Abstract
First identified almost two decades ago as a novel gene differentially expressed in human melanoma cells induced to terminally differentiate, MDA-7/IL-24 has since shown great potential as an anti-cancer gene. MDA-7/IL24, a secreted protein of the IL-10 family, functions as a cytokine at normal physiological levels and is expressed in tissues of the immune system. At supra-physiological levels, MDA-7/IL-24 plays a prominent role in inhibiting tumor growth, invasion, metastasis and angiogenesis and was recently shown to target tumor stem/initiating cells for death. Much of the attention focused on MDA-7/IL-24 originated from the fact that it can selectively induce cell death in cancer cells without affecting normal cells. Thus, this gene originally shown to be associated with melanoma cell differentiation has now proven to be a multi-functional protein affecting a broad array of cancers. Moreover, MDA-7/IL-24 has proven efficacious in a Phase I/II clinical trial in humans with multiple advanced cancers. As research in the field progresses, we will unravel more of the functions of MDA-7/IL-24 and define novel ways to utilize MDA-7/IL-24 in the treatment of cancer.
Keywords: MDA-7, IL-24, Cytokine, Cancer, Apoptosis, Autophagy, Bystander antitumor activity, Cancer terminator virus
6.1 Introduction
The growth and differentiation of an individual cell is controlled by the signals it receives from its surroundings. Based on the stimuli a cell receives, most cells will ultimately differentiate into a specific cell lineage type (besides stem cells). Experimentally, terminal differentiation in human melanoma can be achieved by treating cells with recombinant fibroblast interferon (IFN-β) and the protein kinase C activator, mezerein [26]. While performing subtraction hybridization and screening cDNA libraries for genes that were differentially expressed in melanoma cells before and after terminal differentiation, our laboratory identified MDA-7 (melanoma differentiation associated gene – 7) as one of the transcripts whose expression was induced in terminally differentiated cells [43]. This gene was later designated interleukin-24 (IL-24) by the Human Gene Organisation (HUGO) based on its conserved structure, chromosomal location and cytokine-like properties [9, 73, 81]. Upon examining various cancer cell lines, it was observed that MDA-7/IL-24 protein expression was low or absent in cancer cells as compared to normal tissues [24, 38]. Further studies revealed that MDA-7/IL-24 played a key role in tumor inhibition [44, 48, 56, 69, 92]. Extensive work carried out on MDA-7/IL-24 since then, both in melanoma and other cancers, has revealed several clues to the functioning of MDA-7/IL-24 as an anti-cancer gene. In this chapter, we will first discuss our current knowledge of the MDA-7/IL-24 gene, we will then look at the various ways in which MDA-7/IL-24 exerts its anti-cancer properties, followed by the application of MDA-7/IL-24 in the clinic and conclude with the future prospects of MDA-7/IL-24.
6.2 MDA-7/IL-24
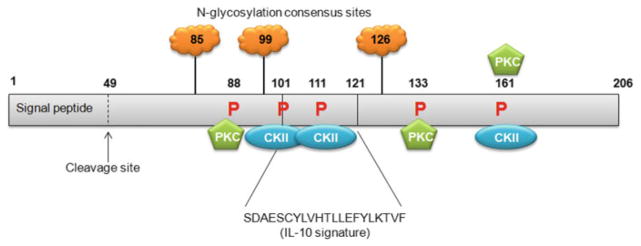
Melanoma differentiation associated gene-7 (mda-7)/interleukin-24 (IL-24) is a secreted cytokine that belongs to the IL-10 gene family [9, 24, 38, 71, 73, 81]. Along with several other IL-10 cytokine family members, mda-7/IL-24 is located on chromosome 1q32-33 in humans and encompasses seven exons and six introns [38]. The mda-7/IL-24 cDNA is 1,718 base pairs in length and encodes a 206 amino acid protein with a predicted molecular weight of 23.8 kDa [38, 43, 44, 71]. mda-7/IL-24 open reading frame is flanked by a 274 bp 5′ untranslated region (UTR) and a 823 bp 3′ UTR. Three consensus elements (AUUUA) involved in mRNA stability and three polyadenylation signals (AAUAAA) are present in the 3′ UTR. A 49-amino acid N terminal hydrophobic signal peptide was identified by sequence analysis and allows the molecule to be cleaved and secreted. Secreted MDA-7/IL-24 protein can have several molecular sizes because of putative N-glycosylation sites [95] at amino acids 85, 99 and 126 (Fig. 6.1) [81]. mda-7/IL-24 sequence analysis also revealed a region of the gene encoding an IL-10 signature sequence from amino acid 101 to 121, three protein kinase C consensus phosphorylation sites at amino acid 88, 133 and 161 and three casein kinase II consensus phosphorylation sites at amino acid 101, 111 and 161 [81]. Structural analysis also revealed the possibility of dimerization of MDA-7/IL-24 due to a potential disulfide bond. MDA-7/IL-24 protein was found to be highly conserved throughout evolution with sequence homology with species such as yeast, cat, dog, cow and monkey.
Fig. 6.1. Schematic representation of MDA-7/IL-24 protein with predicted and established domains and protein modification sites illustrated.
MDA-7/IL-24 contains an IL-10 signature sequence between amino acids 101 and 121. The MDA-7/IL-24 signal peptide is cleaved to allow secretion of MDA-7/IL-24 protein. MDA-7/IL-24 is glycosylated at three sites at amino acid 85, 99 and 126. Protein kinase C consensus phosphorylation sites are present at amino acid 88, 133 and 161, while Casein kinase II (CKII) consensus phosphorylation sites are present at amino acid 101, 111 and 161. Numbers indicate amino acids. Not drawn to scale (Figure modified from Sauane et al. [81], Copyright 2003, with permission from Elsevier)
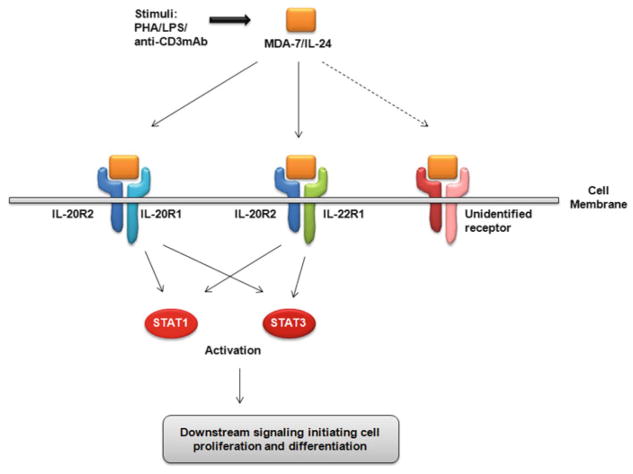
Members of the IL-10 cytokine family signal through receptor dimers consisting of an R1 type receptor (with a long cytoplasmic domain) and an R2 type receptor (with a short cytoplasmic domain). MDA-7/IL-24 can bind to its cognate receptors – IL-20R1 and IL-20R2; or IL-22R1 and IL-20R2 in order to activate downstream STAT1 (signal transducer and activator of transcription 1) and STAT3 signaling (Fig. 6.2) [20, 73, 81, 95].
Fig. 6.2. Schematic representation of MDA-7/IL-24 action mediated through its cognate receptors.
MDA-7/IL-24 can be induced by PHA, LPS and anti-CD3 monoclonal antibody. MDA-7/IL-24 then binds to its receptor dimers IL-20R1 and IL-20R2 or IL-22R1 and IL-20R2 or possibly an as-yet unidentified receptor to activate STAT1 or STAT3 downstream signaling
To gain insight into the regulation of mda-7/IL-24 expression, the mda-7/IL-24 promoter was isolated and characterized [54]. Several recognition binding sites for two transcription factor families, AP-1 and C/EBP, were identified within the mda-7/IL-24 promoter. Overexpression of c-Jun (a member of the AP-1 family), C/EBP-α or β caused an upregulation of mda-7/IL-24 promoter activity indicating their involvement in the transcriptional control of mda-7/IL-24 expression. Electrophoretic mobility shift assays (EMSA) further demonstrated that AP-1 and C/EBP could bind to the mda-7/IL-24 promoter [54]. Since mda-7/IL-24 expression was induced upon treatment with IFN-β and mezerein, mda-7/IL-24 promoter activity was assessed in HO-1 and MeWo melanoma cells with and without the treatment [55]. mda-7/IL-24 promoter activity did not increase above basal levels with and without treatment with IFN-β and mezerein. However, mda-7/IL-24 mRNA was markedly increased in melanoma cells upon treatment with IFN-β and mezerein. Thus, mda-7/IL-24 expression appears to also be regulated at the post-transcriptional level during melanoma cell differentiation and IFN-β and mezerein might somehow be stabilizing mda-7/IL-24 mRNA levels [55]. As mentioned earlier, mda-7/IL-24, like other cytokines, contains AU-rich elements (ARE) in the 3′UTR region that correlate with rapid mRNA turnover and posttranscriptional control and might play a role in the post transcriptional regulation of mda-7/IL-24. mda-7/IL-24 expression is also regulated by p38 MAPK-mediated stabilization of mda-7/IL-24 3′UTR [60]. A recent study showed that IL-2 can upregulate the expression of mda-7/IL-24 [42].
At low concentrations, MDA-7/IL-24 predominantly functions as a cytokine. MDA-7/IL-24 is normally expressed in humans in tissues of the immune system such as the thymus, spleen, peripheral blood leukocytes (PBL) and normal melanocytes [38]. In fact, MDA-7/IL-24 transgenic mice (overexpressing MDA-7/IL-24 specifically in the skin) showed neonatal lethality within hours of birth, and displayed epidermal hyperplasia and abnormality in keratinocyte differentiation [36]. Several cells of the immune system can be induced to upregulate expression of mda-7/IL-24. Megakaryocyte differentiation induced in human hematopoietic cells upon treatment with TPA (12-O-tetradecanoyl phorbol-13-acetate) causes an increase in mda-7/IL-24 expression [38]. Stimulation of peripheral blood mononuclear cells (PBMC) with phytohemagglutinin (PHA) or lipopolysaccharide (LPS) also caused induction of mda-7/IL-24 expression. MDA-7/IL-24 is upregulated in monocytes treated with LPS and in T cells, especially CD4+ naïve and memory cells activated by anti-CD3 monoclonal antibody. Assessment of the secretion profile of PBMC treated with MDA-7/IL-24 protein (purified from conditioned media obtained from HEK 293 cells infected with Ad.mda-7) showed increase in secretion of IL-6, TNF-α and INF-γ at high levels and IL-1β, IL-12 and GM-CSF at low levels within 48 h [9]. Simultaneous administration of IL-10 caused a partial or complete block in these changes mediated by MDA-7/IL-24, probably due to shared receptors with IL-10 having a ten-fold higher affinity for its receptor. However, PBMC proliferation was unaffected by treatment with MDA-7/IL-24. Of note, although IL-10 and MDA-7/IL-24 belong to the same cytokine family, IL-10 is a suppressor of immune response and inflammation while MDA-7/IL-24 is immunomodulatory.
MDA-7/IL-24 plays a role in wound healing [87], in autoimmune diseases such as psoriasis, rheumatoid arthritis and spondyloarthropathy [45], and protection against infectious diseases caused by bacteria such as Pseudomonas aeruginosa [3], Salmonella typhimurium [52] and Mycobacterium tuberculosis [53].
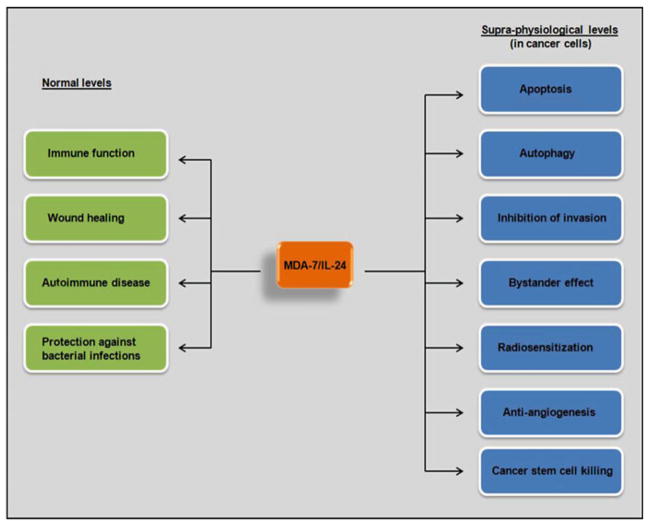
Besides the normal physiological role that MDA-7/IL-24 plays in the immune system, MDA-7/IL-24 has been studied in great detail for its role in cancer. The known functions of MDA-7/IL-24 are schematically represented in Fig. 6.3. The next section describes the role of MDA-7/IL-24 in cancer.
Fig. 6.3. Schematic representation of the currently known functions of MDA-7/IL-24.
Under normal physiological conditions or when triggered by the appropriate signals, MDA-7/IL-24 can function in immune regulation, wound healing, autoimmune disease and in protection against bacterial infections. When expressed at supra-physiological levels in cancer cells, MDA-7/IL-24 exerts several anti-cancer activities
6.3 Role of MDA-7/IL-24 in Cancer
6.3.1 Apoptosis
Apoptosis, also known as programmed cell death, involves a sequential series of events that are responsible for the elimination of unwanted cells from the system. Aberrant apoptosis is considered to be a hallmark of cancer [35]. The ability of cancer cells to escape apoptosis is one of the major contributing factors to chemotherapeutic resistance. Much of the research over the past several years has been dedicated to understand the mechanisms adopted by tumor cells to evade cell death. This has led to the development of novel strategies and therapeutic agents that can modulate cell death pathways in a manner to selectively induce apoptosis in cancer cells.
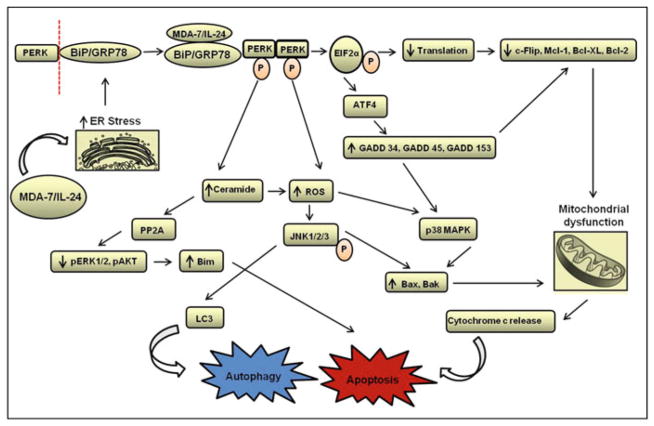
There is abundant evidence in the literature that emphasizes the role of MDA-7/IL-24 as a selective anti-cancer agent. The tumor growth suppressing effects are contributed largely by apoptotic pathways that are triggered in response to MDA-7/IL-24. Previous and ongoing research efforts by our research group and others have shed some light on the key players that are involved in MDA-7/IL-24-mediated apoptosis (Fig. 6.4). Most of these proteins play an important role in the regulation of endoplasmic reticulum (ER) stress and mitochondrial function [16, 17, 32, 49, 50, 61, 73, 74, 80, 99–101]. In earlier reports from our laboratory, it was observed that MDA-7/IL-24 induces cell death by the activation of PKR-like endoplasmic reticulum kinase (PERK), an unfolded protein response (UPR) sensor. MDA-7/IL-24 prevents the interaction between the ER residing chaperone protein BiP/GRP78 and PERK by associating with BiP/GRP78 [33]. Dissociation of PERK from BiP/GRP78 results in the oligomerization and auto phosphorylation of PERK. Phosphorylated PERK in turn, leads to the activation and phosphorylation of EIF2α protein that shuts down the global translation of proteins. This leads to decreased expression of pro-survival proteins like Mcl-1, Bcl-XL, and c-Flip [23, 28, 68]. In other studies, treatment of cancer cells with MDA-7/IL-24 reduced the expression of pro-survival proteins and this reduction was found to be correlated with the increased levels of pro-apoptotic markers like Bax and Bak [28, 91, 92]. Recent studies have also explored the role of second messenger molecules like ceramide and dihydroceramide (generated after PERK activation) upon MDA-7/IL-24 exposure [6, 84, 100]. It was hypothesized that MDA-7/IL-24 possibly functions by stabilizing ceramide synthase 6 protein thus increasing the levels of dihydroceramide in a PERK-dependent manner. Enhanced levels of ceramide result in calcium ion-dependent production of reactive oxygen species (ROS) that impacts diverse signaling pathways and alters mitochondrial integrity [49].
Fig. 6.4. Overview of the molecular pathways involved in MDA-7/IL-24-mediated regulation of cell growth.
GRP-78/BiP chaperone protein represents one of the key molecules targeted by MDA-7/IL-24. The cytokine dissociates the complex formed between GRP-78/BiP and PERK allowing activation of PERK and EIF2α protein. This, in turn, affects the global translation of proteins resulting in loss of pro-survival proteins like c-Flip, Mcl-1, Bcl-XL, and Bcl-2. Phosphorylated PERK also enhances the transcription of certain DNA damage response genes (i.e., GADD 34, GADD 45, GADD 153) in a PERK-dependent manner. Activation of PERK also results in increased levels of ROS and ceramide that affects autophagy pathway and triggers JNK and p38 signaling. Overall, these events disrupt the mitochondrial integrity by affecting the balance between pro-survival and pro-apoptotic proteins and eventually lead to growth inhibition and cell death (Figure modified from Dent et al. [18], Copyright 2010, with permission from Elsevier)
Over the past decade, it has become increasingly clear that MDA-7/IL-24 affects tumor cell viability by inducing cell death, but the exact mode of action varies to certain extents in different cancer types. For instance, upon infection of prostate carcinoma cells DU145, Ad.mda-7 (a non-replicating type 5 adenovirus expressing mda-7/IL-24) induced apoptosis in a Bax-independent manner [49, 50, 80]. In contrast, in primary human glioblastoma cells, the mitochondrial dysfunction and cytotoxicity was largely contributed by the cathepsin-B dependent cleavage of a pro-apoptotic family member Bid [101, 104]. When melanoma cells were infected with Ad.mda-7, a dramatic reduction was observed in the levels of anti-apoptotic proteins Bcl-2 and Bcl-XL, whereas pro-apoptotic proteins like Bax and Bak were upregulated to a modest degree [48]. This suggests that Ad.mda-7 stimulates the apoptotic pathway by affecting the ratio of pro-apoptotic to anti-apoptotic markers in cancer cells. In another study, Dash et al. documented that ER stress response triggered by MDA-7/IL-24 in prostate cancer cells resulted in apoptosis due to the inhibition of an anti-apoptotic myeloid cell leukemia-1 (Mcl-1) protein [17]. Conversely, when Mcl-1 was overexpressed in transformed cells, a significant inhibition of MDA-7/IL-24-mediated toxicity was observed. This data emphasizes the role of Mcl-1 in MDA-7/IL-24-induced cell death.
Sarkar et al. first reported the involvement of p38 mitogen activated protein kinase (MAPK) pathway in cell cycle arrest and cell death in context of Ad.mda-7 or MDA-7/IL-24 [74]. In this study, MAPK activity was shown to be partly dependent on the activation of PERK and subsequent stimulation of a subset of growth arrest and DNA damage (GADD) genes. Other studies also found that in some of the cancer cell types, exposure to MDA-7/IL-24 resulted in the dephosphorylation and inactivation of ERK 1/2 [99]. In others, c-Jun NH2-terminal kinase (JNK) signaling cascade was triggered following treatment with this multipurpose cytokine [99, 103]. This resulted in the stabilization of Bim and activation of Bax and Bak proteins, finally leading to mitochondrial dysfunction and cell death. Additionally, MDA-7/IL-24 also induces cell death via apoptosis selectively in cancer stem cell without affecting normal breast stem cell growth [5]. The underlying mechanism of the unique ability of MDA-7/IL-24 to induce selective apoptosis in cancer cells requires further clarification. Fundamental biochemical differences between normal and cancer cells might be a possible reason for MDA-7/IL-24-mediated selective apoptosis in cancer cells. One of the main mechanisms by which MDA-7/IL-24 induces cell death is by inducing ER stress. Although ER stress activates both pro-survival and pro-apoptotic pathways, a particularly strong ER stress response for prolonged periods could shift the balance toward apoptotic pathways, attenuating tumor growth and metastasis. Since cancer cells have higher levels of ER stress as compared to normal cells, this renders them more susceptible to ER stress-mediated cell death triggered by MDA-7/IL-24. Another plausible explanation why MDA-7/IL-24 mediates differential cell killing effects between normal and cancer cells relies on reactive oxygen species (ROS), which is one of the key mediators of MDA-7/IL-24 toxic responses in cancer cells. Numerous studies have demonstrated that the basal level of ROS in cancer cells is higher than the normal cells [65]. As a consequence, agents like MDA-7/IL-24 that enhance ROS production, could easily overcome the natural antioxidant effects in a more efficient manner in cancer cells as compared to normal cells, resulting in cell death.
6.3.2 Autophagy
Autophagy is an evolutionarily conserved pathway involved in the recycling of cellular components including whole organelles [7, 8, 19, 51]. The process is regulated by a set of autophagy related (ATG) proteins arranged in three different complexes [88]. The autophagy pathway is initially triggered by unc-51 like kinase (ULK1). The kinase is responsible for further stimulation of a signaling pathway that results in the formation of a double membrane bound vesicle called autophagosome. Autophagosome consists of components that are destined for degradation and its formation requires a PI3 kinase complex. In the final step, the autophagosome membrane elongation occurs with the aid of other conjugation systems like LC3 and ATG12 and it fuses with the lysosome resulting in the degradation of its enclosed components. There are three different kinds of autophagy categorized as micro-autophagy, macro-autophagy and chaperone-mediated autophagy (CMA). Micro-autophagy degrades the enclosed components directly by lysosomal invagination [105]. Whereas macro-autophagy sequesters the proteins meant for degradation in autophagosome, which are later on degraded by the lysosomal proteases [19, 105]. CMA is the only form of autophagy that involves no vesicular trafficking. During the process of autophagy, particular proteins are tagged by the CMA substrate chaperone complex, which are then directed to the lysosomes or endosomes for degradation [19, 105].
Numerous studies suggest the dynamic role of autophagy in regulating tumor-igenesis [7, 57, 96, 97]. Autophagy is known to suppress growth of tumors during the early developmental stages but promotes tumor growth in advanced tumors [7, 37]. Based on this, different therapeutic agents may modulate tumor growth and proliferation by affecting the autophagic process. In fact, autophagy-related protein markers have gained significant interest for cancer therapy. There are several proteins implicated in tumor growth and proliferation that also play a role in the regulation of autophagy. For instance, mTOR is reported to negatively regulate the ULK1 complex. Therefore, inhibitors of the mTOR pathway, like PTEN, stimulate autophagy and the activators of mTOR, such as AKT, inhibit the process [51]. Studies from our laboratory and other research groups have demonstrated the involvement of MDA-7/IL-24 in autophagy in different cancer models (Fig. 6.4). In glioblastoma (GBM) and transformed fibroblasts, cytokine-induced ER stress results in the activation and phosphorylation of PERK. Activation of PERK and UPR is generally considered a protective response. However, prolonged PERK activity and stimulation of UPR signaling can lead to cell death. Previous studies have validated the involvement of PERK in MDA-7/IL-24-mediated autophagy [62]. PERK−/− cells or cells that were transformed to carry a dominant negative PERK gene displayed increased resistance to MDA-7/IL-24-induced autophagy and cell death [99, 104]. Likewise, overexpression of BiP/GRP78 blocked MDA-7/IL-24 induced autophagy and cell death by inhibiting PERK activation [104]. Conversely, activation of PERK triggered the vacuolization of LC3 protein and resulted in an increased expression of autophagy markers like ATG5 and Beclin1 [104]. Furthermore, inhibition of autophagy by suppression of important mediators like Beclin1 and ATG5 or treatment with a chemical inhibitor, 3-methyl adenine, dramatically attenuated MDA-7/IL-24-induced cytotoxicity [104].
In prostate cancer cells, autophagy plays a different role upon MDA-7/IL-24 treatment. Unlike GBM cells, blocking autophagy in prostate cancer cells by using 3-methyl adenine or by suppressing the expression of Beclin1 enhanced MDA-7/IL-24 cytotoxicity [6]. In contrast, knockdown of ATG5, an autophagy marker, resulted in decreased MDA-7/IL-24-mediated toxic autophagy and apoptosis [6]. This discrepancy could be explained by the differential role of ATG5 in programmed cell death. In prostate cancer cells, ATG5 is cleaved into a 25-kDa fragment in an autophagy-dependent manner [6]. This small fragment, in turn, induces mitochondrial dysfunction via activation of pro-apoptotic molecules like Bax and Bak leading to enhanced cell death.
In renal and ovarian cancer cells, MDA-7/IL-24-induced autophagy is mainly dependent on CD95 signaling. Treatment of renal carcinoma cells with GST-MDA-7 enhanced ER stress response via CD95 signaling eliciting autophagy and cell death [63]. Previous reports have shown that drug-induced activation of CD95 resulted in a protective form of autophagy that promoted survival in melanoma, hepatoma, renal, and pancreatic cancer cells [64]. Subsequent studies in renal carcinoma have found a direct correlation between suppression of ATG5 and/or Beclin1 expression and MDA-7/IL-24-mediated cell death [61]. Thus, MDA-7/IL-24 induces autophagy in a wide variety of cell types, but the precise function of autophagy in response to MDA-7/IL-24 varies greatly according to the tumor cell type. The differential response induced by MDA-7/IL-24 in a broad range of cancer cells versus normal cells could be attributed to the inherent complexities in these cells. ROS is known to be involved in the autophagic process. We speculate that MDA-7/IL-24-mediated PERK activation might lead to increased ROS production in cancer cells (that have higher basal levels of ROS as compared to normal cells), which leads to toxic autophagy and cell death in these transformed cells without affecting normal cells.
6.3.3 Anti-angiogenesis
Induction of angiogenesis is another hallmark of cancer [35]. Tumors rely on the (tumor) vasculature in order to maintain a source of nutrients and oxygen and to eliminate metabolic wastes and carbon dioxide. In addition to direct targeting of cancer cells, MDA-7/IL-24 also plays a role in inhibiting angiogenesis, thereby further inhibiting tumor growth.
Human umbilical vascular endothelial cells (HUVEC) do not endogenously express mda-7/IL-24. Infection of HUVEC cells with Ad.mda-7 induced sustained mda-7/IL-24 expression from day 1 to 4 with no sign of cytotoxicity. The ability of MDA-7/IL-24 to inhibit endothelial cell differentiation was assessed in HUVEC cells and compared to a known cell cycle regulator p16. Both MDA-7/IL-24 and p16 caused an inhibition of endothelial cell formation; however MDA-7/IL-24 showed a more profound effect with no evidence of tube formation in HUVEC as compared to p16, that allowed formation of full and hemitubes [70]. Injection of Ad.mda-7 into subcutaneous lung tumor xenografts in athymic mice caused an inhibition of angiogenesis (as evidenced by reduced tumor vasculature) [67] with tumors expressing low levels of CD31 expression, a protein highly expressed in neo-angiogenic endothelial cells. Simultaneously the expression of TRAIL, a promoter of apoptosis, was upregulated, indicating that Ad.mda-7 infection caused tumor inhibition by multiple mechanisms [70].
Inhibition of tumor angiogenesis can be brought about by directly suppressing tumor blood vessel formation or indirectly by suppressing production of tumor-derived growth factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and intereukin-8 (IL-8) [46]. Evidence of direct suppression of tumor blood vessel formation was demonstrated using secreted purified MDA-7/IL-24 protein [67]. MDA-7/IL-24 protein inhibited endothelial cell differentiation in vitro in a dose-dependent manner, without any cytotoxic effects. Induction of endothelial cell migration by VEGF and bFGF were also inhibited by MDA-7/IL-24 protein. Further analysis revealed an essential role of IL-22R1, one of the two MDA-7/IL-24 cognate receptors, in MDA-7/IL-24-mediated anti-angiogenic abilities. Introduction of an IL-22 receptor-blocking antibody along with MDA-7/IL-24 protein led to abrogation of inhibition of endothelial differentiation, indicating IL-22 receptors play an important role in MDA-7/IL-24 anti-angiogenesis abilities. The role of IL-20 receptors in MDA-7/IL-24-mediated anti-angiogenic abilities is currently unknown. MDA-7/IL-24-receptor-mediated downstream signaling (such as STAT-1) might also enhance MDA-7/IL-24 anti-angiogenic activity. MDA-7/IL-24 inhibits PI3K/AKT signaling in lung and breast cancer cells. PI3K and AKT have known roles in angiogenesis and their inhibition might further aid in MDA-7/IL-24-mediated anti-angiogenic abilities.
MDA-7/IL-24 also inhibits angiogenesis indirectly by suppressing angiogenic tumor-derived growth factors. Inhibition of tumor-derived growth factors will lead to further suppression of tumor growth by blocking neovascularization [46]. Infection of human lung cancer cells with Ad.mda-7 causes down regulation of the expression of two key modulators of angiogenesis, vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β). Infection of human lung cancer xenografts with Ad.mda-7 caused an inhibition of VEGF, bFGF and IL-8 [59]. MDA-7/IL-24 was shown to regulate VEGF transcription resulting in reduced VEGF protein expression. Src kinase is known to regulate VEGF transcription and expression and MDA-7/IL-24 can directly inhibit Src kinase activity [41]. Thus, MDA-7/IL-24 inhibits tumor angiogenesis by multiple mechanisms.
6.3.4 Inhibition of Invasion and Metastasis
MDA-7/IL-24 also plays a role in inhibiting tumor invasion and metastasis, yet another hallmark of cancer. Treatment options and survival rates are adversely affected once a tumor has metastasized to a distant area in the body. MDA-7/IL-24 was shown to inhibit migration and invasion of several different types of cancer. Human non-small cell lung carcinoma cells (H1299 and A549) infected with Ad.mda-7 showed a decrease in in vitro migration and invasion by downregulating expression of PI3K (phosphatidylinositol 3-kinase)/PKB (protein kinase B), FAK (focal adhesion kinase), MMP-2 (matrix metalloproteinase-2) and MMP-9. Treatment using Ad.mda-7 in an in vivo experimental lung metastasis model also resulted in a decrease in the number of tumors [66]. Infection of human cervical cancer cells (CaSki) with Ad.mda-7 caused a decrease in in vitro cell migration and invasion that was attributed to downregulation of MMP-2 and upregulation of p38 MAPK (mitogen-activated protein kinase) [86]. Over expression of MDA-7/IL-24 in human hepatocellular carcinoma cells (HepG2 and BEL-7402) in vitro inhibited tumor cell adhesion and invasion and induced a G2/M cell cycle arrest [39], but not in normal liver cells (L02) [94]. MDA-7/IL-24 mediated inhibition of adhesion and invasion by inhibiting the expression of metastasis-related genes such as CD44, ICAM-1, MMP-2, MMP-9, Cyclin B1, Twist, Survivin, TGF-β and pAkt and downregulating NFκB transcriptional activity. On the other hand, expression of E-cadherin and p-ERK was increased along with an increase in transcriptional activity of AP-1 [39].
To determine whether MDA-7/IL-24-mediated inhibition of invasion was brought about via binding to its cognate receptors or in a manner independent of receptor signaling, a non-secretable version of MDA-7/IL-24 (lacking the secretory signal peptide) was generated. Infection of prostate cancer cells (C8161) with both secretable and non-secretable versions of Ad.mda-7 inhibited Matrigel invasion to a similar extent [79] suggesting that mda-7/IL-24 could inhibit invasion by both receptor-mediated and receptor-independent pathways.
An interesting discovery regarding MDA-7/IL-24 was made while examining its role in pancreatic cancer. Infection of Ad.mda-7 into a number of different cancer cell lines resulted in cancer cell growth suppression and apoptosis (as discussed in the apoptosis section); however Ad.mda-7 did not show a growth suppressive or apoptotic effect in pancreatic cancer cells. Pancreatic cancer is often associated with genetic changes in a subset of genes and one of the most frequently altered genes in pancreatic cancer is the K-ras oncogene (85–95 %). Silencing the expression of K-ras in pancreatic cancer cells along with simultaneous infection with Ad.mda-7 caused growth suppression and apoptosis, suggesting K-ras mediated translational suppression of mda-7/IL-24 mRNA to protein [90]. Interestingly pancreatic cancer cells (MIA PaCA-2) infected with Ad.mda-7 and transfected with antisense K-ras plasmid before injecting into athymic mice resulted in complete tumor suppression as compared to rapidly growing control Ad.mda-7 infected tumors [90]. Thus, MDA-7/IL-24 secreted from a very small subset of tumor cells was able to inhibit growth of the entire tumor, an effect that is known as a ‘bystander’ antitumor effect. Since then this ‘bystander’ effect has been observed in several cancer types. It was determined that N-glycosylation of MDA-7/IL-24 was not essential for the ‘bystander’ effect [82]. The ability of MDA-7/IL-24 secreted by normal cells to mediate ‘bystander’ effect was also evaluated [89]. Normal cells (primary and immortalized human melanocytes FM516-SV, astrocytes IM-PHFA and prostate epithelial cells P69) infected with Ad.mda-7 were able to secrete MDA-7/IL-24 without adverse effects on normal growth. Secreted MDA-7/IL-24 from these three normal cell lines was able to inhibit anchorage-independent growth of DU-145 prostate cancer cells. To validate that the ‘bystander’ effects were mediated by secreted MDA-7/IL-24, the ability of MDA-7/IL-24 to inhibit anchorage-independent growth and invasion was assessed in cells with and without functional IL-20/IL-22 receptors. MDA-7/IL-24 secreted from normal prostate epithelial cells (P69) inhibited anchorage-independent growth and invasion in prostate cancer cells (DU-145 and BxPC-3) with functional IL-20/IL-22 receptors, but not in lung cancer cells (A549) that lack a complete set of receptors [89]. Evidence for ‘bystander’ activity in vivo was obtained using a xenograft mouse model. Tumors were formed by injecting breast cancer cells into the right and left flanks of athymic mice. Tumors on the left flank were injected with Ad.PEG-E1A-mda-7 (a Cancer Terminator Virus; CTV), a conditionally replication competent adenovirus controlling E1A expression in cancer cells and expressing mda-7/IL-24 when replicating in cancer cells, which eliminated tumors on both flanks of the mice [76]. Purging of tumors on the right untreated flank by MDA-7/IL-24 treatment of the left flank serves as a good model to assess systemic inhibitory potential of MDA-7/IL-24 in vivo on secondary tumors in distant regions of the animal. The CTV, first generation in a serotype 5 adenovirus and a second generation CTV in a tropism-modified Ad.5/3 virus, has also shown profound primary and ‘bystander’ antitumor activity in additional tumor models including breast cancer [76], therapy resistant prostate cancer [2, 72], melanoma [75], glioblastoma multiforme [34], and pancreatic cancer [78, 77].
In conclusion, MDA-7/IL-24 exerts its anti-cancer activities by targeting multiple tumor growth mechanisms making it a very attractive translational molecule for treatment of human cancers.
6.4 Combination Effects of MDA-7/IL-24
Cancer is a multi-factorial disease and involves cross talk between complex molecular pathways [35]. Advances in the biomedical field have broadened our understanding regarding the key effector molecules and aberrant pathways involved but successful application of targeted therapies has been limited. This is due to the presence of alternate or compensatory pathways that cancer cells use to thrive and acquire enhanced resistance to drugs. To address this problem, a combination approach using multiple targeted agents has been successfully used in several pre-clinical and clinical studies. A combination strategy offers multiple advantages. Firstly, combination of therapeutic agents that target similar molecular pathways may provide synergistic effects. Secondly, agents that target different pathways may help to overcome compensatory pathways that are responsible for reducing the sensitivity toward drugs and providing low therapeutic benefit. Thirdly, combination treatments may also circumvent the tumor heterogeneity, particularly in advanced stage cancer patients, where genetic instability gives rise to aggressive variants, each with different characteristics and sensitivity to therapeutic regimens. Tumor heterogeneity may not only result in treatment failure but also increases the number of potential sites that need to be targeted to inhibit tumor growth and proliferation. In this scenario, combinatorial approaches may provide better therapeutic effectiveness as compared to single agents. Also, in the adjuvant setting, combination therapy is considered more favorable as compared to a single therapy due to the survival benefit from a clinical standpoint. MDA-7/IL-24 has been shown to induce synergistic effects in a wide spectrum of human cancers when used in combination with other agents. For example, McKenzie et al. demonstrated significant growth inhibitory effects in Her-2/neu-overexpressing breast cancer cells following treatment with Ad.mda-7 in combination with a monoclonal antibody targeting Her-2/neu receptor [58]. The inhibition in tumor growth was partly mediated by effect of combination agents on the β-catenin, and AKT survival pathways. In another study, mda-7/IL-24 incorporated in an adenoviral vector was used along with bevacizumab in a lung cancer model [40]. The combination treatment induced cell cycle arrest and apoptosis in the in vitro system. Similar effects were recapitulated in the xenograft lung cancer model. The subcutaneously implanted tumors receiving Ad.mda-7 plus bevacizumab showed improved survival rate as compared to their control counterparts with complete tumor regression observed at the completion of the study. Emdad et al. determined the efficacy of combining Ad.mda-7 with a selective EGFR inhibitor, gefitinib, in non-small cell lung cancer [22]. Combination treatment enhanced apoptotic cell death in the treated cells by increasing the expression of a downstream effector molecule, RNA-activated protein kinase (PKR). Zheng et al. combined Ad.mda-7 with an alkylating agent, temozolamide, to overcome the chemoresistance in human melanoma cells [106]. They observed increased cell death in melanoma cells that were treated with optimal doses of Ad.mda-7 and temozolamide. A combinatorial effect was also observed when a bacterially synthesized glutathione S-transferase (GST)-fusion protein of MDA-7/IL-24, GST-MDA-7, was combined with Tarceva in non-small cell lung carcinoma cells [31]. Similarly combining GST-MDA-7 protein with arsenic trioxide effectively controlled growth and proliferation of renal carcinoma cells [102]. The free radicals generated by arsenic trioxide interacted with GST-MDA-7 and induced cell death in the treated cells by a non-apoptotic pathway. An oncolytic adenovirus incorporated with mda-7/IL-24 (ZD55-IL-24) was used in combination with cisplatin to analyze growth inhibitory effects on a panel of cancer cells [98]. The combination approach dramatically enhanced the cytotoxic and apoptotic effects in the tested cancer cells as compared to their normal counterparts. Chimeric adenoviral vector carrying mda-7/IL-24 gene (Ad5/3-MDA7) also mediated enhanced gene transduction to the otherwise refractory colorectal cancer and prostate cancer cells and when used in combination with an apogossypol derivative BI-97C1, Sabutoclax, toxicity to MDA-7/IL-24 was enhanced [2, 14].
In addition to chemotherapeutic agents, monoclonal antibodies, and chemical inhibitors, MDA-7/IL-24 has also been effectively used in conjunction with other conventional therapies. Nishikawa and colleagues observed that in non-small cell lung carcinoma, MDA-7/IL-24 enhanced the sensitization of resistant cancer cells to ionizing radiation and induced cell death by affecting angiogenesis in the supporting tumor endothelial cells [59]. In another study, Chada et al. compared the efficacy of gene transfer when MDA-7/IL-24 was combined with a range of chemotherapy agents, monoclonal antibodies, and radiation [10]. The authors found that combination approach effectively improved the overall sensitivity of the breast cancer cells toward most of the tested therapeutics in an additive or synergistic manner.
Another interesting study explored the idea of targeting both the tumor as well as immunologic components by administering Ad.mda-7 along with a secreted form of an ER resident chaperone protein Grp170 (Ad.sgrp170) [29]. Grp170 acts as a strong immunostimulatory agent and antigen carrier. Combined delivery of both the Ad vectors (Ad.mda-7 and Ad.sgrp170) effectively reduced growth of tumors in TRAMP-C2 prostate cancer model when compared with single agent. Furthermore, it enhanced the antigen and tumor-specific T cell response evident by increase in the production of interferon-gamma and cytolytic activity. The MDA-7/IL-24 and Grp170 combination also provided protection against subsequent tumor challenge and displayed ‘bystander’ effect.
Collectively, these observations suggest that careful design and rational combination of traditional and novel therapeutic agents with MDA-7/IL-24 represents a viable strategy to induce multi-pronged attack on cancer cells and significantly enhance therapeutic outcome.
6.5 Phase I Clinical Trial with Ad.mda-7 (INGN-241)
The efficacy of MDA-7/IL-24 as a cancer therapeutic has been demonstrated in a wide range of tumor models [16, 32, 46]. Based on the unprecedented success in pre-clinical studies, a Phase I clinical trial was initiated to determine the therapeutic potential of mda-7/IL-24 incorporated in an adenoviral vector (Ad.mda-7; INGN 241) [11, 21, 25, 46, 47, 71, 93]. The adenoviral vector used in this study had deletions in the E1 and E3 regions making it replication-incompetent. The human mda-7/IL-24 sequence was cloned into the vector along with the cytomegalovirus immediate-early promoter and SV40 polyadenylation sequence. The Phase I study evaluated the safety profile, pharmacodynamics, pharmacokinetics of vector-specific DNA, mRNA, MDA-7/IL-24 protein distribution and its biological effects, both locally and systemically. Twenty-eight patients diagnosed with squamous cell carcinoma of the head and neck (SCCHN), non-small cell lung carcinoma, melanoma, breast carcinoma, colorectal carcinoma, lymphoma, hepatoma, adeno-carcinoma, sarcoma and carcinomas of the adrenal, bladder, penis, parotid, lip and kidney were enrolled in this dose-escalating study. All the patients included in the study received prior treatment with chemotherapeutic drugs, immunologic agents, radiation, and/or surgery. They were divided into eight cohorts based on the treatment dose of Ad.mda-7 (INGN 241) (ranging from 2 × 1010 vp (viral particles) to 2 × 1012 vp), time of post-treatment biopsy (24 h to 30 days), and frequency/mode of administration (single dose, divided dose or multiple doses) (Table 6.1).
Table 6.1.
Patients cohorts and treatment profile
| Cohorts | No. of patientsa | Diagnosisb | Prior treatmentsc | Dose (vp) | Biopsy time | Adverse eventsd |
|---|---|---|---|---|---|---|
| 1 | 1 (1) | BrCa (1) | S, C, Ca, N, H, G | 2 × 1010 | +24 h | 2 |
| 2 | 1 (1) | CoCa (1) | S, F, I | 2 × 1011 | +24 h | 1 |
| 3 | 3 (3) | SCCHN (1), Mel (1), LCL (1) | S, RT, T, P, C, V, D, Fl, Cl | 2 × 1012 | +24 h | 0/1/1 |
| 4 | 3 (3) | BrCa (1), AdrCa (1), Hep (1) | S, A, C, T, Ta, N, P, RT, E | 2 × 1012 | +48 h | 0/1/0 |
| 5 | 4 (3) | BrCa (1), Mel (1), Mel (1) | S, Ta, RT, I, D | 2 × 1012 | +96 h | 0/2/2 |
| 6 | 1 (1) | BrCa (1) | S, A, T, Ta | 2 × 1012, divided dose | +48 h | 0 |
| 7 | 7 (5) | TCC (1), Mel (1), CoCa (1), SCCHN (2) | S, M, V, A, P, T, G, RT, F, I | 2 × 1012 | Day 30 | 2/0/0/2/2 |
| 8 | 8 (5) | Mel (3), SCCHN (2) | S, RT, IF, IT, P, T, F | 2 × 1012, twice weekly × 3 | Day 30 | 2/2/2/1/1/e |
Number of patients enrolled per cohort is listed. The number of patients completing at least one round of treatment is indicated in parentheses
Diagnosis: BrCa breast carcinoma, CoCa colon carcinoma, SCCHN squamous cell carcinoma of the head and neck, Mel melanoma, LCL large cell lymphoma, AdrCa Adrenal carcinoma, Hep hepatoma, TCC transitional cell carcinoma
Prior treatments: S Surgery, A adriamycin, C cyclophosphamide, Ca capecitabine, Cl chlorambucil, D dacarbazine, E etoposide, F 5-fluorouracil, Fl fludarabine, G gemcitabine, H herceptin, I irinotecan, IF IFN-α, IT immunotherapy, M methotrexate, N navelbine, P platinum, RT radiotherapy, T taxane, Ta tamoxifen, V vincistine
Adverse effects possibly due to Ad.MDA-7 (INGN 241) administration are indicated
One patient from cohort 8 experienced a grade 3 SAE and withdrew from the study (Adapted by permission from Cunningham et al. [11], Copyright 2005)
Cohorts one to three were administered with single intratumoral injection of Ad.mda-7 (INGN 241) and tumors were resected 24 h post-injection. Cohorts four and five were treated with high dose of Ad.mda-7 and tumors were excised 48 h or 96 h after treatment, respectively. Subjects in cohort six received ten injections totaling 2 × 1012 vp and the tumors were resected 48 h post-administration. Cohort seven was injected with single dose of Ad.mda-7 (INGN 241) and biopsies were taken 30 days post-treatment. In cohort eight, patients received high dose of Ad.mda-7 (INGN 241) twice a week for a period of 3 weeks and tumors were biopsied at day 30 (Table 6.1). The biological effects in response to Ad.mda-7 (INGN 241) are summarized according to the tumor type in Table 6.2.
Table 6.2.
Ad.mda-7 (INGN 241) induces biological effects in different tumor types
| Tumor type | Dose (vp) | Apoptosis at central regiona | Apoptosis at peripheral regions | Biological responseb |
|---|---|---|---|---|
| Breast carcinoma | 2 × 1010 | ++ | + | T, b, K |
| Colorectal carcinoma | 2 × 1011 | +++ | + | T, b, I, K |
| Melanoma | 2 × 1012 | ++ | − | T, b, I, C |
| Breast | 2 × 1012 | +++ | ++ | T, b, K |
| Squamous cell carcinoma of the head and neck | 2 × 1012 | +++ | ++ | T, b, C, K |
| Adrenal carcinoma | 2 × 1012 | +++ | + | T, b |
| Hepatoma | 2 × 1012 | + | − | T, b, C |
TUNEL reactivity: −, <5 %; +, 5–19 %; ++, 20–49 %; +++, >50 %
Biological response is mentioned as T TUNEL reactivity, b β-catenin decrease, I iNOS reduction, C CD31 reduction, K Ki-67 staining decrease (Adapted by permission from Tong et al. [93], Copyright 2005)
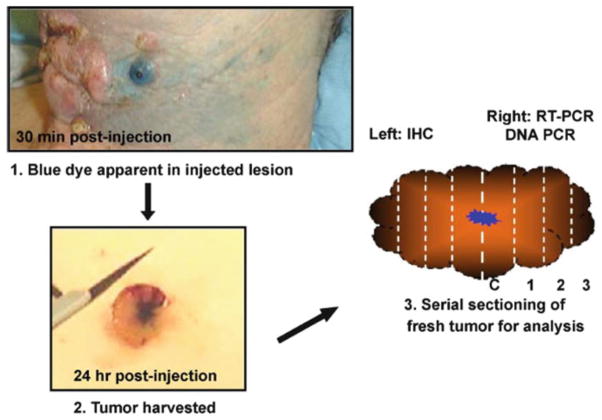
A comprehensive analysis was done to follow the vector biodistribution with time. To identify the injection site, Ad.mda-7 (INGN 241) was mixed with isosulfan blue dye before administration (Fig. 6.5). Maximal levels of vector-specific DNA and mRNA were detected at the site of injection (the center of the injected tumor averaged more than 1 × 108 vector DNA copies/μg) and it decreased in the peripheral areas. Low levels of vector DNA was detectable up to 3 cm from the site of injection. This could be due to the ‘bystander’ activity induced by secreted MDA-7/IL-24 protein in the neighboring tumor cells [82, 83, 85, 89]. The overall levels of vector DNA and RNA varied in a dose- and time-dependent manner with high levels detected in tumors 24–48 h post-injection [11, 21, 71]. The levels dropped by nearly three logs of magnitude in tumors that were resected at day 4. However, vector DNA was present above baseline levels at 30 days.
Fig. 6.5. Representation of excisional biopsy procedure.
1 Ad.mda-7/IL-24 (INGN 241) vector was mixed with isosulfan blue dye to identify the site of injection. 2 Lesion was resected 24 h post-injection. 3 The bisected lesion was processed into serial sections and the left portion is fixed and analyzed by immunohistochemistry. The right half was sectioned and frozen for quantitative PCR analyses (Reprinted by permission from Cunningham et al. [11], Copyright 2005)
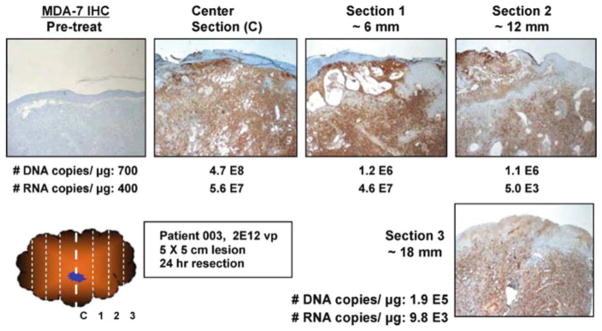
Further, immunohistochemical analysis was done to analyze the expression of MDA-7/IL-24 protein (Fig. 6.6). On an average, 20 % of tumors that were treated with low dose of Ad.mda-7 (INGN 241) displayed positive staining for MDA-7/IL-24 protein [11]. The percentage of lesions showing positive MDA-7/IL-24 staining increased up to 53 % for cohorts that were administered with high dose of Ad.mda-7 (INGN 241). Tumor sections were also evaluated for the expression of β-catenin proto-oncogene [93]. It was found that the expression of β-catenin was dramatically reduced post-treatment with Ad.mda-7 (INGN 241). In addition, the levels of iNOS (inducible nitric oxide synthase) were also decreased in four of the nine treatment subjects [93]. CD31 acts as a marker for angiogenesis. Upon analysis, it was found that CD31 levels decreased by 28 % in the treated lesions [11, 93]. TUNEL assays were performed to detect the apoptotic activity in the tumor sections. Up to 80 % of tumor cells at the injection site showed positive TUNEL staining. A good correlation was found between the areas of TUNEL reactivity and MDA-7/IL-24 staining [11, 93]. The MDA-7/IL-24 staining and TUNEL reactivity dropped down to baseline levels 30 days post treatment of tumor lesions. Ki-67 staining was performed as an indicator of tumor cell proliferation. After evaluation, 67 % of tumors showed reduced expression of Ki-67 following Ad.mda-7 (INGN 241) administration [93].
Fig. 6.6. MDA-7/IL-24 gene expression correlates with Ad vector distribution throughout the tumor.
Half of the tumor was analyzed for MDA-7/IL-24 transgene expression and the remaining half to detect vector specific DNA and RNA levels. The number of DNA copies/μg genomic DNA and number of RNA copies/μg total RNA are indicated for each tumor section (Reprinted by permission from Cunningham et al. [11], Copyright 2005)
Activation of immune response by MDA-7/IL-24 was studied by analyzing the levels of inflammatory cytokines [93]. Most of the patients displayed enhanced serum levels of IL-6, IL-10, and TNF-α. A small number of patients showed a rise in the levels of GM-CSF, and IL-2 [71, 93]. This cytokine profile indicates stimulation of TH-1 type of immune response following Ad.mda-7 (INGN 241) administration [71]. However, observed increase in the levels of different cytokines was a transient response, which subsided within 15–30 days post-injection. There were some mild side effects including pain and erythema at the site of injection. One of the patients suffered from grade 3 serious adverse event (SAE) and was discontinued from the trial.
At the completion of this study, three patients from cohort seven were followed up for clinical evaluation. No change was evident in tumor size in two patients with melanoma and colorectal carcinoma whereas about 23 % reduction in tumor size was observed in one of the patients in same group. Partial regression in tumors was observed in at least two of the five cohort eight patients who received bi-weekly injections of Ad.mda-7 (INGN 241) for 3 weeks. On follow-up, a 64-old female patient with metastatic melanoma showed a dramatic reduction in tumor size after the sixth injection with continued regression for another 2 weeks. Similar response was observed following Ad.mda-7 (INGN 241) injection in the second lesion. Tumor size was decreased by 84 % by the fifth injection. Non-injected lesions also displayed erythema but there was no reduction in tumor size.
Overall, Ad.mda-7’s (INGN 241) entry into the clinic in a Phase I trial employing intratumoral injection was exceptionally successful demonstrating that this therapy was well-tolerated in human subjects with an ~44 % clinical response in injected lesions [11, 21, 71, 93]. Future clinical trials with MDA-7/IL-24 employing improved delivery vectors, such as the CTV [72, 75, 76, 78, 77] and targeted delivery systemically, using ultrasound-targeted microbubble-destruction [14, 15], should elicit even more relevant and enduring clinical responses. The findings from these newer studies will expand our current knowledge and culminate in safer and even more efficient delivery of this therapeutic gene in cancer patients.
6.6 Conclusions and Future Prospectives
As described in this chapter, the encouraging results in the preclinical studies and Phase I clinical trial with MDA-7/IL-24 (INGN 241) highlights the potency of this multifunctional cytokine as an efficient anti-cancer agent. Over the past few years, numerous studies have provided insights into the mechanism of action and diverse signaling pathways that are triggered in response to MDA-7/IL-24. At present, research is focused on expanding our current understanding and enhancing the overall potential of MDA-7/IL-24. This involves development of novel strategies to transfer the therapeutic gene to cancer cells in a selective manner [4, 12, 13]. Our laboratory and others are finding solutions to efficiently deliver the conditionally replicating adenoviruses carrying MDA-7/IL-24 or purified protein using targeted microbubbles by the ultrasound-targeted microbubble-destruction (UTMD) approach [2, 13–15, 30, 1]. This would not only shield adenoviral vectors from neutralizing antibodies following systemic administration but also avoid its sequestration in the liver and other non-specific tissues thus, maximizing the biological outcome. Furthermore, studies involving high throughput screening approaches to develop the next generation of molecules that would augment the expression and/or activity of MDA-7/IL-24 in the target cell/tissue are currently underway. Rational combination of agents that would synergize with MDA-7/IL-24 would help to overcome the resistance of cancer cells toward conventional treatment regimens as well as generate the biological response at low doses. Additionally, novel strategies to define ways of selectively inducing mda-7/IL-24 expression, protein production and secretion using small molecules would also augment the applications of this novel cytokine for therapy of cancer. Accordingly, concerted research efforts in the context of MDA-7/IL-24 will not only augment our understanding in the related field of mechanisms of action of cytokines, but will also pave the way for future translational studies for therapeutic intervention of cancer.
Acknowledgments
The present studies were supported in part by National Institutes of Health grants P01 CA104177, R01 CA097318, R01 CA127641 and R01 CA168517; Department of Defense synergy grant W81XWH-10-PCRP-SIDA; National Foundation for Cancer Research; an A. David Mazzone Prostate Cancer Foundation Challenge Award; NCI Cancer Center Support Grant to VCU Massey Cancer Center; and VCU Massey Cancer Center developmental funds. D.S. and X.-Y.W. are Harrison Scholars in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Contributor Information
Mitchell E. Menezes, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Shilpa Bhatia, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Praveen Bhoopathi, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Swadesh K. Das, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Luni Emdad, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Santanu Dasgupta, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Paul Dent, VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. Department of Neurosurgery, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Xiang-Yang Wang, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Devanand Sarkar, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
Paul B. Fisher, Email: pbfisher@vcu.edu, Department of Human and Molecular Genetics, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Institute of Molecular Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. VCU Massey Cancer Center, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA
References
- 1.Azab B, Dash R, Das SK, Bhutia SK, Shen X-N, Quinn BA, Dent P, Dmitriev IP, Wang X-Y, Pellecchia M, et al. Enhanced prostate cancer gene transfer and therapy using a novel serotype chimera cancer terminator virus (Ad.5/3-CTV) J Cell Physiol. 2013 doi: 10.1002/jcp.24408. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, Sarkar S, Wang XY, Hedvat M, Dmitriev IP, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. J Cell Physiol. 2012;227:2145–2153. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastonero S, Le Priol Y, Armand M, Bernard CS, Reynaud-Gaubert M, Olive D, Parzy D, de Bentzmann S, Capo C, Mege JL. New microbicidal functions of tracheal glands: defective anti-infectious response to Pseudomonas aeruginosa in cystic fibrosis. PLoS One. 2009;4:e5357. doi: 10.1371/journal.pone.0005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Menezes ME, Das SK, Emdad L, Dasgupta S, Wang XY, Sarkar D, Fisher PB. Innovative approaches for enhancing cancer gene therapy. Discov Med. 2013;15:309–317. [PubMed] [Google Scholar]
- 5.Bhutia SK, Das SK, Azab B, Menezes ME, Dent P, Wang XY, Sarkar D, Fisher PB. Targeting breast cancer initiating/stem cells with melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24) Int J Cancer. 2013 doi: 10.1002/ijc.28289. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutia SK, Mukhopadhyay S, Sinha N, Das DN, Panda PK, Patra SK, Maiti TK, Mandal M, Dent P, Wang XY, et al. Autophagy: cancer’s friend or foe? Adv Cancer Res. 2013;118:61–95. doi: 10.1016/B978-0-12-407173-5.00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 9.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 10.Chada S, Mhashilkar AM, Liu Y, Nishikawa T, Bocangel D, Zheng M, Vorburger SA, Pataer A, Swisher SG, Ramesh R, et al. mda-7 gene transfer sensitizes breast carcinoma cells to chemotherapy, biologic therapies and radiotherapy: correlation with expression of bcl-2 family members. Cancer Gene Ther. 2006;13:490–502. doi: 10.1038/sj.cgt.7700915. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Curiel DT, Fisher PB, editors. Applications of viruses for cancer therapy. Adv Cancer Res. 2012;115:1–334. [Google Scholar]
- 13.Das SK, Sarkar S, Dash R, Dent P, Wang XY, Sarkar D, Fisher PB. Chapter one–cancer terminator viruses and approaches for enhancing therapeutic outcomes. Adv Cancer Res. 2012;115:1–38. doi: 10.1016/B978-0-12-398342-8.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, Rahmani M, Wei J, Hedvat M, Dent P, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A. 2011;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash R, Azab B, Shen XN, Sokhi UK, Sarkar S, Su ZZ, Wang XY, Claudio PP, Dent P, Dmitriev IP, et al. Developing an effective gene therapy for prostate cancer: new technologies with potential to translate from the laboratory into the clinic. Discov Med. 2011;11:46–56. [PMC free article] [PubMed] [Google Scholar]
- 16.Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, et al. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev. 2010;21:381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dent P, Yacoub A, Hamed HA, Park MA, Dash R, Bhutia SK, Sarkar D, Wang X-Y, Gupta P, Emdad L, et al. The development of MDA-7/IL-24 as a cancer therapeutic. Pharmacol Ther. 2010;128:375–384. doi: 10.1016/j.pharmthera.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 20.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–3549. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 21.Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8:1633–1643. doi: 10.1517/14712598.8.10.1633. [DOI] [PubMed] [Google Scholar]
- 22.Emdad L, Lebedeva IV, Su ZZ, Gupta P, Sarkar D, Settleman J, Fisher PB. Combinatorial treatment of non-small-cell lung cancers with gefitinib and Ad.mda-7 enhances apoptosis-induction and reverses resistance to a single therapy. J Cell Physiol. 2007;210:549–559. doi: 10.1002/jcp.20906. [DOI] [PubMed] [Google Scholar]
- 23.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 24.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 25.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- 26.Fisher PB, Prignoli DR, Hermo H, Jr, Weinstein IB, Pestka S. Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. J Interferon Res. 1985;5:11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- 27.Fisher PB. Enhancement of viral transformation and expression of the transformed phenotype by tumor promoters. In: Slaga TJ, editor. Tumor promotion and cocarcinogenesis in vitro, mechanisms of tumor promotion. CRC Press, Inc; Boca Raton: 1984. pp. 57–123. [Google Scholar]
- 28.Fritsch RM, Schneider G, Saur D, Scheibel M, Schmid RM. Translational repression of MCL-1 couples stress-induced eIF2 alpha phosphorylation to mitochondrial apoptosis initiation. J Biol Chem. 2007;282:22551–22562. doi: 10.1074/jbc.M702673200. [DOI] [PubMed] [Google Scholar]
- 29.Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, Fisher PB, Wang XY. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68:3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, Miranda M, Brunetti A, Salvatore M, Claudio L, et al. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18:295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta P, Emdad L, Lebedeva IV, Sarkar D, Dent P, Curiel DT, Settleman J, Fisher PB. Targeted combinatorial therapy of non-small cell lung carcinoma using a GST-fusion protein of full-length or truncated MDA-7/IL-24 with Tarceva. J Cell Physiol. 2008;215:827–836. doi: 10.1002/jcp.21369. [DOI] [PubMed] [Google Scholar]
- 32.Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, Valerie K, Sarkar D, Fisher PB. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–8191. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- 34.Hamed HA, Yacoub A, Park MA, Archer K, Das SK, Sarkar D, Grant S, Fisher PB, Dent P. Histone deacetylase inhibitors interact with MDA-7/IL-24 to kill primary human glioblastoma cells. Mol Pharmacology. 2013 doi: 10.1124/mol.113.086553. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.He M, Liang P. IL-24 transgenic mice: in vivo evidence of overlapping functions for IL-20, IL-22, and IL-24 in the epidermis. J Immunol. 2010;184:1793–1798. doi: 10.4049/jimmunol.0901829. [DOI] [PubMed] [Google Scholar]
- 37.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 38.Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 39.Huo W, Li ZM, Zhu XM, Bao YM, An LJ. MDA-7/IL-24 suppresses tumor adhesion and invasive potential in hepatocellular carcinoma cell lines. Oncol Rep. 2013 doi: 10.3892/or.2013.2507. (in press) [DOI] [PubMed] [Google Scholar]
- 40.Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC, Chada S, Ramesh R. mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol Ther. 2007;15:287–294. doi: 10.1038/sj.mt.6300035. [DOI] [PubMed] [Google Scholar]
- 41.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda-7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther. 2005;12:707–715. doi: 10.1016/j.ymthe.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 42.Jen EY, Poindexter NJ, Farnsworth ES, Grimm EA. IL-2 regulates the expression of the tumor suppressor IL-24 in melanoma cells. Melanoma Res. 2012;22:19–29. doi: 10.1097/CMR.0b013e32834d2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- 44.Jiang H, Su ZZ, Lin JJ, Goldstein NI, Young CS, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kragstrup TW, Otkjaer K, Holm C, Jorgensen A, Hokland M, Iversen L, Deleuran B. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine. 2008;41:16–23. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, et al. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience. Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- 47.Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, et al. mda-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 49.Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, Reed JC, Fisher PB. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138–8144. [PubMed] [Google Scholar]
- 50.Lebedeva IV, Washington I, Sarkar D, Clark JA, Fine RL, Dent P, Curiel DT, Turro NJ, Fisher PB. Strategy for reversing resistance to a single anticancer agent in human prostate and pancreatic carcinomas. Proc Natl Acad Sci U S A. 2007;104:3484–3489. doi: 10.1073/pnas.0700042104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Y, Chen H, Wang Q, Luo F, Yan J, Zhang XL. IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells. Eur J Immunol. 2009;39:3357–3368. doi: 10.1002/eji.200939678. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y, Chen HD, Wang Y, Wang Q, Li Y, Zhao Y, Zhang XL. Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection. Microbes Infect Instit Pasteur. 2011;13:1099–1110. doi: 10.1016/j.micinf.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Madireddi MT, Dent P, Fisher PB. AP-1 and C/EBP transcription factors contribute to mda-7 gene promoter activity during human melanoma differentiation. J Cell Physiol. 2000;185:36–46. doi: 10.1002/1097-4652(200010)185:1<36::AID-JCP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 55.Madireddi MT, Dent P, Fisher PB. Regulation of mda-7 gene expression during human melanoma differentiation. Oncogene. 2000;19:1362–1368. doi: 10.1038/sj.onc.1203424. [DOI] [PubMed] [Google Scholar]
- 56.Madireddi MT, Su ZZ, Young CS, Goldstein NI, Fisher PB. Mda-7, a novel melanoma differentiation associated gene with promise for cancer gene therapy. Adv Exp Med Biol. 2000;465:239–261. doi: 10.1007/0-306-46817-4_22. [DOI] [PubMed] [Google Scholar]
- 57.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136:437–442. doi: 10.1016/j.surg.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther. 2004;9:818–828. doi: 10.1016/j.ymthe.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 60.Otkjaer K, Holtmann H, Kragstrup TW, Paludan SR, Johansen C, Gaestel M, Kragballe K, Iversen L. The p38 MAPK regulates IL-24 expression by stabilization of the 3′ UTR of IL-24 mRNA. PLoS One. 2010;5:e8671. doi: 10.1371/journal.pone.0008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, Sarkar D, Rahmani M, Graf M, Yacoub A, et al. MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther. 2009;8:1280–1291. doi: 10.1158/1535-7163.MCT-09-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, et al. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4:513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park MA, Zhang G, Norris J, Hylemon PB, Fisher PB, Grant S, Dent P. Regulation of autophagy by ceramide-CD95-PERK signaling. Autophagy. 2008;4:929–931. doi: 10.4161/auto.6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510–518. doi: 10.1016/j.ymthe.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 67.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- 68.Raven JF, Koromilas AE. PERK and PKR: old kinases learn new tricks. Cell Cycle. 2008;7:1146–1150. doi: 10.4161/cc.7.9.5811. [DOI] [PubMed] [Google Scholar]
- 69.Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7:2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 70.Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, Branch CD, Zumstein L, Meyn RE, Roth JA, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558–4566. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 71.Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, Curiel DT, Fisher PB. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- 72.Sarkar D, Lebedeva IV, Su ZZ, Park ES, Chatman L, Vozhilla N, Dent P, Curiel DT, Fisher PB. Eradication of therapy-resistant human prostate tumors using a cancer terminator virus. Cancer Res. 2007;67:5434–5442. doi: 10.1158/0008-5472.CAN-07-0195. [DOI] [PubMed] [Google Scholar]
- 73.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Dent P, Fisher PB. mda-7 (IL-24): signaling and functional roles. BioTech Suppl. 2002:30–39. [PubMed] [Google Scholar]
- 74.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar S, Azab B, Quinn BA, Shen XN, Dent P, Klibanov AL, Emdad L, Das SK, Sarkar D, Fisher PB. Chemoprevention gene therapy (CGT) of pancreatic cancer using perillyl alcohol and a novel chimeric serotype cancer terminator virus. Curr Mol Med. 2013 doi: 10.2174/1566524013666131118110827. (in press) [DOI] [PubMed] [Google Scholar]
- 78.Sarkar S, Azab BM, Das SK, Quinn BA, Shen X, Dash R, Emdad L, Thomas S, Dasgupta S, Su ZZ, et al. Chemoprevention gene therapy (CGT): novel combinatorial approach for preventing and treating pancreatic cancer. Curr Mol Med. 2012 doi: 10.2174/1566524011313070008. (in press) [DOI] [PubMed] [Google Scholar]
- 79.Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, Dent P, Fisher PB. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene. 2004;23:7679–7690. doi: 10.1038/sj.onc.1207958. [DOI] [PubMed] [Google Scholar]
- 80.Sauane M, Gopalkrishnan RV, Lebedeva I, Mei MX, Sarkar D, Su ZZ, Kang DC, Dent P, Pestka S, Fisher PB. Mda-7/IL-24 induces apoptosis of diverse cancer cell lines through JAK/STAT-independent pathways. J Cell Physiol. 2003;196:334–345. doi: 10.1002/jcp.10309. [DOI] [PubMed] [Google Scholar]
- 81.Sauane M, Gopalkrishnan RV, Sarkar D, Su ZZ, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 82.Sauane M, Gupta P, Lebedeva IV, Su ZZ, Sarkar D, Randolph A, Valerie K, Gopalkrishnan RV, Fisher PB. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” antitumor activity. Cancer Res. 2006;66:11869–11877. doi: 10.1158/0008-5472.CAN-06-1887. [DOI] [PubMed] [Google Scholar]
- 83.Sauane M, Lebedeva IV, Su ZZ, Choo HT, Randolph A, Valerie K, Dent P, Gopalkrishnan RV, Fisher PB. Melanoma differentiation associated gene-7/interleukin-24 promotes tumor cell-specific apoptosis through both secretory and nonsecretory pathways. Cancer Res. 2004;64:2988–2993. doi: 10.1158/0008-5472.can-04-0200. [DOI] [PubMed] [Google Scholar]
- 84.Sauane M, Su ZZ, Dash R, Liu X, Norris JS, Sarkar D, Lee SG, Allegood JC, Dent P, Spiegel S, et al. Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. J Cell Physiol. 2010;222:546–555. doi: 10.1002/jcp.21969. [DOI] [PubMed] [Google Scholar]
- 85.Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi H, Wei LL, Yuan CF, Yang JX, Yi FP, Ma YP, Song FZ. Melanoma differentiation-associated gene-7/interleukin 24 inhibits invasion and migration of human cervical cancer cells in vitro. Saudi Med J. 2007;28:1671–1675. [PubMed] [Google Scholar]
- 87.Soo C, Shaw WW, Freymiller E, Longaker MT, Bertolami CN, Chiu R, Tieu A, Ting K. Cutaneous rat wounds express c49a, a novel gene with homology to the human melanoma differentiation associated gene, mda-7. J Cell Biochem. 1999;74:1–10. [PubMed] [Google Scholar]
- 88.Stipanuk MH. Macroautophagy and its role in nutrient homeostasis. Nutr Rev. 2009;67:677–689. doi: 10.1111/j.1753-4887.2009.00252.x. [DOI] [PubMed] [Google Scholar]
- 89.Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 90.Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, Dent P, Reed JC, Fisher PB. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 92.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 94.Wang CJ, Zhang H, Chen K, Zheng JW, Xiao CW, Ji WW, Yu Y, Hu HY, Li Y, Xue XB. Ad.mda-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncol Res. 2010;18:561–574. doi: 10.3727/096504010x12767359113929. [DOI] [PubMed] [Google Scholar]
- 95.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 96.Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–1527. doi: 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu SY, Lan SH, Cheng DE, Chen WK, Shen CH, Lee YR, Zuchini R, Liu HS. Ras-related tumorigenesis is suppressed by BNIP3-mediated autophagy through inhibition of cell proliferation. Neoplasia. 2011;13:1171–1182. doi: 10.1593/neo.11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu YM, Zhang KJ, Yue XT, Wang YQ, Yang Y, Li GC, Li N, Wang YG. Enhancement of tumor cell death by combining cisplatin with an oncolytic adenovirus carrying MDA-7/IL-24. Acta Pharmacol Sin. 2009;30:467–477. doi: 10.1038/aps.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yacoub A, Gupta P, Park MA, Rhamani M, Hamed H, Hanna D, Zhang G, Sarkar D, Lebedeva IV, Emdad L, et al. Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1-3 pathway signaling. Mol Cancer Ther. 2008;7:314–329. doi: 10.1158/1535-7163.MCT-07-2150. [DOI] [PubMed] [Google Scholar]
- 100.Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, et al. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yacoub A, Liu R, Park MA, Hamed HA, Dash R, Schramm DN, Sarkar D, Dimitriev IP, Bell JK, Grant S, et al. Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-dependent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells. Mol Pharmacol. 2010;77:298–310. doi: 10.1124/mol.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yacoub A, Mitchell C, Brannon J, Rosenberg E, Qiao L, McKinstry R, Linehan WM, Su ZS, Sarkar D, Lebedeva IV, et al. MDA-7 (interleukin-24) inhibits the proliferation of renal carcinoma cells and interacts with free radicals to promote cell death and loss of reproductive capacity. Mol Cancer Ther. 2003;2:623–632. [PubMed] [Google Scholar]
- 103.Yacoub A, Mitchell C, Lebedeva IV, Sarkar D, Su ZZ, McKinstry R, Gopalkrishnan RV, Grant S, Fisher PB, Dent P. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003;2:347–353. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- 104.Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, Hanna D, Sarkar D, Lebedeva IV, Emdad L, et al. Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008;7:297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 106.Zheng M, Bocangel D, Ramesh R, Ekmekcioglu S, Poindexter N, Grimm EA, Chada S. Interleukin-24 overcomes temozolomide resistance and enhances cell death by down-regulation of O6-methylguanine-DNA methyltransferase in human melanoma cells. Mol Cancer Ther. 2008;7:3842–3851. doi: 10.1158/1535-7163.MCT-08-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]