Abstract
Objectives
The objectives of this study were to test the hypothesis that end-systolic volume (ESV), as a marker of severity of left ventricular (LV) remodeling, influences the relationship between myocardial viability and survival in patients with coronary artery disease and LV systolic dysfunction.
Background
Retrospective studies of ischemic LV dysfunction suggest that severity of LV remodeling determines whether myocardial viability predicts improved survival with surgical (CABG) compared to medical (MED) therapy, with CABG only benefitting patients with viable myocardium who have smaller ESV. However, this has not been tested prospectively.
Methods
Interactions of ESV index (ESVI), myocardial viability and treatment with respect to survival were assessed in patients in the prospective randomized STICH trial of CABG vs MED who underwent viability assessment (n=601, age 61±9 years, ejection fraction ≤35%), median follow-up 5.1 years. Median ESVI was 84 ml/m2. Viability was assessed by SPECT or dobutamine echocardiography using prespecified criteria.
Results
Mortality was highest among patients with larger ESVI and non-viability (P<0.001), but no interaction was observed between ESVI, viability status, and treatment assignment (P=0.491). Specifically, the effect of CABG versus MED in patients with viable myocardium and ESVI ≤84 ml/m2 (HR 0.85, 95% CI 0.56,1.29) was no different than in patients with viability and ESVI >84 ml/m2 (HR 0.87, 95% CI 0.57,1.31). Other ESVI thresholds yielded similar results, including ESVI ≤60 ml/m2 (HR 0.87, 95% CI 0.44,1.74). ESVI and viability assessed as continuous rather than dichotomous variables yielded similar results (P=0.562).
Conclusions
Among patients with ischemic cardiomyopathy, those with greater LVESVI and no substantial viability have worse prognosis. However, the effect of CABG relative to MED is not differentially influenced by the combination of these two factors. Lower ESVI does not identify patients in whom myocardial viability predicts better outcome with CABG relative to MED.
Keywords: Heart failure, Coronary artery disease, Coronary artery bypass surgery, Myocardial viability
Despite advances in diagnosis and treatment, heart failure remains a substantial cause of death and disability [1,2], driven importantly by the causal role of coronary artery disease (CAD) in the development of left ventricular (LV) dysfunction [3]. LV systolic dysfunction in the setting of CAD is not always an irreversible process, as LV function may improve substantially with beta blocker therapy, cardiac resynchronization, and revascularization [3–7]. LV function is most likely to improve with medical, device, or surgical therapies in patients with viable myocardium identified using noninvasive imaging [4,8–14]. Many previous studies, primarily retrospective and performed before the advent of beta blockers for LV systolic dysfunction, suggested that myocardial viability also identifies patients in whom survival is enhanced with revascularization compared to medical management [8,15,16]. In contradistinction, the prospective Surgical Treatment for Systolic Heart Failure (STICH) trial, which randomized patients with CAD and LV dysfunction to evidence-based medical therapy or coronary artery bypass surgery (CABG) plus medical therapy, demonstrated no interaction between myocardial viability and treatment strategy with respect to survival [17].
Previous retrospective studies of patients with ischemic LV dysfunction suggest that severity of LV remodeling affects the relation between myocardial viability and survival with CABG, such that patients with marked LV dilation – i.e., large end-systolic volume (ESV) – have developed irreversible remodeling to the extent that viable myocardium, if present, does not contribute to improved LV function or improved survival with revascularization. According to this concept, the beneficial effect of CABG on LV functional recovery and survival would thus be limited to patients with viable myocardium and smaller ESV [18–21]. This theory is plausible but has not been tested prospectively with random allocation of treatment strategies. The current study investigates the impact of LV remodeling on the relationship between myocardial viability, treatment with revascularization versus medical management, and survival in patients enrolled in the STICH trial.
Methods
Patient Enrollment
Design and enrollment criteria for STICH and the STICH viability substudy have been reported in detail [17,22,23]. STICH is a multicenter non-blinded randomized trial funded by the National Heart, Lung, and Blood Institute (NHLBI). The study of revascularization versus medical therapy was conducted at 99 sites in 22 countries. Patients with angiographic documentation of CAD amenable to surgical revascularization and LV ejection fraction (EF) ≤35% were eligible for enrollment. Exclusion criteria included left main coronary stenosis >50%, cardiogenic shock, myocardial infarction within 3 months, and need for aortic valve surgery. All participants provided written informed consent. Patients were randomized to receive medical therapy alone or medical therapy plus CABG. A “risk at randomization” score was calculated for each patient using a statistical model derived in an independent dataset from multiple variables with known power to predict 5-year risk of death without CABG [24]. Medical therapy was excellent with ≥90% of patients receiving statins, beta blockers and either angiotensin converting enzyme inhibitors or angiotensin receptor blockers at 1 year, and 88% receiving aspirin (≥92% received either aspirin or warfarin) [23].
Viability Testing
Of the 1212 total enrolled subjects, 601 underwent viability testing. Details regarding patient selection for imaging have been reported previously [17]. Viability was assessed using single photon computed tomography (SPECT) in 471 patients or dobutamine echocardiography in 280 patients; 150 patients were studied by both techniques. For SPECT, 4 protocols for assessing myocardial viability were permitted at the enrolling sites, including thallium imaging using a rest-redistribution or stress-rest-reinjection protocol, rest-redistribution thallium imaging, or imaging with a technetium-99m tracer at rest after administration of nitroglycerin. For echocardiography, imaging was performed at rest and during staged infusions of dobutamine starting at 5 µg/kg/min and increasing to 10, 20, 30 and 40 µg/kg/min in 3–5 minute intervals.
Independent NHLBI-funded core laboratories [22] blinded to patient details and treatment assignment coordinated data collection and analysis for the SPECT and dobutamine echocardiography studies. Thresholds of viable myocardium were pre-specified to classify patients in a binary fashion as being either with or without substantial myocardial viability. Viability was also evaluated as a continuous variable. Core laboratory measurements were submitted to the Duke Clinical Research Institute, which performed all statistical analyses.
For SPECT, patients with viability were defined as those with ≥11 viable segments based on relative tracer activity using a 17-segment model. A myocardial segment was deemed viable if tracer activity was ≥50% of activity in the segment with maximal activity. For thallium rest-redistribution imaging, a segment with activity <50% of maximal myocardial activity on the redistribution images was also defined as viable if improvement in activity from rest to redistribution images was ≥12%.
For dobutamine echocardiography, patients with viability were defined as those with ≥5 segments with abnormal resting systolic function manifesting contractile reserve with dobutamine, using a 16-segment model. In the 150 patients studied with both techniques, based on the thresholds defined above, when both tests demonstrated viability, the sum of SPECT plus echocardiography scores was ≥16 viable segments; when both tests demonstrated nonviability, the sum was <16. This threshold was then applied for those with discordant results between the two tests; the SPECT viability and echocardiography viability scores were added together, and patients were considered to have viable myocardium when the total segment score was ≥16 [17].
Left Ventricular Function and End-Systolic Volumes
LVEF and ESV were measured by the independent investigators from core laboratories blinded to treatment allocation. As previously described [25], the best available method (based on study quality using a predetermined hierarchical algorithm) was used to measure LVEF and volumes. The ESV index (ESVI) was computed by dividing ESV by body surface area.
Patient Follow-Up
After trial enrollment, patients were followed every 4 months for the first year and every 6 months thereafter [17,23]. The primary outcome was all-cause mortality. Secondary outcomes included cardiovascular mortality and all-cause mortality plus cardiovascular hospitalization. All endpoints were adjudicated by an independent Clinical Events Committee [22].
Statistical Methods
Baseline clinical characteristics of patients were descriptively summarized using means and standard deviations unless otherwise specified. Group characteristics at baseline were compared using the Wilcoxon rank-sum test for continuous variables and the conventional chi-square test or Fisher’s Exact test for categorical variables. Patients were initially subgrouped on the basis of median ESVI (84 ml/m2). The relationships of myocardial viability, ESVI, and treatment with the primary outcome of all-cause mortality were assessed using the Cox proportional hazards regression model and Kaplan-Meier mortality curves [26,27]. Specifically, we examined whether the effect of CABG versus medical therapy on mortality differed depending on viability status and ESVI by assessing the interactions of these factors with treatment using the Cox model. We also produced Kaplan-Meier mortality curves for subgroups of patients defined by viability status and ESVI and descriptively summarized CABG mortality compared to medical mortality using hazard ratios and 95% confidence intervals generated from the Cox model and log-rank assessments of treatment differences. Sensitivity analyses of the interactions between myocardial viability, ESVI, and treatment were also performed using different thresholds of ESVI (≥90 ml/m2, 61–90ml/m2, and ≤60 ml/m2). Similar analyses to those described above were performed for the secondary endpoints of cardiovascular death, and death or cardiac hospitalization. In addition to treatment comparisons of CABG versus medical therapy as randomized (intention-to-treat), supplementary analyses compared the study arms as treated (accounting for treatment crossovers), and per protocol [28]. Finally, Cox model analyses were performed treating viability status and ESVI as continuous rather than binary variables.
Results
Among the 601 patients undergoing viability testing, the median ESVI was 84 ml/m2. Myocardial viability was present in 487 patients (81%) [17]. Patients with viable myocardium had higher LVEF (27.5 ± 8.3 vs 22.9 ± 8.8%, p<0.001) and lower ESVI (84.5 ± 30.9 vs 107.7 ± 43.5 mL/m2, p<0.001) than those without myocardial viability. ESVI did not differ between patients undergoing CABG versus medical therapy (88.7 ± 33.9 vs 89.1 ± 35.7 mL/m2, p=0.820). Baseline characteristics of patients with viable myocardium, comparing those with ESVI above and below the median value, are presented in Table 1 and characteristics of patients without viable myocardium are presented in Table 2. Among patients with myocardial viability, those with ESVI >84 ml/m2 had more severe symptoms, lower LVEF, and higher LV end-diastolic volume index, but otherwise did not differ from those with lower ESVI.
Table 1.
Baseline characteristics of patients with myocardial viability
| Characteristic | Patients with LVESVI ≤ 84 ml/m2 (n=267) |
Patients with LVESVI > 84 ml/m2 (n=220) |
P value |
|---|---|---|---|
| Age, mean ± SD | 61±10 | 60±9 | 0.077 |
| Prior myocardial infarction, no. (%) | 208 (78%) | 165 (75%) | 0.452 |
| Diabetes, no. (%) | 115 (43%) | 83 (38%) | 0.232 |
| Prior stroke, no. (%) | 25 (9%) | 17 (8%) | 0.522 |
| Hypertension, no. (%) | 175 (66%) | 137 (62%) | 0.454 |
| Hyperlipidemia, no. (%) | 177 (67%) | 149 (68%) | 0.782 |
| Current smoker, no. (%) | 53 (20%) | 55 (25%) | 0.173 |
| Chronic renal insufficiency, no. (%) | 19 (7%) | 14 (6%) | 0.734 |
| Atrial flutter/fibrillation, no. (%) | 42 (16%) | 32 (15%) | 0.717 |
| Peripheral vascular disease, no. (%) | 45 (17%) | 30 (14%) | 0.328 |
| RAR score, mean ± SD * | 12±9 | 13±8 | 0.140 |
| Previous CABG, no. (%) | 5 (2%) | 7 (3%) | 0.354 |
| Bypass graft status, no. (%) | |||
| ≥1 stenosed or occluded | 4 (80%) | 7 (100%) | |
| ≥1 occluded | 4 (80%) | 6 (86%) | |
| Previous PCI, no. (%) | 43 (16%) | 34 (16%) | 0.845 |
| CAD distribution, no. (%) | |||
| No. of diseased vessels ≥75% | 0.162 | ||
| None | 6 (2%) | 3 (1%) | |
| One-vessel | 77 (29%) | 47 (22%) | |
| Two-vessel | 91 (34%) | 88 (40%) | |
| Three-vessel | 93 (35%) | 81 (37%) | |
| Proximal LAD stenosis ≥75% | 170 (64%) | 139 (64%) | 0.964 |
| Left main stenosis (≥50%) | 8 (3%) | 4 (2%) | 0.408 |
| Highest NYHA functional class within 3 months, no. (%) | 0.002 | ||
| I | 14 (5%) | 10 (5%) | |
| II | 114 (43%) | 68 (31%) | |
| III | 110 (41%) | 101 (46%) | |
| IV | 29 (11%) | 41 (19%) | |
| Medications at baseline, no. (%) | |||
| Beta blocker | 235 (88%) | 202 (92%) | 0.169 |
| ACE inhibitor | 223 (84%) | 189 (86%) | 0.467 |
| Angiotensin receptor blocker | 21 (8%) | 19 (9%) | 0.758 |
| ACE inhibitor or ARB | 242 (91%) | 204 (93%) | 0.408 |
| Statin | 227 (85%) | 178 (81%) | 0.228 |
| Aspirin | 227 (85%) | 187 (85%) | 0.995 |
| Blood pressure, mean ± SD | |||
| Systolic (mmHg) | 123±19 | 119±16 | 0.029 |
| Diastolic (mmHg) | 75±11 | 75±11 | 0.564 |
| Heart rate, mean ± SD | 72±11 | 75±13 | 0.074 |
| LV ejection fraction, mean ± SD | 33±8 | 23±6 | <0.001 |
| LVEDVI (ml/m2), mean ± SD | 94±21 | 145±31 | <0.001 |
| LVESVI (ml/m2), mean ± SD | 63±15 | 111±24 | --- |
| Hemoglobin (g/dL), mean ± SD | 14±2 | 14±2 | 0.195 |
| Creatinine (mg/dL), mean ± SD | 1.2±1.0 | 1.1±0.3 | 0.573 |
| BUN (mg/dL), mean ± SD | 30±21 | 29±19 | 0.540 |
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; BUN = blood urea nitrogen; CABG = coronary artery bypass graft surgery; CAD = coronary artery disease; EDVI = end-diastolic volume index; ESVI = end-systolic volume index; LV= left ventricular; NYHA = New York Heart Association; RAR = risk at randomization
The RAR score ranges from 1 to 32, with higher numbers indicating a higher predicted rate of death. Among patients receiving medical therapy, a score of 1 predicts a rate of 18% and a score of 32 predicts a rate of 99% over 5 years.
Table 2.
Baseline characteristics of patients without myocardial viability
| Characteristic | Patients with LVESVI ≤ 84 ml/m2 (n=37) |
Patients with LVESVI > 84 ml/m2 (n=77) |
P value |
|---|---|---|---|
| Age, mean ± SD | 64±8 | 60±9 | 0.019 |
| Prior myocardial infarction, no. (%) | 34 (92%) | 74 (96%) | 0.388 |
| Diabetes, no. (%) | 11 (30%) | 15 (20%) | 0.222 |
| Prior stroke, no. (%) | 3 (8%) | 8 (10%) | 1.000 |
| Hypertension, no. (%) | 23 (62%) | 28 (36%) | 0.010 |
| Hyperlipidemia, no. (%) | 30 (81%) | 47 (62%) | 0.039 |
| Current smoker, no. (%) | 4 (11%) | 14 (18%) | 0.312 |
| Chronic renal insufficiency, no. (%) | 3 (8%) | 7 (9%) | 1.000 |
| Atrial flutter/fibrillation, no. (%) | 3 (8%) | 13 (17%) | 0.207 |
| Peripheral vascular disease, no. (%) | 1 (3%) | 15 (20%) | 0.016 |
| RAR score, mean ± SD * | 10±9 | 14±9 | 0.039 |
| Previous CABG, no. (%) | 1 (3%) | 3 (4%) | 1.000 |
| Bypass graft status, no. (%) | |||
| ≥1 stenosed or occluded | 1 (100%) | 3 (100%) | |
| ≥1 occluded | 1 (100%) | 3 (100%) | |
| Previous PCI, no. (%) | 12 (32%) | 15 (20%) | 0.128 |
| CAD distribution, no. (%) | |||
| No. of diseased vessels ≥75% | 0.835 | ||
| None | 0 (0%) | 3 (4%) | |
| One-vessel | 8 (22%) | 20 (26%) | |
| Two-vessel | 17 (46%) | 25 (33%) | |
| Three-vessel | 12 (32%) | 29 (38%) | |
| Proximal LAD stenosis ≥75% | 27 (73%) | 53 (69%) | 0.651 |
| Left main stenosis (≥50%) | 1 (3%) | 1 (1%) | 0.546 |
| Highest NYHA functional class within 3 months, no. (%) | 0.349 | ||
| I | 2 (5%) | 1 (1%) | |
| II | 10 (27%) | 20 (26%) | |
| III | 21 (57%) | 43 (56%) | |
| IV | 4 (11%) | 13 (17%) | |
| Medications at baseline, no. (%) | |||
| Beta blocker | 30 (81%) | 67 (87%) | 0.405 |
| ACE inhibitor | 32 (87%) | 70 (91%) | 0.521 |
| Angiotensin receptor blocker | 2 (5%) | 4 (5%) | 1.000 |
| ACE inhibitor or ARB | 34 (92%) | 74 (96%) | 0.388 |
| Statin | 36 (97%) | 67 (87%) | 0.100 |
| Aspirin | 31 (84%) | 68 (88%) | 0.559 |
| Blood pressure, mean ± SD | |||
| Systolic (mmHg) | 118±14 | 113±14 | 0.050 |
| Diastolic (mmHg) | 75±9 | 73±9 | 0.385 |
| Heart rate, mean ± SD | 71±11 | 75±16 | 0.190 |
| LV ejection fraction, mean ± SD | 31±9 | 20±6 | <0.001 |
| LVEDVI (ml/m2), mean ± SD | 94±23 | 172±44 | <0.001 |
| LVESVI (ml/m2), mean ± SD | 63±14 | 129±36 | ---- |
| Hemoglobin (g/dL), mean ± SD | 14±2 | 14±1 | 0.401 |
| Creatinine (mg/dL), mean ± SD | 1.1±0.3 | 1.2±0.4 | 0.074 |
| BUN (mg/dL), mean ± SD | 26±18 | 28±18 | 0.498 |
Abbreviations as in Table 1
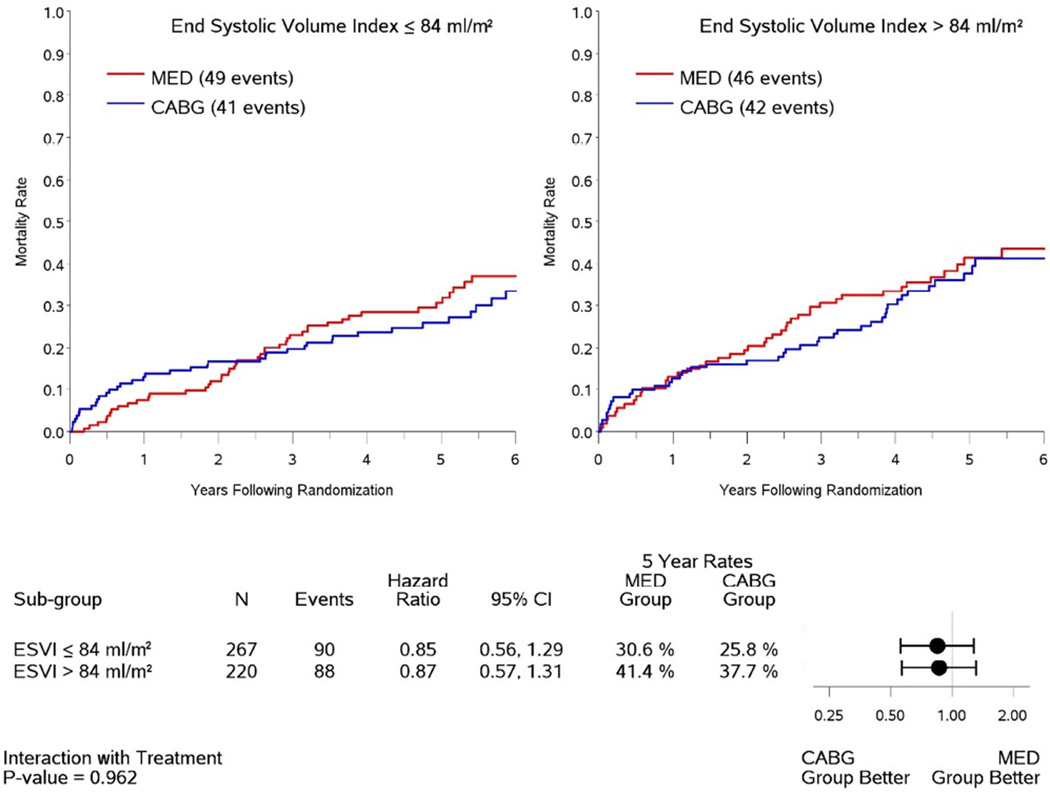
For the entire group, there was no interaction between ESVI, viability status, and treatment assignment to CABG versus medical therapy with respect to survival (P=0.491). Among the 487 patients with viable myocardium (Figure 1), no interaction was observed between ESVI and treatment assignment with respect to survival (P=0.962). Specifically, the effect of CABG compared to medical therapy in patients with ESVI ≤84 ml/m2 (HR 0.85, 95% CI 0.56,1.29) was not different from that of patients with ESVI >84 ml/m2 (HR 0.87, 95% CI 0.57,1.31). Among patients with viability treated surgically, postoperative mortality was higher in those high ESVI compared to low ESVI (37.7% vs 25.8% at 5 years, Fig. 1), but this trend was not significant (HR 1.30, 95% CI 0.85,2.00).
Figure 1. Kaplan-Meier analysis of mortality rates in patients with myocardial viability.
Data are shown for patients with baseline end-systolic volume index (ESVI) above and below the median value of 84 ml/m2 according to treatment with coronary artery bypass surgery plus medical therapy (CABG) or medical therapy alone (MED). The relationship between viability, treatment assignment and survival is not influenced by ESVI (interaction P-value = 0.962).
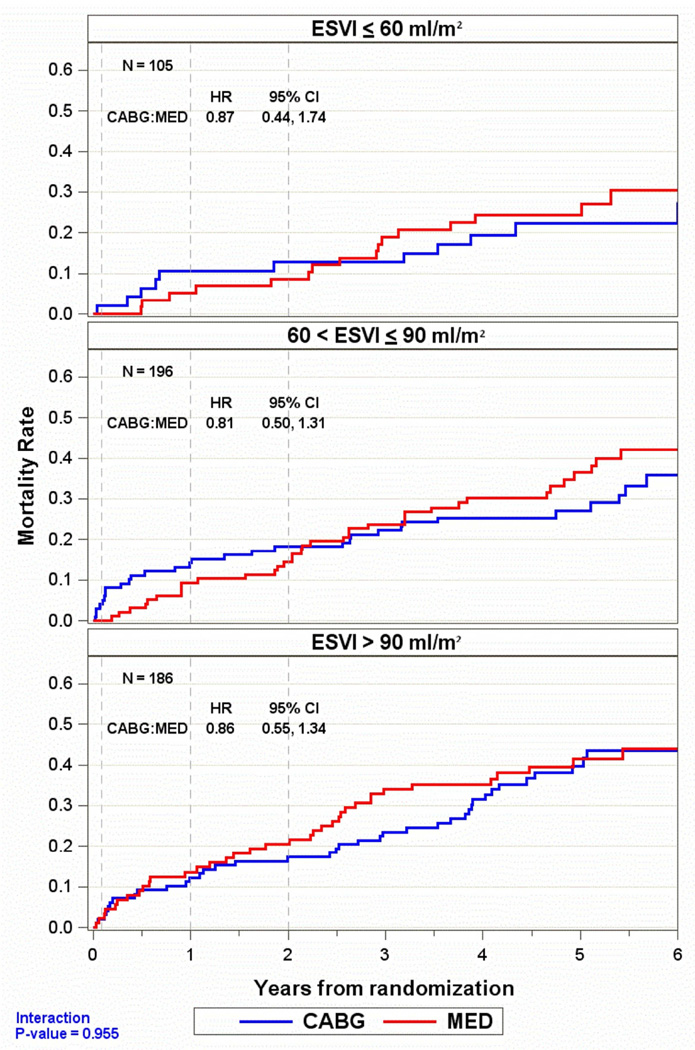
An additional analysis separated the patients with myocardial viability into 3 subgroups of ESVI (≥90 ml/m2, 61–90 ml/m2, and ≤60 ml/m2). There was no difference in the effect of CABG compared to medical therapy on 5-year mortality across the range of ESVI (Figure 2), including the subgroup with the lowest ESVI (interaction P-value 0.955).
Figure 2. Kaplan-Meier analysis of mortality rates in patients with myocardial viability in 3 subgroups of end-systolic volume index (ESVI).
In each subgroup, including patients with lowest values of ESVI (≤60 ml/m2), there was no interaction of ESVI, myocardial viability, treatment with coronary artery bypass surgery plus medical therapy (CABG) versus effects of medical therapy alone (MED), and mortality.
Similarly, in patients with nonviable myocardium, the effect of CABG compared to medical therapy did not differ significantly between patients with ESVI ≤84 ml/m2 (HR 1.30, 95% CI 0.34,5.00) and those with ESVI >84 ml/m2 (HR 0.67, 95% CI 0.38,1.20), although the number of patients with non-viable myocardium was small, particularly among those with lower values of ESVI.
Myocardial viability and End-Systolic Volume as Continuous Variables
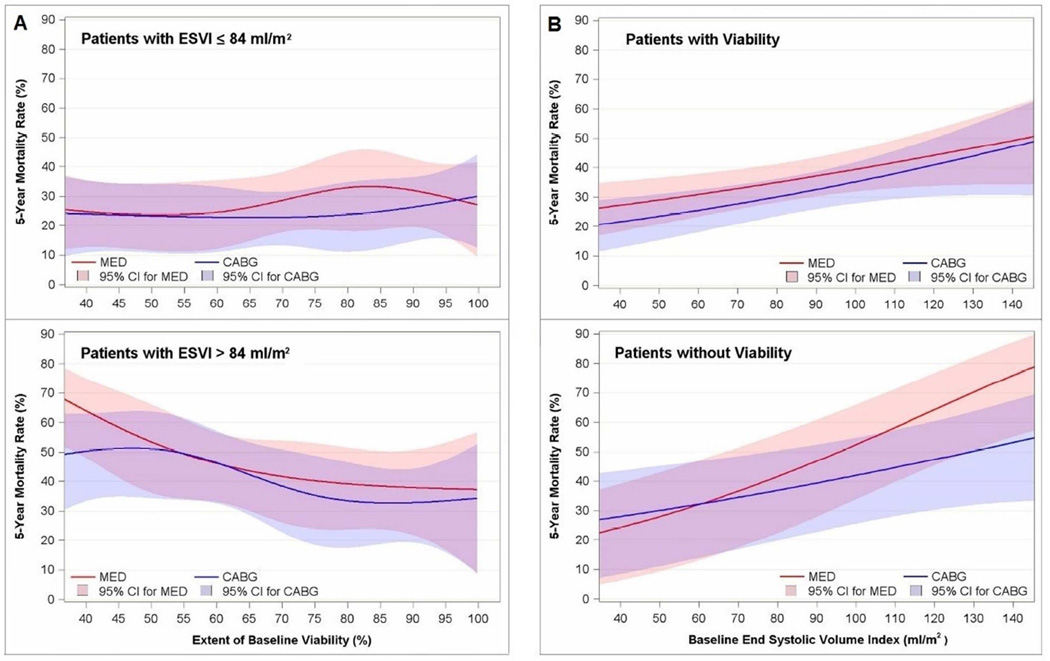
Analyses in which both myocardial viability scores and ESVI were assessed as continuous rather than dichotomous variables did not demonstrate significant interactions of viability, ESVI, and treatment with CABG versus medical therapy on mortality (P=0.562). Mortality rates across the continuum of magnitude of viable myocardium are shown in Figure 3A, and across the continuum of ESVI are shown in Figure 3B. Specifically, patients with greater degrees of myocardial viability and lower values of ESVI did not manifest a significant differential benefit of CABG over medical therapy compared to patients with less viability and/or larger values of ESVI.
Figure 3. Extent of viability and end-systolic volume expressed as continuous variables.
Panel A: Five-year mortality rate plotted as a function of percent of left ventricular myocardium demonstrating viability in patients with end-systolic volume index (ESVI) above and below the median value. Panel B: Five-year mortality rate plotted as a function of end-systolic volume index in patients with and without myocardial viability. Mean and 95% confidence limits are shown for patients treated with coronary artery bypass surgery plus medical therapy (CABG) and medical therapy alone (MED).
Secondary Endpoints
Analysis of secondary endpoints paralleled the primary analysis, showing no significant interactions of myocardial viability, ESVI, treatment and outcome. With respect to cardiovascular mortality, the effect of CABG compared to medical therapy in patients with myocardial viability did not differ significantly between patients with ESVI ≤84 ml/m2 (HR 0.65, 95% CI 0.39,1.08) and those with ESVI >84 ml/m2 (HR 0.88, 95% CI 0.56,1.37, interaction P-value 0.387). Similarly, with the composite endpoint of mortality plus cardiovascular hospitalization, in patients with myocardial viability the effect of CABG compared to medical therapy did not differ between patients with ESVI ≤84 ml/m2 (HR 0.68, 95% CI 0.50,0.92) and those with ESVI >84 ml/m2 (HR 0.67, 95% CI 0.49,0.92, interaction P-value 0.942).
Analysis of Treatment Received
Analysis of actual treatment received provided similar results to the intention-to-treat analysis for both primary and secondary endpoints. For example, for the primary endpoint of all-cause mortality, among patients with myocardial viability the effect of CABG compared to medical therapy did not differ between patients with ESVI ≤84 ml/m2 (HR 0.77, 95% CI 0.51,1.16) and those with ESVI >84 ml/m2 (HR 0.82, 95% CI 0.54,1.24, interaction P-value 0.873).
Discussion
The current report extends the analysis of the prospective STICH myocardial viability study [17] to examine the interaction of ESVI, as a marker of severity of LV remodeling, with extent of myocardial viability and treatment with CABG versus medical therapy with respect to survival in patients with CAD and LV systolic dysfunction. The results indicate that, even after accounting for ESVI, specifically in patients with lower values of ESVI, there was no significant interaction between viability and treatment assignment with respect to survival.
The current study was stimulated by previous reports suggesting that improvement in LV function after CABG occurs in patients with myocardial viability who have less severe LV remodeling, whereas functional recovery is less likely, despite viable myocardium, in patients with severe LV remodeling. Yamaguchi, et al. [18] studied 20 patients undergoing CABG with LVEF <30% and reported improvement in LV function in those with ESVI <100 ml/m2 but not in those with larger ESVI. Three other studies assessing the impact of ESV on recovery of LV function after revascularization did not index ESV for body size. Bax, et al. [19] studied patients with mean LVEF 29% and observed improvement in EF after CABG in patients with smaller ESV and myocardial viability (assessed using 18F-fluorodeoxyglucose [FDG] SPECT), The same investigators subsequently reported comparable results using dobutamine echocardiography to assess viability [20], in which the likelihood of recovery of LVEF decreased proportionally with the increase of ESV despite the presence of viable myocardium. Similarly, Mandegar et al. [21] reported changes in LV function after CABG in 85 patients with EF ≤35% (mean 27%), all of whom manifested myocardial viability by dobutamine echocardiography; patients with ≥6 viable segments manifested improvement in EF postoperatively, whereas patients with <6 viable segments did not increase EF if there was high ESV. Of these 4 studies, only Bax, et al. [19] reported postoperative survival data, in which patients with viable myocardium and small ESV had lower mortality rates after CABG than those with viable myocardium and high ESV (similar to trends we observed in Figures 1–3 in the current study). None of these 4 studies included a comparison cohort of patients treated with medical therapy alone.
Previous studies and meta-analyses indicating improved survival with CABG compared to medical therapy in patients with LV systolic dysfunction and viable myocardium are limited by retrospective design and lack of adjustment for key baseline comorbidities [9,15,16]. Factors influencing recommendations for revascularization in each patient were not considered; hence, the subsequent analyses ignore the biases inherent in therapeutic decisions made by each treating physician. Moreover, the medical therapies employed are often not reported, and when reported would be considered suboptimal by current standards. Specifically, beta blockers were underutilized or not used at all. Treatment with beta blockers has the potential to improve survival in patients with ischemic LV dysfunction [6] and also to improve LV function in those with myocardial viability [10–12]. Although patients in the STICH trial had lower mean LVEF than patients with myocardial viability treated medically in prior reports [9,15,16,17], patients with viable myocardium randomized to medical therapy in STICH had substantially lower annual mortality rates than patients with viability treated medically in the previous studies. This appears to reflect the adherence to guidelines-driven medical therapy in the majority of patients in this prospective trial [29].
When myocardial viability was assessed as a continuous variable in the current analysis (Fig. 3), there was no differential effect of CABG over medical therapy with increasing extent of viable myocardium. These findings are supported by the previous retrospective study of Tarakji et al. [30] who reported survival with medical therapy versus revascularization in 765 patients with LVEF ≤35% (mean 23%). Across the continuum of magnitude of compromised viable myocardium assessed by FDG positron emission tomography (PET), there was no differential effect of CABG with increasing extent of myocardial viability. A subsequent study from the same institution [31] in 648 patients with CAD and LV systolic dysfunction (mean EF 31%) studied with FDG PET did report reduced mortality with early revascularization compared to medical therapy as a function of increasing extent of hibernating myocardium. However, in that study early revascularization was defined as revascularization within 92 days of PET, yet the survival analysis began at 92 days, excluding all deaths before 92 days from the analysis. Thus, early postoperative mortality, the time period of greatest hazard for CABG relative to medical therapy [23,32], was not accounted for in the survival curves. STICH results also demonstrate a differential benefit of CABG over medical therapy once patients survive the first several months, and it is the higher early mortality risk of CABG that produces the overall balance between surgical and medical outcomes [23,32]. In the current analysis, in which early postoperative mortality was included in the mortality analysis, no interaction between myocardial viability, ESVI and survival with CABG or medical therapy was observed across the spectra of myocardial viability and ESVI (Fig. 3).
The STICH viability analysis has several limitations worth noting. Viability assessment with SPECT and dobutamine echocardiography does not incorporate the particular advantages of metabolic imaging with PET or assessment of myocardial fibrosis with cardiac magnetic resonance [31,33,34]. However, in a meta-analysis and other reviews, SPECT and dobutamine echocardiography have had similar prognostic potential to that of PET [9,15,16], a small randomized study of PET versus SPECT for viability assessment failed to show improved event-free survival in patients assigned to PET [35], and a randomized study of PET-guided care versus usual care failed to demonstrate improved outcome with the PET strategy [36]. The STICH protocol was designed in 2000 [22] before the advent of CMR for viability assessment [8], using imaging protocols identical to previous non-randomized studies reporting survival advantages of CABG over medical therapy in patients with viable myocardium [9]. As noted previously, patients with viability data represent roughly 50% of all patients enrolled in STICH, and viability testing was not performed on a randomly selected subset, but depended on test availability and judgment of the recruiting investigator. However, previous analyses did not reveal an interaction between performance of a viability test and treatment assignment [17], which was prospective and randomized. The majority of patients studied were deemed to have viable myocardium based on our prespecified criteria. Although this limits the interpretation of outcomes in patients with nonviable myocardium, it provides sufficient patient numbers in those with myocardial viability to assess the interaction of ESVI on outcomes in patients with viable myocardium. In addition, assessment of viability as a continuous variable (Fig. 3) supports the primary analysis in which viability was assessed as a dichotomous variable. The STICH results pertain only to patients eligible for enrollment in STICH (LVEF ≤35%), and the interaction of ESVI, myocardial viability, and survival with CABG compared to medical therapy may differ in patients with less severe LV dysfunction.
The lack of significant interaction between myocardial viability and survival with surgical versus medical management of patients with severe ischemic LV dysfunction is reflected in the current recommendations for revascularization in the 2013 ACC/AHA guideline for the management of heart failure [37], which indicates that, in the absence of angina, CABG may be considered with the intent of improving survival in patients with ischemic heart disease with severe LV systolic dysfunction (EF <35%) whether or not viable myocardium is present (class IIb, level of evidence: B). In contrast, other guidelines continue to recommend that decisions for revascularization be driven by evidence of myocardial viability [38,39]. The current data should stimulate further discussion of the role of viability testing in determining appropriate candidacy for revascularization.
In summary, the current findings indicate that patients with ischemic LV dysfunction and extensive LV remodeling (manifested by greater ESVI) have a worse prognosis than those with lower ESVI. However, the effect of CABG when added to evidence-based medical therapy is not differentially influenced by the combination of ESVI and extent of myocardial viability. Lower ESVI does not identify patients in whom the presence of viable myocardium predicts a better outcome with CABG relative to medical therapy alone.
Perspectives.
Competency in medical knowledge
Among patients with coronary artery disease and left ventricular (LV) systolic dysfunction, lower LV end-systolic volume index does not identify patients in whom myocardial viability predicts better outcome with surgical relative to medical treatment.
Translational outlook
Future research should determine whether end-systolic volume and myocardial viability interact to affect improvement in LV function with surgical versus medical treatment, and the relation between improvement in function and survival.
Acknowledgments
Support: The STICH trial was funded by the National Heart, Lung, and Blood Institute (ClinicalTrials.gov number, NCT00023595) through cooperative agreement mechanisms: U01 HL-069009, HL-069010, HL-069011, HL-069012, HL-069012-03, HL-069013, HL-069015, HL-070011, HL-072683. The views expressed in this manuscript do not necessarily reflect those of the NHLBI or the NIH.
Dr. Harvey White discloses the following:
Research Grants - Modest: Sanofi Aventis, Eli Lilly, The Medicines Company, Roche, MSD, Astra Zeneca, Daiichi Sankyo Pharma Development
Research Grants – Significant: GSK
Advisory Boards – Modest: Astra Zeneca, MSD, Roche, Regado Bioscience,
Abbreviations
- CABG
coronary artery bypass graft surgery
- CAD
coronary artery disease
- EF
ejection fraction
- ESV
end-systolic volume
- ESVI
end-systolic volume index
- FDG
18F-fluorodeoxyglucose
- LV
left ventricular
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- STICH
Surgical Treatment of Ischemic Heart Failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Daniel Berman discloses that he participates in royalties from Cedars-Sinai Medical Center for computer software.
There are no other conflicts of interest to disclose.
References
- 1.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–1213. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 4.Dilsizian V, Bonow RO. Current diagnostic techniques of assessing myocardial viability in hibernating and stunned myocardium. Circulation. 1993;87:1–20. doi: 10.1161/01.cir.87.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Elefteriades JA, Tolis G, Jr, Levi E, et al. Coronary artery bypass grafting in severe left ventricular dysfunction: excellent survival with improved ejection fraction and functional state. J Am Coll Cardiol. 1993;22:1411–1417. doi: 10.1016/0735-1097(93)90551-b. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Colucci WS, Swedberg K. Beta blockers in chronic heart failure. Circulation. 2003;107:1570–1575. doi: 10.1161/01.CIR.0000065187.80707.18. [DOI] [PubMed] [Google Scholar]
- 7.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 8.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 9.Allman KC, Shaw LJ, Hachamovitch R, MD, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–1158. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 10.Bello D, Farah GM, Di Luzio S, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in heart failure patients undergoing beta-blocker therapy. Circulation. 2003;108:1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 11.Cleland JG, Pennell DJ, Ray SG, et al. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21. doi: 10.1016/s0140-6736(03)13801-9. [DOI] [PubMed] [Google Scholar]
- 12.Seghatol FF, Shah DJ, DiLuzio S, et al. Relation between contractile reserve and improvement in left ventricular function with beta blocker therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2004;93:854–859. doi: 10.1016/j.amjcard.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Ypenburg C, Schalij MJ, Bleeker GB, et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33–41. doi: 10.1093/eurheartj/ehl379. [DOI] [PubMed] [Google Scholar]
- 14.Becker M, Zwicker C, Kaminski M, et al. Dependency of cardiac resynchronization therapy on myocardial viability at the LV lead position. J Am Coll Cardiol Img. 2011;4:366–374. doi: 10.1016/j.jcmg.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Schinkel AFL, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Prob Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Camici PG, MD, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–114. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 17.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi A, Ino T, Adachi H, Mizuhara A, Murata S, Kamio H. Left ventricular endsystolic volume index in patients with ischemic cardiomyopathy predicts postoperative ventricular function. Ann Thorac Surg. 1995;60:1059–1062. doi: 10.1016/0003-4975(95)00488-7. [DOI] [PubMed] [Google Scholar]
- 19.Bax JJ, Schinkel AFL, Boersma E, et al. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation. 2004;110:18–22. doi: 10.1161/01.CIR.0000138195.33452.b0. [DOI] [PubMed] [Google Scholar]
- 20.Schinkel AFL, Poldermans D, Rizzello V, et al. Why do patients with ischemic cardiomyopathy and a substantial amount of viable myocardium not always recover in function after revascularization? J Thorac Cardiovasc Surg. 2004;127:385–390. doi: 10.1016/j.jtcvs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Mandegar MH, Yousefnia MA, Roshanali F, Rayatzadeh H, Alaeddini F. Interaction between two predictors of functional outcome after revascularization in ischemic cardiomyopathy: left ventricular volume and amount of viable myocardium. J Thorac Cardiovasc Surg. 2008;136:930–936. doi: 10.1016/j.jtcvs.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Lee KL, Deja MA, et al. Coronary artery bypass graft surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RH, White H, Velazquez EJ, et al. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–498. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh JK, Velazquez EJ, Menicanti L, et al. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J. 2013;34:39–47. doi: 10.1093/eurheartj/ehs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 28.Doenst T, Cleland JGF, Rouleau JL, et al. Influence of crossover on mortality in a randomized study of revascularization in patients with systolic heart failure and coronary artery disease. Circ Heart Fail. 2013;6:443–450. doi: 10.1161/CIRCHEARTFAILURE.112.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang JC. Underestimating medical therapy for coronary disease…again. N Engl J Med. 2011;364:1671–1673. doi: 10.1056/NEJMe1103414. [DOI] [PubMed] [Google Scholar]
- 30.Tarakji KG, Brunken R, McCarthy PM, et al. Myocardial viability testing and the effect of early intervention in patients with advanced left ventricular systolic dysfunction. Circulation. 2006;113:230–237. doi: 10.1161/CIRCULATIONAHA.105.541664. [DOI] [PubMed] [Google Scholar]
- 31.Ling LF, Marwick TH, Flores DR, et al. Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. doi: 10.1161/CIRCIMAGING.112.000138. [DOI] [PubMed] [Google Scholar]
- 32.Panza JA, Velazquez EJ, She L, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2014;64:553–561. doi: 10.1016/j.jacc.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mielniczuk LM, Beanlands RS. Imaging-guided selection of patients with ischemic heart failure for high-risk revascularization improves identification of those with the highest clinical benefit. Circ Cardiovasc Imaging. 2012;5:262–270. doi: 10.1161/CIRCIMAGING.111.964668. [DOI] [PubMed] [Google Scholar]
- 34.Chareonthaitawee P, Gersh BJ, Panza JA. Is viability imaging still relevant in 2012? J Am Coll Cardiol Img. 2012;5:550–558. doi: 10.1016/j.jcmg.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Siebelink HM, Blanksma P, Crijns H, et al. No difference in cardiac event-free survival between positron emission tomography and single-photon emission computed tomography-guided patient management. J Am Coll Cardiol. 2001;37:81–88. doi: 10.1016/s0735-1097(00)01087-1. [DOI] [PubMed] [Google Scholar]
- 36.Beanlands RSB, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 38.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 39.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]