Abstract
Background
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in the world. There is significant practice variation and uncertainty as to the optimal surgical approach for treating CSM.
Objective
The primary objective is to determine if ventral surgery is associated with superior SF-36 Physical Component Summary (PCS) outcome at one year follow-up compared to dorsal (laminectomy/fusion or laminoplasty) surgery for the treatment of CSM. The study will also investigate whether post-operative sagittal balance is an independent predictor of overall outcome and will compare health resource utilization for ventral and dorsal procedures.
Methods
The study is a randomized, controlled trial with a nonrandomized arm for patients who are eligible but decline randomization. Two hundred fifty patients (159 randomized) with CSM from 11 sites will be recruited over 18 months. The primary outcome is the Short Form-36 PCS score. Secondary outcomes include disease specific outcomes, overall health-related quality of life (EuroQol-5D), and health resource utilization.
Expected Outcomes
This will be the first randomized controlled trial to compare directly the health-related quality of life outcomes for ventral versus dorsal surgery for treating CSM.
Discussion
An NIH-funded (1R13AR065834-01) investigator meeting was held prior to initiating the trial in order to bring multiple stakeholders together to finalize the study protocol. Study investigators, coordinators, and major stakeholders were able to attend and discuss strengths, limitations, and concerns regarding the study. The final protocol was approved for funding by PCORI (CE-1304-6173). The RCT began enrollment on April 1, 2014.
Keywords: Cervical spondylotic myelopathy, randomized clinical trial, outcomes
General Information
Cervical Spondylotic Myelopathy-Surgical (CSM-S) Trial (www.ClinicalTrials.gov; identifier: NCT02076113). Overall study dates: April 2014 to March 2019. Funding agencies: National Institutes of Health (NIH) (1R13AR065834-01) and the Patient-Centered Outcomes Research Institute (PCORI) (CE-1304-6173).
Principal Investigator
Zoher Ghogawala, MD, FACS; Lahey Hospital & Medical Center, 41 Mall Road, Burlington, MA 01805; phone: 781-744-3180, fax: 781-744-5104
Study Sites
Lahey Hospital & Medical Center; Site investigator – Subu Magge, MD
University of Medicine and Dentistry – New Jersey; Site investigator - Robert Heary, MD
University of Utah; Site investigator – Erica Bisson, MD
Cleveland Clinic Foundation; Site investigator – Edward Benzel, MD
Thomas Jefferson University; Site investigator – Todd Albert, MD
Massachusetts General Hospital; Site investigator – Jean-Valery Coumans, MD
Washington University-St. Louis; Site investigator – K. Daniel Riew, MD
Metro Health; Site investigator – Michael Steinmetz, MD
Medical College of Wisconsin; Site investigator – Marjorie Wang, MD
Mount Sinai Medical Center; Site investigator – Tanvir Choudhri, MD
University of Toronto; Site investigator – Michael Fehlings, MD
Rationale and Background Information
Cervical spondylotic myelopathy (CSM) is the most common cause of spinal cord dysfunction in the world.1 The condition presents insidiously and is defined in terms of its clinical symptoms (gait instability, bladder dysfunction, fine finger motor difficulties) and signs (hyperreflexia, Hoffman's sign, ankle clonus, spasticity, alteration of joint position sense). CSM is caused by dynamic repeated compression of the spinal cord from degenerative arthritis of the cervical spine.2 Proposed mechanisms include axonal stretch-associated injury2 and spinal cord ischemia from compression of larger vessels and impaired microcirculation.3,4 Surgery to decompress and stabilize the spine is often advocated for severe or progressive symptoms, with mixed results. About two-thirds of patients improve with surgery, while surgery is not successful in 15%-30% of cases.5 In 2000, over 112,400 cervical spine operations for degenerative spondylosis were performed in the US (100% increase over the past decade),6 with CSM accounting for nearly 20% of cervical spine operations in the United States (US).7 Annual hospital charges for CSM surgery exceeded 2 billion dollars in 2000.6 In addition, CSM is associated with substantial post-surgical outpatient expenses (e.g., physician visits, imaging, physical therapy, medications).
There is significant uncertainty as to the optimal surgical approach for treating CSM. Three alternative surgical approaches currently are widely used in contemporary US surgical practice(ventral decompression and fusion versus dorsal decompression and fusion versus laminoplasty). Recently, the Institute of Medicine designated CSM as one of the top 100 national health research priorities for comparative effectiveness research.8 Our previous work suggests that most American cervical spine experts (both orthopaedic and neurological surgeons) believe that there is sufficient clinical equipoise to support a comparative randomized clinical trial (RCT) if the study population is carefully defined.9 Several other important reasons justify a trial of surgical approaches for CSM at this time. First, the complication rate for CSM surgery is high (17% in a recent prospective study).10 This complication rate is particularly noteworthy in patients greater than 74 years of age,7 who represent a growing segment of the US population.11 Second, clinical outcomes are unsatisfactory in up to 30% of cases.5 Third, ventral surgery might be associated with significantly better health-related quality of life (HR-QOL) outcomes compared to dorsal approaches.12 Finally, the adjusted 5-year re-operation rate for dorsal surgery (17.7%) has been reported to be significantly higher than for ventral surgery (12.1%; P<0.001) by members of our study team.13
The Cervical Spondylotic Myelopathy-Surgical (CSM-S) trial was recently awarded funding from both the National Institutes of Health (NIH) (1R13AR065834-01) and the Patient-Centered Outcomes Research Institute (PCORI) (CE-1304-6173). The purpose of this paper is to describe the rationale for the study and to present the study protocol for the CSM-S trial.
Rationale for CSM-S Study
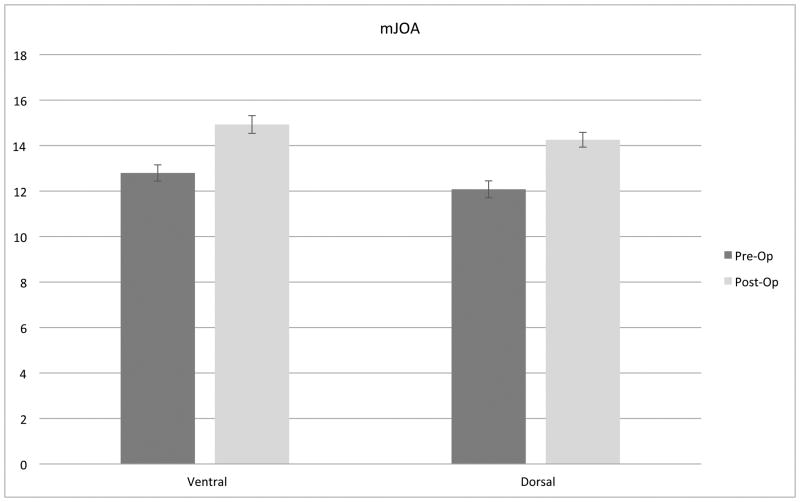
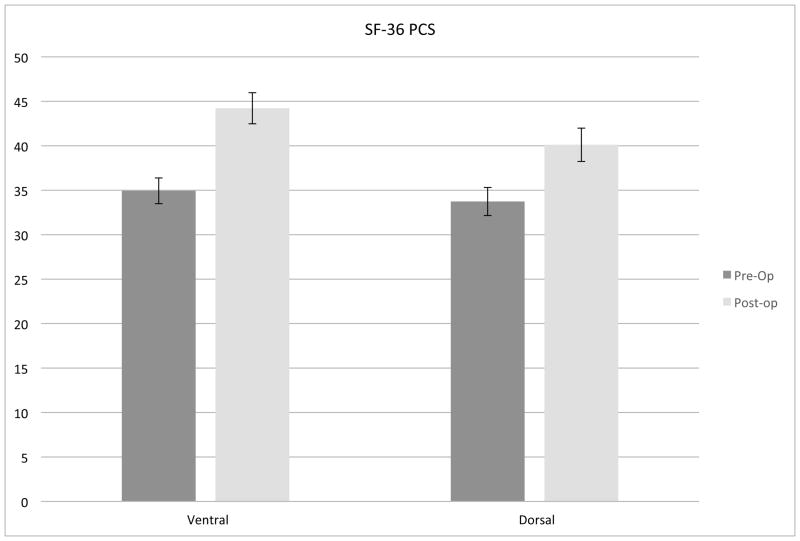
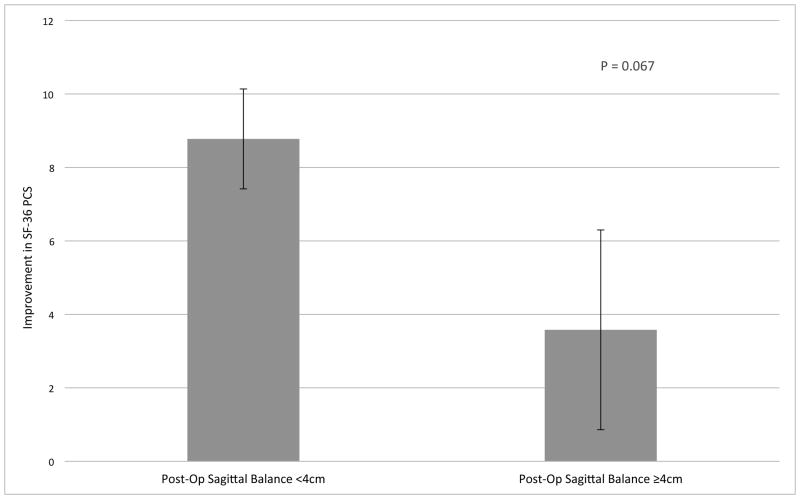
A non-randomized pilot study enrolled 102 patients comparing ventral and dorsal surgery for CSM. Both ventral and dorsal surgeries were associated with improvement in myelopathy scores (mJOA), but ventral surgery was associated with superior HR-QOL outcomes compared to dorsal approaches (Figure 1).12 Poor sagittal balance (C2-C7) was observed more frequently in dorsal surgery cases and was identified as an independent risk factor for poorer HR-QOL outcome following surgery for CSM (Figure 2).
Figure 1.
Both ventral and dorsal surgery were associated with improvement in myelopathy scores (mJOA) (A), but ventral surgery was associated with superior HR-QOL outcomes compared to dorsal approaches (P = 0.07) (B).
Figure 2.
Patients with sagittal balance greater than 4 cm post-operatively did not experience improvement in HR-QOL following surgery. In contrast, patients with relatively normal cervical sagittal balance experienced an 8.8 point improvement in SF-36 PCS at one year post-operatively.
Study Goals and Objectives
The objective of the CSM-S trial is to compare ventral decompression with fusion versus dorsal surgery for patients with multi-level CSM with the following specific aims:
Specific Aim #1
1a. Ventral surgery will be associated with superior Short Form-36 Physical Component Summary (SF-36 PCS) outcome at one-year follow-up compared to dorsal (laminectomy/fusion or laminoplasty) surgery.
1b. Compared to pre-operative baseline status, both ventral and dorsal surgery for CSM will improve symptoms of spinal cord dysfunction using the modified Japanese Orthopedic Association (mJOA) score.
Specific Aim #2
2. From a patient perspective, health resource utilization (out-of-pocket expenses and loss of productivity) for ventral surgery, dorsal fusion, and laminoplasty surgery will be different.
Specific Aim #3
3. Cervical sagittal balance post-operatively will be a significant predictor of SF-36 PCS outcome.
Study Design
The study is a multicenter, randomized, controlled clinical trial (www.ClinicalTrials.gov; identifier: NCT02076113). The study also includes a nonrandomized arm for patients who are eligible for, but refuse randomization. In addition, patients who are eligible but for whom equipoise is not confirmed by the Spinal Experts Panel are entered into the nonrandomized arm.
Methodology
Subjects
Inclusion Criteria
Inclusion criteria for the trial are subjects aged 45-75 years with CSM identified with two or more levels of spinal cord compression from C3 to C7 by imaging with magnetic resonance imaging (MRI) or an equivalent study. Patients will have two or more of the following symptoms or signs: clumsy hands, gait disturbance, hyperreflexia, up-going toes, bladder dysfunction, or ankle clonus, and will be treated with either ventral decompression with fusion, dorsal decompression with fusion, or dorsal laminoplasty. The Central Review Committee will review imaging to evaluate for radiographic exclusion criteria. In addition, subjects will meet inclusion criteria if a majority of the Spinal Expert Network who review the imaging concur that the case meets randomization criteria, and less than 80% would recommend the same procedure in a non-randomized scenario (see below).
Exclusion Criteria
Subjects with C2-C7 kyphosis > 5° (measured in standing neutral cervical spine radiograph), a segmental kyphotic deformity defined as three or more disc-osteophytes that extend dorsal to a C2-C7 dorsal-caudal line measured on cervical spine computerized tomography (CT) or magnetic resonance imaging (MRI), structurally significant ossification of the posterior longitudinal ligament (OPLL), previous cervical spine surgery, or significant active health-related comorbidity (Anesthesia Class IV or higher) will be excluded from the study. The Central Review Committee will review imaging to evaluate for radiographic exclusion criteria.
Randomization
The randomization scheme will be 2:3 ventral to dorsal. There was significant interest in having outcomes data comparing dorsal laminectomy and fusion (a more commonly conducted procedure in the US) with dorsal laminoplasty, the most commonly performed procedure for CSM worldwide. We determined that a three-armed RCT was not feasible. Therefore, we chose to increase the randomization to the dorsal arm and increase the sample size to allow post hoc non-randomized comparisons of these two dorsal procedures.
Spinal Experts Network Review
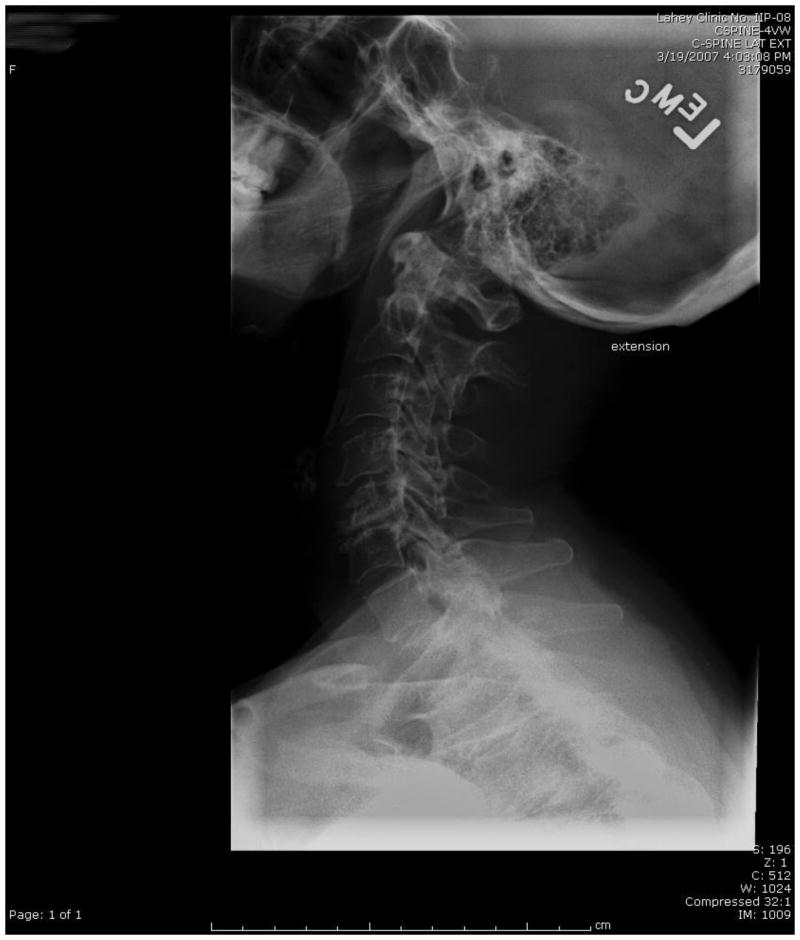
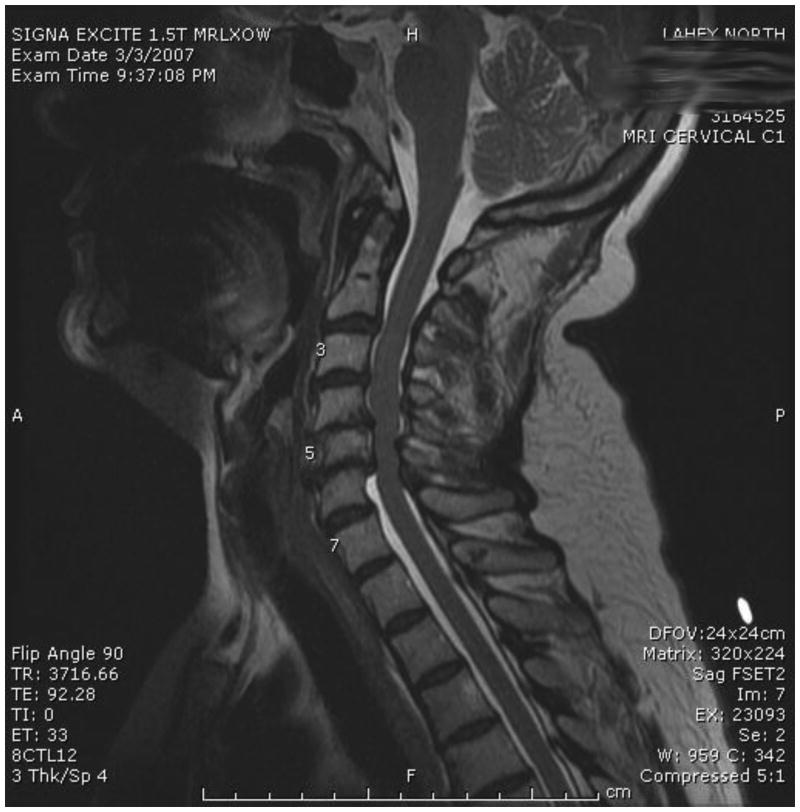
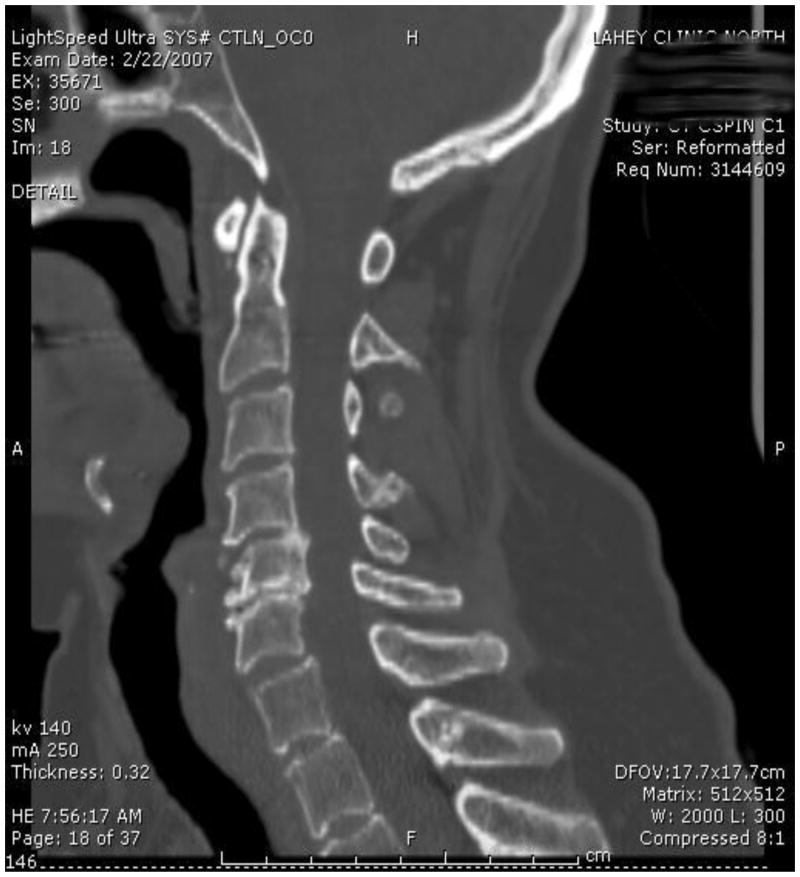
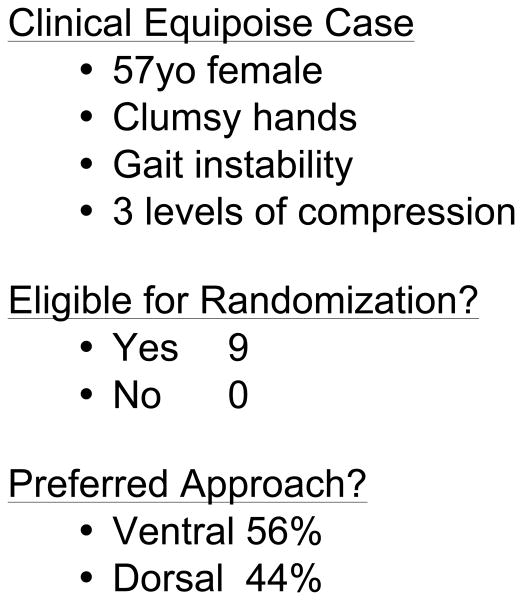
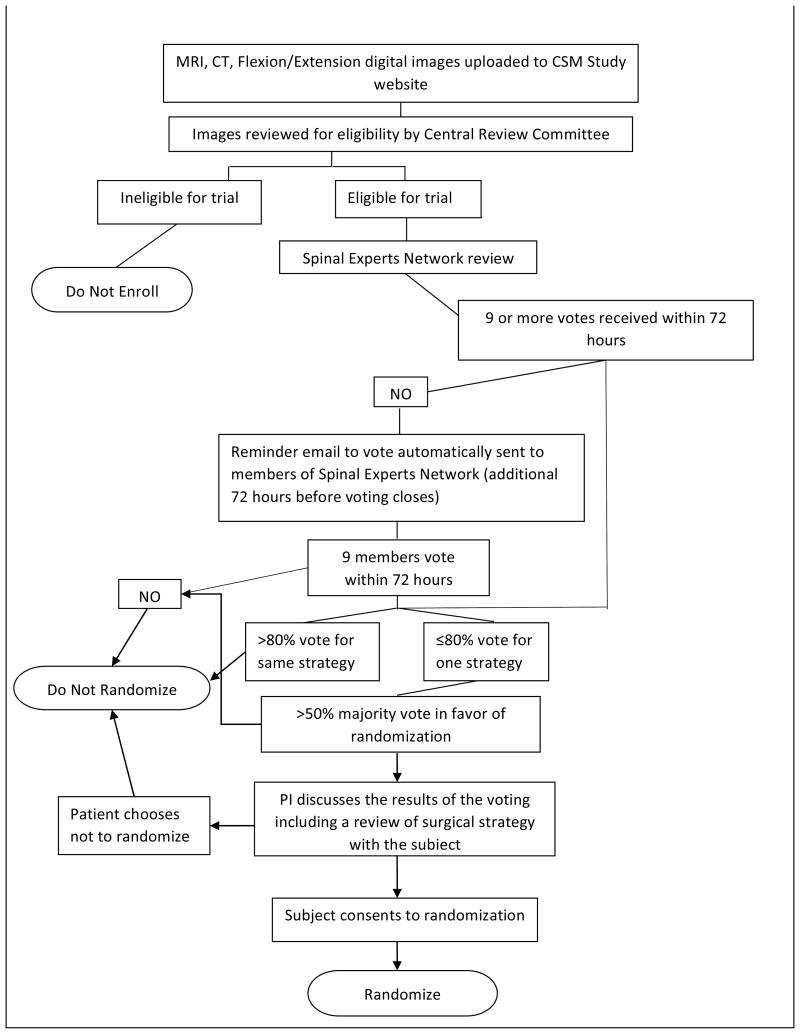
One of the most important barriers to performing high-quality RCTs in surgery is patient accrual. Participation in RCTs often is limited by a lack of sufficient equipoise on the part of both the treating surgeon and the patient. We have taken several proactive and innovative steps to improve patient consent to randomization, including the development of a novel web-based Spinal Experts Network that was demonstrated to facilitate and increase patient enrollment and randomization. In this approach, each expert reviews the radiographic images of eligible patients and makes two assessments: their preferred approach and, whether for this case, there is clinical equipoise for use of either a dorsal or ventral surgical approach (Figure 3). This provides scientific rigor to the definition of equipoise on an individual patient basis. It also provides the patient with multiple “second opinions”, increasing the patient's interest in participating in the RCT and trust in the appropriateness of randomization.
Figure 3.
A-D: Flexion-Extension Images, Sagittal CT and MRI images for CSM case judged eligible for study. E: Spinal Experts' Review. All 9 investigators found the case eligible for randomization. The preferred approach is also shown for each of the voting investigators.
For the CSM-S trial, once a patient has been screened, identified as having CSM, and has agreed to participate in the trial by signing consent, the subject will undergo two reviews. The patient's images will first be reviewed by a Central Review Committee (composed of two surgeon investigators from the primary site) to confirm eligibility. If the patient is deemed eligible, their images will then be reviewed by the Spinal Experts Network. The patient's images will be uploaded onto the web-based platform, and an email will be generated and sent to all 15 members (10 CSM surgeon investigators and five senior non-investigator spine surgeons) of the Spinal Experts Panel. Each surgeon will be asked to vote: ‘randomize’ or ‘do not randomize’ and then to characterize his/her preferred surgical approach as ventral or dorsal. If after 72 hours, a majority (>50%) of the review panel (with at least nine votes for a quorum) favor randomization (equipoise) and less than 80% select one procedure over another, then the patient will be eligible for study randomization (Figure 4).
Figure 4.
Randomization schema for the CSM-S trial.
The results of the voting (the number of votes for “randomize/do not randomize” and the number of ventral or dorsal votes) will be made available to the patient both to increase patient trust and confidence in the recommendation for randomization, as well as to protect patients from undergoing randomization when one approach (ventral or dorsal) might be superior for that patient. Patients will be offered randomization if two conditions are met:
Central Review Committee checked the images and clinical exam findings and found the patient eligible; and
Spinal Experts Panel confirmed patient's eligibility (>50% majority vote “randomize” and less than 80% select one procedure over another).
The patient can either consent to or decline randomization. There will be two categories of patients who have signed an IRB-approved consent to participate in the study:
Randomized cohort: Upon confirmation of eligibility and patient acceptance of randomization, the study coordinator will access the assignment by logging into the secure study website (www.csm-study.org) with a password and will obtain the randomization assignment from the web-based platform with its pre-programmed blocked (5, 10, or 15 subjects/block) stratified site-specific randomization scheme.
Non-Randomized cohort: The non-randomized cohort will consist of all patients who meet entry criteria but for whom equipoise is not confirmed by the Spinal Experts Panel. In addition, patients who do not consent to randomization will be in this cohort.
CSM-S Trial Website
In preparation for this trial, we constructed and tested a HIPAA compliant web-based platform for the study (www.csm-study.org) (Figure 5). In addition to facilitating direct data entry at each site by local study coordinators, this web platform provides the mechanism for the spine experts panel to vote on each case. As each new case is enrolled into the study, the patient images are de-identified prior to being uploaded to the website for review. Members of the Spine Experts Network are automatically sent an email when a patient's images are ready for review. The email will contain a link to the patient case in question, enabling rapid assessment and voting by investigators.
Figure 5.
CSM-S Study Website.
Study Interventions
Surgical Treatment
All patients will undergo surgery as standard clinical care. Decompression of the spinal canal to a diameter of at least 13 mm with restoration of cerebrospinal fluid flow around the spinal cord is the goal, regardless of the approach. Surgical techniques have been standardized, as summarized below:
Ventral Surgery
Ventral decompression and fusion14 will be performed using a multi-level discectomy (including partial or single level corpectomy) with fusion and plating.15,16 Allograft will be used at each disc space and all compressive osteophytes will be removed using the operating microscope. Fixation will be performed with rigid, semi-constrained, or dynamic titanium plates to optimize fusion and minimize complications.17,18
Dorsal Surgery
(Surgeon will choose either dorsal laminectomy + fusion or dorsal laminoplasty)
Dorsal Laminectomy + Fusion
Dorsal decompression and fusion will be performed using midline cervical laminectomy with the application of lateral mass screws and rods for rigid fixation.19 All surgeons will use local bone and allograft as needed to perform a lateral mass fusion, which typically will include one level rostral to the levels decompressed.
Dorsal Laminoplasty
Laminoplasty will be performed using an open-door approach with the application of plates and screws at each treated level. Ceramic or allograft laminar spacers (surgeon's choice) can be used with plates and screws to expand the canal diameter.20,21
Outcome Measures and Follow-up
Primary Outcome Measure
The primary outcome measure is the physical component summary score (PCS) of the SF-36. Physical component summary (PCS) scores will be calculated using population-adjusted norms to generate normalized scores with a mean of 50 ± 10 (standard deviation). The SF-36 (version 2) will be administered in the office by a study coordinator pre-operatively, and at three months, six months and one year post-operatively. A clinically meaningful difference in the SF-36 PCS scores will be defined as five points.22-25
Secondary Outcome Measures
Two validated disease-specific outcomes measures (modified Japanese Orthopedic Association (mJOA)26 and Neck Disability Index (NDI)27) will be measured pre-operatively, and at three months, six months and one year post-operatively. Preference-based health-related quality of life measures reflecting US population values for calculation of Quality Adjusted Life Years (QALYs) will be used. Preference-based outcome measures produce a single outcome score on an interval scale anchored at zero (death) and one (perfect health). The assessment of health state preferences will be performed using the EQ-5D.28 A separate analysis will compare use of SF-6D versus EQ-5D.29,30 Preference-based QOL will be assessed with the EQ-5D28 and SF-6D31 at the same intervals as the primary outcome measure SF-36 PCS, as will return to work status and disability benefits and payments.32 In addition to days missed from work for medical treatments or evaluations, participants will be asked to keep track of days missed from work and days unable to perform usual activities. Major adverse outcomes will be recorded at 30 days and one year post–operatively. Health resource utilization information (including out-of-pocket expenses) will be obtained using patient diaries, along with copies of all medical bills and receipts, at 1 month, 3 months, 6 months, and 12 months post-operatively for all patients. At one year post-operatively, full standing sagittal cervical–thoracic-lumbar-sacral radiographs will be obtained to calculate cervical C2-C7 sagittal balance, overall spinal sagittal balance, and cervical C2-C7 kyphosis or lordosis (in randomized patients only).
Sample Size
Sample size estimates were calculated based on analysis of covariance model with α= 0.05 at 80% and 90% power using Power Analysis and Sample Size software (PASS 2008, NCSS, LLC. Kaysville, Utah). The primary endpoint is the SF-36 PCS. This component of the SF-36 is derived from the sums of scores of 21 items and thus exhibits distributional behavior commensurate with assumptions for parametric analysis. Our preliminary observational data showed a SF-36 PCS difference score of 8.7 for ventral surgery compared with 4.0 for dorsal procedures, with standard deviations between 10 and 12 and correlations of baseline with one year SF-36 PCS between 0.6 and 0.7. Table 1 describes the total sample size (2:3 ventral-dorsal randomization) required to detect a five point difference in SF-36 PCS one year post-surgery for various combinations of power, standard deviation and one year correlations under a fixed design.
Table 1.
A-B. A) Pre- and Post-operative SF-36 PCS scores and correlations based on preliminary data. B) Total sample size (2:3 ventral-dorsal randomization) required to detect a five point difference in SF-36 PCS one year post-surgery for various combinations of power, standard deviation and one year correlations under a fixed design.
| A) | ||||
|---|---|---|---|---|
| Surgery (N) | Pre-op | Post-Op | Correlation between Pre- and Post-Op | Difference |
| Ventral (45) | 35.5 ± 10.3 | 44.2 ± 11.7 | 0.64 | + 8.7 ± 8.2 |
| Dorsal (70) | 35.8 ± 11.3 | 39.8 ± 11.6 | 0.66 | + 4.0 ± 9.5 |
| Dorsal Fusion (42) | 35.0 ± 11.7 | 39.6 ± 12.4 | 0.65 | + 4.6 ± 10.0 |
| Laminoplasty (28) | 37.0 ± 10.9 | 40.1 ± 10.6 | 0.67 | + 3.1 ± 8.8 |
| All patients (115) | 35.7 ± 10.9 | 41.5 ± 11.8 | 0.64 | + 5.8 ± 9.7 |
| B) | |||||||
|---|---|---|---|---|---|---|---|
| Difference / SD | Correlation | 80% Power / 5% Type I error (2-sided) | 90% Power / 5% Type I error (2-sided) | ||||
| NVentral | NDorsal | N | NVentral | NDorsal | N | ||
| 5 / SD = 10 | 0.60 | 38 | 56 | 94 | 50 | 74 | 124 |
| 5 / SD = 10 | 0.65 | 40 | 59 | 99 | 53 | 78 | 131 |
| 5 / SD = 10 | 0.70 | 42 | 62 | 104 | 55 | 82 | 137 |
A minimum sample size assuming a .70 correlation of 137 patients provides at least 90% power. The sample size was inflated by 5% to accommodate multiple significance testing using an Obrien-Fleming stopping boundary. Based on our preliminary data and pilot studies, withdrawal and loss-to-follow-up are not expected to be high. The sample size was further inflated by 10% to accommodate attrition during the follow-up. Thus, 159 total patients will be recruited and randomized. Based on our prior work in terms of both refusal rates and non-eligible subjects, we anticipate 91 nonrandomized patients will be enrolled into the study.
Study Sites and Surgeons
Both orthopaedic and neurological surgeon leaders were selected for inclusion in this study based on clinical volume and expertise as well as their participation in multiple preliminary investigator meetings. In Table 2, the clinical volume for each of the 11 study sites included in this study is summarized. All surgeons submitted radiographic evidence of their clinical quality for each of the surgical procedures.
Table 2.
Annual volume of surgically treated CSM cases at each site.
| Site | PI | Ventral | Dorsal Laminectomy + Fusion | DorsalLaminoplasty |
|---|---|---|---|---|
| 1. Lahey Hospital & Medical Center | Subu Magge, MD | 52 | 38 | 2 |
| 2. University of Medicine and Dentistry- New Jersey | Robert Heary, MD | 15 | 18 | 1 |
| 3. University of Utah | Erica Bisson, MD | 36 | 18 | 9 |
| 4. Cleveland Clinic Foundation | Edward C. Benzel, MD | 23 | 37 | 2 |
| 5. Thomas Jefferson University | Todd Albert, MD | 200 | 75 | 25 |
| 6. Massachusetts General Hospital | Jean-Valery Coumans, MD | 14 | 2 | 10 |
| 7. Washington University- St. Louis | Daniel Riew, MD | 12 | 1 | 7 |
| 8. MetroHealth | Michael Steinmetz, MD | 45 | 33 | 15 |
| 9. Medical College of Wisconsin | Marjorie Wang, MD | 45 | 70 | 1 |
| 10. Mount Sinai Medical Center | Tanvir Choudhri, MD | 90 | 58 | 11 |
| 11. University of Toronto | Michael Fehlings, MD | 57 | 65 | 16 |
| Total | Annual Cases | 589 | 415 | 99 |
Follow-up
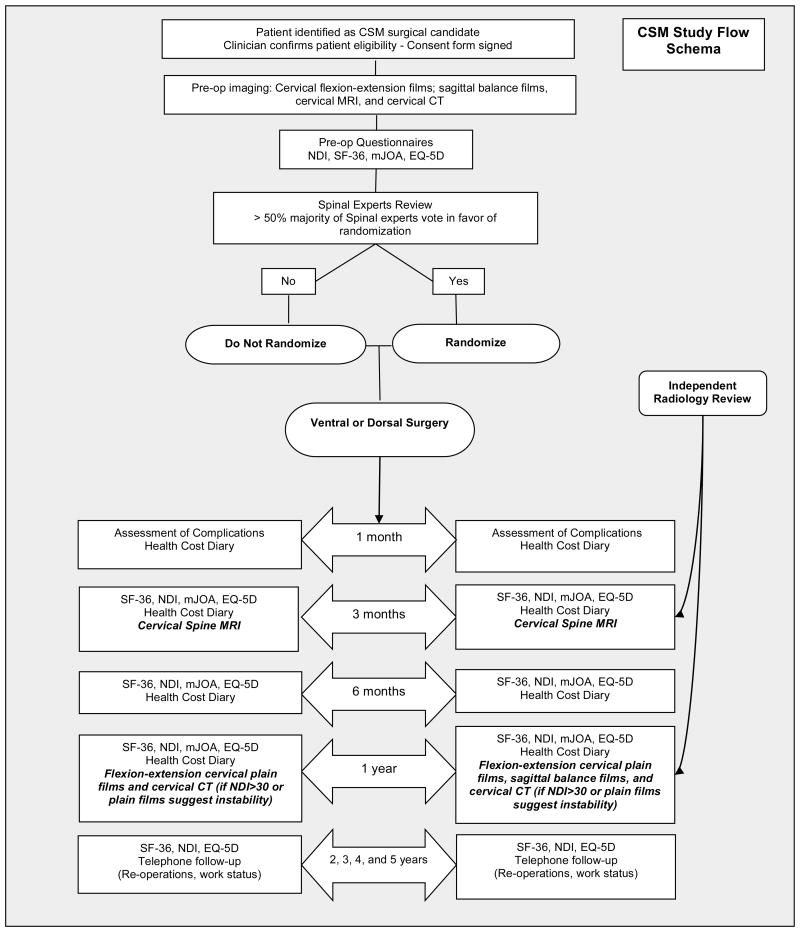
Clinical Follow-Up (one year) – End point assessment (Figure 6)
Figure 6.
CSM-S study schema.
Post-operative clinic visits (outcomes assessment) will occur at one month, three months, six months and one year post-operatively. Health resource utilization diaries will be completed at all time points. All complications will be reported to the central project manager within 48 hours. The study coordinator will record:
30 day complications: death, myocardial infarction, pulmonary embolus, re-hospitalization, recurrent laryngeal nerve injury, new hoarseness, new neurological deficit (e.g. C5 palsy), infection, dysphagia at 30 days resulting in weight loss and/or formal swallow evaluation and therapy, esophageal perforation, and re-operation.
Delayed complications: re-operation, fusion complication, problems with instrumentation, deformity, re-hospitalization.
Post-operative imaging analysis (3 months and one year) (Figure 6)
Post-operative imaging of randomized patients (de-identified and on CD) will undergo independent radiographic review at the central reading site. Post-operative cervical spine MRI will be performed at 3 months to document satisfactory spinal cord decompression. Cervical spine flexion-extension radiographs will be obtained at one year after surgery to assess cervical fusion and/or radiographic complications. Standing cervical-thoracic-lumbar-sacral films will be obtained at one year postoperatively to assess cervical as well as overall sagittal balance. Cervical spine CT scans will be obtained at one year if the patient has an NDI > 30 or if plain films suggest instability.
Five–Year Extended Follow-up (Figure 6)
Long-term follow-up will occur annually in years 2 through 5 post-operatively. It will include the SF-36, NDI, and EQ-5D questionnaires, as well as a long-term follow-up phone questionnaire that addresses complications (including reoperations) and return to work status.
Data Management and Statistical Analysis
All patient questionnaire data will be recorded on IRB-approved case report forms (CRFs) with study ID numbers but without patient identifiers. A HIPAA-compliant data platform website has been created to manage the data for the trial. Hard copies of each CRF will be faxed or scanned and sent to the central data management center (Lahey Comparative Effectiveness Research Institute).
Primary analyses will include all subjects randomized using an intent-to-treat approach.33 The primary endpoint is the SF-36 PCS at one year. A likelihood-based analysis using a mixed model will be used to compare SF-36 PCS between groups.34,35 This model will adjust for baseline SF-36 PCS, as well as study surgeon, using a random effects model. All time points will be included in the model, and each subject will contribute data for the time points at which they were assessed. The model enables a statistical comparison between treatment groups at each time point, though the comparison at the one-year time point will be the primary analysis. The primary advantage of the mixed model, when compared to commonly used methods such as complete case analysis and single imputation (e.g. last observation carried forward), is its flexibility in handling missing data.34 This analysis will assume that missing data occurs at random (i.e. the missing data value can be dependent on observed data, but independent of unobserved data). The inclusion of all follow-up time points in the model, as well as covariates identified to be associated with withdrawal, will assist in meeting this assumption and minimizing the risk of bias. Although the assumption for missing data is weaker under the likelihood based analysis compared to complete case analysis, a non-ignorable missing data mechanism is possible. Sensitivity analysis using selection and pattern mixture models will be employed to evaluate the robustness of conclusions to the missing at random (MAR) assumption.36 Analysis of continuous secondary outcomes, such as the post-operative sagittal balance and a disease specific QOL measure, the NDI, will be performed similarly to that described for the primary outcome measure.
Discussion
The CSM-S RCT is designed to compare the effectiveness of ventral versus dorsal approaches for the surgical management of CSM, a common degenerative condition of the cervical spine associated with significant morbidity, for which the optimal surgical treatment for improved long-term function is unknown. The trial is innovative as are the methods to address clinical equipoise for the individual subject. The Spinal Experts Panel methodology aims to increase trust on the part of patients and in turn increase their willingness to participate in an RCT, and ensures that for a specific patient there is no consensus among experts as to the best treatment approach. The trial is innovative in its use of stakeholder engagement, at the patient, provider, payer and funder level, which we believe will increase the dissemination of the findings to the relevant groups and increase likelihood of the results to have an impact on clinical care.
NIH Funded Initial Investigator Meeting
The National Institutes of Health (NIH) awarded an R13 grant to fund an initial investigator meeting for the CSM-S trial. The objective was to bring together major stakeholders and investigators to review the current evidence, clinical protocol, and agree on a clinical population to be included in the RCT. The conference was held on January 23-24, 2014 in National Harbor, Maryland (Figure 7) and conducted by Zoher Ghogawala MD, National Study Principal Investigator (PI), of Lahey Hospital and Medical Center, Burlington, MA. There were a total of 42 attendees including the study investigators, radiologists, neurological monitoring experts, health economists, the CSM-S advisory board, all study coordinators, two spine patients, a PCORI representative, and a representative from the Centers for Medicare and Medicaid Services (CMS). The environment provided an excellent opportunity to discuss the trial protocol and for attendees to address concerns. As a result of the productive discussion at the end of the conference, several major changes were made in the CSM-S protocol:
Figure 7.
Agenda for the NIH funded (1R13AR065834-01) CSM-S Investigator Meeting.
Exclusion criteria were amended. Specifically, developmental narrowed spinal canal was removed from the exclusion criteria and several other criteria were revised to be more specific.
Required radiographic assessments were amended to reduce patient exposure to radiation. Specifically, a routine post-operative CT scan was removed from the protocol. A post-operative cervical CT scan was added at 1 year if the patient's NDI score is greater than 30 or if plain cervical spine radiographs suggest instability.
Ventral-dorsal randomization was amended to a 2:3 randomization (from a 1:1 randomization). The rationale for the 2:3 randomization is to accrue sufficient numbers of dorsal surgery patients to permit exploratory analysis to compare laminoplasty versus laminectomy and fusion surgery.
This initial investigator meeting was vital for the trial success. It allowed participating members and important stakeholders to express their expert opinion regarding the conduct of the trial. It also allowed an opportunity for site study coordinators to attend and receive formal training.
Trial Status
Patient enrollment was initiated on April 1, 2014. As of April 30, 2014, 6 patients (3 randomized) have been enrolled from 2 sites. Patient enrollment will occur over an 18-month period with 11 sites participating in enrollment. Seventeen orthopaedic spine surgeons and nineteen neurosurgeons will enroll patients in this trial.
Safety Considerations
All of the subjects in this trial are surgical candidates and would have had surgery recommended even if they were not involved in the study. All subjects will receive standard follow-up by the treating surgeon. All surgical procedures are considered standard care and none are considered investigational. Any significant findings regarding the overall safety of any of the procedures at any site will be brought to the attention of all subjects in the study once all of the appropriate IRB committees have been notified. The confidentiality of all subjects will be assured by assigning all subjects a code and by removing all identifying information from questionnaires. Data entered into the study web site will be encrypted using the current industry standard to protect patient confidentiality.
The DSMB committee will be convened by the Tufts Clinical and Translational Science Institute. An interim analysis is planned when 50% of patients have been enrolled and have completed 1 year follow-up. The interim analysis will be performed earlier if the DSMB identifies any compelling safety concerns.
Quality Assurance
There will be several layers of quality control for this study. An NIH-funded initial site investigator meeting was held, which included a training session for all study coordinators. It covered data collection and data entry procedures, as well as an opportunity to review all clinical protocols and answer questions. The data, after being recorded on CRFs and entered into the web-based platform, will be checked at the central data management site, the Lahey Comparative Effectiveness Research Institute (CERI), by the project data manager. In addition, there will be bi-monthly Steering Committee meetings with the Director of Data Management, who will supervise audits of the database bi-monthly with the project data manager. The PI and Biostatistician will supervise the quality control mechanisms at these bi-monthly meetings. There will also be site visits to audit each site's ability to screen and enroll patients and maintain accurate study records.
Expected Outcomes of the Study
This will be the first randomized study to evaluate the optimal surgical approach to CSM. The results of this study will help surgeons and patients understand the outcomes, complications, and costs associated with the three major surgical approaches for this spinal condition.
Duration of the Project
Enrollment is expected to occur over an 18-month period and patients will be followed for a total of 5 years post-operatively.
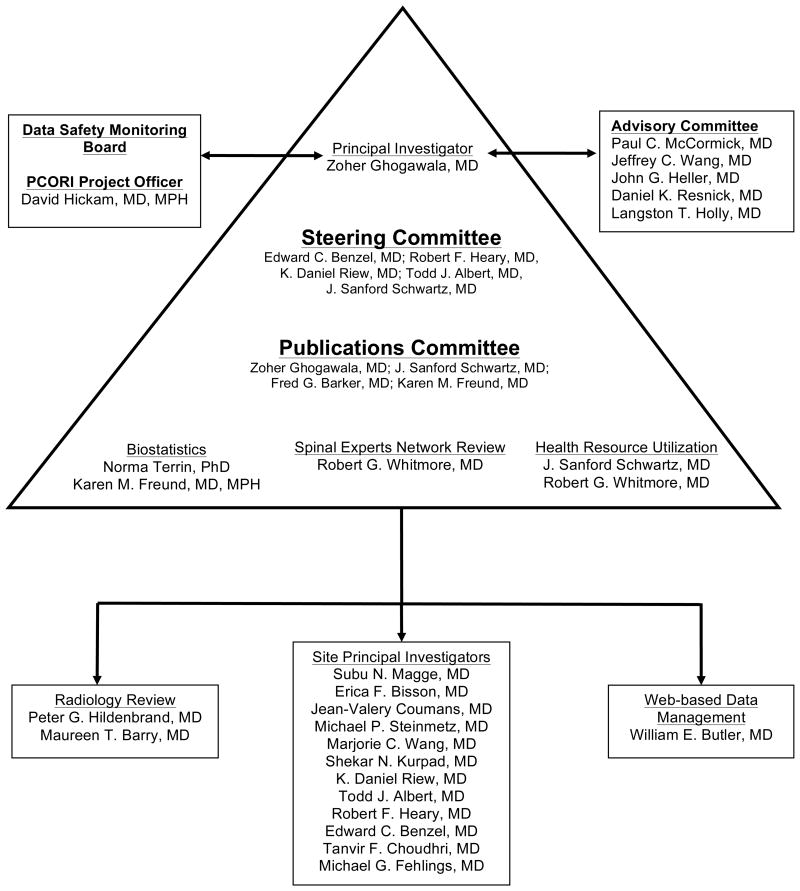
Project Management (Figure 8)
Figure 8.
Administrative organization of the CSM-S study.
The study is being led by the PI, Zoher Ghogawala MD. An advisory committee comprised of 5 senior specialists with expertise in clinical trial design and spinal surgery is responsible for reviewing study progress and advising the PI from time to time as to the potential impact of the study results on the quality of surgical care for patients with CSM. The steering committee, comprised of 5 senior investigators, is responsible for overall direction and general design and conduct of the study, preparation of study documents, and review of study procedures and progress. A separate publications committee will review all manuscripts and set guidelines for authorship.
Study investigators at each site are responsible for recruiting and enrolling patients, reporting adverse events, and completing data collection. Statistical design and analyses are being supervised by Dr. Karen Freund of the Tufts Clinical and Translational Science Institute.
Ethics
The study protocol will undergo review from the institutional review boards at each of the 11 participating sites. No patients will be enrolled until IRB approval has been obtained at the enrolling site. Written informed consent will be obtained from all eligible patients or next of kin for enrollment into the study.
Conclusion
Few surgical treatments are evaluated using RCTs. The CSM-S trial is an innovative study that aims to compare the effectiveness of three major surgical approaches to treating CSM using an RCT design. Using a spine experts panel to establish clinical equipoise for randomization might elevate the ethics of this trial by protecting patient's rights while promoting trust between investigators and patients. All patients that do not consent to randomization will be captured in a non-randomized cohort. By focusing upon patient overall health-related quality of life, this study will identify the optimal procedure for thousands of patients with CSM and will advance the science of surgical treatment for CSM.
Footnotes
Disclosures: National Institutes of Health (NIH) (1R13AR065834-01) and Patient-Centered Outcomes Research Institute (PCORI) (CE-1304-6173) to Zoher Ghogawala. Dr. K. Daniel Riew reports financial interest in Cerapedics, Medtronic, Spinal Dynamics, Amedica, Benvenue, Expanding Orthopedics, Nexgen, Osprey, Paradigm Spine, Spinal Kinetics, Spineology, Vertiflex, and Promethian Surgical Devices. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. American family physician. 2000 Sep 1;62(5):1064–1070. 1073. [PubMed] [Google Scholar]
- 2.Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005 May;56(5):1101–1113. discussion 1101-1113. [PubMed] [Google Scholar]
- 3.al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. Journal of neurosurgery. 1993 Oct;79(4):550–561. doi: 10.3171/jns.1993.79.4.0550. [DOI] [PubMed] [Google Scholar]
- 4.Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007 Jan;60(1 Supp1 1):S35–41. doi: 10.1227/01.NEU.0000215383.64386.82. [DOI] [PubMed] [Google Scholar]
- 5.Rowland LP. Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology. 1992 Jan;42(1):5–13. doi: 10.1212/wnl.42.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Patil PG, Turner DA, Pietrobon R. National trends in surgical procedures for degenerative cervical spine disease: 1990-2000. Neurosurgery. 2005 Oct;57(4):753–758. discussion 753-758. [PubMed] [Google Scholar]
- 7.Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine. 2007 Feb 1;32(3):342–347. doi: 10.1097/01.brs.0000254120.25411.ae. [DOI] [PubMed] [Google Scholar]
- 8.Committee. Initial National Priorities for Comparative Effectiveness Research. [Accessed April 30, 2014];2009 http://www.iom.edu/en/Reports/2009/ComparativeEffectivenessResearchPriorities.aspx. 2014.
- 9.Ghogawala Z, Coumans JV, Benzel EC, Stabile LM, Barker FG., 2nd Ventral versus dorsal decompression for cervical spondylotic myelopathy: surgeons' assessment of eligibility for randomization in a proposed randomized controlled trial: results of a survey of the Cervical Spine Research Society. Spine. 2007 Feb 15;32(4):429–436. doi: 10.1097/01.brs.0000255068.94058.8a. [DOI] [PubMed] [Google Scholar]
- 10.Fehlings MG, Sasso RC, Kopjar B, et al. Anterior vs. Posterior Surgery of Cervical Spondylotic Myelopathy: A large prospective multi-center clinical trial. 24th Annual Meeting of the AANS/CNS Section on Disorders of the Spine and Peripheral Nerves; February 27-March 1, 2008; Orlando, FL. 2008. [Google Scholar]
- 11.Riggs BL, Melton LJ., 3rd The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995 Nov;17(5 Suppl):505S–511S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 12.Ghogawala Z, Martin B, Benzel EC, et al. Comparative effectiveness of ventral vs dorsal surgery for cervical spondylotic myelopathy. Neurosurgery. 2011 Mar;68(3):622–630. doi: 10.1227/NEU.0b013e31820777cf. discussion 630-621. [DOI] [PubMed] [Google Scholar]
- 13.King JT, Jr, Abbed KM, Gould GC, Benzel EC, Ghogawala Z. Cervical spine reoperation rates and hospital resource utilization after initial surgery for degenerative cervical spine disease in 12,338 patients in Washington State. Neurosurgery. 2009 Dec;65(6):1011–1022. doi: 10.1227/01.NEU.0000360347.10596.BD. discussion 1022-1013. [DOI] [PubMed] [Google Scholar]
- 14.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. The Journal of bone and joint surgery American volume. 1958 Jun;40-A(3):607–624. [PubMed] [Google Scholar]
- 15.Hillard VH, Apfelbaum RI. Surgical management of cervical myelopathy: indications and techniques for multilevel cervical discectomy. The spine journal : official journal of the North American Spine Society. 2006 Nov-Dec;6(6 Suppl):242S–251S. doi: 10.1016/j.spinee.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Stewart TJ, Schlenk RP, Benzel EC. Multiple level discectomy and fusion. Neurosurgery. 2007 Jan;60(1 Supp1 1):S143–148. doi: 10.1227/01.NEU.0000217015.96212.1B. [DOI] [PubMed] [Google Scholar]
- 17.Brodke DS, Zdeblick TA. Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine. 1992 Oct;17(10 Suppl):S427–430. doi: 10.1097/00007632-199210001-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kwon BK, Vaccaro AR, Grauer JN, Beiner JM. The use of rigid internal fixation in the surgical management of cervical spondylosis. Neurosurgery. 2007 Jan;60(1 Supp1 1):S118–129. doi: 10.1227/01.NEU.0000249222.57709.59. [DOI] [PubMed] [Google Scholar]
- 19.Huang RC, Girardi FP, Poynton AR, Cammisa FP., Jr Treatment of multilevel cervical spondylotic myeloradiculopathy with posterior decompression and fusion with lateral mass plate fixation and local bone graft. Journal of spinal disorders & techniques. 2003 Apr;16(2):123–129. doi: 10.1097/00024720-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Heller JG, Edwards CC, 2nd, Murakami H, Rodts GE. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: an independent matched cohort analysis. Spine. 2001 Jun 15;26(12):1330–1336. doi: 10.1097/00007632-200106150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Park AE, Heller JG. Cervical laminoplasty: use of a novel titanium plate to maintain canal expansion--surgical technique. Journal of spinal disorders & techniques. 2004 Aug;17(4):265–271. doi: 10.1097/01.bsd.0000095401.27687.c0. [DOI] [PubMed] [Google Scholar]
- 22.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. 2nd. Lincoln, RI: QualityMetric, Inc; 2001. [Google Scholar]
- 23.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. The spine journal : official journal of the North American Spine Society. 2008 Nov-Dec;8(6):968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Glassman S, Gornet MF, Branch C, et al. MOS short form 36 and Oswestry Disability Index outcomes in lumbar fusion: a multicenter experience. The spine journal : official journal of the North American Spine Society. 2006 Jan-Feb;6(1):21–26. doi: 10.1016/j.spinee.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Birkmeyer NJ, Weinstein JN, Tosteson AN, et al. Design of the Spine Patient outcomes Research Trial (SPORT) Spine. 2002 Jun 15;27(12):1361–1372. doi: 10.1097/00007632-200206150-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suda K, Abumi K, Ito M, Shono Y, Kaneda K, Fujiya M. Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine. 2003 Jun 15;28(12):1258–1262. doi: 10.1097/01.BRS.0000065487.82469.D9. [DOI] [PubMed] [Google Scholar]
- 27.Coric D, Finger F, Boltes P. Prospective randomized controlled study of the Bryan Cervical Disc: early clinical results from a single investigational site. Journal of neurosurgery Spine. 2006 Jan;4(1):31–35. doi: 10.3171/spi.2006.4.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Suhonen R, Virtanen H, Heikkinen K, et al. Health-related quality of life of day-case surgery patients: a pre/posttest survey using the EuroQoL-5D. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2008 Feb;17(1):169–177. doi: 10.1007/s11136-007-9292-3. [DOI] [PubMed] [Google Scholar]
- 29.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2005 Aug;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 30.Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health economics. 2005 Nov;14(11):1169–1189. doi: 10.1002/hec.1006. [DOI] [PubMed] [Google Scholar]
- 31.Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 Health Survey. Journal of clinical epidemiology. 1998 Nov;51(11):1115–1128. doi: 10.1016/s0895-4356(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 32.Gold M, Siegel J, Russell L, Weinstein M. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 33.Lachin JM. Statistical considerations in the intent-to-treat principle. Controlled clinical trials. 2000 Jun;21(3):167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 34.Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004 Jul;5(3):445–464. doi: 10.1093/biostatistics/5.3.445. [DOI] [PubMed] [Google Scholar]
- 35.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of Clinical Trials using SAS: A Practical Guide. Cary, NC: SAS Institute, Inc; 2005. [Google Scholar]
- 36.Michiels B, Molenberghs G, Bijnens L, Vangeneugden T, Thijs H. Selection models and pattern-mixture models to analyse longitudinal quality of life data subject to drop-out. Statistics in medicine. 2002 Apr 30;21(8):1023–1041. doi: 10.1002/sim.1064. [DOI] [PubMed] [Google Scholar]