Abstract
Chronic liver disease and liver cancer associated with chronic hepatitis B (CHB) are leading causes of death among adults in China. Although newborn hepatitis B immunization has successfully reduced the prevalence of CHB in children, about 100 million Chinese adults remain chronically infected. If left unmanaged, 15–25% will die from liver cancer or liver cirrhosis. Antiviral treatment is not necessary for all patients with CHB, but when it is indicated, good response to treatment would prevent disease progression and reduce disease mortality and morbidity, and costly complications. The aim of this study is to analyze the cost-effectiveness of generic and brand antiviral drugs for CHB treatment in China, and assessing various thresholds at which a highly potent, low resistance antiviral drug would be cost-saving and/or cost-effective to introduce in a national treatment program. We developed a Markov simulation model of disease progression using effectiveness and cost data from the medical literature. We measured life-time costs, quality adjusted life years (QALYs), incremental cost-effectiveness ratios (ICERs), and clinical outcomes. The no treatment strategy incurred the highest health care costs ($12,932-$25,293) per patient, and the worst health outcomes, compared to the antiviral treatment strategies. Monotherapy with either entecavir or tenofovir yielded the most QALYs (14.10–19.02) for both HBeAg-positive and negative patients, with or without cirrhosis. Threshold analysis showed entercavir or tenofovir treatment would be cost saving if the drug price is $32–75 (195–460 RMB) per month, highly cost-effective at $62–110 (379–670 RMB) per month and cost-effective at $63–120 (384–734 RMB) per month. This study can support policy decisions regarding the implementation of a national health program for chronic hepatitis B treatment in China at the population level.
Introduction
China has the greatest disease burden of chronic hepatitis B (CHB) in the world, with an estimated 350,000–500,000 deaths each year from hepatitis B virus (HBV) related diseases, including hepatocellular carcinoma (HCC) and hepatic failure [1]. Approximately 80% of HCC, the most common type of liver cancer, is due to chronic HBV infection in China [2, 3]. Many Asian adults with CHB infection develop HCC at a rate of about 5% per decade, which is 100-fold higher than the rate among uninfected persons. Without monitoring or appropriate treatment, 15–25% of those chronically infected will die from liver cancer or liver cirrhosis. In comparison to HIV, which affects 600,000 Chinese, an estimated 100 million Chinese are living with chronic hepatitis B, making it the most prevalent life threatening chronic infection in China [1]. Major progress has been made in China to reduce the prevalence of chronic hepatitis B in children through a robust new born immunization program, and a recent nationwide catch up vaccination program for unprotected children [4, 5]. Although hepatitis B vaccination clearly contributed to the reduction of new cases, it does not address the healthcare needs of the chronically-infected individuals who are at risk of disease progression leading to the development of HCC and cirrhosis. There is currently no curative treatment for CHB, but good response to approved treatments could prevent disease progression and reduce deaths and costly complications.
According to both international and Chinese professional guidelines, treatment is indicated for those with chronic hepatitis B who are hepatitis B e-antigen (HBeAg)-positive and HBeAg antigen negative with active hepatitis (high HBV DNA and ALT levels) or cirrhosis. Current therapies fall into two categories: immune modulators and antiviral agents. The immune modulators such as pegylated interferon alfa are given over 6–12 months by subcutaneous injection and can induce remission of liver disease in a fraction of patients, but the remission may not be permanent. Many patients cannot tolerate interferon treatment because of the associated side effects. Antiviral agents such as nucleoside or nucleotide analogues that suppress viral replication are well tolerated and as simple as a pill a day, but likely need to be taken indefinitely. The antiviral therapies vary in terms of costs, effectiveness in suppressing viral replication and risk of drug resistance.
Despite the availability of CHB treatment, the proportion of patients actually receiving treatment is low in China [1]. The main obstacle to treatment is often the cost. In recent years, many cities have begun providing partial coverage for CHB treatment but the choice of covered treatment is limited in the rural health plans. Treatment coverage also varies in the different provinces. Currently, there is no national policy to cover CHB treatment nationwide.
This study is a comprehensive analysis of the cost-effectiveness of treatment therapies for CHB in China, and assessing various thresholds at which a highly potent low resistance drug would be cost-saving and/or cost-effective. We cover all major drugs that would be used for treatment and evaluate several different potential patient groups according to HBeAg status with or without cirrhosis. This analysis is intended to help guide discussions particularly for policy makers and health professionals about national coverage for the treatment of CHB and also provide insights into the potential cost-effectiveness of the various treatment options in China.
Methods
Overview
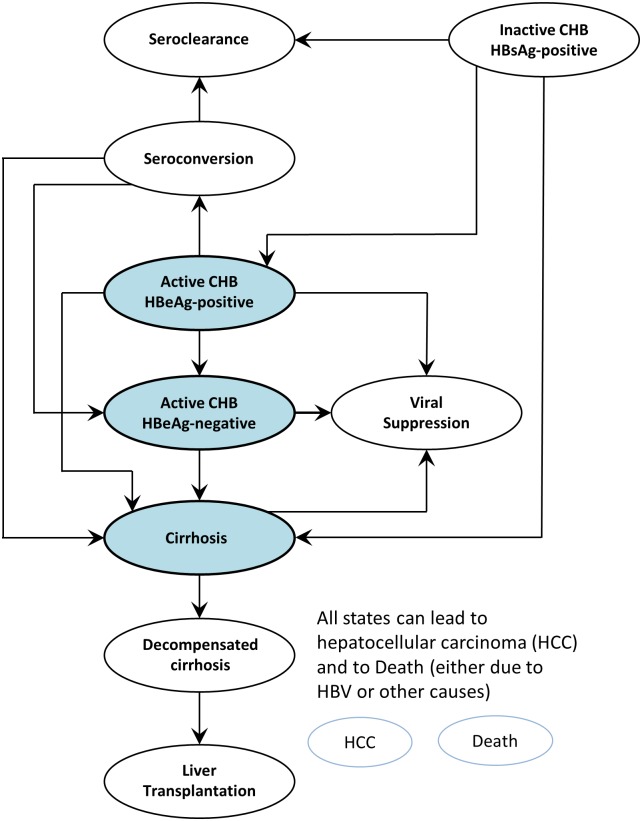
We used a Markov model that describes disease progression to evaluate the long-term outcomes for patients under various treatment strategies. The model used is similar to our previous study on CHB in Shanghai, China [6]. Treatment-naïve, chronic HBV, HBeAg-positive or HBeAg-negative patients eligible for treatment under international treatment guidelines enter the model either in the cirrhotic or non-cirrhotic health state (Fig 1). Patients can progress to compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, and would be eligible to receive a liver transplantation. All patients face age-specific mortality plus increased mortality if they have cirrhosis, HCC, or a liver transplant. If patients receive treatment, they can develop drug resistance or sustained virologic response.
Fig 1. Markov model schematic with entry points active HBeAg-positive, active HBeAg-negative disease, and cirrhosis, and transition between states according to annual transition estimates.
Strategies
Eight different strategies were analysed in this study.
“No antiviral treatment.”
Chronic hepatitis B patients progress according to the natural history, following annual disease progression estimates (Table 1). The disease progression estimates were derived from recent age-specific cohort studies on inactive and active CHB in Asia [7–15]. We assumed that patients received best supportive care, except for drug treatment. Patients followed the natural history according to their HBeAg and disease status (with or without cirrhosis). Spontaneous virologic response was defined as seroconversion to anti-HBe in HBeAg-positive patients, and as persistent HBV DNA suppression and ALT normalization in HBeAg-negative patients. We assumed that a proportion of patients with decompensated cirrhosis and HCC became eligible for liver transplantation.
Table 1. Annual transition estimates governing natural history of chronic hepatitis B by initial state.
| Transition | Age group | Estimate (%) | Range | References |
|---|---|---|---|---|
| From inactive CHB, HBsAg-positive | ||||
| To seroclearance | <30 years | 0.8 | (0.38–1.15) | 7 |
| 30–39 years | 1.1 | (0.53–1.60) | ||
| 40–49 years | 1.7 | (0.82–2.47) | ||
| 50+ years | 1.8 | (0.91–2.74) | ||
| To active CHB, HBeAg-positive | <30 years | 0.9 | (0.4–1.3) | 7,12 |
| 30–39 years | 1.4 | (0.7–2.1) | ||
| 40–49 years | 2.8 | (1.4–4.1) | ||
| 50+ years | 2.0 | (1.0–3.0) | ||
| To cirrhosis | <30 years | 0.038 | (0.019–0.057) | 12 |
| 30–39 years | 0.049 | (0.024–0.073) | ||
| 40–49 years | 0.068 | (0.034–0.102) | ||
| 50+ years | 0.150 | (0.052–0.202) | ||
| To HCC | All ages | 0.168 | (0.001–0.25) | 9,13,14 |
| From active CHB, HBeAg-positive | ||||
| To seroconversion | All ages | 7.0 | (2.0–23) | 8 |
| To active CHB, HBeAg-negative | All ages | 1.9 | (1.0–3.8) | 15 |
| To cirrhosis | All ages | 2.4 | (2.1–2.6) | 9 |
| To HCC | All ages | 0.8 | (0.5–1.0) | 9 |
| To HBV-related death | All ages | 0.6 | (0.2–0.9) | 9 |
| From active CHB, HBeAg-negative | ||||
| To inactive CHB, HBsAg-positive | All ages | 1.6 | (0.0–11) | 8 |
| To cirrhosis | All ages | 2.4 | (1.3–3.4) | 9 |
| To HCC | All ages | 0.8 | (0.5–1.0) | 9 |
| To HBV-related death | All ages | 0.6 | (0.2–0.9) | 9 |
| From seroconversion | ||||
| To active CHB, HBeAg-negative | <30 years | 2.9 | (1.4–4.3) | 14 |
| 31–40 years | 3.8 | (1.9–5.7) | ||
| 40+ years | 8.6 | (4.3–12.9) | ||
| To cirrhosis | <30 years | 0.2 | (0.1–0.3) | |
| 31–40 years | 1.0 | (0.5–1.5) | ||
| 40+ years | 4.2 | (2.1–6.3) | ||
| To HCC | <30 years | 0.1 | (0.05–0.15) | |
| 31–40 years | 0.2 | (0.1–0.3) | ||
| 40+ years | 0.6 | (0.3–0.9) | ||
| To seroclearance | <30 years | 0.8 | (0.4–1.2) | |
| 31–40 years | 0.7 | (0.3–1.0) | ||
| 40+ years | 0.3 | (0.1–0.4) | ||
| From seroclearance | ||||
| To HCC | 50+ years | 1.0 | (0.0–2.0) | 11 |
| From cirrhosis | ||||
| To decompensated cirrhosis | All ages | 3.9 | (3.2–4.6) | 9 |
| To HCC | All ages | 5.0 | (3.0–7.0) | |
| To HBV-related death | All ages | 5.6 | (3.1–8.0) | |
| From decompensated cirrhosis | ||||
| To liver transplantation | All ages | 12.0 | (6.0–18.0) | * |
| To HCC | All ages | 7.1 | (3.5–10.0) | 9 |
| To HBV-related death | All ages | 15.0 | (9.9–20.0) | 9 |
| From HCC | ||||
| To liver transplantation | All ages | 4.7 | (2.3–7.0) | * |
| To HBV-related death | All ages | 54.5 | (20.0–60.0) | 9 |
| From liver transplantation | ||||
| To HBV-related death | All ages | 6.6 | (2.0–12) | 8 |
* Authors’ estimates based on consultation with experts in liver transplantation in China.
Table adapted from Toy et al. [6].
“Lamivudine mono-therapy” (LAM)
Patients receive 100mg orally once daily of the first licensed antiviral HBV drug. This drug is known to be associated with a high incidence of resistance [16]. Such mono-therapy is still commonly prescribed in China [17]. We assigned different rates of virologic response under long-term therapy between resistant and non- resistant patients (Table 2) [16, 18–20].
Table 2. Treatment transition estimates.
| Annual probability, % (range) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Transition | Lamivudine monotherapy* | Entecavir monotherapy|| | Adefovir salvage¶ | Tenofovir** | Tenofovir salvage†† | |||||
| e-positive | e-negative | e-positive | e-negative | e-positive | e-negative | e-positive | e-negative | e-positive | e-negative | |
| From active CHB, initial treatment † | ||||||||||
| To sustained virologic response‡ | 20 (15–25) | 10 (5.0–17) | 22 (17–27) | 11 (5.5–22) | 12 (5.0–17) | 10 (5.0–17) | 23 (11.5–34.5) | 11 (5.5–22) | 19 (9.5–28.5) | 11 (5.5–16.5) |
| To cirrhosis§ | 0.5 (0.2–1.0) | 1.2 (0.9–2.1) | 0.2 (0.1–0.5) | 0.6 (0.3–1.2) | 0.5 (0.2–1.0) | 1.2 (0.9–2.1) | 0.2 (0.1–0.5) | 0.6 (0.3–1.2) | 0.5 (0.25–0.75) | 1.2 (0.6–1.8) |
| To HCC‡‡ | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) |
| From active CHB, long-term treatment, drug-sensitive | ||||||||||
| To sustained virologic response‡ | 24 (19–29) | 10 (5.0–17) | 27 (17–27) | 11 (5.5–22) | 12 (5.0–17) | 10 (5.0–17) | 27 (17–27) | 11 (5.5–22) | 19 (9.5–28.5) | 11 (5.5–16.5) |
| To cirrhosis§ | 0.5 (0.2–1.0) | 1.2 (0.9–2.1) | 0.2 (0.1–0.5) | 0.6 (0.3–1.2) | 0.5 (0.2–1.0) | 1.2 (0.9–2.1) | 0.2 (0.1–0.3) | 0.6 (0.3–0.9) | 0.5 (0.25–0.75) | 1.2 (0.6–1.8) |
| To active CHB, drug resistant year 1‡ | 23 (18–28) | 23 (18–28) | 1 (0.0–2.0) | 1 (0.0–2.0) | 6 (1.0–12) | 6 (1.0–12) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| To active CHB, drug resistant year 2‡ | 42 (37–45) | 42 (37–45) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0 (0–0) | 0 (0–0) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To active CHB, drug resistant year 3‡ | 53 (48–58) | 53 (48–58) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0.4 (0.2–0.6) | 0.4 (0.2–0.6) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To active CHB, drug resistant year 4‡ | 70 (65–75) | 70 (65–75) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0.8 (0.4–1.2) | 0.8 (0.4–1.2) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To active CHB, drug resistant year 5‡ | 74 (69–79) | 74 (69–79) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 1 (0.0–2.0) | 1 (0.0–2.0) | 1 (0.0–2.0) | 1 (0.0–2.0) |
| To HCC‡‡ | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) | 0.2 (0.1–0.5) |
| From active CHB, long-term treatment, drug resistant | ||||||||||
| To sustained virologic response‡ | 4.5 (3.3–7.8) | 0 | 5 (2–7) | 0.5 (0.2–1.0) | 4.5 (3.3–7.8) | 0 | 5 (2–7) | 0.5 (0.2–1.0) | 5 (2–7) | 0.5 (0.2–1.0) |
| To cirrhosis | 2.7 (1.6–3.8) | 6.2 (2.8–9.7) | 2.7 (1.6–3.8) | 6.2 (2.8–9.7) | 2.7 (1.6–3.8) | 6.2 (2.8–9.7) | 2.7 (1.6–3.8) | 6.2 (2.8–9.7) | 2.7 (1.6–3.8) | 6.2 (2.8–9.7) |
| To HCC‡‡ | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) | 0.4 (0.3–0.6) |
| From cirrhosis, initial treatment † | ||||||||||
| To sustained virologic response‡ | 20 (15–25) | 10 (5.0–17) | 22 (17–27) | 11 (5.5–22) | 12 (5.0–17) | 10 (5.0–17) | 23 (11.5–34.5) | 12 (6–18) | 19 (9.5–28.5) | 11 (5.5–16.5) |
| To HCC‡‡ | 0.9 (0.3–1.4) | 1.5 (0.7–3.0) | 0.9 (0.3–1.4) | 1.5 (0.7–3.0) | 0.9 (0.3–1.4) | 1.5 (0.7–3.0) | 0.9 (0.3–1.4) | 1.5 (0.7–3.0) | 0.9 (0.3–1.4) | 1.5 (0.7–3.0) |
| From cirrhosis, long-term treatment, drug-sensitive | ||||||||||
| To sustained virologic response‡ | 24 (19–29) | 10 (5.0–17) | 27 (17–27) | 11 (5.5–22) | 12 (5.0–17) | 10 (5.0–17) | 27 (17–27) | 11 (5.5–22) | 19 (9.5–28.5) | 11 (5.5–16.5) |
| To cirrhosis, drug-resistant year 1 | 23 (18–28) | 23 (18–28) | 1 (0.0–2.0) | 1 (0.0–10) | 6 (1.0–12) | 6 (1.0–12) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| To cirrhosis, drug-resistant year 2 | 42 (37–45) | 42 (37–45) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0 (0–0) | 0 (0–0) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To cirrhosis, drug-resistant year 3 | 53 (48–58) | 53 (48–58) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0.4 (0.2–0.6) | 0.4 (0.2–0.6) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To cirrhosis, drug-resistant year 4 | 70 (65–75) | 70 (65–75) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 0.8 (0.4–1.2) | 0.8 (0.4–1.2) | 1 (0.5–1.5) | 1 (0.5–1.5) |
| To cirrhosis, drug-resistant year 5 | 74 (69–79) | 74 (69–79) | 1 (0.0–2.0) | 1 (0.0–2.0) | 21 (16–27) | 21 (16–27) | 1 (0.0–2.0) | 1 (0.0–2.0) | 1 (0.0–2.0) | 1 (0.0–2.0) |
| To decompensated cirrhosis | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) | 1.9 (0.9–3.8) |
| To HCC‡‡ | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) | 1.6 (0.8–3.2) |
| To HBV-related death | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) | 2.4 (1.2–4.8) |
| From cirrhosis, long-term treatment, drug-resistant | ||||||||||
| To sustained virologic response‡ | 4.5 (3.3–7.8) | 0 | 5 (2–7) | 0.5 (0.2–1) | 4.5 (3.3–7.8) | 0 | 5 (2–7) | 0.5 (0.2–1) | 5 (2–7) | 0.5 (0.2–1) |
| To decompensated cirrhosis | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) | 7.9 (4–15) |
| To HCC‡‡ | 1.8 (0.9–3.8) | 2.9 (1.0–5.6) | 1.8 (0.9–3.8) | 2.9 (1.0–5.6) | 1.8 (0.9–3.8) | 2.9 (1.0–5.6) | 1.8 (0.9–3.8) | 2.9 (1.0–5.6) | 1.8 (0.9–3.8) | 2.9 (1.0–5.6) |
| To HBV-related death | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) | 3.1 (3.1–3.8) |
* Estimates from Kanwal 2005 [8].
† Initial therapy is 12 months (48 weeks) of therapy.
‡ Estimates calculated by the author, based on the assumption that the natural progression rates of chronic hepatitis B are reduced by antiviral therapy. Estimates derived from natural history estimate similar to Kanwal’s assumption of no progression of disease in HBeAg seroconversion, we assume no progression of disease in case HBV DNA is undetectable by PCR. In the papers from Chang and Lai full suppression of HBV DNA was observed in 80% with a high resistance profile drug, and 90% with a low resistance profile drug. We took these percentages for our calculations [18,19].
|| Estimates for Entecavir from Chang et al. 2006, Lai et al. 2006 and Colonno 2007, [18,19,25] and resistance from Colonno et al. 2006, Colonno et al. 2007 and Tenny et al. 2009. [24–26].
** Estimates for tenofovir from Heathcote et al. 2011 [31].
†† Tenofovir salvage scenario estimates from van Bommel et al., Reijnders et al., Ke et al., Gordom et al., Patterson et al., Lee et al. [32–37].
‡‡ Estimates based on reduction of progression rates by nucleoside analogue therapy of 50% [16].
“Adefovir salvage” (LAM→ADV)
Patients initially receive lamivudine, but switched to adefovir when they develop lamivudine resistance. Patients without resistance continue to receive the initial drug lamivudine [16, 21–23].
“Entecavir monotherapy” (ETV)
Patients in this strategy received 0.5mg entecavir once daily. The treatment related probability estimates for responding and resistant patients are shown in Table 2 [24–26]. Since entecavir and lamivudine share cross-resistance, it is not a recommended salvage therapy for lamivudine resistant patients.
“Pegylated Interferon” (PEG-IFN)
Patients receive 180mcg of pegylated interferon administered subcutaneously once a week for 48 weeks. If the patient does not respond or relapses in the second year after treatment, they follow the transitions in the natural history (no treatment) strategy.
“Pegylated Interferon, followed by entecavir” (PEG-IFN→ETV)
Patients receive 180 mcg of pegylated interferon once a week for 48 weeks. If the patient does not respond or relapses in the second year after treatment, they start entecavir at 0.5 mg/day (S1 Table) [27–30].
“Tenofovir monotherapy” (TDF)
Patients receive 300mg of tenofovir once daily. The annual probability of resistance in this scenario was 0% for the first and second year of treatment [31–34].
“Tenofovir salvage” (LAM→TDF)
In this strategy, patients who develop resistance during lamivudine monotherapy are switched to tenofovir [32, 35–37].
Assumptions
Causes of death not related to liver disease associated with CHB were included in the model, based on age-specific mortality rates from the National Bureau of Statistics China [38]. The annual probabilities of receiving a liver transplant for decompensated cirrhosis and HCC (12% and 4.7%, respectively) in China were calculated based on data from China Liver Transplant Registry [39].
If progression rates were reported, these were transformed into annual probabilities using a standard formula (P = 1-e -r×t), where P is the probability, e is the base of the natural logarithm, r is the event rate, and t is the time interval [40]. We assumed that it was possible to develop cirrhosis and hepatocellular carcinoma while on treatment, but with a 50% reduction in the rate decrease from the natural history [41].
Treatment guidelines often recommend a finite period of therapy with oral nucleoside analogs for patients with HBeAg-positive CHB who undergo HBeAg seroconversion. However, prolonged therapy is to be considered for patients with evolving HBeAg-negative CHB and active HBV DNA replication (> log4 IU/ml) and for patients with cirrhosis [42–46]. A more recent publication [47] suggests continuation of long-term nucleos(t)ide analogue treatment, irrespective of the occurrence of HBeAg seroconversion in HBeAg-positive patients. Following these recent findings, our model assumes continued antiviral therapy for HBeAg-positive patients even if seroconversion occurs. Also our model assumes that the resistance rate for ETV and TDF remains as low as recent studies report [33, 48]. The scenario for PEG-IFN assumes that non-responders and relapsers continue with long-term ETV treatment both in HBeAg-positive and negative patients.
Cost and utility estimates
We used generic antiviral drug costs for the base case analysis and examined brand drug costs in the sensitivity analysis. Generic drugs are widely available in China, and whether they are prescribed often depends on the physician’s preference and the choice of the patient based on their health insurance coverage and out of pocket costs. The generic and brand drug costs were obtained from official prices approved by the Shanghai Municipal Bureau of Pricing. Prices were available for all generic antiviral drugs except for PEG-IFN and TDF which are not yet available in generic form in China. The medical management costs for CHB and other related costs were derived from the retrospective analysis of the medical records of patients with CHB by Zhiqiang et al. [49].
All costs were inflated to 2014 prices using China National Healthcare Index from the National Bureau of Statistics of China and converted to US dollars using the exchange rate as of 2014 (1 USD = 6.23 RMB). The age-specific utilities were obtained from a multinational study on hepatitis B [50] (Table 3). Following the World Health Organization guidelines for cost-effectiveness estimates [51], we regarded the incremental ratio of less than one times the gross domestic product (GDP) per capita for each QALY gained as an cost-effective intervention, and an ICER between one and three times GDP per capita per QALY as a potential cost-effective intervention, in which the GDP per capita in China in 2013 was $6,800 (41,775 RMB) [52].
Table 3. Annual base case cost and utility estimates (2014).
| Variable | Annual base-case estimate (range) | |
|---|---|---|
| Generic drug costs | Yuen (RMB) | Dollar |
| Lamivudine | 3,650 (2,730–4,215) | $585 (415–790) |
| Adefovir | 3,423 (2,610–4,100) | $549 (395–751) |
| Entecavir (0.5mg) | 7,662 (5,990–9,330) | $1,229 (972–2,003) |
| Pegylated Interferon | N.A | N.A |
| Tenofovir (300 mg) | N.A | N.A |
| Branded drug costs | ||
| Lamivudine | 4,360 (3,266–5,446) | $699 (524–873) |
| Adefovir | 4,088 (3,060–5,108) | $656 (491–819) |
| Entecavir (0.5mg) | 14,645 (10,984–18,306) | $2,349 (1,762–2,937) |
| Pegylated Interferon | 69,761 (52,318–87,196) | $11,191 (8,393–13,988) |
| Tenofovir (300 mg) | 18,202 (13,652–22,753) | $2,920 (2,190–3,650) |
| Annual medical management costs | ||
| Annual monitoring* | 126 (95–158) | $20 (15–25) |
| Chronic hepatitis B | 1,332 (1,000–1,665) | $214 (160–267) |
| Cirrhosis | 1,736 (1,301–2,170) | $278 (209–348) |
| Decompensated cirrhosis | 15,974 (11,980–19,968) | $2,562 (1,922–3,203) |
| Hepatocellular carcinoma | 44,499 (33,374–55,623) | $7,139 (5,354–8,923) |
| Liver transplantation | 573,230 (429,923–716,538) | $91,961 (68,970–114,951) |
| Health state utilities ± | Utility | Range |
| Viral suppression | 1 | (0.95–1.00) |
| Seroclearance | 0.99 | (0.90–1.00) |
| Inactive CHB | 0.95 | (0.90–0.99) |
| Active CHB | 0.85 | (0.68–0.90) |
| Cirrhosis | 0.69 | (0.66–0.71) |
| Decompensated cirrhosis | 0.35 | (0.32–0.37) |
| Hepatocellular carcinoma | 0.38 | (0.36–0.41) |
| Liver transplantation | 0.67 | (0.64–0.69) |
* Annual monitoring costs include ALT and HBV DNA tests.
± See Levy et al. [50] for age-specific utilities.
Sensitivity analysis
A Monte Carlo simulation was conducted, assuming that all variables followed a triangular distribution, with base case, low and high range values. We simulated 10,000 trials and plotted the results on willingness to pay threshold acceptability curves. We also analyzed at which threshold annual drug price at which ETV and TDF (since both these antivirals are considered highly potent with low resistance) annual drug price would be cost-effective or cost-saving. In addition we also analyzed the cost-effectiveness of branded costs for the all drugs.
Results
Base case
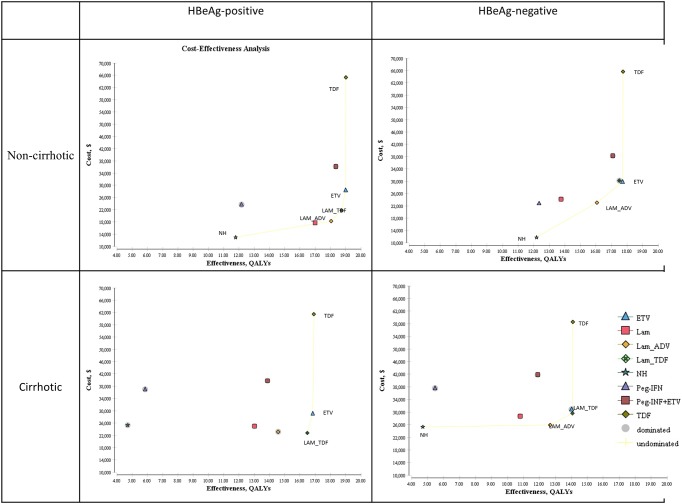
A cost-effectiveness plot of the various scenarios according to HBeAg status with or without cirrhosis is shown in Fig 2. For the base-case analysis, the lowest available prices were taken for the drugs (generic prices except for PEG-IFN and TDF, where the generic form of the drugs are not yet available in China). The CHB treatment costs, long-term health care costs, total costs, QALYs, and ICERs compared to the next best therapy for all subgroups are shown in Table 4 and for the branded price outcomes in S2 Table. The health outcomes for each sub-group in terms of morbidity (decompensated cirrhosis, hepatocellular carcinoma, and liver transplantation) and mortality are shown in Fig 3.
Fig 2. Results plotted on a cost-effectiveness plane stratified by hepatitis B e-antigen (HBeAg) with or without cirrhosis.
The x-axis represents the gain in QALYs with each strategy, and the y-axis represents the total costs (year 2014 values).
Table 4. Base Case Results.
| Therapy | HBV Antiviral or Interferon Treatment Costs | Long-Term Other Healthcare Costs | Total Costs ($) | Quality Adjusted Life Years | ICER compared to next-best therapy ($/QALY)* | ICER compared to no treatment ($/QALY)** |
|---|---|---|---|---|---|---|
| Non-cirrhotic HBeAg-positive | ||||||
| No Treatment | - | 12,932 | 12,932 | 11.79 | ||
| (generic) Lamivudine | 11,855 | 5,875 | 17,730 | 16.99 | ext. dominated | 1,042 |
| (generic)Lamivudine with Adefovir (generic) Salvage | 15,050 | 3,155 | 18,205 | 18.04 | 844 | 1,008 |
| (generic) Lamivudine with (branded) Tenofovir Salvage | 19,490 | 2,267 | 21,757 | 18.73 | 5,103 | 1,161 |
| (generic) Entecavir | 26,807 | 1,554 | 28,361 | 19.00 | 25,058 | 1,492 |
| (branded) Peg-Interferon-alfa | 11,191 | 12,174 | 23,365 | 12.35 | abs. dominated | 18,670 |
| (branded) Peg-Interferon-alfa with Entecavir (generic) Salvage | 33,744 | 2,387 | 36,131 | 18.35 | abs. dominated | 3,535 |
| (branded) Tenofovir | 63,720 | 1,518 | 65,238 | 19.02 | 1,900,948 | 3,429 |
| Non-cirrhotic HBeAg-negative | ||||||
| No Treatment | - | 11,735 | 11,735 | 12.19 | ||
| (generic) Lamivudine | 10,095 | 14,100 | 24,195 | 13.76 | abs. dominated | 1,757 |
| (generic)Lamivudine with Adefovir (generic) Salvage | 16,511 | 6,559 | 23,070 | 16.05 | 2,933 | 1,436 |
| (generic) Lamivudine with (branded) Tenofovir Salvage | 26,126 | 4,071 | 30,197 | 17.50 | abs. dominated | 1,725 |
| (generic) Entecavir | 26,078 | 3,709 | 29,787 | 17.71 | 4,066 | 1,681 |
| (branded) Peg-Interferon-alfa | 11,191 | 11,613 | 22,804 | 12.34 | ext. dominated | 71,701 |
| (branded) Peg-Interferon-alfa with Entecavir (generic) Salvage | 33,734 | 4,536 | 38,270 | 17.08 | abs. dominated | 5,426 |
| (branded) Tenofovir | 62,032 | 3,640 | 65,672 | 17.73 | 1,492,068 | 3,703 |
| Cirrhotic HBeAg-positive | ||||||
| No Treatment | - | 25,293 | 25,293 | 4.70 | ||
| (generic) Lamivudine | 8,530 | 16,490 | 25,020 | 13.02 | abs. dominated | 1,920 |
| (generic)Lamivudine with Adefovir (generic) Salvage | 11,015 | 11,752 | 22,767 | 14.64 | 0 | 1,554 |
| (generic) Lamivudine with (branded) Tenofovir Salvage | 16,214 | 6,580 | 22,794 | 16.48 | 15 | 1,382 |
| (generic) Entecavir | 23,498 | 5,606 | 29,104 | 16.84 | 17,497 | 1,727 |
| (branded) Peg-Interferon-alfa | 11,191 | 25,808 | 36999 | 5.85 | abs. dominated | 10,236 |
| (branded) Peg-Interferon-alfa with Entecavir (generic) Salvage | 27,424 | 12,348 | 39772 | 13.87 | abs. dominated | 1,579 |
| (branded) Tenofovir | 56,019 | 5,470 | 61,489 | 16.90 | 538,474 | 3,637 |
| Cirrhotic HBeAg-negative | ||||||
| No Treatment | - | 25,293 | 25,293 | 4.70 | ||
| (generic) Lamivudine | 7,122 | 21,571 | 28,693 | 10.79 | abs. dominated | 2,658 |
| (generic)Lamivudine with Adefovir (generic) Salvage | 10,192 | 15,733 | 25,925 | 12.67 | 79 | 2,045 |
| (generic) Lamivudine with (branded) Tenofovir Salvage | 18,727 | 10,843 | 29,570 | 14.08 | 2,583 | 2,099 |
| (generic) Entecavir | 20,038 | 10,848 | 30,886 | 14.02 | abs. dominated | 2,202 |
| (branded) Peg-Interferon-alfa | 11,191 | 26,365 | 37,556 | 5.47 | abs. dominated | 16,036 |
| (branded) Peg-Interferon-alfa with Entecavir (generic) Salvage | 25,715 | 16,122 | 41,837 | 11.90 | abs. dominated | 2,299 |
| (branded) Tenofovir | 47,865 | 10,681 | 58,546 | 14.10 | 1,876,422 | 4,151 |
ICER, Incremental Cost-Effectiveness Ratio.
* Calculated as the incremental cost compared to the next-best undominated alternative divided by the incremental QALYs compared to the next-best undominated alternative.
** Calculated as the incremental cost compared to no treatment divided by the incremental QALYs compared to no treatment.
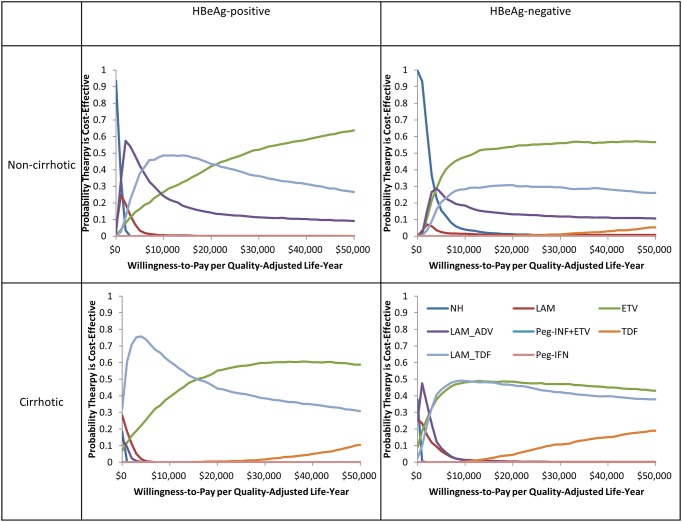
Fig 3. Cost-effectiveness acceptability curves showing the probabilities of net benefits achieved by each strategy for different willingness to pay thresholds stratified by hepatitis B e-antigen (HBeAg) with or without cirrhosis.
Non-cirrhotic HBeAg-positive patients in the no CHB treatment strategy would likely incur the highest health care costs, and the worst health outcomes, compared to the other strategies. With no antiviral treatment, each HBeAg-positive patient had $12,932 in total healthcare costs, 11.8 QALYs and 65% of them would die of hepatitis-B related liver disease, and 32% will develop HCC over their lifetime. When comparing the different therapies, some therapies appeared better than others. LAM→ADV salvage gave patients 18.0 QALYs at a cost of only $844 per QALY gained. Both ETV and TDF monotherapy offered the highest benefit, 19.0 QALYs, and 7.2 QALYs gained over the no treatment strategy. LAM monotherapy, PEG-IFN monotherapy, and the PEG-IFN→ETV salvage therapy did not appear cost-effective compared to the other therapies, so were all dominated (which means the intervention costs more and is less effective than the comparator). LAM monotherapy provides fewer QALYs at a higher cost-effectiveness ratio than LAM→ADV salvage (known as “dominated by extended dominance” in health-economics). PEG-IFN→ETV salvage therapy had fewer QALYs at a higher cost than ETV alone (known as “absolutely dominated” in health economics). If a low resistance, highly potent drug such as ETV or TDF is used for treatment of HBeAg positive patients who met the treatment criteria, approximately 60% of HBV-related mortality and 31% of HCC can be prevented.
Non-cirrhotic HBeAg-negative patients in the no treatment strategy had $11,735 in costs, 12.2 QALYs and 59% of them would die of hepatitis-B related liver disease, and 30% will develop HCC over their lifetime. When comparing the various therapies, both ETV and TDF offered the highest benefits, 17.7 QALYs and 5.5 QALY gained over no treatment. With no generic TDF and the high cost of brand TDF in the Chinese market, the ICER for generic ETV is $4,066 while the ICER for TDF is $1.5 million per QALY in China. Approximately 49% of HBV-related deaths, and 26% of HCC can be prevented if a low resistance, highly potent drug such as ETV or TDF is used.
If CHB cirrhotic patients are left untreated, 95% would die of HBV related liver disease, and 39% would develop HCC over their lifetime.
For cirrhotic HBeAg-positive cirrhotic patients, LAM→ADV salvage was the lowest cost alternative, it dominated no treatment and LAM therapy alone. LAM→TDF salvage was the next-best option, which added 1.9 QALYs over LAM→ADV salvage therapy at a cost of $15 per QALY. PEG-IFN monotherapy and PEG-IFN→ETV was dominated by ETV monotherapy. Nearly 79% of HBV-related mortality and 33% of HCC in HBeAg-positive patients can be prevented if they are treated with a low resistance, highly potent drug such as ETV or TDF.
For cirrhotic HBeAg-negative cirrhotic patients, LAM→ADV salvage would provide 12.67 QALYs and has the lowest ICER, $79 per QALY. ETV monotherapy or TDF monotherapy had the best health outcomes with 14.0 QALYs, at an additional cost of $2,583, and $1.8 million per QALY, respectively. Approximately 61% of HBV-related mortality and 26% of HCC in HBeAg-negative cirrhotic patients can be prevented if they are treated with a low resistance, highly potent antiviral drug such as ETV or TDF.
Sensitivity analysis
The results of the probabilistic sensitivity analysis (Fig 4) indicated that unless the cost of TDF drops, among all the therapies, generic ETV is likely the most cost effective therapy for both HBeAg-positive and negative patients with or without cirrhosis in China. For non- cirrhotic HBeAg-positive patients, ETV was 24% likely to be cost-effective at a willingness to pay threshold of $6,800 (1xGDP), and increased to 42% likely to be cost-effective at $20,400 (3xGDP). For the non-cirrhotic HBeAg-negative patients, ETV was 43% likely to be cost-effective at a threshold of $6,800 and further increased to 54% likely to be cost-effective, when compared to other treatments, at a threshold of $20,400. For cirrhotic HBeAg-positive patients, ETV was 32% likely to be cost-effective at a threshold of $6,800 and 55% likely to be cost-effective at a threshold of $20,400. For cirrhotic HBeAg-negative patients, ETV was 45% likely to be cost-effective at a threshold of $6,800 and 48% likely to be cost-effective at a threshold of $20,400. This shows the ETV is most likely to be cost-effective at commonly cited thresholds for cost-effectiveness.
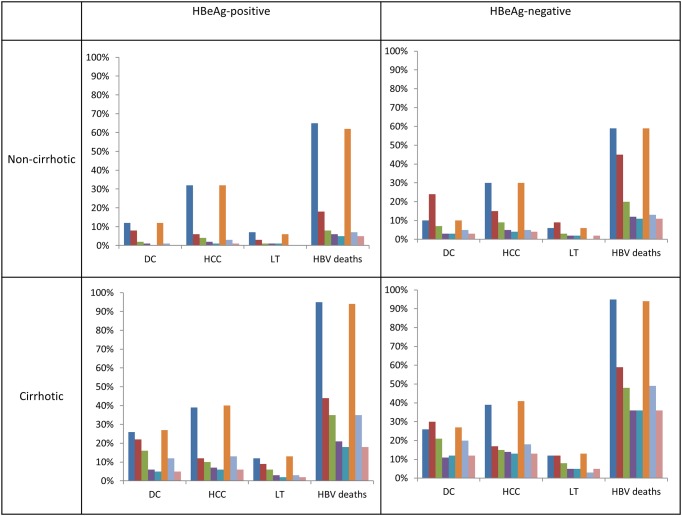
Fig 4. Clinical health outcomes for each strategy stratified by hepatitis B e-antigen (HBeAg) with or without cirrhosis.
The bars in order from left to right are: no treatment, LAM, LAM→ADV, LAM→TDF, ETV, PEG-IFN, PEG-IFN→ETV, and TDF.
We did an additional analysis on the costs of the two highly potent and low resistance profile drugs, ETV and TDF, to determine the thresholds at which treatment becomes cost-saving (with a willingness to pay (WTP) threshold of $0), highly-cost-effective (WTP $6,800, 1xGDP) and cost-effective (WTP $20,400, 3xGDP) for a national procurement and treatment program in China, compared to no treatment, for all sub-groups (Table 5). We chose ETV and TDF since they were the most effective strategies and had the highest health outcomes in all subgroups. In non-cirrhotic HBeAg-positive patients, ETV and TDF are cost-saving if the drug cost is below $523/year ($44/month), and considered highly cost-effective at $1,008/year ($84/month), and cost-effective at $1,173/year ($98/month). In non-cirrhotic HBeAg-negative patients, ETV and TDF are cost-saving if the drug cost is below $381/year ($32/month), and considered highly cost-effective at $1,313 year ($109/month) and cost-effective at $1,442 ($120/month). For cirrhotic HBeAg-positive patients, ETV and TDF are cost-saving below $899 ($75/month), and considered highly cost-effective at $1,027/year ($85/month), and cost-effective at $1,284/year ($107/month). For cirrhotic HBeAg-negative patients, ETV and TDF are cost-saving below $886 ($74/month), and considered highly cost-effective at $1,120/year ($93/month), and cost-effective at $1,065/year ($89/month).
Table 5. Annual Cost Thresholds for Entecavir and Tenofovir.
| Population | Cost-saving (WTP $0) | Highly cost-effective (WTP $6,800) | Cost-effective (WTP $20,400) | |||
|---|---|---|---|---|---|---|
| ETV | TDF | ETV | TDF | ETV | TDF | |
| Non-cirrhotic HBeAg-positive | $522 | $523 | $1,008 | $788 | $1,173 | $986 |
| Non-cirrhotic HBeAg-negative | $378 | $381 | $1,313 | $866 | $1,442 | $1,051 |
| Cirrhotic HBeAg-positive | $899 | $697 | $1,027 | $861 | $1,284 | $1,190 |
| Cirrhotic HBeAg-negative | $886 | $738 | $1,120 | $746 | $1,065 | $762 |
ETV, entecavir; TDF, tenofovir; WTP, willingness to pay.
Threshold between $6,800–20,400 according to the WHO 1-3x GDP for China.
Discussion
We examined the cost-effectiveness of treatment for four sub-groups of CHB patients; HBeAg-positive and HBeAg-negative patients with or without cirrhosis. We found neither pegylated interferon with or without salvage therapy were cost effective. Lamivudine monotherapy was also not cost effective. For patients who were previously on lamivudine treatment and developed drug resistance, salvage therapy by switching to generic adefovir was cost saving in cirrhotic, HBeAg-positive patients, and cost effective in cirrhotic, HBeAg-negative patients and in non-cirrhotic patients. Salvage therapy by switching to tenofovir was cost effective in cirrhotic patients and in non-cirrhotic, HBeAg-positive patients.
In our study we found entecavir or tenofovir monotherapy treatment in non-cirrhotic patients would prevent 49–69% of liver related deaths and 26–31% of HCC, and would prevent 61–79% of liver related deaths and 26–33% of HCC in cirrhotic patients. The QALYs gained from entercavir or tenofovir treatment compared to no treatment ranged from 5.5–7.3 for non-cirrhotic patients, and 9.3 to 12.1 for cirrhotic patients. These QALY gains are impressive, since few treatments for chronic non-communicable diseases or chronic infectious diseases result in such a gain in healthy life years. The QALYs gained by treatment is similar to that reported in HIV.
Among the two highly potent, low resistance drugs, entecavir is available as a branded drug at about $196/month (1,204 RMB) or as the less costly generic drug at about $102/month (626 RMB) for CHB treatment. Branded tenofovir is available to the public health system in China for the treatment of HIV/AIDS at the cost of less than $30/month (184 RMB) [53]. Even lower-cost generic tenofovir priced at as little as $5/month (30 RMB) for HIV treatment is available for low-income countries through the Global Fund [53, 54]. Recently, several low-income countries in Asia also have access to generic tenofovir for CHB treatment. Unfortunately, following approval of tenofovir for CHB treatment in China, there is no generic tenofovir available and the price of branded tenofovir for CHB treatment in China is high at $240/month (1,474 RMB), making it out of reach for most infected persons and the public health treatment programs in China.
Our sensitivity analysis indicated that at current prices of the available CHB treatment in China, entecavir is highly likely to be the preferred therapy. According to our threshold analysis, in order for entecavir to be cost-saving, meaning that using this strategy will have the lowest total costs with the best health outcomes, the drug price needs to drop to $671/year ($56/month (344 RMB)) from its current price of $1,229/year ($102/month (626 RMB)) in China. If the price of branded tenofovir for CHB treatment drop to the same level as the price afforded for HIV treatment in China (at less than $30/month), tenofovir treatment would be cost saving.
According to Li et al., a majority of physicians in China still prescribe lamivudine as first line CHB therapy and was prescribed by 54% of physicians in urban areas and 37% of physicians in rural areas, whereas, entecavir was prescribed by 5.4% of physicians in urban and 10% of those in rural areas [55]. Although several studies on the cost effectiveness of CHB treatments in China have been published, our study is the first to include tenofovir, the first to compare nucleoside, nucleotide and interferon therapies and include all four treatment subgroups, and the first to calculate cost thresholds for the most effective treatments. Another strength of our study is that we use age-specific transition estimates taken specifically from recent Chinese cohort studies to capture disease progression in this population as closely as possible. One limitation of our study is that we were unable to find literature on the rate of progression to cirrhosis and HCC for individuals who experience sustained virological response, so we assumed there is a 50% decrease in the rate of disease progression compare with the estimate of patients on treatment without sustained viral response [41, 56].
For a treatment program to be cost-saving, the price of the two highly potent low resistance drugs, entecavir and tenofovir would need to cost between $32-75/month (195–460 RMB). For it to be highly cost-effective or cost-effective, the drug price would need to be lowered to $62-120/month (380–737 RMB). Our study was undertaken to potentially support policy decisions regarding implementation of a national public health treatment program for CHB in China at the population level. This study not only compares the health outcomes but the impact of a reduction in the price of antiviral therapy. Our study could also be adapted to provide similar cost benefit estimates for decision makers in other endemic countries in developing their national viral hepatitis action plan.
Supporting Information
(DOCX)
(DOCX)
Acknowledgments
This study was supported by Stanford Asian Liver Center and CJ Huang and Ha Lin Yip Huang research funds at Stanford University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Stanford Asian Liver Center and the CJ Huang and Ha Lin Yip Huang research funds from Stanford University.
References
- 1. Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369(9573):1582–3. Epub 2007/05/15. S0140-6736(07)60723-5 [pii] 10.1016/S0140-6736(07)60723-5 . [DOI] [PubMed] [Google Scholar]
- 2.Globocan. 2012. Available: http://globocan.iarc.fr.
- 3. Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Seminars in cancer biology. 2011;21(1):59–69. Epub 2010/12/15. 10.1016/j.semcancer.2010.11.002 . [DOI] [PubMed] [Google Scholar]
- 4. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–7. Epub 2009/09/05. 10.1016/j.vaccine.2009.08.048 . [DOI] [PubMed] [Google Scholar]
- 5. Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–14. Epub 2009/10/20. 10.1002/hep.23310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toy M, Salomon JA, Jiang H, Gui H, Wang H, Wang J, et al. Population health impact and cost-effectiveness of monitoring inactive chronic hepatitis B and treating eligible patients in Shanghai, China. Hepatology. 2014;60(1):46–55. Epub 2014/07/06. 10.1002/hep.26934 . [DOI] [PubMed] [Google Scholar]
- 7. Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45(5):1187–92. Epub 2007/04/28. 10.1002/hep.21612 . [DOI] [PubMed] [Google Scholar]
- 8. Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Intern Med. 2005;142(10):821–31. . [DOI] [PubMed] [Google Scholar]
- 9. Lin X, Robinson NJ, Thursz M, Rosenberg DM, Weild A, Pimenta JM, et al. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: review of disease progression. J Gastroenterol Hepatol. 2005;20(6):833–43. . [DOI] [PubMed] [Google Scholar]
- 10. Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51(2):435–44. Epub 2009/11/18. 10.1002/hep.23348 . [DOI] [PubMed] [Google Scholar]
- 11. Yuen MF, Wong DK, Fung J, Ip P, But D, Hung I, et al. HBsAg Seroclearance in chronic hepatitis B in Asian patients: replicative level and risk of hepatocellular carcinoma. Gastroenterology. 2008;135(4):1192–9. Epub 2008/08/30. 10.1053/j.gastro.2008.07.008 . [DOI] [PubMed] [Google Scholar]
- 12. Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104(7):1693–9. Epub 2009/05/21. 10.1038/ajg.2009.187 . [DOI] [PubMed] [Google Scholar]
- 13. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295(1):65–73. . [DOI] [PubMed] [Google Scholar]
- 14. Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138(5):1747–54. Epub 2010/02/02. 10.1053/j.gastro.2010.01.042 . [DOI] [PubMed] [Google Scholar]
- 15. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52. [DOI] [PubMed] [Google Scholar]
- 16. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–31. . [DOI] [PubMed] [Google Scholar]
- 17. Kennedy PT, Phillips N, Chandrasekhar J, Jacobs R, Jacobs M, Dusheiko G. Potential and limitations of lamivudine monotherapy in chronic hepatitis B: evidence from genotyping. Liver Int. 2008;28(5):699–704. 10.1111/j.1478-3231.2008.01717.x [DOI] [PubMed] [Google Scholar]
- 18. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–10. . [DOI] [PubMed] [Google Scholar]
- 19. Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36(6):687–96. . [DOI] [PubMed] [Google Scholar]
- 20. Moskovitz DN, Osiowy C, Giles E, Tomlinson G, Heathcote EJ. Response to long-term lamivudine treatment (up to 5 years) in patients with severe chronic hepatitis B, role of genotype and drug resistance. J Viral Hepat. 2005;12(4):398–404. . [DOI] [PubMed] [Google Scholar]
- 21. Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43(6):1385–91. . [DOI] [PubMed] [Google Scholar]
- 22. Chen CH, Wang JH, Lee CM, Hung CH, Hu TH, Wang JC, et al. Virological response and incidence of adefovir resistance in lamivudine-resistant patients treated with adefovir dipivoxil. Antivir Ther. 2006;11(6):771–8. . [PubMed] [Google Scholar]
- 23. Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55(10):1488–95. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colonno RJ, Rose RE, Pokornowski K, Baldick CJ, Eggers B, Xu D, et al. Four year assessment of entecavir resistance in nucleoside naïve and lamivudine refractory patients. J Hepatol. 2007;46(Suppl. 1):S294. [Google Scholar]
- 25. Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, et al. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology. 2006;44(6):1656–65. . [DOI] [PubMed] [Google Scholar]
- 26. Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–14. 10.1002/hep.22841 [DOI] [PubMed] [Google Scholar]
- 27. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352(26):2682–95. . [DOI] [PubMed] [Google Scholar]
- 28. Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, et al. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136(7):2169–79 e1–4. 10.1053/j.gastro.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 29. Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat. 2003;10(4):298–305. . [DOI] [PubMed] [Google Scholar]
- 30. Piratvisuth T, Lau G, Chao YC, Jin R, Chutaputti A, Zhang QB, et al. Sustained response to peginterferon alfa-2a (40 kD) with or without lamivudine in Asian patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. Hepatol Int. 2008;2(1):102–10. 10.1007/s12072-007-9022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–43. Epub 2010/10/20. S0016-5085(10)01499-X [pii] 10.1053/j.gastro.2010.10.011 . [DOI] [PubMed] [Google Scholar]
- 32. Reijnders JG, Janssen HL. Potency of tenofovir in chronic hepatitis B: mono or combination therapy? J Hepatol. 2008;48(3):383–6. 10.1016/j.jhep.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 33. Ke W, Liu L, Zhang C, Ye X, Gao Y, Zhou S, et al. Comparison of efficacy and safety of tenofovir and entecavir in chronic hepatitis B virus infection: a systematic review and meta-analysis. PloS one. 2014;9(6):e98865 Epub 2014/06/07. 10.1371/journal.pone.0098865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505–13. Epub 2013/02/01. 10.1002/hep.26277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, et al. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut. 2011;60(2):247–54. Epub 2010/11/03. 10.1136/gut.2010.223206 . [DOI] [PubMed] [Google Scholar]
- 36. Lee CI, Kwon SY, Kim JH, Choe WH, Lee CH, Yoon EL, et al. Efficacy and safety of tenofovir-based rescue therapy for chronic hepatitis B patients with previous nucleo(s/t)ide treatment failure. Gut and liver. 2014;8(1):64–9. Epub 2014/02/12. 10.5009/gnl.2014.8.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Bommel F, Wunsche T, Mauss S, Reinke P, Bergk A, Schurmann D, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40(6):1421–5. . [DOI] [PubMed] [Google Scholar]
- 38.National Bureau of Statistics of China. Available: http://www.stats.gov.cn/english [cited 2010].
- 39.China Liver Transplant Registry. Available: http://www.cltr.org [cited 2010].
- 40. Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6. . [DOI] [PubMed] [Google Scholar]
- 41. Cho JY, Paik YH, Sohn W, Cho HC, Gwak GY, Choi MS, et al. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut. 2014. Epub 2014/03/13. 10.1136/gutjnl-2013-306409 . [DOI] [PubMed] [Google Scholar]
- 42. European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50(2):227–42. 10.1016/j.jhep.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 43. Liaw YF, Leung N, Kao J- H. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;(2):263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. 10.1002/hep.23190 [DOI] [PubMed] [Google Scholar]
- 45. Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int. 2009;3(3):425–33. 10.1007/s12072-009-9140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: The case for continuous antiviral therapy. J Hepatol. 2009;50(2):289–95. 10.1016/j.jhep.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 47. Reijnders JG, Perquin MJ, Zhang N, Hansen BE, Janssen HL. Nucleos(t)ide Analogues Only Induce Temporary Hepatitis B e Antigen Seroconversion in Most Patients with Chronic Hepatitis B. Gastroenterology. 2010;139(2):491–8. 10.1053/j.gastro.2010.03.059 [DOI] [PubMed] [Google Scholar]
- 48. Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DK, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139(4):1218–29. Epub 2010/07/06. 10.1053/j.gastro.2010.06.042 . [DOI] [PubMed] [Google Scholar]
- 49. Zhiqiang G, Zhaohui D, Qinhuan W, Dexian C, Yunyun F, Hongtao L, et al. Cost of chronic hepatitis B infection in China. J Clin Gastroenterol. 2004;38(10 Suppl):S175–8. [DOI] [PubMed] [Google Scholar]
- 50. Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11(3):527–38. 10.1111/j.1524-4733.2007.00297.x [DOI] [PubMed] [Google Scholar]
- 51.World Health Organization. WHO guide to cost-effectiveness. 2003.
- 52.T. WB. China Overview. 2013.
- 53. Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future virology. 2013;8(4):371–80. Epub 2013/05/11. 10.2217/fvl.13.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yin WY, Zhang FJ, Juniper N, Wu ZY. Improving China's antiretroviral treatment program: assessing current and future performance using the principals of ethics. Chinese medical journal. 2009;122(11):1346–51. Epub 2009/07/02. . [PubMed] [Google Scholar]
- 55. LI N, Yu B, Zhou L, Liu J, Xu A. Analysis of length of stay and clinical prognosis and influencing factors for inpatients with hepatitis B under different health insurance schemes. Chinese Journal of Helath Policy. 2012;5(1). [Google Scholar]
- 56. Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53(2):348–56. Epub 2010/05/21. 10.1016/j.jhep.2010.02.035 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.