythms in the pathogenesis of critically illness
While prophylactic maintenance of normal circadian rhythms is indicated in all patients, one population is particularly vulnerable to sleep disturbances. Critically-ill patients in the intensive care unit (ICU) suffer disproportionately from sleep deprivation and frequent sleep disturbances.20 Continuous lighting, noise, overnight patient-care interactions, mechanical ventilation, pain, surgery,21 fatigue, stress, sedation, and critical illness itself, all disrupt the normal circadian rhythm (Figure 2).
Figure 2. Disrupted circadian rhythms and its consequences.
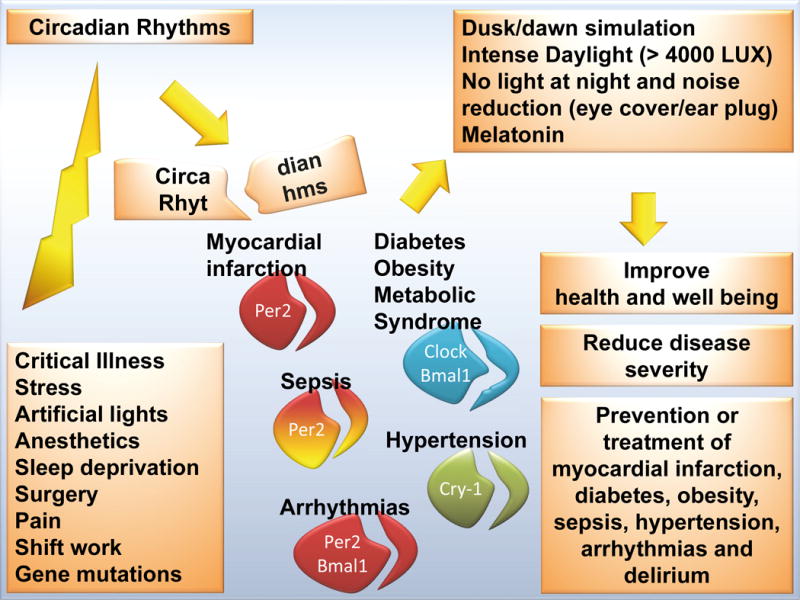
Many factors in a clinical setting lead to a disrupted circadian rhythm. As proper sleep is a reflection of a functional circadian rhythm, in particular sleep deprived patients are at risk (e.g. intensive care unit [ICU]). In addition, many common clinical scenarios disrupt our circadian rhythm, such as severe illness, stress, noise, surgery, sepsis, drugs, light at night etc. Based on current literature this could increase the risk for myocardial infarction, stroke, sepsis and obesity. The use of intense daylight (at least 4000 LUX, reflecting a sunny day outside) in conjunction with quiet and dark nights in hospitals could represent a future strategy to restore circadian rhythms and to benefit the overall health of inpatients; the circadian clock is composed of a primary negative-feedback loop involving the genes Clock, Bmal1, Period homologue 1 (Per1), Per2, Cryptochrome 1 (Cry1) and Cry2; disruption of these genes in animals models leads to the diseases indicated.
A dysfunctional circadian rhythm leads to a specific metabolic phenotype often encountered in critically ill patients. Patients in the ICU, and in particular those with sepsis, experience mitochondrial and endothelial dysfunction, as well as derangements of nitric oxide synthesis and pyruvate dehydrogenase activity.22 These cellular functions are all regulated by circadian proteins (Figure 1C).14 Thus, restoration of a misaligned circadian rhythm will help balance these metabolic disruptions.
An altered circadian system in association with sleep interruptions also plays an important role in the development of delirium.23 A robust body of research has now demonstrated that the development of ICU delirium is associated with poor clinical outcomes, including an increased risk of morbidity and mortality. Because serum melatonin levels correlate with high-quality sleep and functional circadian rhythms, this relationship has become an essential field for critical care research.24–26 Recently, a multicenter randomized controlled trial demonstrated efficacy in the use of ramelteon, a synthetic melatonin agonist, for the prevention of delirium.26
Based on clinical evidence that disruptions in circadian rhythms are an important factor influencing critical illness, as well as new knowledge about the mechanisms by which daylight exposure regulates circadian rhythms, re-establishment of natural day-night cycles using daylight sources and minimizing light and sleep interruptions overnight in ICUs needs to become an important priority (Figure 2). The potential health benefit of synchronizing the circadian rhythm to a natural cycle has been reported in multiple recent studies1,19,20,27 and correlates with basic molecular research demonstrating that circadian proteins are strong regulators of metabolism and protect from ischemia and other disease states.14
Future Directions and Conclusions
Both environmental (sleep disruption, artificial lighting, illness etc.) and genetic factors (polymorphisms or mutations in circadian rhythm genes) result in disruption of the circadian rhythm (Figure 2). Biologically, circadian rhythms are controlled by a cyclical expression of circadian genes. Mutations or polymorphisms in these genes result in a modification or disruption of the circadian oscillator and therefore it is important to analyze genetic factors that may contribute to circadian disruption.2 The discovery of genes involved in circadian rhythm-related diseases could open up new opportunities for therapy and might also yield new biomarkers for diagnosis and prognosis.5,14 Proteomic analysis and high-temporal resolution measurement of candidate proteins over a 24-hour cycle in hospitalized human patients will be necessary to understand the complex kinetics of a circadian-rhythm-guided system. Melatonin or Per2 seem to be promising candidates in this context, as indicted by many studies. In addition, as the circadian proteins are strongly involved in the regulation of metabolic pathways (Figures 1 and 2), metabolic assays (lactate levels, glucose levels, liver enzymes or tests for mitochondrial function) in relation to circadian protein levels in these patient samples could give new insights into circadian rhythm protein function in humans. This could be useful to monitor the efficacy of a treatment such as daylight exposure to restore circadian rhythm functionality.
Many characteristics of human behavior and their underlying molecular biochemical processes are driven by circadian rhythms. In the last few years, new evidence has become available that points to explicit connections between disrupted circadian rhythms and numerous clinical disorders. Clinically, evidence suggests that if the circadian rhythm is experimentally disrupted in mice or men, metabolic syndrome and obesity, premature aging, diabetes, cardiac arrhythmias, immune deficiencies, hypertension and abnormal sleep cycles develop (Figure 2).14 While the links between disrupted circadian rhythms and the pathogenesis of cardiovascular disease is relatively well-described in recent medical literature, the evidence for diseases such as hypertension or diabetes may be less well-known. It will be important for physicians to understand that a disrupted circadian rhythm and poor-quality sleep are associated with insulin resistance, high glucose levels and elevated blood pressures.28,29
Although available data describe the clinical effects of long-term circadian disturbances,1,18 the specific clinical consequences of short-term circadian disturbances, as occur during surgery and anesthesia are currently less clear. Better defining these short-term effects, and their association with morbidity and mortality, are an important goal of future circadian research.
We now have emerging data on the molecular changes associated with circadian rhythms and exposure to daylight. Oscillating Per2 and melatonin expression represent examples of this direct link and it appears that many other important regulators exist. Long prescribed for psychiatric illness, therapeutic daylight has emerged as a potential non-invasive and low-risk therapy for the prevention and treatment of critical illness. Several hospitals and centers in the United Kingdom, Germany, Sweden and the Netherlands are presently investigating inpatient daylight therapy, including the installation of LED-based lighting that simulates a day and night sky. Analysis from a study in Maastricht demonstrated that subjective well-being and sleep patterns, a mirror of a functional circadian rhythm, were significantly improved in 171 cardiac patients.27 Future clinical studies are needed to define specific protocols for the use of light in treatment and prevention of circadian-related illness, including source of light, intensity, timing and duration of therapy. However, based on the available evidence, a reasonable first goal should be to reduce continuous lighting in hospitals to a minimum. As this can be difficult in the hospital environment, alternative strategies have successfully used earplugs and eye-covers to improve sleep and circadian rhythms in patients.19 We envision a near future in which use of daylight (real or simulated) to entrain circadian rhythms will become standard of care for patients in the ICU, but also in the operating room and post anesthesia care unit. It is certainly intriguing that findings from recent biomedical research may challenge us to restore ancient patterns of exposure to daylight, under which life has evolved for the last four billion years.
Acknowledgments
Source of financial support for the work: National Heart, Lung, and Blood Institute (NIH-NHLBI), Bethesda, MD, USA, Grant 1K08HL102267-01 and 1R01HL122472-01A1 to T.E.
Footnotes
The authors declare no competing interests.
Publisher's Disclaimer: Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98:337–44. doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr Biol. 2007;17:R44–5. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93:107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braunwald E. On circadian variation of myocardial reperfusion injury. Circ Res. 2012;110:6–7. doi: 10.1161/CIRCRESAHA.111.260265. [DOI] [PubMed] [Google Scholar]
- 9.Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian Dependence of Infarct Size and Left Ventricular Function After ST Elevation Myocardial Infarction. Circ Res. 2012;110:105–10. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011;97:970–6. doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery Am J Physiol Cell Physiol. 2011;301:C550–2. doi: 10.1152/ajpcell.00176.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 14.Bonney S, Hughes K, Harter PN, Mittelbronn M, Walker L, Eckle T. Cardiac Period 2 in myocardial ischemia: Clinical implications of a light dependent protein. Int J Biochem Cell Biol. 2013;45:667–71. doi: 10.1016/j.biocel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379:540–2. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 16.Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Carlson DE, Chiu WC. The absence of circadian cues during recovery from sepsis modifies pituitary-adrenocortical function and impairs survival. Shock. 2008;29:127–32. doi: 10.1097/shk.0b013e318142c5a2. [DOI] [PubMed] [Google Scholar]
- 18.Tuchsen F, Hannerz H, Burr H. A 12 year prospective study of circulatory disease among Danish shift workers. Occup Environ Med. 2006;63:451–5. doi: 10.1136/oem.2006.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14:R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 21.Wright MC, Phillips-Bute B, Mark JB, Stafford-Smith M, Grichnik KP, Andregg BC, Taekman JM. Time of day effects on the incidence of anesthetic adverse events. Qual Saf Health Care. 2006;15:258–63. doi: 10.1136/qshc.2005.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin. 2010;26:567–75. doi: 10.1016/j.ccc.2010.04.007. , x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellapart J, Boots R. Potential use of melatonin in sleep and delirium in the critically ill. Br J Anaesth. 2012;108:572–80. doi: 10.1093/bja/aes035. [DOI] [PubMed] [Google Scholar]
- 24.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 25.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 26.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H, DELIRIA-J Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry. 2014;71:397–403. doi: 10.1001/jamapsychiatry.2013.3320. [DOI] [PubMed] [Google Scholar]
- 27.Giméneza MC, G L, Versteylen M, Leffers P, Meekesa GJBM, Herremansa H, de Ruyterd B, Schlangen LJM. Light and Sleep within Hospital Settings, Sleep-Wake Research. In: Enschede Boer Td., editor. The Netherlands, Annual Proceedings of the Dutch Society for Sleep-Wake Research (NSWO) Ipskamp Drukkers BV; 2011. pp. 56–59. [Google Scholar]
- 28.Depner CM, Stothard ER, Wright KP., Jr Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int. 2014;31:779–86. doi: 10.3109/07420528.2014.900501. [DOI] [PubMed] [Google Scholar]


