Abstract
Patients with on-line game addiction (POGA) and professional video game players play video games for extended periods of time, but experience very different consequences for their on-line game play. Brain regions consisting of anterior cingulate, thalamus and occpito-temporal areas may increase the likelihood of becoming a pro-gamer or POGA. Twenty POGA, seventeen pro-gamers, and eighteen healthy comparison subjects (HC) were recruited. All magnetic resonance imaging (MRI) was performed on a 1.5 Tesla Espree MRI scanner (SIEMENS, Erlangen, Germany). Voxel-wise comparisons of gray matter volume were performed between the groups using the two-sample t-test with statistical parametric mapping (SPM5). Compared to HC, the POGA group showed increased impulsiveness and perseverative errors, and volume in left thalamus gray matter, but decreased gray matter volume in both inferior temporal gyri, right middle occipital gyrus, and left inferior occipital gyrus, compared with HC. Pro-gamers showed increased gray matter volume in left cingulate gyrus, but decreased gray matter volume in left middle occipital gyrus and right inferior temporal gyrus compared with HC. Additionally, the pro-gamer group showed increased gray matter volume in left cingulate gyrus and decreased left thalamus gray matter volume compared with the POGA group. The current study suggests that increased gray matter volumes of the left cingulate gyrus in pro-gamers and of the left thalamus in POGA may contribute to the different clinical characteristics of pro-gamers and POGA.
Keywords: Cingulate gyrus, Functional magnetic resonance imaging, On-line game addiction, Pro-gamer, Thalamus
1. Introduction
1.1. The comparison between patients with on-line game addiction, pro-gamers, and healthy comparison subjects
Recently, there have been several studies suggesting that Internet addiction may be harmful enough to be categorized as a “psychiatric disorder” (Grant et al., 2010; Shapira et al., 2000; Dell'Osso et al., 2008). Grant et al. (2010) have proposed that Internet addiction should be classified as one category of `behavioral addiction' considering its natural course, clinical symptoms, tolerance, comorbidity, and neurobiological aspects. Other authors have referred to Internet addiction as a subtype of impulse control disorder (Shapira et al., 2000; Dell'Osso et al., 2008). Among Asian countries with a high incidence of Internet addiction (Fitzpatrick, 2008), South Korea is one of the most endemic areas.
In parallel with patients who suffer from on-line game addiction, there is another population in South Korea characterized by extensive on-line game playing, professional gamers (pro-gamers), who do not meet criteria for addiction. There are currently 259 StarCraft pro-gamers on twelve StarCraft teams in South Korea (KeSPA, 2010). As with other professional sports players, pro-gamers have contracts, an annual salary, and belong to teams in a gamer's village. To become a pro-gamer, candidates must complete a two step processing including a competition involving 3000 aspirants to select 1 pro-gamer. During team practice, pro-gamers practice StarCraft 7–10 h/day (9:00AM–5:00PM, 7:00PM–10:00PM). Each team has one general manager and two or three strategy coaches. As noted above, there are definite differences between patients with on-line addiction and pro-gamers in terms of regular life patterns, meeting criteria for addiction, and game playing ability (as evidenced by the difficult process of becoming a pro-gamer).
Given the above findings, we thought that differences in the regional brain volumes might play a critical role in determining whether an individual game player developed into a pro-gamer or a POGA. In the 12 month follow up study with 75 patients alcohol dependence and 43 healthy controls, patients with relapse showed smaller volumes than controls in superior frontal gyrus, amygdala, and hippocampus (Durazzo et al., 2011). In functional brain imaging studies of alcohol use, different patterns of brain activation have also demonstrated differences between individuals with alcohol dependence/abuse and sommeliers. After a sip of alcohol, in response to alcohol cues, patients with alcohol dependence had increased activation in the left dorsolateral prefrontal cortex and anterior thalamus, compared to social drinkers (George et al., 2001). However, in sommeliers, brain activation was found bilaterally in the insular and dorsolateral prefrontal cortex (Castriota-Scanderbeg et al., 2005), which is implicated in high-level cognitive processes such as working memory and selection of behavioral strategies (Huettel et al., 2004). The different patterns of brain volumes between POGA and pro-gamers provide insights into the vulnerability and resistance to gaming addiction in POGA and healthy controls.
1.2. The neural circuitry mediating on-line game playing
In a comparison of brain circuitry between patients with on-line game addiction, pro-gamers, and healthy comparison subjects we might expect to see changes within brain cortex related to (1) addiction to on-line game play, (2) the visuospatial system (visual system and attentive ability), and (3) comorbid disorders including major depression, attention deficit hyperactivity disorder, and impulse control disorder (Table 1).
Table 1.
The neural circuitry mediating on-line game playing.
| Cingulate | ||
| Ko et al., 2009; Miedl et al., 2010 | fMRI: activity | ↑ AC and PC in POGA |
| Grusser et al., 2004; Goldstein et al., 2007 | ↑ AC in AD/Drug | |
| Chua et al., 2009 | ↑ PC in smokers | |
| Zhou et al., 2011 | VBM: volume | ↓ AC and PC in POGA |
| Konarski et al., 2008 | ↓ AC in MDD | |
| Westlye et al., 2010 | Cortical Thickness | Thick of AC ∝ VSFx |
| Mayberg et al., 2000 | PET | ↓Glc in AC of MDD |
| Green and Bavelier, 2003 | Cog test | ↑ VSFx in healthy on-line game player |
| Sun et al., 2008 | ↓ POGA may be related to VSFx | |
| Fineberg et al., 2010 | Review | ↓ AC in PG |
| Thalamus | ||
| George et al., 2001 | fMRI: activity | ↑ Thalamus in AD |
| Potenza et al., 2003 | ↑ Thalamus in PG | |
| Wang et al., 2007 | VSFx circuitry ∝ cravings for smoking | |
| Due et al., 2002 | ↑ VSFx circuitry and attention in smokers | |
| Tomasi et al., 2007 | ↓ VSFx in cocaine abuser | |
| Raine et al., 1998 | ↑activity of thalamus in impulsive behavior | |
| Dibbets et al., 2010 | ↑ conflict detection in thalamus in ADHD | |
| Ivanov et al., 2010 | VBM: volume | ↑ lat. thalamus in ADHD |
| Asensio et al., 2010 | PET | ↓D2R availability in cocaine abuser |
| Occipito-temporal cortex | ||
| Herrman et al., 2007 | fMRI: activity | ↓ OC in AD |
| Pereverzeva and Murray, 2008 | VSFx ∝visual cortex | |
| Sasayama et al., 2010 | VBM: volume | ↓ temporal and occipital cortex in ADHD |
| Park et al., 2010 | PET | ↓ Glc metabolism of OC in POGA |
| Wang et al., 2010 | MRS | ↓Glc oxidation of OC in smokers |
| Harding and Jeavons, 1994 | EEG | Photosensitivity seizure in temporal-occipital cortex |
AC: anterior cingulate, PC: posterior cingulate, OC: occipital cortex, Cort Thick: cortical thickness, VSFx: visuospatial function, Cog test: cognitive test, MDD: major depressive disorder, POGA: patients with on-line game addiction, AD: alcohol dependence, ADHD: attention deficit hyperactivity disorder, PG: pathologic gambler, Glc: glucose, VBM: voxel-based morphometry, fMRI: functional magnetic resonance imaging, EEG: eletroencephalogram.
1.2.1. Cingulate gyrus: executive function, salience, and visuospatial attention
The anterior cingulate has frequently been mentioned in studies of substance addiction because of its role in conflict resolution (executive function) and attention maintenance (salience) (Goldstein and Volkow, 2002), as well as in pathologic gambling (Camchong et al., 2007). The executive function of the anterior cingulate appears to be involved in dealing with conflict, response inhibition, performance monitoring, completion of behavior, arousal/drive state, and error monitoring (Paus, 2001; Bush et al., 2002). Impairment of these functions could potentially damage an individual's ability to check inappropriate behavior (Goldstein et al., 2007). Within a pathway from the thalamus to the hippocampus, the cingulate gyrus is known to be responsible for focusing attention (attention maintenance) on emotionally significant events and regulating addictive behaviors (Mayberg, 1997). Previous studies of Internet addiction have reported decreased volume within the cingulate gyrus and hyperactivity of the anterior cingulate in response to on-line game stimulation (Ko et al., 2009; Zhou et al., 2011).
Cortical maturation in the anterior cingulate, lateral prefrontal, and right inferior frontal gyrus is thought to regulate visuospatial attention in fronto-striatal-thalamo attention circuits (Westlye et al., 2010). Interestingly, visuospatial attention is reported to be closely linked with on-line game play (Green and Bavelier, 2003; Dye et al., 2009). Ten days of video game playing has been reported to enhance visuospacial capacity in healthy subjects (Green and Bavelier, 2003). Expert video game players demonstrated superior cognitive control abilities in a performance switching task, compared to non-video game players (Karle et al., 2010).
Deficits of anterior cingulate function are well known to be associated with major depressive disorder, ADHD, and impulse control disorders (Mayberg et al., 2000; Ha et al., 2006). Several studies of Internet addiction have noted the significant comorbidity of major depressive disorder, attention deficit hyperactivity disorder, and impulse control disorder (Ha et al., 2006; Dell'Osso et al., 2008).
1.2.2. Thalamus: reinforcement and alerting
In substance addiction, the thalamus is thought to be associated with conditioned responses (expectation) (Volkow et al., 2003). Interactions between drug effects and conditioned responses (expectation) are likely to produce the reinforcing effects of drug abuse (Robinson and Berridge, 1993). The thalamus also demonstrates increased activity in response to higher dopamine availability in the striatum (caudate and putamen) due to monetary reward (Asensio et al., 2010). Interestingly, on-line video game playing has been reported to be associated with dopamine release in striatum and activation of thalamus (Koepp et al., 1998; Han et al., 2008).
Tomasi et al. (2007) have suggested a deficit of three components of attention, including alertness, orienting, and executive function, in cocaine abusers. The thalamus, an essential brain area for visual, auditory, and somatosensory information processing, is an important structure in the alerting component of attention (Fan et al., 2005), including spatial attention (Christian et al., 2006). The lateral geniculate body of the thalamus, a primary processor of visuospatial information in the central nervous system, is located within the major pathway from the retina to the visual cortex (Horvath, 1998).
In an analysis of conventional volumes and surface morphology of the thalamus, Ivanov et al. (2010) suggested that hyperactivity scores would be associated with smaller regional volumes on the lateral thalamic surface and inattention scores would be associated with larger regional volumes on the medial thalamic surface in patients with ADHD.
1.2.3. Occipital cortex and inferior temporal cortex: damage of visual cortex
The temporal-occipital junction has also been reported to be associated with substance and behavioral addiction (Park et al., 2010; Wang et al., 2010; Hermann et al., 2007; Fehr et al., 2008). In a comparison of patients with alcohol dependence and healthy comparison subjects, patients with alcohol dependence showed a lower blood oxygen level dependent (BOLD) signal bilaterally in the occipital area (Hermann et al., 2007). Acute administration of nicotine decreased brain glucose oxidation, glutamate-glutamine neurotransmitter cycling, and GABA synthesis in the occipital cortex in smokers (Wang et al., 2010). In an 18F-fluorodeoxyglucose positron emission tomography study, patients with internet addiction showed decreased metabolism bilaterally in the occipital cortex compared to healthy volunteers (Park et al., 2010).
Human visual cortex could be sensitive to and possibly damaged by large amounts of visual stimuli during on-line game playing (Lyskov et al., 1998; Herrmann, 2001; Pereverzeva and Murray, 2008). A number of functional imaging studies have evaluated the posterior inferior temporal cortex, posterior fusiform gyrus, and posterior superior temporal cortex, which are involved in the processing of human motion (Grossman and Blake, 2002; Peuskens et al., 2005).
1.3. Hypothesis
In line with the published addiction literature, we thought that brain regions consisting of anterior cingulate, thalamus and occpito-temporal areas would increase the likelihood of becoming a pro-gamer or POGA (Fig. 1). In these circuits, decreased volumes of anterior cingulate and increased volumes of thalamus (reciprocal with hyperfunctional striatum) would reinforce impulsive behavior (addictive behavior) in patients with on-line game addiction (POGA). In contrast to POGA, increased volumes of anterior cingulate (healthy anterior cingulate) in pro-gamers would regulate thalamus by modulating visual and auditory stimulation and reduce the development of addiction.
Fig. 1.
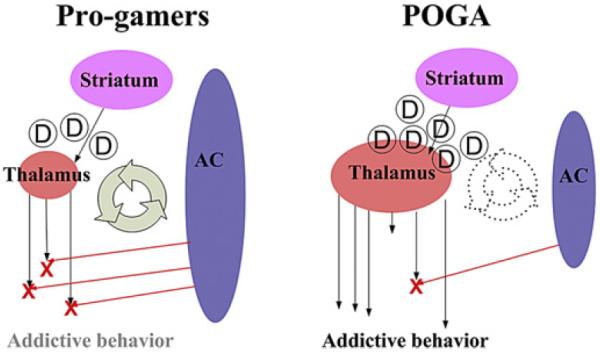
The neural circuit mediating on-line game playing. PVC: primary visual cortex, ITC: inferior temporal cortex, AC: anterior cingulate.
2. Methods
2.1. Subjects
Twenty patients with on-line game addiction, seventeen pro-gamers and eighteen healthy comparison subjects were recruited at Chung Ang University Medical Center. All subjects were screened with the Structured Clinical Interview for DSM-IV and the Beck Depression Inventory (BDI) (Beck et al., 1961). The research protocol was approved by the Chung Ang University Hospital Institutional Review Board. Written informed consent was provided by all participants. There were no significant differences in age (F(2,52) = 0.04, p = 0.96), education years (F(2,52) = 0.05, p = 0.95), economic state (χ2 = 2.24, p = 0.69), BDI scores (F(2, 52) = 1.19, p = 0.31) or alcohol drinking (χ2 = 0.13, p = 0.94)/smoking habit (χ2 = 0.83, p = 0.66) between patients with on-line game addiction, pro-gamers, and healthy comparisons. However, there were significant differences in terms of on-line game playing time (F(2, 52) = 65.4, p < 0.01), total internet use time (F(2, 52) = 108.9, p < 0.01), and Young's internet addiction scale (YIAS) (F(2, 52) = 64.4, p < 0.01) between the three groups (Table 2).
Table 2.
Demographic characteristics.
| 1. POGA (20) | 2. Pro-gamer (17) | 3. Healthy (18) | F/χ2, p | Duncan | |
|---|---|---|---|---|---|
| Age (years) | 20.9 ± 2.0 | 20.8 ± 1.5 | 20.9 ± 2.1 | 0.04, 0.96 | 1 = 2 = 3 |
| Education (years) | 12.0 ± 1.7 | 12.1 ± 1.1 | 12.1 ± 1.1 | 0.05, 0.95 | 1 = 2 = 3 |
| Career/duration of illness (years) | 4.9 ± 0.9 | 4.9 ± 1.7 | – | – | |
| Handedness (right/left) | 19/1 | 16/1 | 17/1 | 0.01, 0.99 | 1 = 2 = 3 |
| Smoking (smoker/non-smoker) | 7/13 | 4/13 | 4/14 | 0.83, 0.66 | 1 = 2 = 3 |
| Drinking (drinking/non-drinking) | 9/11 | 8/8 | 9/9 | 0.13, 0.94 | 1 = 2 = 3 |
| Economic state High | 2 | 1 | 1 | 2.24, 0.69 | 1 = 2 = 3 |
| Middle | 14 | 10 | 14 | ||
| low | 4 | 6 | 3 | ||
| Internet use (hours/day) | 13.1 ± 2.9 | 11.6 ± 2.1 | 2.8 ± 1.1 | 108.9, <0.01 | 1 = 2 > 3 |
| Online game (hours/day) | 9.0 ± 3.7 | 9.4 ± 1.6 | 1.0 ± 0.7 | 65.4, <0.01 | 1 = 2 > 3 |
| YIAS score | 81.2 ± 9.8 | 40.8 ± 15.4 | 41.6 ± 10.6 | 64.4, <0.01 | 1 > 2 = 3 |
| BDI score | 9.4 ± 5.2 | 7.2 ± 4.1 | 7.5 ± 6.6 | 1.19, 0.31 | 1 = 2 = 3 |
| BIS | |||||
| Total | 61.5 ± 6.4 | 51.8 ± 8.4 | 50.1 ± 6.9 | 13.6, <0.01 | 1 > 2 = 3 |
| Cognitive | 15.5 ± 1.8 | 13.4 ± 2.2 | 13.1 ± 1.8 | 8.7, <0.01 | 1 > 2 = 3 |
| Motor | 21.4 ± 2.3 | 18.1 ± 3.1 | 17.4 ± 2.3 | 12.6, <0.01 | 1 > 2 = 3 |
| Non-planning | 24.4 ± 2.7 | 20.3 ± 3.1 | 19.6 ± 2.9 | 15.1, <0.01 | 1 > 2 = 3 |
| WCST | |||||
| Total trials to complete 6 categories | 101.9 ± 17.9 | 76.5 ± 12.1 | 97.8 ± 17.6 | 12.5, <0.01 | 2 < 1 = 3 |
| Total error | 29.5 ± 18.7 | 11.3 ± 6.9 | 24.8 ± 12.5 | 8.2, <0.01 | 2 < 1 = 3 |
| Perseverative error | 16.3 ± 9.7 | 7.1 ± 2.9 | 11.3 ± 3.6 | 8.7, <0.01 | 1 > 2 = 3 |
POGA: patients with on-line game addiction, YIAS: Young Internet Addiction Scale, BDI: Beck Depression Inventory, Career: career of pro-gamer, Economic state (NTS, 2009) high: family income >$30,000/year; middle $12,000–$30,000: low < $12,000, BIS: Barratt Impulsiveness Scale, WCST: Wisconsin Card Sorting Test.
2.2. Patients with on-line game addiction (POGA) group
The criteria for on-line game addiction in the current study are similar to those used in a previous study (Han et al., 2010). These criteria are also consistent with DSM-IV criteria for substance abuse: 1) extensive game play time (more than 4 h per day/30 h per week) (Ko et al., 2009); 2) a score of more than 50 on the YIAS (Young, 1996; Ha et al., 2010); and 3) impaired behaviors or distress due to a maladaptive pattern of internet video game play. Exclusion criteria were: 1) BDI scores >19; 2) other axis I psychiatric disorders including substance abuse; and 3) head injury or trauma history. The mean age of the POGA group was 20.9 ± 2.0 years old and mean education level was 12.0 ± 1.7 years. Duration of illness (problematic on-line game play) was 4.9 ± 0.9 years. These subjects played on-line games 9.0 ± 3.7 h/day and used the Internet 13.1 ± 2.9/day. YIAS scores for the POGA group were 81.2 ± 9.8. During the most recent 12-month period, on-line game play in all subjects had gradually increased. In addition, all patients in the POGA group reported a persistent desire for on-line gaming everyday as well as unsuccessful efforts to cut down or control on-line game play. School grades and work performance had decreased. All POGAs also showed disruption of their daily routine (sleeping during the day and gaming at night, irregular meals, and poor hygiene) and were irritable, aggressive, and violent when family members asked them to stop playing on-line games. Seven POGAs were absent from school for more than 6 months due to on-line game playing in Internet cafés and six POGAs were expelled from school for frequent absences and for stealing money in order to participate in on-line game play. Three POGAs only participated in on-line game playing after finishing school and did not obtain a job or participate in a significant social role. Two POGAs borrowed $30,000 over three months from an usurer for on-line game playing. Two POGAs lost his job due to frequent absences from work without notice.
2.3. Pro-gamer group
Pro-gamers on the 000 StarCraft pro-game team who are the members of the Korea eSports Association (KeSPA) were recruited. Exclusion criteria were: 1–3 same with POGA group; and 4) impaired behaviors or distress due to maladaptive pattern of on-line game play. The age of the pro-gamers was 20.8 ± 1.5 years and education level was 12.0 ± 1.1 years. The average career length of the pro-gamers was 4.9 ± 1.7 years. Thirteen of the pro-gamers graduated from high school or university. Two pro-gamers were student gamers, which is similar to being a student athlete. Two pro-gamers quit high school to become a pro-gamer with the consent of teachers and parents. Pro-gamers played StarSraft an average of 9.4 ± 1.6 h/day and used the Internet an average of 11.6 ± 2.1 h/day. The mean YIAS score of pro-gamers was 40.8 ± 15.4.
2.4. Healthy comparison group
Age and education matched male subjects who used the Internet and played on-line games less than 3 h/day and less than 3 day/week were recruited as healthy comparison subjects (HC). The age of HCs was 20.9 ± 2.1 years and education level was 12.1 ± 1.1 years. Three HCs were high school students, eight were university students, and seven were office workers. HC subjects played on-line games an average of 1.0 ± 0.7 h/day and used the Internet an average of 2.8 ± 1.1 h/day. The mean YIAS score of healthy comparison subjects was 41.6 ± 10.6.
2.5. Impulsiveness scale and executive function assessment
The Barratt Impulsiveness Scale-Korean version (BIS-K) was used for the assessment of impulsiveness in patients and subjects (Barratt, 1965; Patton et al., 1995; Lee, 1992). This scale is a 23-item self-report questionnaire consisting of three subscales: cognitive, motor, and non-planning. Internal consistency of this scale has been reported to have a Cronbach alpha coefficient = 0.82. For assessing executive function involving strategic planning, organized searching, and the ability to use environmental feedback (Marazziti et al., 2008), a computerized version of the Wisconsin Card Sorting Test (CNT4.0, Maxmedica Inc) was used (Kim et al., 2006).
2.6. MR imaging processing and data analysis
All MR imaging was performed on a 1.5 Tesla Espree MRI scanner (SIEMENS, Erlangen, Germany). 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) data were collected with the following parameters: TR = 1500 ms; TE = 3.00 ms; Inversion time = 1100 ms; FOV = 256 × 256 mm; Flip angle = 15°; 128 slices; 1.0 × 1.0 × 1.33 mm voxel size.
Image processing was conducted using the voxel-based morphometry toolbox (VBM5.1) (http://dbm.neuro.uni-jena.de/vbm/) implemented in SPM5 running on Matlab 7.5. VBM in SPM5 consists of tissue segmentation, bias correction, and spatial normalization in a unified model (Ashburner and Friston, 2005). The segmented images were smoothed with a 12 mm full-width half-maximum Gaussian kernel for the subsequent statistical analysis.
Voxel-wise comparisons of gray matter volume were performed between the groups using the two-sample t-test with SPM5. The significance of group differences was estimated by the theory of random Gaussian fields, and significance levels were set at uncorrected P ≤ 0.001, while the cluster size was set at >100 voxels.
3. Results
3.1. Impulsivity and executive function
Eighteen of 20 POGA, fifteen of 17 pro-gamers, and seventeen of 18 HC completed the BIS-K and the Wisconsin Card Sorting Test. The POGA group showed higher mean scores on the BIS-K-total (F(2, 48) = 13.6, p < 0.01), cognitive (F(2, 48) = 8.7, p < 0.01), motor (F(2, 48) = 12.6, p < 0.01) and non-planning (F(2, 48) = 15.1, p < 0.01) scales, compared to pro-gamers and HC. There was no significant difference between pro-gamers and HC. In the Wisconsin Card Sorting Test, the pro-gamer group required fewer trials to complete six categories (F(2, 48) = 12.5, p < 0.01) and total errors (F(2, 48) = 8.2 p < 0.01), compared to the POGA and HC. POGA showed increased perseverative errors, compared to pro-gamers and HC (F(2, 48) = 8.7, p < 0.01).
YIAS scores of the POGA subjects were positively correlated with scores on the BIS-K-total (r = 0.56, p = 0.02), motor (r = 0.58, p = 0.01), and non-planning (r = 0.54, p = 0.02) scales. There was no significant correlation between the scores of Wisconsin Card Sorting Test measures and YIAS scores.
3.2. The regions in the comparison of gray matter volume between patients with on-line game addiction (POGA), pro-gamer, and healthy comparisons
3.2.1. Patients with on-line game addiction (POGA) vs. healthy comparison (HC)
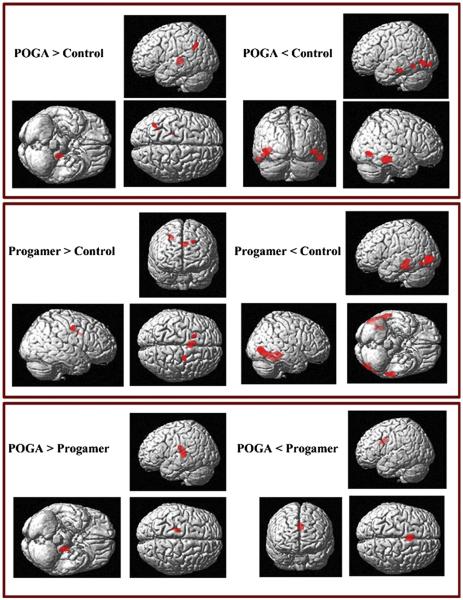
Compared to HCs, the POGA group showed increased gray matter volume in the left thalamus (−20, −31, 3) (κE = 595 (>200), t = 3.45, uncorrected p = 0.001) and decreased gray matter volume in the right inferior temporal gyrus (60, −37, −19, BA 20) (κE = 1534, t = 4.95, uncorrected p < 0.001), right posterior cingulate gyrus (1, −50, 9, BA 29) (κE = 415, t = 4.37, uncorrected p < 0.001), right middle occipital gyrus (49, −72, −4, BA 19) (κE = 463, t = 4.20, uncorrected p < 0.001), left inferior occipital gyrus (−45, −77, −3, BA 19) (κE = 689, t = 4.15, uncorrected p < 0.001), left inferior occipital gyrus (−45, −92, −8, BA 18) (κE = 246, t = 4.06, uncorrected p < 0.001), Right middle occipital gyrus (49, −72, −4, BA19) (κE = 2514, t = 4.20, uncorrected p < 0.001), and left inferior temporal gyrus (−56, −27, −21, BA 20) (kE = 371, t = 3.94, uncorrected p < 0.001) (Fig. 2) (Table 3).
Fig. 2.
Comparison of gray matter volume between patients with on-line game addiction (POGA), pro-gamer, and healthy comparisons. POGA: Patients with on-line game addiction.
Table 3.
Regional comparison of gray matter volume between patients with on-line game addiction (POGA), pro-gamer, and healthy comparison subjects.
| Talairach |
κ E | t | p value | Regions | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Patients with on-line game addiction vs Healthy comparison subjects | ||||||
| Patients with on-line game addiction > Healthy comparison subjects | ||||||
| −20 | −29 | 2 | 1527 | 4.25 | puncorrected < 0.0001 | left thalamus |
| −37 | −60 | 35 | 602 | 4.60 | puncorrected < 0.0001 | left parietal angular gyrus BA39 |
| Patients with on-line game addiction < Healthy comparison subjects | ||||||
| 59 | −38 | −19 | 2421 | 6.45 | PFDR-corr = 0.001 | right inferior temporal gyrus, BA 20 |
| 48 | −71 | −5 | 1146 | 5.75 | PFDR-corr = 0.001 | right middle occipital gyrus, BA 19 |
| −44 | −76 | −4 | 2067 | 4.15 | PFDR-corr = 0.002 | left inferior occipital gyrus, BA 19 |
| −47 | −55 | −10 | 703 | 4.06 | PFDR-corr = 0.002 | left temporal fusiform gyrus, BA 37 |
| −57 | −27 | −21 | 1056 | 3.94 | PFDR-corr = 0.002 | left inferior temporal gyrus, BA20 |
| Pro-gamers vs healthy comparison subjects | ||||||
| Pro-gamers > healthy comparison subjects | ||||||
| −18 | 11 | 35 | 461 | 6.83 | PFDR-corr = 0.02 | left cingulate gyrus, BA 32 |
| −3 | 5 | 31 | 1247 | 4.57 | puncorrected < 0.0001 | left cingulate gyrus, BA 24 |
| 23 | −8 | 43 | 743 | 4.21 | puncorrected < 0.0001 | right middle frontal gyrus, BA 6 |
| Pro-gamers < healthy comparison subjects | ||||||
| −46 | −82 | −1 | 4123 | 7.90 | PFDR-corr < 0.001 | left middle occipital gyrus, BA 19 |
| −62 | −34 | −10 | 2486 | 4.94 | PFDR-corr = 0.003 | left middle temporal gyrus, BA21 |
| 50 | −74 | −1 | 3580 | 7.35 | PFDR-corr < 0.001 | right inferior temporal gyrus, BA 19 |
| Pro-gamer vs Patients with on-line game addiction | ||||||
| Pro-gamer > Patients with on-line game addiction | ||||||
| −3 | 6 | 31 | 3310 | 5.09 | PFDR-corr = 0.02 | left cingulate gyrus, BA 24 |
| Pro-gamer < Patients with on-line game addiction | ||||||
| −18 | −26 | 11 | 2550 | 4.67 | puncorrected < 0.0001 | left thalamus |
PFDR-corr: corrected FDR<0.05, puncorrected: uncorrected.
3.2.2. Pro-gamer vs. healthy comparison (HC)
Relative to healthy comparison subjects, the pro-gamer group showed increased gray matter volume in left cingulate gyrus (−18, 11, 35, BA 32) (kE = 213, t = 5.97, uncorrected p < 0.001) and decreased gray matter volume in left middle occipital gyrus (−46, −81, 0, BA 19) (kE = 1280, t = 7.00, corrected p = 0.009, FDR<0.05) and right inferior temporal gyrus (50, −74, −1, BA19) (kE = 262, t = 6.75, corrected p = 0.009, FDR<0.05) (Fig. 2) (Table 3).
3.2.3. Pro-gamer vs. patients with on-line game addiction (POGA)
Compared to the POGA group, the pro-gamer group showed increased gray matter volume in left cingulate gyrus (−2, 5, 30, BA24) (kE = 3310, t = 5.60, uncorrected p < 0.001) and decreased gray matter volume in left thalamus (−19, −26, 11) (kE = 684, t = 3.37, uncorrected p = 0.001) (Fig. 2) (Table 3).
3.3. Correlation between clinical variables and brain regions
The gray matter volume of left cingulate gyrus in POGA and progmaers was negatively correlated with the scores of YIAS (r = −0.61, p < 0.01) and BIS total (r = −0.49, p < 0.01). The gray matter volume of thalamus in POGA and pro-gamers was positively correlated with the score of YIAS (r = 0.39, p = 0.02). However, there was the trace of the correlation between thalamus and the score of BIS in POGA and pro-gamers (r = 0.31, p = 0.07).
4. Discussion
The results of this study suggest that clinically, increased impulsivity and perseverative errors may contribute to on-line game addiction. In addition, increased gray matter volumes of the left cingulate gyrus in pro-gamers and increased gray matter volumes of the left thalamus in POGA may also contribute to the differences that exist between pro-gamers and individuals with POGA. Moreover, the differences in terms of brain volumes between the Pro-gamer and POGA groups were lateralized to the left hemisphere. The left-hemisphere is thought to be associated with verbal working memory (Petrides et al., 1993) while the right hemisphere is linked with spatial working memory. Given these facts, we can speculate that right prefrontal cortex would be relatively preserved due to enhanced spatial working memory in both POGA and pro-gamer groups. Green and Bavelier (Green and Bavelier, 2003) found that 10 days video game playing enhanced visuospatial working memory in healthy adults. However, there are differences in playing styles between POGA and pro-gamers. Pro-gamers may regard playing games in competition as a narrative, which is derived from as an effect of practice to learn different strategies in their training sessions. In sports psychology, elite sports players usually use narrative mental intervention such as imagery and mental rehearsal, targeting, and self-feedback (Whelan et al., 1991). In addition, the use of oral narrative tasks is thought to be associated with creativity in performance (Alberts & Kormos. 2004). However, relative to healthy comparison subjects, both POGA and pro-gamer groups had decreased gray matter volumes in the occipital cortex and inferior temporal cortex.
4.1. Cingulate gyrus
There was a significant difference in gray matter volume in the left anterior cingulate gyrus between POGA and pro-gamers in the current results. In addition, the gray matter volume of the left cingulate gyrus was negatively correlated with measures of impulsivity and perseverative errors. Other studies of Internet addiction that reported different patterns of brain activation and gray matter volume in the prefrontal cortex (PFC), including the anterior cingulate and dorsolateral PFC, between patients with internet addiction and healthy comparison subjects (Ko et al., 2009; Zhou et al., 2011). An important difference between POGA and pro-gamers may lie in the ability of left anterior cingulate to control executive functioning and attention maintenance (salience) (MacDonald et al., 2000). Disrupted function of conflict resolution (executive function) in anterior cingulate of POGA could impair an individual's ability to monitor and inhibit inappropriate behavior (Goldstein et al., 2007). Alternatively, the larger left cingulate in the pro-gamers may be a predisposing factor for successful on-line game play (Karle et al., 2010; Westlye et al., 2010). In contrast to POGA, increased left anterior cingulate activity in pro-gamers may defuse reinforced behaviors induced by an overactive thalamus in response to dopamine discharge in the striatum. In addition, methylphenidate treatment, which acts on the cingulate (Udo de Haes et al., 2007), has been shown to improve attention symptoms and reduce on-line game playing time and the severity of internet addiction (Han et al., 2009).
In the comparison of Young's Internet Addiction Scale scores, POGA showed higher scores than pro-gamers and healthy comparison subjects. This may be correlated with incentive salience (craving) for internet use or on-line game playing. A study of alcohol cue-induced activation of the brain showed that brain areas such as the anterior cingulate, medial prefrontal cortex and striatum might be associated with incentive salience to alcohol-related stimuli (Grusser et al., 2004). Franken (2003) further emphasized the role of salience in addiction, which may lead to increases in craving and episodes of drug use, resulting in repeated drug use and drug seeking. The regulated lifestyle of the pro-gamer, with restricted behavioral disturbances, the endurance of a tight training schedule, and the pressure to win might be related to increased anterior cingulate activity (even better than healthy controls), compared to POGA.
4.2. Thalamus
In the current study, POGA also had differences in terms of regional volumes (increased GMV of thalamus and decreased GMV of middle frontal gyrus compared with healthy controls). In addition, the gray matter volume of the thalamus in POGA and pro-gamers was positively correlated with YIAS scores. The thalamus is a key target for dopamine and plays a major role in conditioned reinforcement and reward expectation (Sanchez-Gonzalez et al., 2005). In addition, striatal dopamine receptor availability has been associated with thalamic activity (Asensio et al., 2010). We presumed that the thalamus in POGAs would be tolerant to tremendous visual and auditory stimulation, which are increased during on-line game play. Furthermore, higher dopamine availability in the striatum during on-line game play makes the thalamus more active, which reinforces the effects of on-line game play in POGA. An imbalance of mesolimbic circuits (orbitofrontal gyri, anterior cingulate, and ventral striatum, amygdala and hippocampus) is thought to be associated with a distorted reward system (Luman et al., 2008). Taken together, we hypothesize that an unbalanced reward system (increased thalamus and decreased cingulate function) would influence someone who played extended periods of time to become either pro-gamers or POGA.
4.3. Occipital cortex and inferior temporal cortex
The gray matter volumes of occipital and inferior temporal cortex were reduced both in POGA and pro-gamers, compared to healthy volunteers. In addition, this area can be damaged by harmful visual stimuli (Lyskov et al., 1998; Herrmann, 2001; Pereverzeva and Murray, 2008; Harding and Fylan, 1999). Early adolescence has consistently been reported to be a critical period for the maturation of visual cortex (Prusky and Douglas, 2003; Crews et al., 2004). Early adolescence is also the critical period for synaptic pruning and myelination of the visual cortex in rodents (Gordon and Stryker, 1996). Pereverzeva and Murray (2008) suggested that neural activity in the human primary visual cortex might be correlated with the retinal information or perception. The human visual system is very sensitive to subtle details in movements, even in response to weakened stimuli such as point light walkers (Blake and Shiffrar, 2007). In response to match/mismatch conditions, greater brain activity in the right inferior occipital-temporal cortex was observed in non-problem gamblers compared with problem gamblers (Goodie, 2005). An increased prevalence of internet addiction or excessive on-line game play has mainly been reported in early adolescents (Yang et al., 2005; Ha et al., 2006). The decreased volume of the occipital cortex (primary visual cortex) may be associated with the excessive exposure to visual stimulation (computer monitor) during on-line game play in both POGA and pro-gamers.
5. Limitation
There are several limitations to the current study. First, the number of subjects may not be enough to fully document the differences between groups. Replication of the present results will be important in order to demonstrate their generalizability. Second, we did not assess clinical evaluation including impulse controlling, conflict resolution, executive function which may represent the characters of POGA, pro-gamers, and healthy controls. Future studies should be interested in these differences between them. Finally, because the current study was a cross sectional study, it is difficult to say whether the results represent the cause or result of on-line game play. A larger sample would be important in future studies.
6. Conclusion
The current study reports morphometric abnormalities in the gray matter volumes of the left anterior cingulate, both inferior temporal cortices, and both occipital cortices in patients with online game addiction and pro-gamers. These findings provide new insights into the pathogenesis of on-line game addiction.
Acknowledgment
This work was supported by Korean Game Culture Foundation and NIDA grant (K24DA015116-09). We thank professional game team, MBC game heroes for providing data and analysis.
Role of funding sources Funding for this study was provided by Korean Game Culture Foundation and NIDA grant (K24DA015116-09); the Korean Game Culture Foundation and NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors Authors Doug Hyun Han and Perry F. Renshaw designed the study and wrote the protocol. Authors Perry Renshaw and In Kyoon Lyoo have participated in the analysis and interpretation of the work. All authors contributed to and have approved the final manuscript.
Conflict of interest All other authors declare that they have no conflicts of interest.
References
- Alberts A, Kormos J. Creativity and narrative task performance: an exploratory study. Journal of Research in Language Studies. 2004;54:277–310. [Google Scholar]
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychology Report. 1965;16:547–54. doi: 10.2466/pr0.1965.16.2.547. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blake R, Shiffrar M. Perception of human motion. Annual Review of Psychology. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Goodie AS, McDowell JE, Gilmore CS, Clementz BA. A cognitive neuroscience approach to studying the role of overconfidence in problem gambling. Journal of Gambling Studies. 2007;23:185–99. doi: 10.1007/s10899-006-9033-5. [DOI] [PubMed] [Google Scholar]
- Castriota-Scanderbeg A, Hagberg GE, Cerasa A, Committeri G, Galati G, Patria F, et al. The appreciation of wine by sommeliers: a functional magnetic resonance study of sensory integration. Neuroimage. 2005;25:570–5. doi: 10.1016/j.neuroimage.2004.11.045. [DOI] [PubMed] [Google Scholar]
- Christian BT, Lehrer DS, Shi B, Narayanan TK, Strohmeyer PS, Buchsbaum MS, et al. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31:139–52. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Chua HF, Liberzon I, Welsh RC, Strecher VJ. Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biological Psychiatry. 2009;65:165–8. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: when, where and why? Alcoholism: Clinical and Experimental Research. 2004;28:350–64. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Dell'Osso B, Hadley S, Allen A, Baker B, Chaplin WF, Hollander E. Escitalopram in the treatment of impulsive-compulsive internet usage disorder: an open-label trial followed by a double-blind discontinuation phase. Journal of Clinical Psychiatry. 2008;69:452–6. doi: 10.4088/jcp.v69n0316. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Evers EA, Hurks PP, Bakker K, Jolles J. Differential brain activation patterns in adult attention-deficit hyperactivity disorder (ADHD) associated with task switching. Neuropsychology. 2010;24:413–23. doi: 10.1037/a0018997. [DOI] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, et al. Cortical thickness, surface area, and volume of the brain reward system in alcohol depdendence: relationships to release and extended abstinence. Alcoholism: Clinical and Experimental Research. 2011;35:1187–200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye MW, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–9. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–9. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. American Journal of Psychiatry. 2008;165:507–14. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick JJ. Internet addiction: recognition and interventions. Archives of Psychiatric Nursing. 2008;22:59–60. doi: 10.1016/j.apnu.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:563–79. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58:345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–9. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodie AS. The role of perceived control and overconfidence in pathological gambling. Journal of Gambling Studies. 2005;21:481–502. doi: 10.1007/s10899-005-5559-1. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. Journal of Neuroscience. 1996;16:3274–86. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. American Journal of Drug and Alcohol Abuse. 2010;36:233–41. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Bavelier D. Action video game modifies visual selective attention. Nature. 2003;423:534–7. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–75. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berlin) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for internet addiction. Journal of Clinical Psychiatry. 2006;67:821–6. doi: 10.4088/jcp.v67n0517. [DOI] [PubMed] [Google Scholar]
- Han DH, Daniels MA, Bolo N, Arenella LS, Lyoo IK, Renshaw PF. The correlation between cue-induced craving for internet video game play and brain activation CPDD 70th ANNUAL SCIENTIFIC MEETING; San Juan, Puerto Rico: NIDA (National Institute on Drug Abuse); 2008. [Google Scholar]
- Han DH, Lee YS, Na C, Ahn JY, Chung US, Daniels MA, et al. The effect of methylphenidate on internet video game play in children with attention-deficit/hyperactivity disorder. Comprehensive Psychiatry. 2009;50:251–6. doi: 10.1016/j.comppsych.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Han DH, Hwang JW, Renshaw PF. Bupropion sustained release treatment decreases craving for video games and cue-induced brain activity in patients with internet video game addiction. Experimental and clinical psychopharmacology (Berlin) 2010;18:297–304. doi: 10.1037/a0020023. [DOI] [PubMed] [Google Scholar]
- Harding GF, Fylan F. Two visual mechanisms of photosensitivity. Epilepsia. 1999;40:1446–51. doi: 10.1111/j.1528-1157.1999.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Harding GFA, Jeavons PM. Photosensitive epilepsy. MacKeith Press; London: 1994. [Google Scholar]
- Hermann D, Smolka MN, Klein S, Heinz A, Mann K, Braus DF. Reduced fMRI activation of an occipital area in recently detoxified alcohol-dependent patients in a visual and acoustic stimulation paradigm. Addiction Biology. 2007;12:117–21. doi: 10.1111/j.1369-1600.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- Herrmann CS. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Experimental Brain Research. 2001;137:346–53. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- Horvath TL. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. Journal of Neuroscience. 1998;18:1546–58. doi: 10.1523/JNEUROSCI.18-04-01546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Misiurek J, Jurkowski AJ, McCarthy G. Dynamic and strategic aspects of executive processing. Brain Research. 2004;1000:78–84. doi: 10.1016/j.brainres.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2010;167:397–408. doi: 10.1176/appi.ajp.2009.09030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle JW, Watter S, Shedden JM. Task switching in video game players: Benefits of selective attention but not resistance to proactive interference. Acta Psychologica (Amsterdam) 2010;134:70–8. doi: 10.1016/j.actpsy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Korea eSport Association (KeSPA) Team and players. 2010 https://www.e-sports.or.kr.
- Kim SW, Shin IS, Kim JM, Yang SJ, Shin HY, Yoon JS. Association between attitude toward medication and neurocognitive function in schizophrenia. Clinical Neuropharmacology. 2006;29:197–205. doi: 10.1097/01.WNF.0000228173.08885.65. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. Journal of Psychiatric Research. 2009;43:739–47. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–8. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disorder. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Lee HS. Impulsivess scale. Korea Guidance; Seoul: 1992. [Google Scholar]
- Luman M, Oosterlaan J, Knol DL, Sergeant JA. Decision-making in ADHD: sensitive to frequency but blind to the magnitude of penalty? Journal of Child Psychology and Psychiatry. 2008;49:712–22. doi: 10.1111/j.1469-7610.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Lyskov E, Ponomarev V, Sandstrom M, Mild KH, Medvedev S. Steady-state visual evoked potentials to computer monitor flicker. International Journal of Psychophysiology. 1998;28:285–90. doi: 10.1016/s0167-8760(97)00074-3. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Catena Dell'osso M, Conversano C, Consoli G, Vivarelli L, Mungai F. Executive function abnormalities in pathological gamblers. Clinical Practice and Epidemiology in Mental Health. 2008;27:4–7. doi: 10.1186/1745-0179-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Miedl SF, Fehr T, Meyer G, Herrmann M. Neurobiological correlates of problem gambling in a quasi-realistic blackjack scenario as revealed by fMRI. Psychiatry Research. 2010;30:165–73. doi: 10.1016/j.pscychresns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- National Tex Service (NTS) National income. 2009 http://www.nts.go.kr.
- Park HS, Kim SH, Bang SA, Yoon EJ, Cho SS, Kim SE. Altered regional cerebral glucose metabolism in internet game overusers: a 18F-fluorodeoxyglucose positron emission tomography study. CNS Spectrum. 2010;15:159–66. doi: 10.1017/s1092852900027437. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. National Review of Neuroscience. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pereverzeva M, Murray SO. Neural activity in human V1 correlates with dynamic lightness induction. Journal of Vision. 2008;8:1–10. doi: 10.1167/8.15.8. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:878–82. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. European Journal of Neuroscience. 2005;21:2864–75. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:828–36. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. European Journal Neuroscience. 2003;17:167–73. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS. Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behavioral Science and the Law. 1998;16:319–32. doi: 10.1002/(sici)1099-0798(199822)16:3<319::aid-bsl311>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. Journal of Neuroscience. 2005;25:6076–83. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hayashida A, Yamasue H, Harada Y, Kaneko T, Kasai K, et al. Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry and Clinical Neuroscience. 2010;64:394–402. doi: 10.1111/j.1440-1819.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- Sun DL, Ma N, Bao M, Chen XC, Zhang DR. Computer games: a double-edged sword? Cyberpsychology and Behavioral Network. 2008;11:545–8. doi: 10.1089/cpb.2007.0145. [DOI] [PubMed] [Google Scholar]
- Shapira NA, Goldsmith TD, Keck PE, Jr, Khosla UM, McElroy SL. Psychiatric features of individuals with problematic internet use. Journal of Affective Disorder. 2000;57:267–72. doi: 10.1016/s0165-0327(99)00107-x. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Research. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo de Haes JI, Maguire RP, Jager PL, Paans AM, den Boer JA. Methylphenidate-induced activation of the anterior cingulate but not the striatum: a [15O] H2O PET study in healthy volunteers. Human Brain Mapping. 2007;28:625–35. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. Journal of Neuroscience. 2003;23:11461–8. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. Journal of Neuroscience. 2007;27:14035–40. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM, Mason GF. Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. Journal of Neurochemistry. 2010;113:1447–58. doi: 10.1111/j.1471-4159.2010.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cerebral Cortex. 2010;113:1447–58. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Whelan JP, Mahoney MJ, Meyers AW. Performance enhancement in sport: a cognitive behavioral domain. Behavior Therapy. 1991;22:307–27. [Google Scholar]
- Yang CK, Choe BM, Baity M, Lee JH, Cho JS. SCL-90-R and 16PF profiles of senior high school students with excessive internet use. Canadian Journal of Psychiatry. 2005;50:407–14. doi: 10.1177/070674370505000704. [DOI] [PubMed] [Google Scholar]
- Young KS. Psychology of computer use: XL. Addictive use of the Internet: a case that breaks the stereotype. Psychological Reports. 1996;79:899–902. doi: 10.2466/pr0.1996.79.3.899. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lin FC, Du YS, Qin LD, Zhao ZM, Xu JR, et al. Gray matter abnormalities in internet addiction: a voxel-based morphometry study. European Journal of Radiology. 2011;79:92–5. doi: 10.1016/j.ejrad.2009.10.025. [DOI] [PubMed] [Google Scholar]