Abstract
Purpose
We have shown previously that Bruch's membrane (BM) aging decreases retinal pigment epithelium (RPE) phagocytosis. Herein, we determine the effects of BM reengineering on RPE phagocytosis.
Methods
BM explants were dissected from young and old donor eyes. Some old BM explants were reengineered by cleaning with Triton X-100 and/or coating with extracellular matrix (ECM) ligands. ARPE-19 cell–derived ECM (ARPE-ECM) modified (“aged”) by sodium nitrite was subjected to similar treatments. ARPE-19 cells were then cultured to confluence onto the different surfaces. Fluorescently-labeled bovine rod outer segments (ROS) were fed to cells with or without αVβ5 integrin antibody. Image acquisition and phagocytosis quantification was performed by fluorescence microscopy and ImageJ analysis.
Results
Cleaning old donor–derived BM with detergent does not increase the uptake of ROS, but a combination of cleaning and coating with ECM ligands significantly increases RPE phagocytosis (54.9 ± 6.2 vs. 83.5 ± 6.5 arbitrary units; P < 0.05) to levels closer to young donor BM (123.6 ± 9.9 arbitrary units). Similar effects were observed on nitrite-modified ARPE-ECM subjected to the same treatments. Incubation of αVβ5 blocking antibody with ROS significantly decreased RPE phagocytosis.
Conclusions
The detrimental effects of aging BM on RPE phagocytosis can be reversed by reengineering the BM surface with detergent cleaning and recoating with ECM ligands.
Translation Relevance
These results demonstrate that the therapeutic success of transplanted RPE cells may require, at least in part, reengineering of diseased BM to make it a more suitable environment for attachment, survival and proper functioning of the RPE.
Keywords: age-related macular degeneration, phagocytosis, RPE, retinal pigment epithelium, RPE transplantation, αVβ5 integrin, ARPE-19
Introduction
In the healthy human eye, the retinal pigment epithelium (RPE) forms a contiguous hexagonal monolayer of cells that lines the inner aspect of Bruch's membrane (BM) and separates the neural retina from the choriocapillaris.1,2 The apical side of the RPE surrounds the distal tip of the rod and cone outer segments, while the RPE basal side is in contact with its basement membrane, which is the innermost layer of a thin multilaminar structure adjacent to the choriocapillaris.1,2 The RPE performs several functions important for maintaining the neural retina, including phagocytosis of photoreceptor outer segments, visual pigment processing and renewal, providing a barrier function, and transferring nutrients to the neural retina.2–4 In order for the integrity of the RPE monolayer to be maintained, there must be proper attachment of the RPE to BM, and between adjacent RPE cells.4,5 The attachment between the RPE and inner aspects of BM are mediated by integrin receptors on the RPE and ligands within the basal lamina that include laminin, fibronectin, and collagen type IV.6–8
Age-related macular degeneration (AMD) is a degenerative disease that affects the outer retina, RPE, BM, and choriocapillaris.9,10 The disorder is characterized by age-related ultrastructural changes within BM that include diffuse thickening, accumulation of drusen, basal laminar and basal linear deposits, collagen cross-linking in the inner and outer collagen layer, calcification, fragmentation of the elastin layer, and lipidization.11–15 Cellular changes in advanced AMD include atrophy of the RPE, choriocapillaris and outer retina in nonexudative AMD, and the development of choroidal neovascularization in exudative AMD.16–18 At the current time, any connection between ultrastructural changes observed in BM with age (mentioned above) and cellular changes that develop in AMD is not known. However, it is known that the structural changes that are due to aging within BM precede cellular changes in the RPE by one or two decades, well before there are signs of early AMD on clinical examination and imaging.11,14,19,20
In the human eye in vivo, the RPE and BM age together and therefore it is impossible to separate the effects of BM aging from the primary effects of cellular aging. Thus, we have developed a system in our laboratory to separate the effects of aging within BM from the effects of aging in the RPE.21–23 We have used this system to demonstrate that aging BM leads to a decrease in human RPE attachment to the inner aspects of BM, survival after attachment, proliferation, as well as an alteration of RPE gene expression.21–25 Recently, we have shown that BM aging decreases the ability of an RPE monolayer cultured on the BM surface to ingest rod outer segments (ROS).26 Nitrite treatment of RPE-derived extracellular matrix mimics the effects of aging BM.26 Interestingly, the effects of BM-extracellular matrix (ECM) aging on RPE attachment, survival, and proliferation of the RPE can be reversed by cleaning the BM surface with Triton X-100 and then coating the surface with a mixture of laminin, fibronectin, and vitronectin.27 Herein, we determine if reengineering of old donor–derived human BM can lead to an improvement in the phagocytic ability of RPE cells cultured on this surface.
Methods
Culture and Propagation of Immortalized ARPE-19 Cells
Immortalized human RPE cells (ARPE-19 cell line) obtained from the American Type Culture Collection (ATCC, # CRL2302; Manassas, VA) were cultured and propagated as specified by ATCC (All reagents from Life Technologies; Carlsbad, CA). ARPE-19 medium contained Dulbecco's modified Eagles medium - F12 (DMEM-F12) with N-2-hydroxyethyl-1-piperazine-N′-2-ethanesulfonic acid (HEPES) buffer (#11330) supplemented with 10% fetal bovine serum (#16000-044), and 1X Antibiotic-Antimycotic (#15240). ARPE-19 cells were plated at a density of 35 to 50 × 103 cells per well of a 96-well plate (Franklin Lakes, NJ) and allowed to reach confluence before conducting experiments. Cells were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C and the culture medium was changed 3 times per week. All of the experiments described herein were performed using the ARPE-19 cell line from ATCC.
Isolation of Bruch's Membrane (BM) Explants
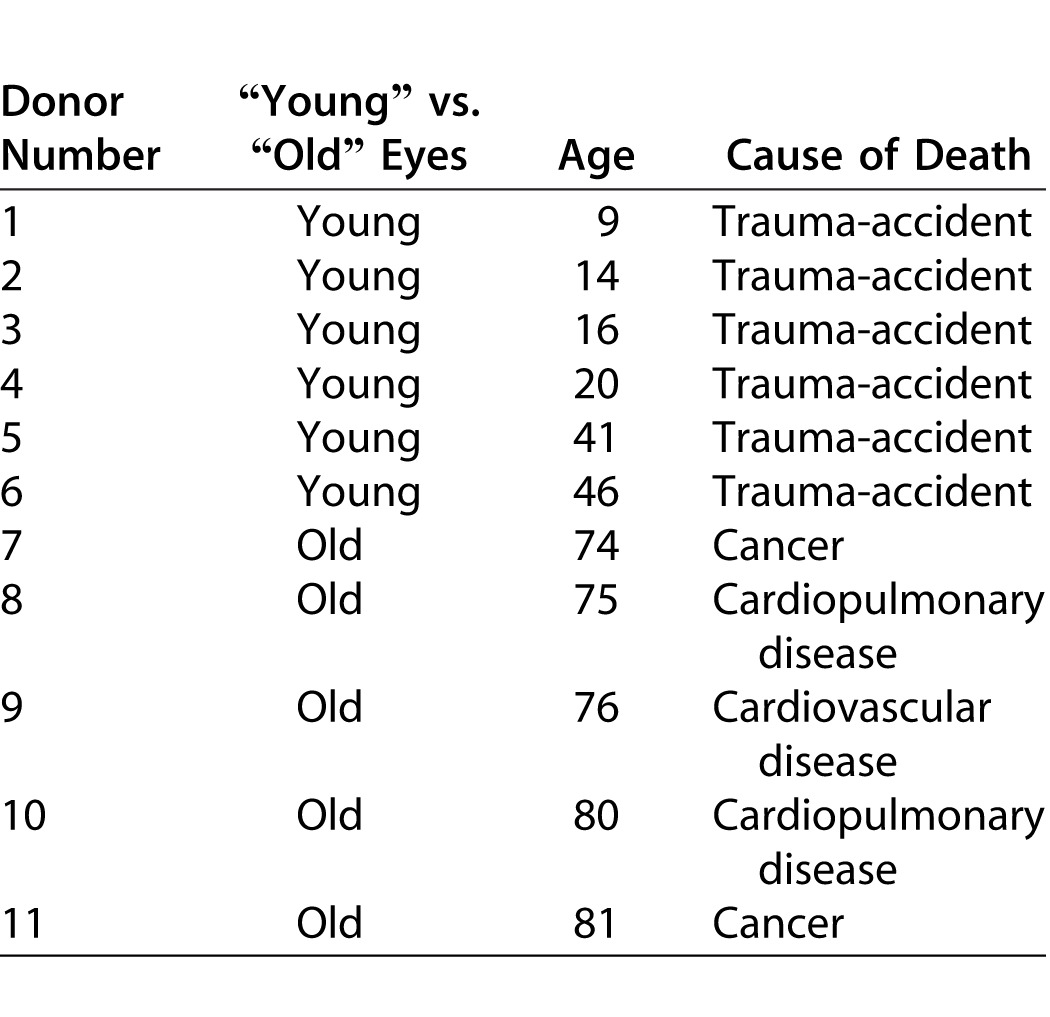
BM explants were obtained from human donors through the National Disease Research Interchange (Philadelphia, PA). All donor eyes (Table) were enucleated within 8–10 hours and shipped to the laboratory within 48 hours of death in a sterile container at 4°C. BM explants were harvested upon receipt and processed as previously described.26 Briefly, BM explants were obtained from young (9- to 46-years old) and old (74- to 81-years old) donors (Table). Two explants were harvested from each donor with the basal lamina layer of BM on the apical surface23,28 as follows: eyes were placed in carbon dioxide-free media and the choroid-BM-RPE complex was dissected away from the rest of the eye (sclera, anterior segment, and vitreous were discarded). Native RPE cells were removed by incubation in 0.02 M ammonium hydroxide for 20 minutes at room temperature followed by washing in phosphate buffered saline (PBS). Each explant was then floated in medium over a hydrophobic 125- to 175-μm thick polytetrafluoroethylene membrane with 0.5-μm pores (the basal lamina of the RPE was facing the membrane). Subsequently, 4% agarose was poured onto the BM-choroid complex from the choroidal side and immediately placed at 4°C to allow the agarose to solidify. Next, the polytetrafluoroethylene membrane was peeled off; 6-mm circular buttons were trephined on a Teflon sheet from peripheral BM and placed on 4% agarose at 37°C in untreated polystyrene wells of a 96-well plate. The agarose was allowed to solidify at room temperature, rinsed gently with PBS three times before sterilization with 20,000 rad of gamma radiation, and stored at 4°C.
Table.
Donor Eyes Were Enucleated Within 8 Hours and Processed in Our Laboratory for BM Isolation Within 48 Hours of Death
Isolation of Bovine Rod Outer Segments
Bovine ROS were isolated as previously described.26 Briefly, fresh bovine eyes were obtained from a local slaughterhouse and transported on ice to the laboratory. Eyes were washed with Hanks' basal salt solution (1X HBSS; Thermo Scientific, Waltham, MA) containing 100 U/mL penicillin and 0.1 mg/mL streptomycin (Pen/Strep; Life Technologies). The anterior half of the eye was removed and discarded and the retina/RPE were dissected out and separated from each other by gently pipetting HBSS into the subretinal space. The retina was then put into a glass tube on ice that contained isolation medium composed of 20% sucrose, 20 mM Tris-Cl, 2 mM MgCl2, and 130 mM NaCl (pH 7.2), and homogenized. One milliliter of the retinal homogenate was layered on top of a 10% to 60% sucrose gradient and centrifuged at 141,000g for 2 hours at 4°C in a Beckman SW-40 rotor. ROS were sedimented between a 27% and 50% sucrose gradient. Bands containing ROS were collected, pelleted by centrifugation at 7700g for 10 minutes at 4°C, suspended in the buffered salt solution, and again pelleted at 800g for 10 minutes. Purified ROS were stored at 4°C in DMEM containing 17% sucrose and Pen/Strep. Prior to use, the ROS were centrifuged at 9000g for 10 minutes to remove storage medium. Outer segment concentration was assessed from the rhodopsin concentration measured spectrophotometrically in 0.1% Ammonyx LO.29
Fluorescent Labeling of Isolated Bovine Rod Outer Segments
Isolated ROS were labeled with fluorescein isothiocyanate (FITC, #F7250; Sigma Aldrich, St. Louis, MO). ROS were pelleted in HBSS solution and suspended in 0.1 M sodium bicarbonate buffer pH 9 to 9.5. FITC was solubilized in dimethyl sulfoxide (DMSO) to a concentration of 2 mg/mL, and added to ROS to a final concentration of 10 to 50 ng/mL and subsequently incubated for 1 hour at room temperature in the dark with gentle shaking. FITC-stained ROS were washed twice, pelleted in a microcentrifuge tube (4 minutes at 4500g), and resuspended in growth medium at a concentration of 0.4 μg/μL. Fluorescently-labeled ROS were then seeded onto cultured cells in 96-well plates at a concentration of 4 to 5 μg/cm2, which corresponds to 1.5 × 105 particles/cm2 (5 × 104 outer segment particles/well). Microscopic examination of the labeled ROS showed intact outer segments after labeling (data not shown).
Preparation of Nitrite-Modified Extracellular Matrix (ECM)
The generation of RPE-derived ECM and subsequent nitrite treatment was described previously.26 In summary, 30 to 50 × 104 ARPE-19 cells were plated onto 96-well plates and grown to confluence for approximately 6 to 8 weeks to allow sufficient time for cells to form ECM. Subsequently, cells were removed by adding 100 μL/well of 20 mM ammonium hydroxide buffer for 20 minutes, followed by washing with PBS three times and air drying inside the culture hood to obtain 96-well plates coated with ARPE-19–derived ECM. Sodium nitrite–treated ECM was prepared by adding 100 μL/well of 100 mM sodium nitrite in pH 4 acetate buffer for 7 days. Wells were then washed at least four times with PBS, incubated with PBS for 4 hours, and washed again at least two additional times to completely remove the nitrite.30
Detergent Cleaning and/or Coating of Human Bruch's Membrane and Extracellular Matrix
RPE-derived ECM damaged by nitrite treatment or human BM explants were processed, as described previously,26,27 to create the following conditions: (1) cleaned BM/ECM, treated with 0.1% Triton-X/0.1% sodium citrate solution for 20 minutes at 4°C, (2) cleaned and coated BM/ECM (i.e., cleaned first with 0.1% Triton-X/0.1% sodium citrate, as above, then coated with an ECM-protein mixture containing laminin, 330 μg/mL; fibronectin, 250 μg/mL; vitronectin, 33 μg/mL, at 37°C for 30 minutes), or (3) untreated surfaces without cleaning or coating, which were used as controls. After the cleaning and/or coating, surfaces were washed three times with PBS for 5 minutes and stored at 4°C to be used within 48 hours or frozen at −20°C, to be thawed immediately prior to use.
Incubation of Cultured RPE Cells with FITC-Labeled Rod Outer Segments
ARPE-19 cells (30–50 × 104) were seeded onto either BM explants or RPE-derived ECM in 96-well plates and cultured to confluence (10–14 days). Subsequently, ARPE-19 cells were fed FITC-labeled ROS in DMEM-F12 with 20% FBS and incubated at 37°C for approximately 16 hours. Cells were then rinsed several times with DMEM-F12 to remove FITC-labeled ROS and counterstained with Hoechst nuclear staining (#62249; Thermo Scientific, Rockford, IL) prior to fixation. Fixation was carried out in 2% paraformaldehyde for 10 to 15 minutes, and rinsed several times with 1X Dulbecco's PBS (Life Technologies). Immediately after fixation, fluorescent images were acquired using an In Cell Analyzer 2000 machine (GE Healthcare Life Sciences, Piscataway, NJ) with a ×40 objective using ultraviolet and FITC filters. Alternatively, for the time course experiment, FITC-labeled ROS were seeded onto cells and a new set of duplicate wells was washed sequentially every 2 hours up to 12 hours, followed by counterstaining, fixation, and image acquisition as described above. To block phagocytosis, cells were incubated with anti-αVβ5 integrin antibody in addition to the FITC-labeled ROS for 3 hours, as described in detail elsewhere.31
Statistical Analysis
All experiments were run in triplicates and repeated at least three times. Values were plotted using Excel spread sheets and graphs generated to indicate the mean ± SD. The significance of the values was determined using the Student's t-test.
Results
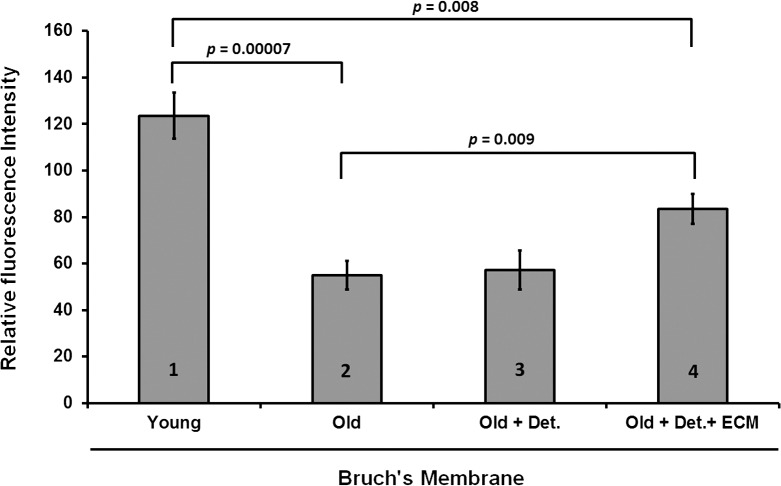
We have shown previously that BM derived from eyes of older individuals decreases the ability of the RPE cultured on its surface to phagocytize ROS in vitro.26 In addition, we have also reported that the combined effect of cleaning old donor–derived BM with detergent and then recoating it with ECM proteins increases the rate of survival, proliferation, and attachment of the overlying RPE cells to levels close to the controls.27 Herein, we sought to investigate whether cleaning alone, or cleaning combined with coating of impaired surfaces (either nitrite-damaged ECM or old donor–derived BM) affected the ability of ARPE-19 cells to phagocytose ROS to levels similar to that seen for young BM. Treating old BM with detergent alone did not change the functional ability of the ARPE-19 monolayer to phagocytize ROS as compared with controls (Fig. 1). However, detergent cleaning followed by recoating BM with ECM-specific proteins increased the capacity of the ARPE-19 cells to phagocytize ROS (Fig. 1: 54.9 ± 6.2 vs. 83.5 ± 6.5 arbitrary units; P = 0.009). Although phagocytosis levels were not brought back to the levels of cells on young BM, the values still represented a 25% increase above old BM, suggesting that ECM proteins played a key role in this essential RPE function (Fig. 1: Young, 123.6 ± 9.9 arbitrary units; Old, 54.9 ± 6.2 arbitrary units; old + cleaned and coated, 83.5 ± 6.5 arbitrary units; N = 11 eyes; and Table demographics).
Figure 1.
ARPE-19 cell phagocytosis on young, old and re-engineered BM. Phagocytosis was higher on young BM (123.6 ± 9.9 arbitrary units) versus older BM (54.9 ± 6.2 arbitrary units) (bar 1 versus bar 2; P < 0.001). Cleaning of old BM surface with detergent and sodium citrate (57.2 ± 8.5 arbitrary units) did not increase phagocytosis (bar 2 versus bar 3; P = 0.45). However, re-engineering of old BM by cleaning and coating with ECM proteins increased phagocytosis (83.5 ± 6.5 arbitrary units) compared with old BM (bar 2 versus bar 4; P = 0.009). Nevertheless, BM re-engineering did not improve phagocytosis in a manner similar to the high levels observed with young BM (bar 1 versus bar 4; P = 0.008). Det, detergent cleaning; ECM, addition of laminin, vitronectin, and fibronectin.
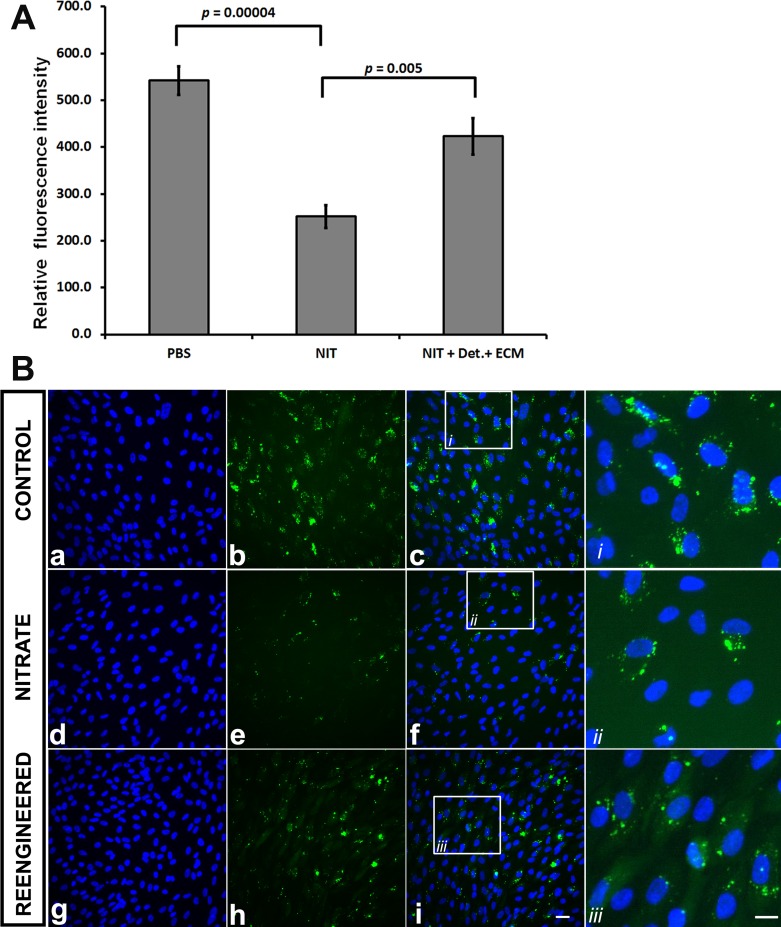
We then tested the effects of simulated aging on isolated ECM derived from ARPE-19 cells. To do this, we “aged” the ECM nonenzymatically by treating it with sodium nitrite, which crosslinks the proteins and serves as an in vitro model of basement membrane aging.28 As expected, nitrite treatment decreases the phagocytosis of ROS in ARPE-19 cells.26 A combination of detergent washing and recoating with a mixture of ECM proteins increased the phagocytic capacity of cultured ARPE-19 cells by approximately 33% as compared with nitrite-treated samples (Fig. 2: sodium nitrite (NIT), 252.0 ± 30.5 versus PBS, 423.0 ± 38.5 arbitrary units; P = 0.005). As with human BM, detergent treatment of nitrite-modified ECM alone was not sufficient to improve phagocytosis (not shown).
Figure 2.
ARPE-19 cell phagocytosis on ARPE-19–derived ECM. (A) ECM treated with sodium nitrite (NIT) decreased the phagocytic capacity of the overlying ARPE-19 cells as compared with untreated (phosphate buffer saline [PBS]) control (252 ± 24.2 vs. 542 ± 30.5 arbitrary units, respectively; P < 0.001). Washing with detergent (Det.) and resurfacing the ECM (NIT + Det. + ECM) with specific proteins (laminin, fibronectin, vitronectin) increased ARPE-19 phagocytic ability (252 ± 24.2 vs. 423 ± 24.2 arbitrary units, respectively; P = 0.005). (B) Representative images of the conditions in panel A. Hoechst nuclear stain (first column), FITC-labeled ROS (second column), and nuclear stain with FITC images superimposed (third column). Controls (b, c) showed basal levels of phagocytosis. There was a decrease in FITC-labeled ROS phagocytosis cells grown on nitrite-treated samples (compare e, f with b, c). Reengineered (h, i) ECM showed an apparent increase in FITC-labeled ROS phagocytosis as compared with nitrite-treated samples (e, f). Scale bar for a to i, 10 μm. Insets and fourth right column (i, ii, iii) depict representative areas of c, f, and i (small squares) showing cells at high magnification from each treatment group. Scale bar in far right-lower corner, 5 μm (for all three insets in the fourth column [i, ii, iii]).
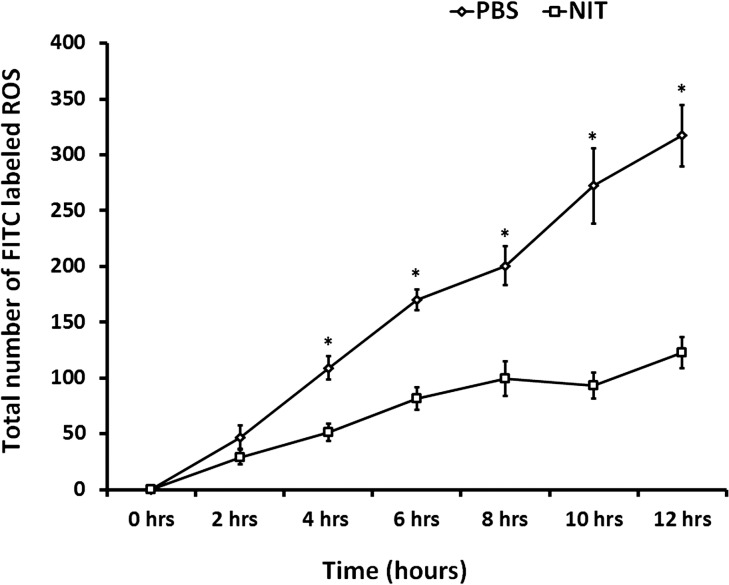
Previous studies have shown that 2 hours after feeding ROS to ARPE-19 cells almost all ROS remain associated with the cell membrane receptor (αVβ5), and by 5 hours about 50% of it is still bound to surface receptors while the remainder is internalized.31 To investigate the kinetics of ROS processing by ARPE-19 cells in our system, we fed FITC-labeled ROS and performed fluorescence measurements every 2 hours for the next 12 hours in nitrite-treated versus control RPE-derived ECM. There was no statistically significant difference between the two groups by 2 hours; but after 4 hours, differences became significant in ROS processing between the nitrite and control treatments, and persisted for up to 12 hours (Fig. 3).
Figure 3.
Time-course analysis of ARPE-19 phagocytosis on control (PBS) and nitrite-treated (NIT) ECM. Differences between the nitrite-treated and control groups were observable within the first 2 hours post ROS delivery, but were not statistically significant (NIT, 29 ± 6.8 versus PBS, 47 ± 10.3; P = 0.16). By 4 hours post ROS delivery, phagocytosis differences became significant between the nitrite-treated and control groups (NIT, 51 ± 7.8 versus PBS, 109 ± 10.3; *P < 0.001). These differences remained significant for the remainder of the experiment (at 6 hours NIT, 82 ± 10.2 versus PBS, 170 ± 9.2, *P < 0.001; 8 hours, NIT, 99 ± 15.7 versus PBS, 201 ± 17.6, *P = 0.001; 10 hours, NIT, 93 ± 11.6 versus PBS, 272 ± 33.7, *P < 0.001; 12 hours, NIT, 123 ± 14.0 versus PBS, 317 ± 27.3, *P < 0.001).
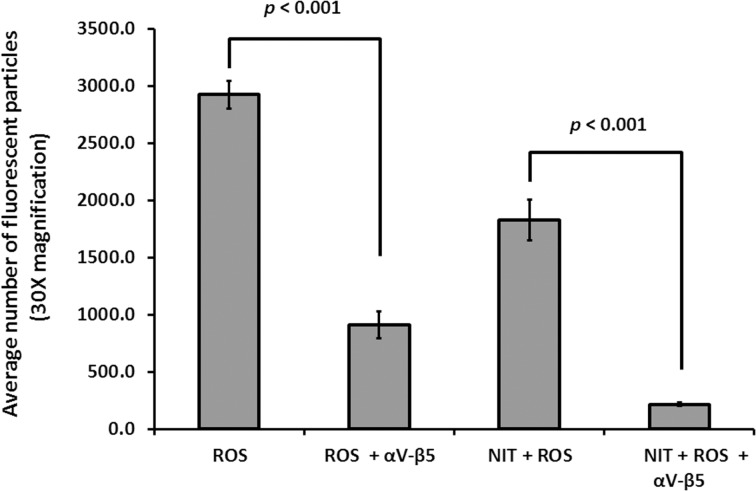
Subsequently, we sought to test whether the internalization of ROS particles involved the αVβ5 integrin receptor on the surface of ARPE-19 cells, which is the predominant receptor associated with the initial step of recognition and binding of ROS.31 To do this we added anti-αVβ5 antibody together with FITC-labeled ROS in the culture medium. As shown in Figure 4, αVβ5 antibody addition causes a 70% reduction in binding/internalization of ROS as compared with samples devoid of blocking antibody (ROS, 2925.6 ± 120.8 versus ROS + αVβ5, 910.9 ± 114.8 arbitrary units; P < 0.001). Nitrite-treated samples appeared more affected by αVβ5 antibody blocking, showing a decrease of about 90% when compared with its unchallenged counterpart (NIT + ROS, 1825.1 ± 177.2 versus NIT + ROS + αVβ5, 215.0 ± 17.3 arbitrary units; P < 0.001). Addition of a nonspecific Immunoglobulin G did not have any substantial effects (not shown).
Figure 4.
ROS processing was blocked by antibody to αVβ5 integrins in ARPE-19 cells. Addition of 50 μg/mL of anti-αVβ5 antibody to media containing ROS for 3 hours decreased levels of ROS binding and ingestion in ARPE-19 cells (ROS + αVβ5) relative to samples devoid of blocking antibody (910.9 ± 114.8 vs. 2925.6 ± 120.8 arbitrary units, respectively; P < 0.001 [P = 1.4 × 10−13]). Similarly, addition of blocking antibody with ROS to ARPE-19 cells on NIT-treated samples (NIT + ROS + αVβ5) decreased levels of ROS binding and ingestion by 90% when compared with samples (NIT + ROS) devoid of antibody (215.0 ± 17.3 vs. 1825.1 ± 177.2 arbitrary units, respectively; P < 0.001 [P = 2.7 × 10−9]).
Discussion
In this study, we investigated whether we could reverse the deleterious effects of BM aging on the phagocytic function of ARPE-19 cells. We demonstrated that treating old BM with detergent alone did not increase the ability of this RPE cell line to phagocytose ROS; instead, a combination of detergent and coating with ECM proteins appeared to rejuvenate BM, and thereby increased the phagocytic capacity of these cells. Similar results are seen using a model of ECM aging that we developed previously26 in which we mimic BM aging by treatment of ECM with sodium nitrite, a nonenzymatic crosslinking agent. Treating nitrite-modified basement membrane with detergent alone does not increase the ability of the RPE to phagocytose ROS. Instead, a combination of detergent cleaning followed by coating with ECM proteins appears to rejuvenate nitrite-modified ECM, and thereby increases the phagocytic capacity of ARPE-19 cells. There is a statistically significant difference in ROS internalization starting at 4 hours between controls and nitrite-treated samples, which continues for at least 12 hours (Fig. 3). Addition of anti-αVβ5 antibody reduced cellular fluorescent intensity by 70% to 95% compared with samples devoid of antibody (Fig. 4).
The primary cause of AMD is not known, but several lines of investigation have been directed at elucidating the connection between BM aging and cellular changes seen in the disease, such as RPE atrophy and photoreceptor degeneration.10,11,14,18,20 Age-related changes within BM occur several decades before cellular ones, thus suggesting that BM dysfunction can precede and may induce changes in surrounding cells.14,15,19,20 In our system, previously developed to isolate the effects of basement membrane aging from the effects of cellular aging, we have shown that BM aging affects proper attachment and proliferation of RPE cells and increases their rate of apoptosis while decreasing the rate of phagocytosis.27,28 We have also shown that reengineering human BM can improve the attachment, survival, and proliferation of the RPE.27 In this report, we showed that reengineering of old donor–derived human BM with detergent cleaning, and subsequent recoating with ECM proteins, can increase the phagocytic ability of ARPE-19 cells cultured on its surface. However, this increase did not reach levels compared with young BM, indicating that other factors are also involved in the aging process and in the functional decrease in phagocytosis of cells cultured onto old BM. It is likely that other deleterious changes occur at the molecular level within BM as a function of age, such as other forms of enzymatic or nonenzymatic protein crosslinking (e.g., nonreducing sugars, reactive oxygen species, and reactive nitrogen species).10 In addition, aging of BM may also affect other proteins (e.g., heparan sulfate) that may play an important role in the overall function of the RPE.20 These results are not surprising, since the molecular changes that occur within BM with aging are protean in nature, and not completely understood. Nevertheless, the fact that there is an increase in proliferation, survival, and RPE function by providing fresh ECM protein may also be important in other systems designed to reproduce an artificial BM.32 A recent report describes how primary RPE cells were able to grow and proliferate as polarized monolayers in three-dimensional scaffolds made of collagen type 1 and a material called poly(lactic-co-glycolic acid) (PLGA).32 This fibrillary network resembles the fibrillar architecture of the collagenous layer of BM, and cells grow looking morphologically and molecularly similar to RPE while displaying a correctly polarized monolayer.32 Additional coating of these scaffolds with ECM proteins may have a long-term positive impact in the overall function of transplanted RPE monolayers.
The RPE is one of the most active phagocytic epithelia in the human body. In vivo, approximately 3% to 5% of the distal tips of photoreceptor outer segments are shed on a daily basis and proper RPE phagocytosis is necessary to maintain the health and integrity of the neural retina and the choriocapillaris.33,34 RPE phagocytosis uses αVβ5 integrin receptors on the RPE surface for initial outer segment cell surface binding, the receptor MerTK for internalization, and the scavenger receptor CD36.31,35,36 Disruption of any step in this process can lead to pathology. For example, mice lacking αVβ5 fail to internalize outer segments leading to retinal degeneration,37–39 while deficiencies in MerTK lead to retinal degeneration in the Royal College of Surgeons rat and in transgenic mice.38,40 In humans, decreased clearance of outer segment material may lead to outer segment accumulation within the RPE, which has been associated with AMD.41,42
We have shown previously that sodium nitrite modification of ECM decreases the phagocytic ability of the RPE cells to an extent comparable to that of aging BM.26 In addition, nitrite modification decreases RPE attachment to ECM, inhibits cell proliferation, and increases apoptosis.43 Nitrite causes these effects by nonenzymatic cross linking of collagen in vitro as well as in vivo.30,44,45 Here we reported that, similar to BM, treating ARPE-19–derived ECM with detergent (Triton X-100) and reconstituting its ECM proteins ameliorated the effects of nonenzymatic nitration as evidenced by an increased phagocytosis relative to the nitrite-treated group. These results suggested that aging of human BM, which can be mimicked by nonenzymatic nitration of ARPE-19–derived ECM in vitro, can decrease phagocytosis of outer segments by ARPE-19 cells, and this decline can be reversed at least partially by combined detergent cleaning and recoating of the surface with ECM ligands. The method we employed to denature the native structure of the BM ECM proteins may well also indirectly cause RPE dysfunction by creating reactive nitrogen species which in turn can exert deleterious effects on cellular proteins.45–47 However, age-related accumulation of nitrite-modified moieties in aging BM48 and their impact on protein dysfunction49 suggest nitration plays a major role in the age-related decline in RPE survival and function. Reversal of this functional loss by rejuvenation of the ECM constitutes an important step in reversing RPE dysfunction leading to AMD. Lastly, we showed that most (if not all) of the phagocytosis observed was mediated through the αVβ5 receptor, which is a key player involved in the initial specific recognition of ROS in RPE cells.34,35 Additional studies are needed to determine whether primary RPE or RPE derived from either human embryonic stem cells or induced pluripotent stem cells (iPSC) would behave similarly on spontaneously aged or nitrite-modified ECM surfaces. Ongoing experiments in our laboratory are aimed at understanding the effects of BM-aging on iPSC-derived RPE cells derived from AMD patients and unaffected controls. These experiments will shed light into understanding the importance of the ECM in the physiological maintenance and functional properties of RPE cells derived from different phenotypic and genotypic backgrounds.
Aging is a complex phenomenon involving alterations within cells and their external milieu, including the ECM, throughout the body. In the eye, accumulation of residual bodies is manifested by intracellular accumulation of lipofuscin within the RPE, and there is an age-dependent increase in the rate of RPE apoptosis in the macula of human eyes; similar changes are not seen in the periphery.41,50 Pathological changes within cells can be accelerated by ultraviolet light and other agents that induce DNA damage.51–53 In addition to cellular changes, aging changes can occur in BM including nonenzymatic nitration and glycation, collagen cross-linking, and elastin fragmentation.14,19,43,44 In our model, age-related changes in human BM, which may include, but are not limited to nonenzymatic nitration, could lead to extensive disruption of RPE function, including RPE phagocytosis. Our in vitro studies demonstrate proof of concept that these changes can be reversed with cleaning and coating of the surface. Additional studies will be necessary to develop treatments to produce the same effects in vivo without toxicity.
Acknowledgments
Supported in part by an unrestricted grant to the Medical University of South Carolina, Storm Eye Institute, from Research to Prevent Blindness, New York, NY; the Macula Society, Cleveland, OH; and the Foundation Fighting Blindness, Owings Mills, MD.
Disclosure: E.F. Moreira, None; H. Cai, None; T.H. Tezel, None; M.A. Fields, None; L.V. Del Priore, None
References
- 1. Zinn K,, Marmor M. The Retinal Pigment Epithelium. Cambridge: Harvard University Press; 1979. [Google Scholar]
- 2. Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005; 85: 845–881. [DOI] [PubMed] [Google Scholar]
- 3. Hsu YC,, Chuang JZ,, Sung CH. Light regulates the ciliary protein transport and outer segment disc renewal of mammalian photoreceptors. Dev Cell. 2015; 32: 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizzolo LJ. Barrier properties of cultured retinal pigment epithelium. Exp Eye Res. 2014; 126: 16–26. [DOI] [PubMed] [Google Scholar]
- 5. Campbell M,, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012; 763: 70–84. [PubMed] [Google Scholar]
- 6. Ho TC,, Del Priore LV. Reattachment of cultured human retinal pigment epithelium to extracellular matrix and human Bruch's membrane. Invest Ophthalmol Vis Sci. 1997; 38: 1110–1118. [PubMed] [Google Scholar]
- 7. Afshari FT,, Fawcett JW. Improving RPE adhesion to Bruch's membrane. Eye (Lond). 2009; 23: 1890–1893. [DOI] [PubMed] [Google Scholar]
- 8. Fang IM,, Yang CH,, Yang CM,, Chen MS. Overexpression of integrin alpha6 and beta4 enhances adhesion and proliferation of human retinal pigment epithelial cells on layers of porcine Bruch's membrane. Exp Eye Res. 2009; 88: 12–21. [DOI] [PubMed] [Google Scholar]
- 9. Lim LS,, Mitchell P,, Seddon JM,, Holz FG,, Wong TY. Age-related macular degeneration. Lancet. 2012; 379: 1728–1738. [DOI] [PubMed] [Google Scholar]
- 10. Zarbin MA,, Casaroli-Marano RP,, Rosenfeld PJ. Age-related macular degeneration: clinical findings histopathology and imaging techniques. Dev Ophthalmol. 2014; 53: 1–32. [DOI] [PubMed] [Google Scholar]
- 11. Abdelsalam A,, Del Priore L,, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999; 44: 1–29. [DOI] [PubMed] [Google Scholar]
- 12. Mullins RF,, Aptsiauri N,, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond). 2001; 15: 390–395. [DOI] [PubMed] [Google Scholar]
- 13. Buschini E,, Piras A,, Nuzzi R,, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol. 2011; 95: 14–25. [DOI] [PubMed] [Google Scholar]
- 14. Booij JC,, Baas DC,, Beisekeeva J,, Gorgels TG,, Bergen AA. The dynamic nature of Bruch's membrane. Prog Retin Eye Res. 2010; 29: 1–18. [DOI] [PubMed] [Google Scholar]
- 15. Beattie JR,, Pawlak AM,, Boulton ME,, et al. Multiplex analysis of age-related protein and lipid modifications in human Bruch's membrane. FASEB J. 2010; 24: 4816–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majji AB,, de Juan E,, Jr. Retinal pigment epithelial autotransplantation: morphological changes in retina and choroid. Graefes Arch Clin Exp Ophthalmol. 2000; 238: 779–791. [DOI] [PubMed] [Google Scholar]
- 17. Bird AC,, Phillips RL,, Hageman GS. Geographic atrophy: a histopathological assessment. JAMA Ophthalmol. 2014; 132: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whitmore SS,, Sohn EH,, Chirco KR,, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015; 45: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chong NH,, Keonin J,, Luthert PJ,, et al. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. 2005; 166: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keenan TD,, Pickford CE,, Holley RJ,, et al. Age-dependent changes in heparan sulfate in human Bruch's membrane: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014; 55: 5370–5379. [DOI] [PubMed] [Google Scholar]
- 21. Tezel TH,, Del Priore LV. Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 1997; 235: 41–47. [DOI] [PubMed] [Google Scholar]
- 22. Kaplan HJ,, Tezel TH,, Del Priore LV. Retinal pigment epithelial transplantation in age-related macular degeneration. Retina. 1998; 18: 99–102. [PubMed] [Google Scholar]
- 23. Tezel TH,, Kaplan HJ,, Del Priore LV. Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch's membrane. Invest Ophthalmol Vis Sci. 1999; 40: 467–476. [PubMed] [Google Scholar]
- 24. Gullapalli VK,, Sugino IK,, Van Patten Y,, Shah S,, Zarbin MA. Retinal pigment epithelium resurfacing of aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc. 2004; 102: 123–137. [PMC free article] [PubMed] [Google Scholar]
- 25. Cai H,, Del Priore LV. Bruch's membrane aging alters the gene expression profile of human retinal pigment epithelium. Curr Eye Res. 2006; 31: 181–189. [DOI] [PubMed] [Google Scholar]
- 26. Sun K,, Cai H,, Tezel TH,, Paik D,, Gaillard ER,, Del Priore LV. Bruch's membrane aging decreases phagocytosis of outer segments by retinal pigment epithelium. Mol Vis. 2007; 13: 2310–2319. [PubMed] [Google Scholar]
- 27. Tezel TH,, Del Priore LV,, Kaplan HJ. Reengineering of aged Bruch's membrane to enhance retinal pigment epithelium repopulation. Invest Ophthalmol Vis Sci. 2004; 45: 3337–3348. [DOI] [PubMed] [Google Scholar]
- 28. Del Priore LV,, Tezel TH,, Kaplan HJ. Maculoplasty for age-related macular degeneration: reengineering Bruch's membrane and the human macula. Prog Retin Eye Res. 2006; 25: 539–562. [DOI] [PubMed] [Google Scholar]
- 29. Fong SL,, Tsin AT,, Bridges CD,, Liou GI. Detergents for extraction of visual pigments: types, solubilization, and stability. Methods Enzymol. 1982; 81: 133–140. [DOI] [PubMed] [Google Scholar]
- 30. Paik DC,, Dillon J,, Galicia E,, Tilson MD. The nitrite/collagen reaction: non-enzymatic nitration as a model system for age-related damage. Connect Tissue Res. 2001; 42: 111–122. [DOI] [PubMed] [Google Scholar]
- 31. Finnemann SC,, Bonilha VL,, Marmorstein AD,, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997; 94: 12932–12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warnke PH,, Alamein M,, Skabo S,, et al. Primordium of an artificial Bruch's membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013; 9: 9414–9422. [DOI] [PubMed] [Google Scholar]
- 33. Nguyen-Legros J,, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000; 196: 245–313. [DOI] [PubMed] [Google Scholar]
- 34. Mazzoni F,, Safa H,, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res. 2014; 126: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kevany BM,, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda). 2010; 25: 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nandrot EF,, Silva KE,, Scelfo C,, Finnemann SC. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of alphavbeta5 integrin. Biol Cell. 2012; 104: 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nandrot EF,, Kim Y,, Brodie SE,, Huang X,, Sheppard D,, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004; 200: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nandrot EF,, Dufour EM. Mertk in daily retinal phagocytosis: a history in the making. Adv Exp Med Biol. 2010; 664: 133–140. [DOI] [PubMed] [Google Scholar]
- 39. Law AL,, Nandrot EF. On your marks...get bound...internalize! Adv Exp Med Biol. 2012; 723: 717–722. [DOI] [PubMed] [Google Scholar]
- 40. Peng YW,, Senda T,, Hao Y,, Matsuno K,, Wong F. Ectopic synaptogenesis during retinal degeneration in the royal college of surgeons rat. Neuroscience. 2003; 119: 813–820. [DOI] [PubMed] [Google Scholar]
- 41. Petrukhin K. Pharmacological inhibition of lipofuscin accumulation in the retina as a therapeutic strategy for dry AMD treatment. Drug Discov Today Ther Strateg. 2013; 10: e11–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conlon TJ,, Deng WT,, Erger K,, et al. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013; 24: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Z,, Paik DC,, Del Priore LV,, Burch RL,, Gaillard ER. Nitrite-modified extracellular matrix proteins deleteriously affect retinal pigment epithelial cell function and viability: a comparison study with nonenzymatic glycation mechanisms. Curr Eye Res. 2005; 30: 691–702. [DOI] [PubMed] [Google Scholar]
- 44. Paik DC,, Wen Q,, Airiani S,, Braunstein RE,, Trokel SL. Aliphatic beta-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res. 2008; 87: 279–285. [DOI] [PubMed] [Google Scholar]
- 45. Drew B,, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann N Y Acad Sci. 2002; 959: 66–81. [DOI] [PubMed] [Google Scholar]
- 46. Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004; 11: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 47. Figueira TR,, Barros MH,, Camargo AA,, et al. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal. 2013; 18: 2029–2074. [DOI] [PubMed] [Google Scholar]
- 48. Murdaugh LS,, Wang Z,, Del Priore LV,, Dillon J,, Gaillard ER. Age-related accumulation of 3-nitrotyrosine and nitro-A2E in human Bruch's membrane. Exp Eye Res. 2010; 90: 564–571. [DOI] [PubMed] [Google Scholar]
- 49. Martinez A,, Portero-Otin M,, Pamplona R,, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010; 20: 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Del Priore LV,, Kuo YH,, Tezel TH. Age-related changes in human RPE cell density and apoptosis proportion in situ. Invest Ophthalmol Vis Sci. 2002; 43: 3312–3318. [PubMed] [Google Scholar]
- 51. Rubio MA,, Kim SH,, Campisi J. Reversible manipulation of telomerase expression and telomere length. Implications for the ionizing radiation response and replicative senescence of human cells. J Biol Chem. 2002; 277: 28609–28617. [DOI] [PubMed] [Google Scholar]
- 52. Boulton M,, Rozanowska M,, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001; 64: 144–161. [DOI] [PubMed] [Google Scholar]
- 53. Rozanowski B,, Cuenco J,, Davies S,, et al. The phototoxicity of aged human retinal melanosomes. Photochem Photobiol. 2008; 84: 650–657. [DOI] [PubMed] [Google Scholar]