Abstract
Introduction
Melanoma differentiation-associated gene – 9 (MDA-9)/Syntenin has become an increasingly popular focus for investigation in numerous cancertypes. Originally implicated in melanoma metastasis, it has diverse cellular roles and is consistently identified as a regulator of tumor invasion and angiogenesis. As a potential target for inhibiting some of the most lethal aspects of cancer progression, further insight into the function of MDA-9/Syntenin is mandatory.
Areas covered
Recent literature and seminal articles were reviewed to summarize the latest collective understanding of MDA-9/Syntenin’s role in normal and cancerous settings. Insights into its participation in developmental processes are included, as is the functional significance of the N- and C-terminals and PDZ domains of MDA-9/Syntenin. Current reports highlight the clinical significance of MDA-9/Syntenin expression level in a variety of cancers, often correlating directly with reduced patient survival. Also presented are assessments of roles of MDA-9/Syntenin in cancer progression as well as its functions as an intracellular adapter molecule.
Expert opinion
Multiple studies demonstrate the importance of MDA-9/ Syntenin in tumor invasion and progression. Through the use of novel drug design approaches, this protein may provide a worthwhile therapeutic target. As many conventional therapies do not address, or even enhance, tumor invasion, an anti-invasive approach would be a worthwhile addition in cancer therapy.
Keywords: angiogenesis, breast cancer, c-Src, EGFR, exosomes, glioblastoma, glioma, integrin, invasion, melanoma, melanoma differentiation-associated gene – 9, metastasis, PDZ, small cell lung carcinoma, syndecan binding protein, syntenin, urothelial cell carcinoma, uveal melanoma
1. Introduction
Since its discovery, melanoma differentiation-associated gene – 9 (MDA-9)/ Syntenin has been linked to an expanding list of cellular functions, including cell–cell and cell–matrix adhesion, signal transduction, as well as intracellular and secreted lipid trafficking. The average number of citations per year that reference ‘MDA-9’ or ‘syntenin’ more than doubled in 2012 – 2013 compared to the previous 7 years. Central to its cellular roles is its ability to bind numerous intracellular molecules, including proteins, glycoproteins and lipids. These promiscuous binding interactions positions MDA-9/Syntenin in an abundance of complexes that regulate a range of activity in many cell types. A common theme among these intracellular activities is their direct or indirect relation to cancer invasion. Here, we explore the properties of MDA-9/Syntenin in the setting of tumor cell invasion, a complex process dependent on the integration of a myriad of signaling pathways.
2. MDA-9/Syntenin: background on a diverse scaffolding protein
2.1 Discovery and cloning
MDA-9/Syntenin was cloned through a subtraction hybridization approach that was designed to identify genes differentially regulated between untreated melanoma cells, and their terminally differentiated counterparts [1–4]. Briefly, human melanoma cells were treated with a combination of fibroblast IFN-β and mezerein, a protein kinase C activating antileukemic agent, to induce a terminally differentiated state [1,5–12]. Comparing the gene expression of the resulting temporally spaced, subtracted libraries at various time points led to the discovery of a number of important genes, termed ‘melanoma differentiation-associated’ (mda) genes. MDA-9/Syntenin was unique in this group in that it did not have a sustained induction pattern, rather it displayed biphasic kinetics with a peak at 8 – 12 h post-treatment followed by a return to baseline expression, which suggested a dissociation from a growth suppression role [13]. Follow-up studies indicated that MDA-9/Syntenin was induced in human melanoma cells by treatment with IFN-γ [13]. Additional yeast-two hybrid studies showed that MDA-9/Syntenin is an interacting partner of the syndecan family of heparan sulfate proteoglycans, cell surface molecules involved in cell–cell and cell–matrix adhesion, signal transduction, trafficking of lipoproteins and cell surface receptors, as well as activity as co-receptors [14].
2.2 Structure and regulation
2.2.1 The PDZ domains
MDA-9/Syntenin is a 2.1-kb gene located on 8q12 with an open reading frame of 894-bp, encoding a 298-aa protein of about 33 kDa [13–15]. Cross-species analysis shows that MDA-9/Syntenin highly conserved with homologues in rat, mouse, zebrafish and Xenopus [16–18]. A distinguishing feature of MDA-9/Syntenin is its inclusion in the family of proteins with PDZ domains. These motifs, (so named for discovery in post-synaptic density protein PSD95/SAP90, drosophila tumor suppressor DLGA, and tight junction protein ZO-1) are well-described regions of 80 – 100 residues organized into six β strands and two α helices that form compact, globular domains of 25 – 30-Å. PDZ domains often mediate the assembly of multiprotein complexes by binding the C-terminal of their targets at the plasma membrane as well as intracellular membranes [19–21]. Target peptide sequence divides the PDZ proteins into three groups: I (−S/T-X-Φ), II (Φ-X-Φ) and III (D/E-X-Φ), of which MDA-9/Syntenin has been shown to bind class I, II and other groups with a low-to-moderate affinity [22,23].
During syndecan binding, MDA-9/Syntenin’s PDZ-2 motif serves as a high-affinity domain, whereas PDZ-1, although necessary for binding, acts as a complementary, low-affinity domain [24]. This pattern is also observed in the binding pattern of MDA-9/Syntenin to c-Src [25].
2.2.2 The N- and C-terminals
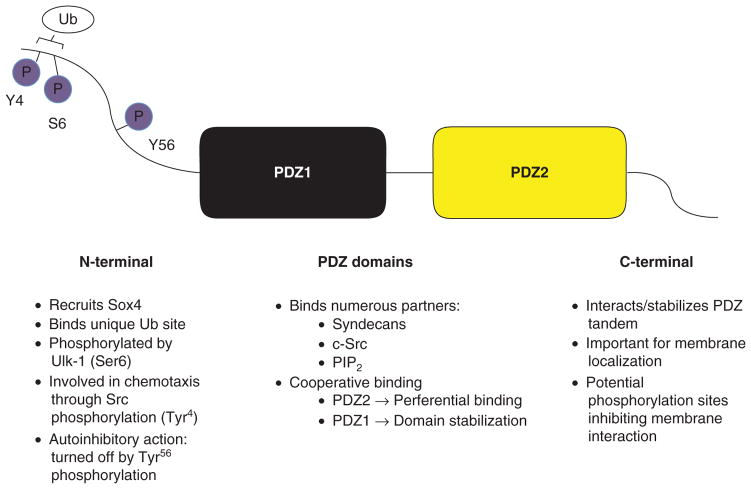
While the majority of activity as a scaffolding protein occurs through the PDZ domains, the amino- and carboxyterminals of MDA-9/Syntenin influence its structure and stability and have recently been implicated in a growing number of unique functions (Figure 1). The N-terminal of MDA-9/Syntenin has been linked notably to recruiting transcription factor SOX4 as well as eukaryotic translation initiation factor 4A to signaling complexes [19,26,27]. A focus on possible phosphorylation sites in the N-terminal has resulted in the discovery of a number of interactions and layers of regulation. Phosphorylation at tyrosine sites was shown to prevent interaction with receptor type protein tyrosine phosphates (rPTPnu) CD148 [28], and recent work pointed to an interesting interaction with ubiquitin (Ub), regulated by the N-terminal [29].
Figure 1. MDA-9/Syntenin domain structure.
Recent studies have shed light on the various activities and functions of the domains comprising MDA-9/Syntenin. Domain structure is depicted and select activities listed beneath each.
MDA-9: Melanoma differentiation-associated gene – 9.
The N-terminal of MDA-9/Syntenin binds Ub and may have an important role in the Ub-dependent sorting of transmembrane cargo [29]. MDA-9/Syntenin was also shown to interact with Ub with an affinity (KD) of 27.3 μM, relatively tight compared to most Ub interactions. A conserved LYPSL sequence in the N terminus of MDA-9/Syntenin binds a unique site on the C terminus of Ub, interacting equally well with Lys48- or Lys63-linked poly-Ub chains. These studies implicate MDA-9/Syntenin in binding a set of ubiquitylated proteins that it links to its transmembrane partners, thus forming ‘Ub-based molecular hubs’. This is particularly important because several transmembrane interacting partners of MDA-9/Syntenin are regulated through Ub-dependent endocytic trafficking including syndecan-4 [30], GlyT2 [31] and IL-5R [32].
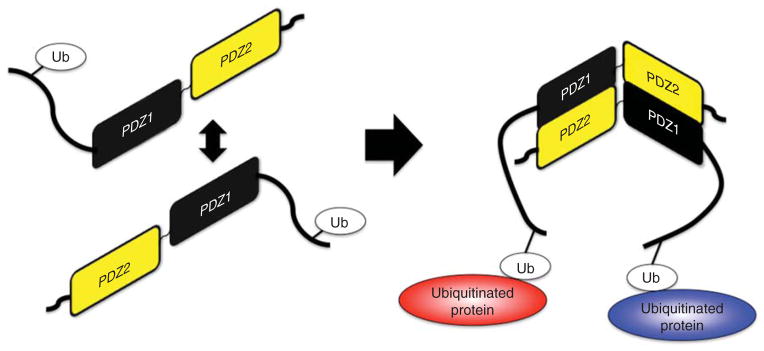
This interaction with Ub requires the C terminus of MDA-9/Syntenin to be intact and is regulated through MDA-9/Syntenin dimerization, as dimerization-defective mutants of MDA-9/Syntenin failed to bind Ub. The head-to-tail dimerization of MDA-9/Syntenin mediated by its PDZ domains allows it to bind two Ub molecules, or two ubiquitinated proteins, in its dimerized state. Further, the principal binding site on Ub for deubiquitinating enzymes overlaps with MDA-9/Syntenin’s binding. Thus, monoubiquitylated partners in complex with MDA-9/Syntenin could be shielded from deubiquitination, thereby leading to prolongation of MDA-9/Syntenin-dependent pathways [29]. This has interesting implications of mediating the interactions of disparate proteins, as well as amplification of cellular pathways regulated by this interaction (Figure 2).
Figure 2. MDA-9/Syntenin-Ubiquitin binding.
The N-terminal of MDA-9/Syntenin has been shown to bind a unique site on ubiquitin (Ub) [29]. As described in this work, the dimerization of MDA-9/Syntenin enhances Ub binding. The possible ramification of this insight is another method by which MDA-9 can facilitate interactions, now between two Ub-bound proteins.
Ulk1, an interacting partner and S/T kinase with roles in autophagy and regulating both clathrin-dependent and clathrin-independent endocytosis, phosphorylates MDA-9/ Syntenin on the N-terminal. When Ulk-1 phosphorylates Ser [6] in the N-terminal LYPSL sequence, this prevents MDA-9/Syntenin interaction with Ub. When MDA-9/Syntenin mutants were expressed that lose the ability to bind Ub, they demonstrated reduced co-localization with CD63, a marker for late endosomes and lysosomes [29].
Further evidence is emerging supporting the importance of the previously unexplored N-terminal domain of MDA-9/Syntenin and its involvement in cell motility programs. Recently, a study showed that MDA-9/Syntenin was a crucial element for immune cell polarization and chemotaxis [33]. MDA-9/Syntenin was vital for forming polarized actin structures as seen in the leading edge and contact zone with antigen-presenting cells. It accomplishes this after phosphorylation by Src at Tyr4, leading to the activation of small GTPase Rac by specific association with myosin phosphatase Rho interacting protein. Integrin and chemokine receptor activation is crucial in T-cell migration and producing functional asymmetry in these cells in forming areas of cell–cell contact referred to as the ‘immunological synapse’ [33].
Further investigation of the N-terminal region suggests that MDA-9/Syntenin exists in equilibrium between a closed and open state, possibly regulated by the phosphorylation of an auto-inhibitory domain in this region [34]. N-terminal deletion mutants of > 57 amino acids lead to an enrichment of this mutant form at the plasma membrane [16,34]. Additionally, a mutant mimicking phosphorylation at Tyr56 (Y56E) was strongly enriched at the plasma membrane, indicating that N-terminal phosphorylation negates autoinhibition and leads to enhanced plasma membrane association [34].
Data show that the C-terminal domain is also functionally important in MDA-9/Syntenin, as NMR results indicate that the C-terminal domain includes structural segments that interact in tandem with PDZ domains [35]. A deletion mutant lacking the entire C-terminal completely lost plasma membrane localization, while positively charged residues were found to be important for promoting membrane targeting. Further, a mutant mimicking phosphorylation in the C-
2.3 Regulation of expression
Genetic regulation of MDA-9 has not been thoroughly elucidated and is likely a complex, multifactorial process, but some clues have been uncovered. Early work showed that MDA-9/ Syntenin was inducible through IFN treatment, and TNF-α treatment can generate expression as early as 10 min post-treatment in umbilical arterial endothelial cells [36]. Nonetheless, a precise picture of the transcriptional regulation of MDA-9/Syntenin has not been established.
MDA-9/Syntenin expression and protein kinase C – α (PKCα) activity were shown to be interdependent following fibronectin (FN)-induced PKCα activation [37]. Inhibition of PKCα suppressed both endogenous and FN-induced MDA-9/Syntenin expression; therefore, a positive feedback loop incorporating PKCα activation may be involved, but the detailed mechanism for this has not been revealed [37]. Recently, Raf kinase inhibitor protein (RKIP) was found to be strongly downregulated in multiple metastatic melanoma cell lines and inversely related to MDA-9/Syntenin expression. Co-immunoprecipitation experiments demonstrated that RKIP could bind MDA-9/Syntenin and when overexpressed, inhibited MDA-9/Syntenin’s interaction with cSrc/ focal adhesion kinase (FAK) complexes leading to decreased invasion, less anchorage independent growth and reduced ability to seed lung tissue in vivo [38].
2.4 Roles in development and neural function
In studies of developmental expression, including those of mouse fetal development, significant expression was noted in the fetal kidney, liver, lung and brain as well as the placenta, adult spleen and heart [2,16,39]. MDA-9/Syntenin was recently shown to be essential for normal development in zebrafish, in which it has two homologues: syntenin-a and syntenin-b [18]. Lack of syntenin-a expression resulted in the death of over 80% of embryos 24 h postfertilization. Zebra-fish embryos that did survive displayed a markedly shorter body axis [18]. This is similar to an earlier finding in Xenopus that found knockdown of syntenin homologues resulted in a shorter body axis [17]. The studies in zebrafish found that syntenin-a was crucial for a central process to gastrulation and epiboly – the spreading and thinning of blastoderm to cover the yolk cell and close the blastosphere in fish embryos. Through interaction with syndecans, notably syndecan-4, phosphatidylinositol 4,5-bisphosphate (PIP2) and small GTPase ADP-ribosylation factor 6 (Arf6), MDA-9/Syntenin regulates epiboly progression through actin cytoskeleton rearrangement [18]. Syntenin-a is also upregulated in the zebrafish spinal cord following injury. Knockdown of syntenin-a results in reduced regrowth of descending axons from brainstem neurons and significant inhibition of locomotor recovery 6 weeks post-injury [40].
Synaptic function is tightly regulated, and dysfunction can be associated with a variety of neurological pathology, including neurodegenerative states such as Alzheimer’s, and psychiatric disorders such as schizophrenia [41]. The matrix of proteins underlying synaptic membranes is rich in scaffolding proteins, including MDA-9/Syntenin. MDA-9/Syntenin has roles in maintaining stable synaptic structures through interaction with adhesion molecules such as synaptic cell adhesion molecule, neurexin and neurofascin [19,24,42–44]. The polarized protein composition at the synaptic plasma membrane can be stabilized by MDA-9/Syntenin’s recruitment of a number of intracellular regulators and formation of multimeric complexes through interaction with ERC2/ CAST1, a cell surface molecule [45]. Additionally, MDA-9/ Syntenin promotes an increase in the numbers and branching patterns of neurites. In PC12 cells, a model for neuron-like cells, Akt inhibition, either through dominant negative (DN) expression or through pharmacological inhibition, led to an increase in MDA-9/Syntenin expression and improvement in neurite outgrowth [46]. Taken together, these findings serve as foundation for a key role of MDA-9/ Syntenin in directional cell movements during early development and regeneration.
Among the receptors that interact with MDA-9/Syntenin at the synaptic cleft are glutamate receptors, involved in the transport of the main excitatory neurotransmitter in the CNS [42,47]. Exogenous expression of MDA-9/Syntenin leads to an increase in the number of dendritic protrusions in both young and mature neurons. This supports the view that MDA-9/Syntenin is involved as a key effector of glutamate-induced membrane protrusions, which establish connections in the developing brain [47]. Additionally, MDA-9/Syntenin can bind with Unc51.1 and Rab5 to initiate axon outgrowth through scaffold formation and endocytic machinery. Unc51.1 is a serine/threonine kinase shown to be important in neurite extension, whereas Rab5 is a member of the Ras-like small GTPases and found in early endosomes [48]. While further studies are needed, including the consequences of MDA-9/Syntenin downregulation, MDA-9/Syntenin’s multifaceted roles in membrane-associated activities and actin cytoskeleton rearrangement are evident in developmental processes such as these.
2.5 Localization
MDA-9/Syntenin is commonly found in areas of cell–cell contact, co-localizing with F-actin, syndecan-1, E-cadherin, β-catenin and α-catenin [16]. In fibroblasts, MDA-9/Syntenin is localized to focal adhesions and stress fibers. MDA-9/Syntenin is also involved in regulating the rearrangement of the actin cytoskeleton as its overexpression leads to the formation of distinct structures such as ruffles, lamellipodia, fine extension and neurite-like structures [16]. The variety of interactions with important adhesion proteins provides the foundation for MDA-9/Syntenin’s involvement in invasion regulation. Activation of cellular programs leading to altered cell–cell and cell–matrix interactions is necessary for cancerous cells to invade and migrate away from the primary tumor. Further, cellular locomotion relies heavily on actin cytoskeleton rearrangement, another area of MDA-9/Syntenin influence.
MDA-9/Syntenin is associated with membranes throughout the cell, anchored to the plasma membrane by interaction with PIP2 and phospholipase Cγ [16]. It also localizes to the early secretory pathway: the endoplasmic reticulum, intermediate compartment, cis-Golgi, as well as apical endosomes, while facilitating the trafficking of cell-surface molecules [20,49–51].
2.6 Syntenin-2
Two isoforms of MDA-9/Syntenin have been identified: syntenin 2α and 2β, cloned from a library of fetal human brain cDNA [19]. Syntenin 2α shares > 70% homology in the PDZ domains as MDA-9/Syntenin, whereas syntenin 2β is a shorter isoform of 2α that lacks 85 residues at the N-terminal [19]. To date, relatively few studies have focused on this isoform, yet like MDA-9/Syntenin, it has also been shown to interact with PIP2 [52]. One study found lower expression of syntenin-2 in colorectal cancer as compared to normal tissue but was not found to be a significant prognostic marker [53]. In other work, syntenin-2 was found in higher amounts in bile from biliary stenosis related to malignant causes, such as pancreatic adenocarcinoma or cholangiocarcinoma, compared to nonmalignant samples such as chronic pancreatitis or biliary stones [54]. Initial studies suggest roles for syntenin-2 in cell division, nuclear PIP2 organization and cell survival [52], although these interesting areas remain relatively unexplored.
3. Involvement in tumor progression and invasion
3.1 Clinical correlations
Tumor cell invasion and metastasis is a complex process requiring the cell’s successful execution of numerous essential steps [55]. MDA-9/Syntenin has repeatedly been found to be expressed at higher levels in more invasive, metastatic cell lines of multiple cancer types compared with their less invasive, less aggressive counterparts (Table 1) [20]. Furthermore, genetic manipulation of cancers forcing elevated expression in cells with lower baseline levels of MDA-9/Syntenin consistently leads to increased migration and invasion. Along with these observations come reports of more polarized distribution of F-actin and increased pseudopodia formation when MDA-9/Syntenin is overexpressed [14,15,47].
Table 1.
MDA-9/Syntenin in cancer: observed clinical correlations.
| Cancer | MDA-9 enhanced invasion? | Tumor grade | Survival correlation | Years | Ref. |
|---|---|---|---|---|---|
| Melanoma | Yes | ↑ with ↑ MDA-9 | Not reported | 2004– 2013 | [15,25,38,67,107] |
| Glioma/GBM | Yes | ↑ with ↑ MDA-9 | ↓ with ↑ MDA-9 | 2012 – 2014 | [50,57] |
| Breast | Yes | ↑ with ↑ MDA-9 | ↓ with ↑ MDA-9 | 2002 – 2013 | [51,58,59] |
| UCC | Yes | ↑ with ↑ MDA-9 | Not reported | 2013 | [49] |
| SCLC | Yes | ↑ MDA-9 in advanced disease | Not reported | 2014 | [62] |
| Uveal melanoma | Yes | ↑ MDA-9 in recurrent cases | ↓ with ↑ MDA-9 | 2012 | [60] |
| Gastric | Yes | Only compared to control tissue | Not reported | 2002 | [58] |
↑: Positively correlates (tumor grade) or elevated (MDA-9 expression); ↓: Negatively correlates (survival); GBM: Glioblastoma; MDA-9: Melanoma differentiation-associated gene – 9; SCLC: Small cell lung cancer; UCC: Urothelial cell carcinoma.
This is clearly outlined in clinical examples of melanoma invasion. As melanoma progresses, the prevailing hypothesis is that it develops from a benign nevi to a radial growth phase primary melanoma, followed by a vertical growth phase primary melanoma as it invades downwards through the dermis, eventually to become a metastatic melanoma. MDA-9/Syntenin expression increases as these phases advance [15,56].
MDA-9/Syntenin has been shown to be a marker of higher aggression and tumor grade in numerous cancers. In melanoma, breast cancer, glioma and urothelial cell carcinoma (UCC), MDA-9/Syntenin correlates with advancing tumor grade [20,49–51,57], is overexpressed in gastric cancer [58] and in breast cancer and glioma, higher MDA-9/Syntenin expression portends shorter survival in patients [50,59]. In addition to being a marker of higher tumor grade in breast cancer, MDA-9/Syntenin was also more highly expressed as estrogen receptor expression is lost [51]. Furthermore, silencing MDA-9/Syntenin led to an accumulation of cells in G1 along with enhance p21 and p27 expression. Separate work in breast cancer showed that MDA-9/Syntenin expression correlated positively with tumor size, lymph node metastasis and tumor recurrence. Furthermore, overall survival and disease-free survival were shorter in patients with high MDA-9/Syntenin tumor expression [59].
In UCC, MDA-9/Syntenin was found to be associated with advanced stages and higher grades of tumors, which can rapidly progress to invade surrounding muscle tissue. Ectopic overexpression in nontumorigenic cells enhanced proliferation and invasion, while MDA-9/Syntenin inhibition led to fewer lung metastases in an in vivo model [49]. MDA-9/ Syntenin also appears to play a role in uveal melanoma, the most common intraocular tumor in adults and a particularly aggressive cancer with survival times of about 5 – 7 months following metastasis. This tumor expresses elevated levels of MDA-9/Syntenin and even higher levels in recurrent cases [60]. Uveal melanoma metastasizes to the liver in nearly 50% of patients, and patients with higher expression of MDA-9/-Syntenin showed significantly shorter disease-free survival. Additionally, knockdown of MDA-9/Syntenin in vitro inhibited HGF-induced invasion in matrigel [60]. Further, MDA-9/ Syntenin was identified in secretomes of uveal melanoma in patients with metastatic tumors [61].
With results similar to other studies, MDA-9/Syntenin was found to be an important regulator of invasion in small cell lung cancer (SCLC). SCLC is another particularly aggressive cancer, with a median survival of 8 months and a 2-year survival of < 5%, and is known to be highly invasive and metastatic. In these patients, high expression of MDA-9/Syntenin correlated with more advanced and extensive disease at diagnosis [62].
3.2 Binding partners/cellular mechanics
Central to MDA-9/Syntenin’s ability to induce invasive behavior in tumor cells is its interaction with key binding partners. Among these are known oncogenic proteins as well as those that have well-demonstrated activity in cell motility and invasion patterns. Studying the mechanisms of these interactions will allow us to understand the full scope of MDA-9/Syntenin’s involvement in tumor invasion (Figure 3).
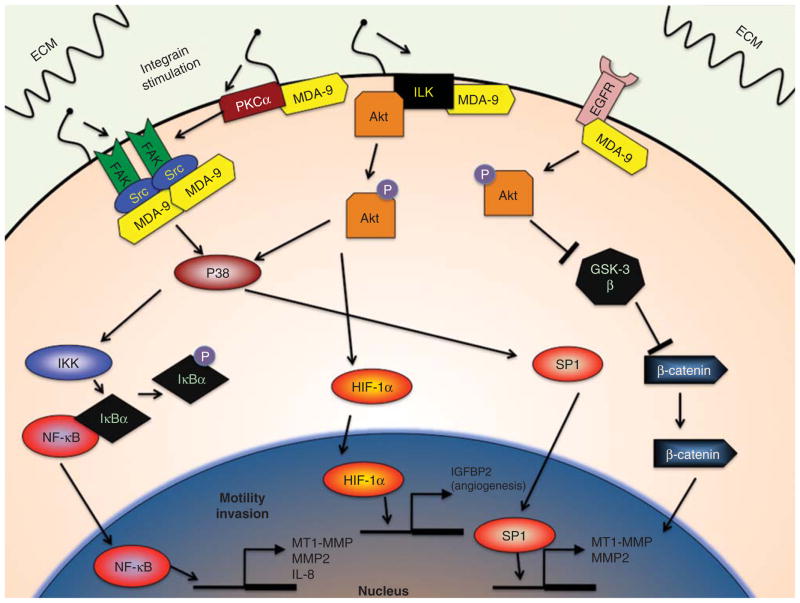
Figure 3. MDA-9/Syntenin invasion signaling.
A summary of important signaling pathways affected by MDA-9/Syntenin that impact cancer progression, particularly motility, invasion and angiogenesis.
ECM: Extracellular matrix; FAK: Focal adhesion kinase; GSK3β: Glycogen synthase kinase 3β; HIF-1α: Hypoxia inducible factor-1α; IGFBP-2: Insulin-like growth factor binding protein – 2; IKK: IκB-kinase; MDA-9: Melanoma differentiation-associated gene – 9; PKCα: Protein kinase C – α.
3.2.1 Src
c-Src is a member of the Src family tyrosine kinases (SFKs), known to have fundamental roles in cell motility, invasiveness and survival and have been implicated in the pathology and progression of numerous cancer types including breast, prostate, glioma and melanoma [63–66]. MDA-9/Syntenin co-localizes with Src in metastatic melanoma, especially in areas corresponding to focal adhesion [67], and interacts with c-Src in glioma [50]. MDA-9/Syntenin-Src complexes have also been found diffusely within the cytoplasm as well as in and near the nucleus [3,4,68], suggesting a heterogeneity of complexes in which they participate and that MDA-9/Syntenin-Src may be involved in promoting transcriptional activities [3,4,20,68]. c-Src is a crucial component of MDA-9/Syntenin-induced invasion. Overexpression of MDA-9/Syntenin can induce invasion in normal or weakly metastatic tumor cell types of diverse tissue origins, yet these invasion gains are lost when c-Src is inhibited either genetically or pharmacologically [50,67].
c-Src interaction is mediated through the PDZ domains of MDA-9/Syntenin, as deletion mutants lacking PDZ1 or PDZ2 dramatically reduce c-Src binding [25]. PDZ-2 was found to be essential for c-Src binding, whereas PDZ-1 was not [19,69]. This is consistent with experimental data that describe PDZ functional units mutually chaperoning each other, enabling the full function of tandem PDZ domains that are necessary to mediate specific interactions with binding partners [3,24,70]. The PDZ1 and PDZ2 domains of MDA-9/Syntenin have been shown to be structurally associated and undergo cooperative denaturation [22]. Thus, PDZ-2 can primarily bind c-Src, whereas PDZ-1 promotes proper folding such that MDA-9/Syntenin successfully assembles into a more stable, multimeric complex [20].
3.2.2 Focal adhesion stimulation
Integrin stimulation leads to the autophosphorylation of FAK at Tyr397, which creates a binding site for SFKs. This leads to the formation of FAK-c-Src dual kinase complexes, further phosphorylating FAK and resulting in the coordination of signaling through multiple pathways that influence the regulation of migration, tumor growth and invasion [65,71]. Increased MDA-9/Syntenin expression correlates with higher levels of FAK-c-Src complexes as well as active, phosphorylated FAK in melanoma cells [15]. Following MDA-9/Syntenin small interfering RNA (siRNA) treatment in highly invasive melanoma cells, p-FAK levels are decreased [3,4,15,25,67]. DN FAK expression significantly reduces the MDA-9/Syntenin-induced migration of weakly metastatic melanoma cells on FN-coated plates [15].
Another protein involved in focal adhesion activation, PKCα, plays important roles in migration and is essential for integrin-mediated signaling, especially through association with integrin β1-associated complexes [71–73]. For example, integrin α5β1 binding to FN activates PKC-α. Inhibiting PKC-α in this scenario suppresses focal adhesion formation and cell migration, critical steps in cancer cell invasion. Both knockdown of MDA-9/Syntenin or DN PKC-α expression abrogates FN-induced FAK phosphorylation in metastatic breast and melanoma cells [37]. Additionally, FN-stimulation also increases FAK association with β1 integrins, c-Src and MDA-9/Syntenin, which can be abrogated by MDA-9/-Syntenin siRNA treatment or PKC-α DN expression [37]. Furthermore, FN stimulation led to increased plasma membrane association of MDA-9/Syntenin and PKCα, whereas knockdown of MDA-9/Syntenin reduced membrane targeting of PKCα [37]. As MDA-9/Syntenin also binds recognized membrane occupants such as syndecan-4 [24] and PIP2 [74] through its PDZ domains, it may be responsible for facilitating binding of PKC-α to PIP2 by forming a complex at the plasma membrane following FN attachment.
3.2.3 Downstream effectors
c-Src/FAK signaling leads to the activation of the NF-κB pathway, which has been repeatedly demonstrated to be involved in invasion-related transcription activity. The NF-κB p50-p65 complex is normally maintained in an inactive state bound to IκBα in the cytoplasm. Upstream activation leads to IκB-kinase (IKK)-mediated phosphorylation of IκBα, targeting it for degradation. Thus, liberated NF-κB translocates to the nucleus, where it binds target DNA sequences in the promoter of an array of genes, enhancing their transcription [75]. The p38MAPK pathway is a known activator of NF-κB, and MDA-9/Syntenin inhibition can reduce the levels of phosphorylated p38MAPK in melanoma and glioma [25,50].
A key step in tumor cell invasion is degradation of the ECM via MMPs, and MDA-9/Syntenin-related signaling has been shown to lead to such expression in multiple cancer indications. MMP-2 is a crucial member of the MMP family often involved in tumor cell invasion [76]. MDA-9/Syntenin signaling leads to enhanced MMP2 transcript and protein expression in melanoma and glioblastoma (GBM) [15,50]. MMP2 activation occurs through the activity of MT1-MMP, a transmembrane-bound MMP that cleaves and activates pro-MMP2 in conjunction with the activity of tissue inhibitor of metalloproteinase-2 [15,20]. Both these processes can be initiated by NF-κB signaling downstream of MDA-9/Syntenin-c-Src activation and p38MAPK signaling in multiple tumor types [15,50]. Nonetheless, there is evidence that MDA-9/Syntenin can be involved in additional tissue-specific signaling.
Although FN engagement and integrin stimulation were found to be important for MDA-9/Syntenin activation of invasion in melanoma and glioma [15,50], Ras, Rho, Rac and PI3K/Akt signaling mediates MDA-9/Syntenin actions in HEK293T cells [20]. In SCLC, MDA-9/Syntenin led to the activation of p38MAPK and Akt and production of MT1-MMP and MMP2. Additionally, the transcription factor SP1, which can promote MT1-MMP and MMP2 production, was activated by MDA-9/Syntenin, adding to the growing number of pathways that MDA-9/Syntenin influences [62].
Collagen binding in breast cancer cells was also found to prompt MDA-9/Syntenin-related signaling events. MDA-9/Syntenin was shown to regulate the action of Akt by facilitating integrin-linked kinase (ILK) adapter function during breast cancer cell adhesion to type-I collagen [77]. Inhibition of MDA-9/Syntenin abrogated the translocation of both ILK and Akt to the plasma membrane, while collagen-I stimulation increased the association of ILK and MDA-9/Syntenin at the plasma membrane. Thus, MDA-9/Syntenin was found to control the membrane targeting of the ILK-Akt complex, thus mediating the activation of Akt during collagen-I adhesion [77]. Further, ILK is also involved in a signaling platform for integrins along with PINCH1 and α-parvin (making up the IPP complex) [78]. Inhibiting MDA-9/Syntenin led to a decrease in plasma membrane translocation of the IPP complex upon collagen-1 binding, leading to decreased assembly of integrin β1-IPP signaling complexes [77]. MDA-9/Syntenin-related effects on transmembrane proteins and Akt activation was also found in UCC cells, where MDA-9/Syntenin inhibition led to decreased EGFR and Akt activation as well as reduced expression of epithelial to mesenchymal transition markers [49].
4. Directing cellular traffic: role as intracellular adapter
Adaptor molecules are often involved in crucial protein–protein interactions that lead to the assembly of multimeric complexes, which can play key roles in propagating extracellular signals to their designated intracellular targets [79]. The PDZ family of proteins is known to control diverse, centrally important physiological processes [80], and MDA-9/Syntenin is no exception. It interacts with an impressive host of binding partners, regulating a plethora of molecular outcomes, which often can be related directly or indirectly to invasion signaling [20].
4.1 Syndecans
Syndecans are a family of abundant type I transmembrane proteins with heparan sulfate side chains on their extracellular domains. The MDA-9/Syntenin–syndecan interaction was the first characterized functional interaction of this protein [14] and was further defined when studies demonstrated MDA-9/ Syntenin altered PIP2 binding led to trapping of syndecans in the perinuclear recycling endosomes. This highlights the importance of MDA-9/Syntenin in syndecan trafficking and recycling back to the plasma membrane [80]. Syndecans can bind and influence the actions of numerous extracellular receptors, influencing their expression through regulation of uptake and trafficking. For example, integrin trafficking was influenced by the phosphorylation of syndecan-4 at Tyr180 by Src [81]. This enhanced MDA-9/Syntenin binding, increased Arf6 suppression, stabilized focal adhesions and promoted recycling of αVβ3 integrin to the plasma membrane at the expense of α5β1. Since the Tyr180 residue and the ability to bind MDA-9/Syntenin are conserved among syndecans, similar processes could be utilized in many cell contexts by any of the numerous syndecan-cell surface receptor interactions [81]. A similar interaction is employed in the recycling of fibroblast growth factor receptor, as it has been shown to accumulate in syndecan-MDA-9/Syntenin-PIP2 endosomes in an FGF-dependent fashion [82].
MDA-9/Syntenin’s interaction with the cytoplasmic domain of syndecan-2 leads to increases in cell migration. When this interaction is abolished, or MDA-9/Syntenin expression is inhibited, cell migration is reduced. Furthermore, MDA-9/Syntenin was shown to mediate Rac activation induced by syndecan-2 [83]. Heparanase can cleave heparan sulfate and is shown to be involved in cancer invasion, angiogenesis and metastasis [84,85]. Its processing and activation is dependent on MDA-9/Syntenin’s interaction with syndecan-1, and knockdown of MDA-9/Syntenin can inhibit heparanase processing by > 50% [86].
4.2 Exosomes
An emerging role for MDA-9/Syntenin function is a role in the regulation of exosome biology. Recent work has shown that syntenin can form a complex with ALIX, which can be recruited to the cytoplasmic tails of syndecans and subsequently support membrane budding [21]. This leads to the formation of early endosomes, which can themselves undergo invaginations that lead to intraluminal vesicles (ILVs) in what are then termed multivesicular bodies (MVBs). These ILVs can be released as exosomes upon fusion of MVBs to the plasma membrane. MDA-9/Syntenin knockdown led to both reduced numbers and average size of exosomes detected, while overexpression of MDA-9/Syntenin could increase the number of exosomes approximately twofold in breast cancer cells. Formation of MDA-9/Syntenin exosomes requires ESCRTs (endosomal-sorting complexes required for transport), as well as syndecan oligomerization and cleavage [21]. Exosome biology has wide applicability and can have implications in numerous processes that utilize extracellular signaling, including inflammation, cancer biology and tumor invasion.
CD63 is a tetraspannin that is abundantly expressed on the plasma membrane and in late endocytic organelles [35] as well as a marker for exosomes [21]. Tetraspannins are found in the plasma membrane and can associate with numerous receptors and cell-surface molecules, including RTKs and integrins, and regulate their maturation, activity and processing. MDA-9/ Syntenin has been shown to bind the cytoplasmic tail of CD63 using biochemical and NMR, indicating that the interaction occurs through its PDZ domains [35]. When MDA-9/Syntenin is overexpressed, the constitutive rapid internalization of CD63 is slowed, and an N-terminal-lacking deletion mutant of MDA-9/Syntenin blocked the internalization of CD63. Additionally, MDA-9/Syntenin can inhibit AP-2 dependent internalization by competing for binding in the CD63-AP-2 interaction [87]. This demonstrates yet another role of MDA-9/Syntenin activity at membrane-associated structures within the cell.
In comparing exosomes of melanoma cells to normal melanocytes, MDA-9/Syntenin was reduced about twofold in exosomes from melanoma cells [88]. This is a similar finding to that shown in analysis of melanoma secretomes, showing that MDA-9/Syntenin was downregulated in the secreted profile of more metastatic B16 melanoma cells compared to those that did not form lung metastases [89]. Taken together, these findings suggest differential roles for intracellular versus secreted MDA-9/Syntenin.
4.3 Notch signaling and regulation of stemness
Analysis of human epidermal stem cells revealed that a more proliferative and adhesive population of stem cells marked by high delta-like 1, a binding partner of MDA-9/Syntenin, had over 13-fold higher MDA-9/Syntenin expression. Consistent with MDA-9/Syntenin’s known actions, this group had a transcriptional profile that associated with active endocytosis, integrin-mediated adhesion and receptor tyrosine kinase signaling [90]. Research conducted in keratinocytes show that MDA-9/Syntenin interacts near cell–cell borders with delta1, a ligand of Notch that leads to Notch cleavage and translocation to the nucleus. In the mammalian epidermis, Notch signaling can have a tumor suppressor function that prompts adjacent cell differentiation [91]. When the C-terminal of Delta1 is mutated in the region of its PDZ-binding motif, or if MDA-9/Syntenin expression is downregulated, Notch-driven transcriptional activation was dramatically increased [82]. Both these approaches decreased plasma membrane expression of delta1, thus indicating that normal MDA-9/Syntenin activity promotes a less differentiated state [82]. This is consistent with the finding that MDA-9/Syntenin is highly expressed in the reservoir of interfollicular stem cells [82]. However, the relationship between MDA-9/Syntenin and Notch signaling has not been fully explored in other settings, including breast cancer, in which this pathway can be a promoter of tumor stem cell activity and invasion [92,93].
4.4 Ephrin family interactions
Ephrin receptors and their respective ligands have been characterized as crucial regulators of neuronal development. Moreover, they have been shown to be involved in cell–cell repulsion both in the developmental environment regulating axons [94] and in cancer cell repulsion, a first step in invasion [95]. Additionally, ephrins are involved in the motility of neural crest cells, fusion of epithelial sheets that close the palate, as well as angiogenesis [96,97].
During synaptic development, Ephrins can recruit a variety of adaptor and signaling complexes that support normal synaptic function, including SFKs, guanine nucleotide exchange factors and PDZ proteins [98]. Ephrin-B is a transmembrane-bound ligand for EphB, and this pair can signal between dendrites and axons in a forward (in the receptor [EphB]-expressing cell) or in reverse (the ligand [ephrin-B]-expressing cell). These signals are important for promoting assembly, maturation and plasticity, as well as axon guidance, pruning and presynaptic development. MDA-9/Syntenin and another PDZ protein, PICK1, were implicated in regulating the number of functional synapses. MDA-9/Syntenin was shown to be expressed in the developing hippocampus and was involved in ephrinB3-related reverse signaling that enabled dendrite pruning, synaptic maturation and formation of neural circuits [99]. Other reports support this role for MDA-9/Syntenin as EphB1 and EphB2 have been shown to bind the PDZ domains of MDA-9/Syntenin to enable synaptic development and inhibition of this partnership prevents presynaptic development [98].
4.5 Sox4 activity
Sox4 is aberrantly expressed in many human tumors, but generally has a short half-life of < 1 h, being degraded by the proteasome in a polyubiquitin-independent fashion. MDA-9/ Syntenin binds to the C-terminal of Sox4 and stabilizes its expression, providing a mechanism for the observation that DNA damage increases Sox4 protein expression independently of Sox4 mRNA levels [100]. P53 binds to the C-terminal of Sox4, and Sox4 is critical for p53 stabilization. It is unknown if Sox4 C-terminal binding proteins, including p53, may act in parallel or compete with MDA-9/Syntenin in regulating stability or functions [100].
Furthermore, IL-5 interaction with its receptor, IL-5R, can result in Sox4 activation, regulating the development and differentiation of B cells [101]. The IL-5Rα subunit interacts with MDA-9/Syntenin and mediates IL-5-induced Sox4 activation [101], yet another demonstration of the wide variety of -cell-specific actions that can be modulated through this important adapter protein.
4.6 Immune cell modulation and viral trafficking
MDA-9/Syntenin has especially high expression in the germinal centers of normal lymph nodes and is robust in follicular dendritic cells (FDCs). An in vitro proxy of FDC cells derived from tonsil tissue (the HK cell line) showed that knockdown of MDA-9/Syntenin reduced FAK activation, similar to observations in cancer-derived cell lines [102].
During dendritic cell-T-cell interactions, MDA-9/Syntenin was shown to be responsible for linking activated leukocyte cell adhesion molecule (ALCAM) to the actin cytoskeleton, stabilizing it as part of a supramolecular complex engaged to CD6 [103]. Interestingly, ALCAM has also been shown to be involved in a multitude of interactions including neural and hematopoietic development, immune responses and osteogenesis. Further, ALCAM has been implicated in the progression of breast cancer, bladder cancer, colorectal cancer and melanoma. It is also involved in GBM tumor invasion and is expressed on GBM progenitor cells [104].
MDA-9/Syntenin is recruited to the plasma membrane during HIV-1 attachment and associates with CD4, the main HIV-1 receptor. When MDA-9/Syntenin is knocked down in T cells, actin polymerization is decreased, while PIP2 production is increased along with HIV-1 entry. Conversely, MDA-9/Syntenin overexpression reduces HIV-1 production and HIV-mediated cell fusion [105]. Additionally, Nef, an HIV-1 accessory protein, was demonstrated to reduce the expression of MDA-9/Syntenin [106].
5. Angiogenesis and inflammation
In addition to numerous examples of MDA-9/Syntenin as a mediator of invasive pathways, this protein has also been shown to be involved in inflammation and angiogenesis, a process that overlaps with invasion in numerous respects. In both melanoma and glioma, MDA-9/Syntenin was shown to increase angiogenic potential in tumor cells [50,107]. In melanoma, MDA-9/Syntenin was found to induce angiogenesis by activating Akt, leading to hypoxia inducible factor-1α induction and transcription of IGF binding protein – 2 (IGFBP-2). Subsequent secretion of IGFBP-2 induces endothelial cell production of VEGF-A and angiogenesis [107]. In glioma, MDA-9/Syntenin induced NF-κB activation and the production of the prominent angiogenic chemokine IL-8 at both the transcript and protein expression levels. Furthermore, knockdown of MDA-9/Syntenin reduced microvessel branching in in vivo assays, and reduced tumor vascularity in an orthotopic xenograft mouse model [50]. MDA-9/Syntenin was also shown to help maintain the blood–brain barrier (BBB) integrity, as miR-155 targeting of MDA-9/Syntenin can lead to downregulation and higher measures of BBB permeability [108].
Inflammatory pathways can be important for numerous normal and pathogenic states, and MDA-9/Syntenin’s role in regulating these processes could prove that it represents a highly useful therapeutic target. MDA-9/Syntenin partners with syndecan-4 regulating exosome-dependent secretion of angiopoietin-2 (Ang2), a crucial Tie2 ligand that influences vascular integrity and inflammation. A recent study demonstrated that excessive Ang2 secretion could be rescued by syndecan-4 knockout or syntenin inhibition. Notably, knockdown of MDA-9/Syntenin, which can bind all syndecans, had a larger reductive effect in Ang2 secretion than single syndecan knockdown [109]. MDA-9/Syntenin was found to be significantly elevated in the plasma of diabetic patients compared to healthy donors as well as upregulated in liver cells cultured in high-glucose media [110].
6. Conclusions
MDA-9/Syntenin displays an impressive diversity of interacting partners, indicating it has a number of flexible roles within the cell. It forms a variety of complexes, some specific to a particular cell type and others to a subcellular compartment, and is involved in numerous intracellular pathways. As noted, MDA-9/Syntenin is frequently identified as being integral in regulating cell migration, invasion and metastasis in a variety of cancer types. This presents the unique opportunity of developing novel and worthwhile cancer therapeutics that target MDA-9/Syntenin.
7. Expert opinion
A continued focus of the cancer field on invasion research is evident as numerous studies pursue the underlying mechanisms of cellular pathways commandeered by tumors. This effort will have a significant impact in enhancing the quality and quantity of life in patients because tumor progression and invasion lead to the deadliest sequelae of cancer: damage of nearby normal tissue and the formation of distant metastases. MDA-9/Syntenin is now widely recognized to be a significant lynchpin in invasive pathways. As more is uncovered about the mechanics of tumor invasion, the more these pathways overlap with crucial developmental programs, which are normally under tight regulation in stem and progenitor cells. Cancer cells successful in invasion have circumvented the regulatory pathways preventing this process and utilize relevant molecules to break away from the primary tumor. The involvement of MDA-9/Syntenin as a facilitator of key developmental processes in a range of tissues thus supports its role observed in invasive cancer cells.
Early studies of MDA-9/Syntenin focused on its interaction partners, mainly through its PDZ domains. Recent data has shed light on the structure and function of its N- and C-terminal domains and on the roles they play in regulating conformation and binding patterns. This provides much needed information on how MDA-9/Syntenin is regulated post-transcriptionally. However, the details of transcriptional regulation have not yet been elucidated. Continued work into that area will be especially useful as we learn more about this versatile cellular adapter. To gain a more complete understanding of MDA-9/Syntenin’s functions and relationships within different cellular contexts, the development and evaluation of transgenic and knockout mice will prove highly useful. Crossing these mice with spontaneous models of tumor formation could prove particularly beneficial for analyzing the role of MDA-9/Syntenin in early tumorigenesis. Even combination with syngeneic models could be valuable as this could isolate effects on tumor growth and pathogenesis inside a tumor microenvironment lacking or overexpressing MDA-9/Syntenin.
Ultimately, MDA-9/Syntenin could prove to be a valuable target for inhibiting invasion and metastasis in a wide variety of cancer indications. Published results in numerous tumors utilizing genetic inhibition of MDA-9/Syntenin have thus far supported this view. Nonetheless, pharmacological inhibition of MDA-9/Syntenin’s invasion-promoting attributes would be ideal. To date, the targeting of pharmaceuticals to PDZ domains has not been widely successful. However, newer methods in generating inhibitors capable of targeting specific protein domains have made advances in recent years. Approaches that enable probing of large libraries of potential molecules, such as mRNA display [111,112] and fragment-based drug discovery coupled with NMR analysis [113–119], will aid in the quest to inhibit difficult structures like the PDZ domain. Initial molecules can then be altered in a series of rational approaches to targeting that could yield promising candidates for drug design. Of course, a challenge during this process will be the relatively slower pace of in vitro and in vivo validation of these candidates compared to timeline of their development. Another significant hurdle will be identifying molecules that avoid rapid clearance from the body, are sufficiently distributed to tumor cells and have minimal observed toxicities.
Notably, the recent observations of a role of MDA-9/Syntenin in exosome biology could be an area of focus in the near future. Tumor cells have been shown to induce invasion through secreted factors, and some cancer therapies, including radiation, can enhance the release of exosomes. This can be through targeting both adjacent tumor cell populations, or influencing the behavior of the surrounding microenvironment. Uncovering related roles of MDA-9/Syntenin in the context of different cancers will be useful in understanding the full extent of this protein’s influence on invasion.
As is evident by the results of years of cancer drug discovery and development, there is unlikely to be a single ‘magic bullet’ that can completely eliminate aggressive tumors. Nonetheless, targeting MDA-9/Syntenin could serve as an ideal complement to many conventional and newer therapy strategies. While inhibiting MDA-9/Syntenin can reduce the proliferation rate of some cancer types, the level to which it slows growth is not nearly as dramatic as true cytotoxic therapies. Therefore, MDA-9/Syntenin targeting could be part of a combination approach that utilizes chemotherapy or radiotherapy. It could be particularly well suited to this task in light of the observation that some current treatments, such as radiation and bevacizumab, can actually enhance the invasive potential of surviving cells. In both cases, key MDA-9/Syntenin interacting partners, such as c-Src and FAK, are activated in these invasive cells.
Due to the overlapping nature of angiogenesis and invasion, it is not surprising that MDA-9/Syntenin can be involved in this crucial tumor phenotype as well. Further investigation of MDA-9/Syntenin’s role in upregulating pro-angiogenic factors, and its influence on tumor endothelial cells and vasculature could prove valuable in this respect. Furthermore, the role of MDA-9/Syntenin in regulating cancer stem cell populations in the heterogeneous tumor environment requires investigation. Targeting this population is the focus of numerous current efforts, as these cells are often responsible for recurrence and metastatic events. Further investigation into the plasticity of tumor cells, or their ability to de-differentiate, and how MDA-9/Syntenin may play a decisive role could yield another aspect of this protein that makes it an attractive focus for drug development.
Overall, MDA-9/Syntenin is an emerging protein in the world of cancer biology. It is unique in its involvement in facilitating both intracellular and extracellular effectors of invasion. This, coupled with its expression and function in numerous cancer types, makes it an exciting potential therapeutic target that warrants further in-depth study. The use of anti-invasive agents combined with cytotoxic therapies may provide a novel approach for effectively treating both primary tumors and metastases. In these contexts, inhibitors of MDA-9/Syntenin, both direct and those that block its interaction with partner proteins, or its critical downstream pathways may usher in new approaches for successfully treating and potentially preventing tumor spread and metastasis.
Article highlights.
Melanoma differentiation-associated gene – 9 (MDA-9)/ Syntenin displays myriad roles in development and cellular function, often involving cell motility and adhesion.
Cancers with reported MDA-9/Syntenin overexpression include: melanoma, glioma, breast cancer, urothelial cell carcinoma, small cell lung carcinoma, uveal melanoma and gastric cancer.
MDA-9/Syntenin has significant effects on invasion, and inhibiting its expression decreases invasion in multiple cancer types.
Numerous binding partners and downstream effectors have been identified, many known to be involved in cancer progression including: c-Src, focal adhesion kinase, Akt, p38MAPK, NF-κB and MMPs.
Exosomes are a growing frontier in cancer progression and MDA-9/Syntenin is a crucial part of exosome biology.
Targeting inhibition of MDA-9/Syntenin expression has significant effects on cancer cells, supporting the development of pharmacological inhibitors that may display profound therapeutic activity.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
The present study was supported in part by P01 CA104177 and R01 CA134721 given to Paul Fisher, R01 CA138540 given to Swadesh Das, R01 CA168517 to Maurizio Pellecchia and Paul Fisher; and a DoD grant W81XWH-10-PCRP-SIDA given to Paul Fisher and Wang Xiang-Yang. Paul Fisher and Swadesh Das were supported by the Samuel Waxman Cancer Research Foundation and Paul fisher was supported by the National Foundation for Cancer Research also. Wang Xiang-Yang and Swadesh Das are Harrison Scholars in the VCU Massey Cancer Center. Paul Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Jiang H, Fisher P. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Differ. 1993;1(3):285–99. [Google Scholar]
- 2••.Lin J, Jiang H, Fisher P. Characterization of a novel melanoma differentiation-associated gene, mda-9, that is down-regulated during terminal cell differentiation. Mol Cell Differ. 1996;4(4):317–33. The above paper provides the initial definition of melanoma differentiation-associated gene – 9 (MDA-9)/Syntenin as a differentially regulated gene in melanoma. [Google Scholar]
- 3.Sarkar D, Boukerche H, Su ZZ, et al. Mda-9/syntenin: recent insights into a novel cell signaling and metastasis-associated gene. Pharmacol Ther. 2004;104(2):101–15. doi: 10.1016/j.pharmthera.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4••.Sarkar D, Boukerche H, Su Z, et al. Mda-9/syntenin: more than just a simple adapter protein when it comes to cancer metastasis. Cancer Res. 2008;68(9):3087–93. doi: 10.1158/0008-5472.CAN-07-6210. Two excellent earlier reviews on the functions of MDA-9/Syntenin. [DOI] [PubMed] [Google Scholar]
- 5.Fisher PB, Weinstein IB, Pestka S. Modulation of differentiation in murine and human-melanoma cells by phorbol ester tumor promoters and interferon. Yale J Biol Med. 1984;57(3):357–8. [Google Scholar]
- 6.Fisher PB, Grant S. Effects of interferon on differentiation of normal and tumor cells. Pharmacol Ther. 1985;27(2):143–66. doi: 10.1016/0163-7258(85)90067-1. [DOI] [PubMed] [Google Scholar]
- 7•.Fisher PB, Prignoli DR, Hermo H, Jr, et al. Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. J Interferon Res. 1985;5(1):11–22. doi: 10.1089/jir.1985.5.11. The above three papers provide foundation for the differentiation approach used in melanoma that led to the discovery of numerous differentially regulated genes discovered through subtraction hybridization, including (MDA-9)/Syntenin. [DOI] [PubMed] [Google Scholar]
- 8.Graham GM, Guarini L, Moulton TA, et al. Potentiation of growth suppression and modulation of the antigenic phenotype in human-melanoma cells by the combination of recombinant human fibroblast and immune interferons. Cancer Immunol Immunother. 1991;32(6):382–90. doi: 10.1007/BF01741333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Lin JJ, Su ZZ, et al. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11(12):2477–86. [PubMed] [Google Scholar]
- 10.Leszczyniecka M, Roberts T, Dent P, et al. Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther. 2001;90(2–3):105–56. doi: 10.1016/s0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 11.Huynh KM, Kim G, Kim DJ, et al. Gene expression analysis of terminal differentiation of human melanoma cells highlights global reductions in cell cycle-associated genes. Gene. 2009;433(1–2):32–9. doi: 10.1016/j.gene.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Staudt MR, Depass AL, Sarkar D, et al. Model cell culture system for defining the molecular and biochemical events mediating terminal differentiation of human melanoma cells. J Cell Physiol. 2009;218(2):304–14. doi: 10.1002/jcp.21602. [DOI] [PubMed] [Google Scholar]
- 13•.Lin JJ, Jiang H, Fisher PB. Melanoma differentiation associated gene-9, mda-9, is a human gamma interferon responsive gene. Gene. 1998;207(2):105–10. doi: 10.1016/s0378-1119(97)00562-3. This paper further describes mda-9/ syntenin and shows it is inducible by IFNγ. [DOI] [PubMed] [Google Scholar]
- 14••.Grootjans JJ, Zimmermann P, Reekmans G, et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94(25):13683–8. doi: 10.1073/pnas.94.25.13683. This paper defined MDA-9/Syntenin as a binding partner with syndecans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Boukerche H, Su ZZ, Emdad L, et al. Mda-9/syntenin: a positive regulator of melanoma metastasis. Cancer Res. 2005;65(23):10901–11. doi: 10.1158/0008-5472.CAN-05-1614. This paper was the first demonstration that MDA-9/Syntenin could function as a positive mediator of melanoma metastasis. [DOI] [PubMed] [Google Scholar]
- 16••.Zimmermann P, Tomatis D, Rosas M, et al. Characterization of syntenin, a syndecan-binding PDZ protein, as a component of cell adhesion sites and microfilaments. Mol Biol Cell. 2001;12(2):339–50. doi: 10.1091/mbc.12.2.339. This paper gives thorough descriptions of the intracellular localization patterns of MDA-9/Sytnenin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luyten A, Mortier E, Van Campenhout C, et al. The postsynaptic density 95/disc-large/zona occludens protein syntenin directly interacts with frizzled 7 and supports noncanonical wnt signaling. Mol Biol Cell. 2008;19(4):1594–604. doi: 10.1091/mbc.E07-08-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Lambaerts K, Van Dyck S, Mortier E, et al. Syntenin, a syndecan adaptor and an Arf6 phosphatidylinositol 4,5-bisphosphate effector, is essential for epiboly and gastrulation cell movements in zebrafish. J Cell Sci. 2012;125(5):1129–40. doi: 10.1242/jcs.089987. This paper demonstrates the importance of MDA-9/Syntenin in the normal development of zebrafish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koroll M, Rathjen FG, Volkmer H. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J Biol Chem. 2001;276(14):10646–54. doi: 10.1074/jbc.M010647200. [DOI] [PubMed] [Google Scholar]
- 20••.Das S, Bhutia S, Kegelman T, et al. MDA-9/syntenin: a positive gatekeeper of melanoma metastasis. Front Biosci. 2012;17:1–15. doi: 10.2741/3911. This review focuses on MDA-9/ Syntenin as a metastatic driver for metastasis in melanoma, among other notable functions. [DOI] [PubMed] [Google Scholar]
- 21••.Baietti M, Zhang Z, Mortier E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85. doi: 10.1038/ncb2502. This paper gives an elegant description of the role of MDA-9/Syntenin in exosome formation and secretion. [DOI] [PubMed] [Google Scholar]
- 22.Kang B, Cooper D, Jelen F, et al. PDZ tandem of human syntenin: crystal structure and functional properties. Structure. 2003;11(4):459–68. doi: 10.1016/s0969-2126(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 23.Grembecka J, Cierpicki T, Devedjiev Y, et al. The binding of the PDZ tandem of syntenin to target proteins. Biochemistry. 2006;45(11):3674–82. doi: 10.1021/bi052225y. [DOI] [PubMed] [Google Scholar]
- 24••.Grootjans JJ, Reekmans G, Ceulemans H, et al. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J Biol Chem. 2000;275(26):19933–41. doi: 10.1074/jbc.M002459200. This paper further describes MDA-9/ Syntenin-syndecan binding, and the importance of the PDZ domains in this interaction. [DOI] [PubMed] [Google Scholar]
- 25••.Boukerche H, Aissaoui H, Prvost C, et al. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene. 2010;29(21):3054–66. doi: 10.1038/onc.2010.65. This paper rigorously describes the function of MDA-9/Syntenin in binding c-Src and activating the NF-κ B pathway in enhancing metastasis in melanoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geijsen N, Uings I, Pals C, et al. Cytokine-specific transcriptional regulation through an IL-5R alpha interacting protein. Science. 2001;293(5532):1136–8. doi: 10.1126/science.1059157. [DOI] [PubMed] [Google Scholar]
- 27.Li A, Li H, Jin B, et al. A novel eIF5A complex functions as a regulator of p53 and p53-dependent apoptosis. J Biol Chem. 2004;279(47):49251–8. doi: 10.1074/jbc.M407165200. [DOI] [PubMed] [Google Scholar]
- 28.Harrod T, Justement L. Evaluating function of transmembrane protein tyrosine phosphatase CD148 in lymphocyte biology. Immunol Res. 2002;26(1–3):153–66. doi: 10.1385/ir:26:1-3:153. [DOI] [PubMed] [Google Scholar]
- 29••.Rajesh S, Bago R, Odintsova E, et al. Binding to syntenin-1 protein defines a new mode of ubiquitin-based interactions regulated by phosphorylation. J Biol Chem. 2011;286(45):39606–14. doi: 10.1074/jbc.M111.262402. This paper describes the ubiquitin interaction dynamics of MDA-9/ Syntenin, describing a new role for the N-terminal domain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvallo L, Munoz R, Bustos F, et al. Non-canonical wnt signaling induces ubiquitination and degradation of syndecan. J Biol Chem. 2010;285(38):29546–55. doi: 10.1074/jbc.M110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Juan-Sanz J, Zafra F, Lopez-Corcuera B, et al. Endocytosis of the neuronal glycine transporter GLYT2: role of membrane rafts and protein kinase C-dependent ubiquitination. Traffic. 2011;12(12):1850–67. doi: 10.1111/j.1600-0854.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Moczygemba M, Huston DP, Lei JT. JAK kinases control IL-5 receptor ubiquitination, degradation, and internalization. J Leukoc Biol. 2007;81(4):1137–48. doi: 10.1189/jlb.0706465. [DOI] [PubMed] [Google Scholar]
- 33.Sala-Valdes M, Gordon-Alonso M, Tejera E, et al. Association of syntenin-1 with M-RIP polarizes rac-1 activation during chemotaxis and immune interactions. J Cell Sci. 2012;125(5):1235–46. doi: 10.1242/jcs.094912. [DOI] [PubMed] [Google Scholar]
- 34•.Wawrzyniak AM, Vermeiren E, Zimmermann P, et al. Extensions of PSD-95/discs large/ZO-1 (PDZ) domains influence lipid binding and membrane targeting of syntenin-1. FEBS Lett. 2012;586(10):1445–51. doi: 10.1016/j.febslet.2012.04.024. This paper proposes an equilibrium between closed and open states regulated by N-terminal phosphorylation of MDA-9/Syntenin, which affects the intracellular localization of the protein. [DOI] [PubMed] [Google Scholar]
- 35.Latysheva N, Muratov G, Rajesh S, et al. Syntenin-1 is a new component of tetraspanin-enriched microdomains: mechanisms and consequences of the interaction of syntenin-1 with CD63. Mol Cell Biol. 2006;26(20):7707–18. doi: 10.1128/MCB.00849-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stier S, Totzke G, Grnewald E, et al. Identification of syntenin and other TNF-inducible genes in human umbilical arterial endothelial cells by suppression subtractive hybridization. FEBS Lett. 2000;467(2–3):299–304. doi: 10.1016/s0014-5793(00)01177-7. [DOI] [PubMed] [Google Scholar]
- 37••.Hwangbo C, Kim J, Lee J, et al. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase calpha and the PDZ adapter protein mda-9/syntenin. Cancer Res. 2010;70(4):1645–55. doi: 10.1158/0008-5472.CAN-09-2447. This paper describes the interdependence of MDA-9/Syntenin and PKCα. [DOI] [PubMed] [Google Scholar]
- 38••.Das SK, Bhutia SK, Sokhi UK, et al. Raf kinase inhibitor RKIP inhibits MDA-9/ syntenin-mediated metastasis in melanoma. Cancer Res. 2012;72(23):6217–26. doi: 10.1158/0008-5472.CAN-12-0402. This paper was the first to identify the inverse relationship between MDA-9/ Syntenin and Raf kinase inhibitor protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Jeon HY, Das SK, Dasgupta S, et al. Expression patterns of MDA-9/syntenin during development of the mouse embryo. J Mol Histol. 2013;44(2):159–66. doi: 10.1007/s10735-012-9468-1. This paper notes the high expression of MDA-9/Syntenin in the developing heart, liver and brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Yu Y, Schachner M. Syntenin-a promotes spinal cord regeneration following injury in adult zebrafish. Eur J Neurosci. 2013;38(2):2280–9. doi: 10.1111/ejn.12222. This paper is the first to implicate MDA-9/Syntenin as important in nervous system regeneration. [DOI] [PubMed] [Google Scholar]
- 41.Bertram L, Tanzi RE. Thirty years of alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9(10):768–78. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 42.Jannatipour M, Dion P, Khan S, et al. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J Biol Chem. 2001;276(35):33093–100. doi: 10.1074/jbc.M105792200. [DOI] [PubMed] [Google Scholar]
- 43.Biederer T, Sara Y, Mozhayeva M, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297(5586):1525–31. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 44.Enz R, Croci C. Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem J. 2003;372:183–91. doi: 10.1042/BJ20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko J, Yoon C, Piccoli G, et al. Organization of the presynaptic active zone by ERC2/CAST1-dependent clustering of the tandem PDZ protein syntenin. J Neurosci. 2006;26(3):963–70. doi: 10.1523/JNEUROSCI.4475-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ro Y, Jang B, Shin CY, et al. Akt regulates the expression of MafK, synaptotagmin I, and syntenin-1, which play roles in neuronal function. J Biomed Sci. 2010;17:18. doi: 10.1186/1423-0127-17-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirbec H, Martin S, Henley J. Syntenin is involved in the developmental regulation of neuronal membrane architecture. Mol Cell Neurosci. 2005;28(4):737–46. doi: 10.1016/j.mcn.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Tomoda T, Kim J, Zhan C, et al. Role of Unc51. 1 and its binding partners in CNS axon outgrowth. Genes Dev. 2004;18(5):541–58. doi: 10.1101/gad.1151204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Dasgupta S, Menezes M, Das SK, et al. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res. 2013;19(17):4621–33. doi: 10.1158/1078-0432.CCR-13-0585. This paper is the first to describe a role of MDA-9/Syntenin in urothelial cell carcinoma as well as inducing EGFR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Kegelman TP, Das SK, Hu B, et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014;16(1):50–61. doi: 10.1093/neuonc/not157. This paper was the first to show that high MDA-9/Syntenin expression in glioma correlates with shorter patient survival, and that MDA-9/Syntenin can be a regulator of both invasion and angiogenesis in glioblastoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian X, Li Y, Yu B, et al. Syndecan binding protein (SDCBP) is overexpressed in estrogen receptor negative breast cancers, and is a potential promoter for tumor proliferation. PLoS One. 2013;8(3):e60046. doi: 10.1371/journal.pone.0060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geeraerts A, Hsiu-Fang F, Zimmermann P, et al. The characterization of the nuclear dynamics of syntenin-2, a PIP2 binding PDZ protein. Cytometry A. 2013;83(9):866–75. doi: 10.1002/cyto.a.22246. [DOI] [PubMed] [Google Scholar]
- 53.Eisenach PA, Soeth E, Roeder C, et al. Dipeptidase 1 (DPEP1) is a marker for the transition from low-grade to high-grade intraepithelial neoplasia and an adverse prognostic factor in colorectal cancer. Br J Cancer. 2013;109(3):694–703. doi: 10.1038/bjc.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lukic N, Visentin R, Delhaye M, et al. An integrated approach for comparative proteomic analysis of human bile reveals overexpressed cancer-associated proteins in malignant biliary stenosis. Biochim Biophys Acta. 2014;1844(5):1026–33. doi: 10.1016/j.bbapap.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 55.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Helmke B, Polychronidis M, Benner A, et al. Melanoma metastasis is associated with enhanced expression of the syntenin gene. Oncol Rep. 2004;12(2):221–8. [PubMed] [Google Scholar]
- 57.Zhong D, Ran J, Tang W, et al. Mda-9/ syntenin promotes human brain glioma migration through focal adhesion kinase (FAK)-JNK and FAK-AKT signaling. Asian Pac J Cancer Prev. 2012;13(6):2897–901. doi: 10.7314/apjcp.2012.13.6.2897. [DOI] [PubMed] [Google Scholar]
- 58.Koo T, Kim E, Kim K, et al. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene. 2002;21(26):4080–8. doi: 10.1038/sj.onc.1205514. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Hong Q, Shi P, et al. Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res. 2013;15(3):R50. doi: 10.1186/bcr3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gangemi R, Mirisola V, Barisione G, et al. Mda-9/syntenin is expressed in uveal melanoma and correlates with metastatic progression. PLoS One. 2012;7(1):e29989. doi: 10.1371/journal.pone.0029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardo M, Garcia A, Antrobus R, et al. Biomarker discovery from uveal melanoma secretomes: identification of gp100 and cathepsin D in patient serum. J Proteome Res. 2007;6(7):2802–11. doi: 10.1021/pr070021t. [DOI] [PubMed] [Google Scholar]
- 62•.Kim WY, Jang JY, Jeon YK, et al. Syntenin increases the invasiveness of small cell lung cancer cells by activating p38, AKT, focal adhesion kinase and SP1. Exp Mol Med. 2014;46:e90. doi: 10.1038/emm.2014.1. This paper was the first to implicate MDA-9/Syntenin in small cell lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnekow A, Paul E, Schartl M. Expression of the C-src protooncogene in human-skin tumors. Cancer Res. 1987;47(1):235–40. [PubMed] [Google Scholar]
- 64.Budde RJ, Ke S, Levin VA. Activity of pp60c-src in 60 different cell lines derived from human tumors. Cancer Biochem Biophys. 1994;14(3):171–5. [PubMed] [Google Scholar]
- 65.Irby R, Yeatman T. Role of src expression and activation in human cancer. Oncogene. 2000;19(49):5636–42. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 66.Ishizawar R, Parsons S. C-src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 67••.Boukerche H, Su Z, Prvot C, et al. Mda-9/syntenin promotes metastasis in human melanoma cells by activating c-src. Proc Natl Acad Sci USA. 2008;105(41):15914–19. doi: 10.1073/pnas.0808171105. This paper rigorously describes the function of MDA-9/Syntenin in binding c-Src and enhancing metastasis in melanoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponting C, Phillips C, Davies K, et al. PDZ domains: targeting signalling molecules to sub-membranous sites. Bioessays. 1997;19(6):469–79. doi: 10.1002/bies.950190606. [DOI] [PubMed] [Google Scholar]
- 69.Meerschaert K, Bruyneel E, De Wever O, et al. The tandem PDZ domains of syntenin promote cell invasion. Exp Cell Res. 2007;313(9):1790–804. doi: 10.1016/j.yexcr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Q, Fan J, Zhang M. Interdomain chaperoning between PSD-95, dlg, and zo-1 (PDZ) domains of glutamate receptor-interacting proteins. J Biol Chem. 2001;276(46):43216–20. doi: 10.1074/jbc.M105996200. [DOI] [PubMed] [Google Scholar]
- 71.Joo NE, Watanabe T, Chen C, et al. NG2, a novel proapoptotic receptor, opposes integrin alpha 4 to mediate anoikis through PKC alpha-dependent suppression of FAK phosphorylation. Cell Death Differ. 2008;15(5):899–907. doi: 10.1038/cdd.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mostafavi-Pour Z, Askari J, Parkinson S, et al. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161(1):155–67. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim K, Lee JJ, Yang Y, et al. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29(4):704–12. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 74••.Zimmermann P, Meerschaert K, Reekmans G, et al. PIP2-PDZ domain binding controls the association of syntenin with the plasma membrane. Mol Cell. 2002;9(6):1215–25. doi: 10.1016/s1097-2765(02)00549-x. This paper describes the interaction of MDA-9/Sytnenin with membrane-associated proteins and lipids. [DOI] [PubMed] [Google Scholar]
- 75.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappa B activation and survival pathway. EMBO J. 2003;22(1):109–20. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dai B, Kang SH, Gong W, et al. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26(42):6212–19. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- 77••.Hwangbo C, Park J, Lee J. Mda-9/ syntenin protein positively regulates the activation of akt protein by facilitating integrin-linked kinase adaptor function during adhesion to type I collagen. J Biol Chem. 2011;286(38):33601–12. doi: 10.1074/jbc.M110.206789. This paper demonstrates the versatility of MDA-9/Syntenin in influencing Akt activation through integrin linked kinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Chen K, Tu Y, et al. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci. 2002;115(24):4777–86. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 79.Hung A, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277(8):5699–702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 80.Zimmermann P, Zhang Z, Degeest G, et al. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev Cell. 2005;9(3):377–88. doi: 10.1016/j.devcel.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Morgan MR, Hamidi H, Bass MD, et al. Syndecan-4 phosphorylation is a control point for integrin recycling. Dev Cell. 2013;24(5):472–85. doi: 10.1016/j.devcel.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Estrach S, Legg J, Watt FM. Syntenin mediates Delta1-induced cohesiveness of epidermal stem cells in culture. J Cell Sci. 2007;120(16):2944–52. doi: 10.1242/jcs.016253. This paper indicates that MDA-9/ Syntenin is highly expressed in the population of interfollicular stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H, Kim Y, Choi Y, et al. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Biophys Res Commun. 2011;409(1):148–53. doi: 10.1016/j.bbrc.2011.04.135. [DOI] [PubMed] [Google Scholar]
- 84.Parish C, Freeman C, Hulett M. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471(3):M99–M108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 85.Vlodavsky I, Friedmann Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest. 2001;108(3):341–7. doi: 10.1172/JCI13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shteingauz A, Ilan N, Vlodavsky I. Processing of heparanase is mediated by syndecan-1 cytoplasmic domain and involves syntenin and alpha-actinin. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-014-1629-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Janvier K, Bonifacino J. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell. 2005;16(9):4231–42. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao D, Ohlendorf J, Chen Y, et al. Identifying mRNA, MicroRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7(10):e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rondepierre F, Bouchon B, Bonnet M, et al. B16 melanoma secretomes and in vitro invasiveness: syntenin as an invasion modulator. Melanoma Res. 2010;20(2):77–84. doi: 10.1097/CMR.0b013e32833279f2. [DOI] [PubMed] [Google Scholar]
- 90.Tan DWM, Jensen KB, Trotter MWB, et al. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development. 2013;140(7):1433–44. doi: 10.1242/dev.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefort K, Dotto G. Notch signaling in the integrated control of keratinocyte growth/differentiation and tumor suppression. Semin Cancer Biol. 2004;14(5):374–86. doi: 10.1016/j.semcancer.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Zhao F, Lu J, et al. Notch-1 signaling promotes the malignant features of human breast cancer through NF-kappaB activation. PLoS One. 2014;9(4):e95912. doi: 10.1371/journal.pone.0095912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Peng G, Tian Y, Lu C, et al. Effects of notch-1 down-regulation on malignant behaviors of breast cancer stem cells. J Huazhong Univ Sci Technolog Med Sci. 2014;34(2):195–200. doi: 10.1007/s11596-014-1258-4. [DOI] [PubMed] [Google Scholar]
- 94.Flanagan J, Vanderhaeghen P. The ephrins and eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 95.Sugiyama N, Gucciardo E, Tatti O, et al. EphA2 cleavage by MT1-MMP triggers single cancer cell invasion via homotypic cell repulsion. J Cell Biol. 2013;201(3):467–84. doi: 10.1083/jcb.201205176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drescher U, Kremoser C, Handwerker C, et al. In-vitro guidance of retinal ganglion-cell axons by rags, a 25 kda tectal protein related to ligands for eph receptor tyrosine kinases. Cell. 1995;82(3):359–70. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 97.Pandey A, Shao H, Marks R, et al. Role of B61, the ligand for the eck receptor tyrosine kinase, in tnf-alpha-induced angiogenesis. Science. 1995;268(5210):567–9. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 98.McClelland AC, Sheffler-Collins SI, Kayser MS, et al. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proc Natl Acad Sci USA. 2009;106(48):20487–92. doi: 10.1073/pnas.0811862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu N, Sun S, Gibson JR, et al. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci. 2011;14(11):1421–U92. doi: 10.1038/nn.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beekman JM, Vervoort SJ, Dekkers F, et al. Syntenin-mediated regulation of Sox4 proteasomal degradation modulates transcriptional output. Oncogene. 2012;31(21):2668–79. doi: 10.1038/onc.2011.445. [DOI] [PubMed] [Google Scholar]
- 101.Iuliano R, Trapasso F, Sama I, et al. Rat protein tyrosine phosphatase eta physically interacts with the PDZ domains of syntenin. FEBS Lett. 2001;500(1–2):41–4. doi: 10.1016/s0014-5793(01)02580-7. [DOI] [PubMed] [Google Scholar]
- 102.Cho W, Kim H, Lee JH, et al. Syntenin is expressed in human follicular dendritic cells and involved in the activation of focal adhesion kinase. Immune Netw. 2013;13(5):199–204. doi: 10.4110/in.2013.13.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tudor C, Te Riet J, Eich C, et al. Syntenin-1 and ezrin proteins link activated leukocyte cell adhesion molecule to the actin cytoskeleton. J Biol Chem. 2014;289(19):13445–60. doi: 10.1074/jbc.M113.546754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kijima N, Hosen N, Kagawa N, et al. CD166/activated leukocyte cell adhesion molecule is expressed on glioblastoma progenitor cells and involved in the regulation of tumor cell invasion. Neuro Oncol. 2012;14(10):1254–64. doi: 10.1093/neuonc/nor202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gordon-Alonso M, Rocha-Perugini V, Alvarez S, et al. The PDZ-adaptor protein syntenin-1 regulates HIV-1 entry. Mol Biol Cell. 2012;23(12):2253–63. doi: 10.1091/mbc.E11-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan R, Patni H, Tandon P, et al. Nef interaction with actin compromises human podocyte actin cytoskeletal integrity. Exp Mol Pathol. 2013;94(1):51–7. doi: 10.1016/j.yexmp.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107••.Das SK, Bhutia SK, Azab B, et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013;73(2):844–54. doi: 10.1158/0008-5472.CAN-12-1681. This paper was the first to define the influence of MDA-9/Syntenin on IGF binding protein – 2 signaling and the consequences of such in melanoma angiogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lopez-Ramirez MA, Wu D, Pryce G, et al. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 2014;28(6):2551–65. doi: 10.1096/fj.13-248880. [DOI] [PubMed] [Google Scholar]
- 109.Ju R, Zhuang ZW, Zhang J, et al. Angiopoietin-2 secretion by endothelial cell Exosomes Regulation by the phosphatidylinositol 3-kinase (Pi3k)/akt/ endothelial nitric oxide synthase (enos) and syndecan-4/syntenin pathways. J Biol Chem. 2014;289(1):510–19. doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J, Chou H, Chen Y, et al. High glucose-induced proteome alterations in hepatocytes and its possible relevance to diabetic liver disease. J Nutr Biochem. 2013;24(11):1889–910. doi: 10.1016/j.jnutbio.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Ma Z, Hartman MC. In vitro selection of unnatural cyclic peptide libraries via mRNA display. Methods Mol Biol. 2012;805:367–90. doi: 10.1007/978-1-61779-379-0_21. [DOI] [PubMed] [Google Scholar]
- 112.White ER, Reed TM, Ma Z, et al. Replacing amino acids in translation: expanding chemical diversity with non-natural variants. Methods. 2013;60(1):70–4. doi: 10.1016/j.ymeth.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 113.Becattini B, Sareth S, Zhai D, et al. Targeting apoptosis via chemical design: inhibition of bid-induced cell death by small organic molecules. Chem Biol. 2004;11(8):1107–17. doi: 10.1016/j.chembiol.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 114.Pellecchia M, Becattini B, Crowell KJ, et al. NMR-based techniques in the hit identification and optimisation processes. Expert Opin Ther Targets. 2004;8(6):597–611. doi: 10.1517/14728222.8.6.597. [DOI] [PubMed] [Google Scholar]
- 115.Becattini B, Culmsee C, Leone M, et al. Structure-activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting bid. Proc Natl Acad Sci USA. 2006;103(33):12602–6. doi: 10.1073/pnas.0603460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen J, Zhang Z, Stebbins JL, et al. A fragment-based approach for the discovery of isoform-specific p38alpha inhibitors. ACS Chem Biol. 2007;2(5):329–36. doi: 10.1021/cb700025j. [DOI] [PubMed] [Google Scholar]
- 117.Pellecchia M, Bertini I, Cowburn D, et al. Perspectives on NMR in drug discovery: a technique comes of age. Nat Rev Drug Discov. 2008;7(9):738–45. doi: 10.1038/nrd2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rega MF, Wu B, Wei J, et al. SAR by interligand nuclear overhauser effects (ILOEs) based discovery of acylsulfonamide compounds active against bcl-x(L) and mcl-1. J Med Chem. 2011;54(17):6000–13. doi: 10.1021/jm200826s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hedvat M, Emdad L, Das SK, et al. Selected approaches for rational drug design and high throughput screening to identify anti-cancer molecules. Anticancer Agents Med Chem. 2012;12(9):1143–55. doi: 10.2174/187152012803529709. [DOI] [PMC free article] [PubMed] [Google Scholar]