Abstract
Functionalized polymeric nanocarriers have been recognized as drug delivery platforms for delivering therapeutic concentrations of chemotherapies. Of this category, star-shaped multiarm polymers are emerging candidates for targeted delivery of anti-cancer drugs, due to their compact structure, narrow size distribution, large surface area and high water solubility. In this study, we synthesized a multi-arm poly(acrylic acid) star polymer via MADIX/RAFT polymerization and characterized it using NMR and size exclusion chromatography. The poly(acrylic acid) star polymer demonstrated excellent water solubility and extremely low viscosity, making it highly suited for targeted drug delivery. Subsequently, we selected a hydrophilic drug, cisplatin, and a hydrophobic nitric oxide-donating prodrug, O2-(2,4-dinitrophenyl) 1-[4-(2-hydroxy)ethyl]-3-methylpiperazin-1-yl]diazen-1-ium-1,2-diolate, as two model compounds to evaluate the feasibility of using poly(acrylic acid) star polymers for delivery of chemotherapeutics. After synthesizing and characterizing two poly(acrylic acid) star polymer-based nanoconjugates, poly(acrylic acid)-cisplatin (acid-Pt) and poly(acrylic acid)-nitric oxide prodrug (acid-NO), the in vitro drug release kinetics of both acid-Pt and acid-NO were determined at physiological conditions. In summary, we have designed and evaluated a polymeric nanocarrier for sustained-delivery of chemotherapies, either as a single treatment or a combination therapy regimen.
Keywords: Conjugated polymer, drug delivery systems, reversible addition fragmentation chain transfer (RAFT), star polymer, water-soluble polymer
Graphical Abstract
We successfully developed multi-arm, star-shaped polymeric carriers for the delivery of nitric oxide-donating prodrugs and cisplatin. The carriers release nitric oxide prodrugs or cisplatin in a sustained fashion, inhibiting the growth of cancer cells in vitro.
Introduction
Star polymer architectures are a new generation of branched polymeric materials that consist of a single core and multiple connecting arms or chains. Star polymers usually have highly condensed and globular structures, tailorable size and large surface areas for conjugation of drugs and imaging agents, which makes them suited as potential delivery platforms. Various star polymer carriers have been synthesized and utilized for the delivery of small-molecule chemotherapeutics, including doxorubicin 1 (conjugation) and paclitaxel 2 (encapsulation), proteins, such as insulin 3, as well as genetic materials, such as DNAs 4 and siRNAs 5. Herein, we developed a multi-arm poly(acrylic acid) star polymer (Fig. 1) via MADIX/RAFT polymerization for long sustained release of multiple chemotherapeutics simultaneously as a chemo ‘cocktail’.
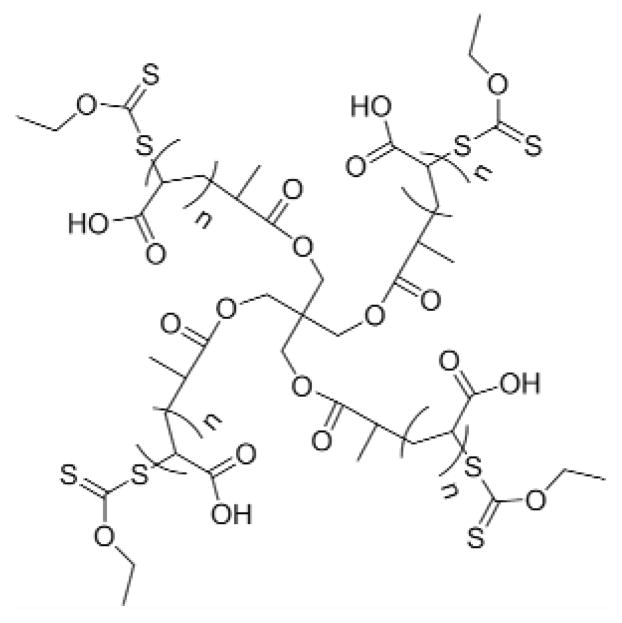
Figure 1.
Chemical structure of multiarm poly(acrylic acid) star polymer
Cisplatin is a water soluble, platinum-based chemotherapeutic that has been widely used for many years in the clinic as a first-line chemotherapy for head and neck squamous cell carcinoma, ovarian cancer, and non-small cell lung cancer. The major side effect of cisplatin infusion chemotherapy is renal toxicity, which limits its use. The toxicity of cisplatin is dependent on the maximum concentration of free drug in the plasma. Slow and confined release of cisplatin into tumors can largely alleviate these toxicities. In our previous studies, we synthesized a hyaluronan-cisplatin conjugate for the controlled release of subcutaneously delivered cisplatin using the biodegradable and biocompatible polymer hyaluronan 6. The conjugates achieved superior pharmacokinetics, reduced systemic toxicity, and enhanced anti-cancer efficacy in rodents compared to unbound intravenous cisplatin 7–10. However, a major drawback of this hyaluronan-based delivery platform and other linear polymers is the relatively high intrinsic viscosity of the vehicle, which makes it challenging to administer a high concentration of the chemotherapy within a small injection volume. Therefore, we sought to develop another drug carrier with desired solubility and viscosity characteristics that could sustain cisplatin over several days.
Polymer carriers can overcome problems with drug solubility and stability. We have developed a nitric oxide-donating prodrug, similar to other drugs in this class, it releases nitric oxide (NO) in vivo upon activation by glutathione-s-transferase in cells. But it is both very poorly water soluble and unstable in serum. Nitric oxide has been shown to effectively inhibit the proliferation of many cancer cells, including breast cancer 11, colon cancer 12, non-small cell lung cancer 13, and melanoma 14. It is a gaseous small molecule with a half life of only seconds 15. To deliver therapeutic concentrations of nitric oxide to tumorigenic tissues, a controlled release strategy is critical. A number of sustained release carriers have been designed for the delivery of nitric oxide, including NO-donor incorporated ethylene/vinyl acetate polymers 16, S-nitrosothiol conjugated interpolymers 17, as well as block copolymer of N-acryloylmorpholine and N-acryloyl-2, 5-dimethylpiperazine 18. To date, no combinational NO/cisplatin delivery platforms have been developed for delivering these two chemotherapeutics simultaneously. Therefore, we designed a nanocarrier-based system to generate active nitric oxide molecules and DNA-crosslinking platinum in a sustained release manner that maintains a relatively steady level of NO and platinum within the therapeutic window for a longer period of time during treatment.
Experimental
Materials
Unless noted otherwise, all reagents and solvents were purchased from Sigma Aldrich (St Louis, MO) or Fisher Scientific (Pittsburgh, PA) and used without further purification. 1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectra were collected on a Bruker DRX 400 spectrometer using compounds dissolved in CDCl3, MeOD or D2O. Chemical shifts were referenced to δ7.28 and 77.0 ppm for 1H-NMR and 13C-NMR spectra, respectively. High-resolution mass spectrometry (HRMS) data were generated after flow injection analysis (FIA) and manually matching peaks on an Applied Biosystems Mariner TOF spectrometer with a turbo-ionspray source. The cell culture media were purchased from MediaTech Inc (Manassas, VA).
Synthesis
Synthesis of MADIX/RAFT agent
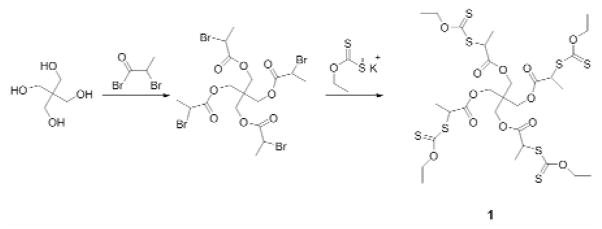
The MADIX/RAFT agent was synthesized based on the procedure reported previously (Fig. 2) 19. The core starting material, pentaerythritol, was esterified with 2-bromopropionyl bromide and O-ethylxanthic acid (potassium salt), successively, to form a four-arm sulfur compound that was used as the MADIX/RAFT initiator agent.
Figure 2.
Synthesis of MADIX/RAFT agent
4-arm core initiator 1
Pentaerithol (1.36 g, 10 mmol) was dissolved in dry chloroform (25 mL) and pyridine (2.5 mL) and cooled to 0 °C. The 2-bromo propionyl bromide (10.01 g, 45 mmol) was added dropwise, and the reaction proceeded at ambient temperature (ca. 23°C) for 48 hours. The mixture then was neutralized with aqueous HCl (10 wt %). Then the organic phases were washed with water, sodium bicarbonate solution (5%) and then brine, followed by drying with sodium sulfate. The solvent was evaporated under reduced pressure, which yielded the desired compound. The molecular structure was verified by 1H-NMR and compared with the reported data.
A solution of the intermediate compound (2.028 g, 3.0 mmol) in chloroform (45 mL) was treated with a 10-fold excess of O-ethylxanthic acid, potassium salt (5.01 g, 30 mmol). The mixture was stirred at ambient temperature for 3 days, and the resulting suspension was filtered and washed with chloroform. Evaporation of the solvent followed by purification through a flash column on silica gel using 3:7 ethyl acetate:hexanes as eluents yielded the desired compound, and the molecular structure was verified by 1H-NMR and compared with the reported data.
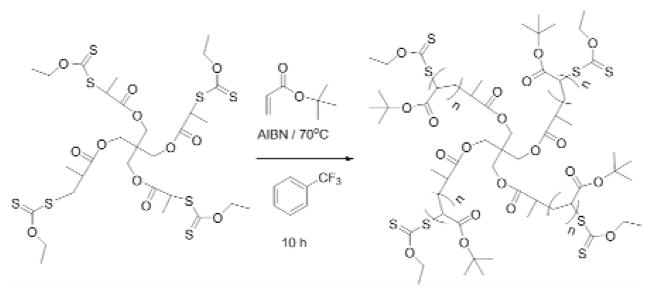
Synthesis of 4-arm tert-butyl acrylate star polymer
The multiarm tert-butyl acrylate star polymer was synthesized based on a modification of Ting et al.’s procedure 20. The tert-butyl acrylate (2.50 g, 19.5 mmol) was treated with basic alumina and dry α, α, α-trifluorotoluene (10 mL) in a 50-mL round bottom flask. The RAFT agent 1 (27 mg, 3.25×10−2 mmol) was added to the solution, followed by the addition of AIBN (0.53 mg, 3.25×10−3 mmol), and the reaction flask was placed on ice and purged with argon for 30 min. The flask then was transferred to a thermostatic oil bath at 70 °C for ca. 12 hours. The mixture was cooled in an ice bath and poured into cold diethyl ether to obtain the precipitate followed by concentration under reduced pressure (Fig. 3). The molecular structure and molecular weight of the resulting polymer were determined by 1H-NMR and size exclusion chromatography (SEC), respectively.
Figure 3.
Synthesis of multiarm tert-butyl acrylate star polymers
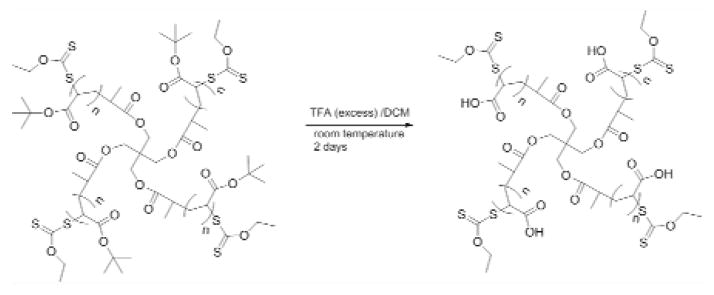
Synthesis of multiarm acrylic acid star polymers
The tert-butyl acrylate star polymer (2.50 g, 19.5 mmol) was dissolved in dichloromethane (200 mL), and trifluoroacetic acid (TFA, 75 mL, 975 mmol) was then added. The mixture was stirred vigorously until the generation of white precipitate ceased. The precipitate was filtered and washed with dichloromethane and diethyl ether, and then dried under reduced pressure overnight. The molecular structures and weights of the resulting polymers (Fig. 4) were determined by 1H-NMR and SEC, respectively.
Figure 4.
Synthesis of multiarm acrylic acid star polymer from tert-acrylate star polymer
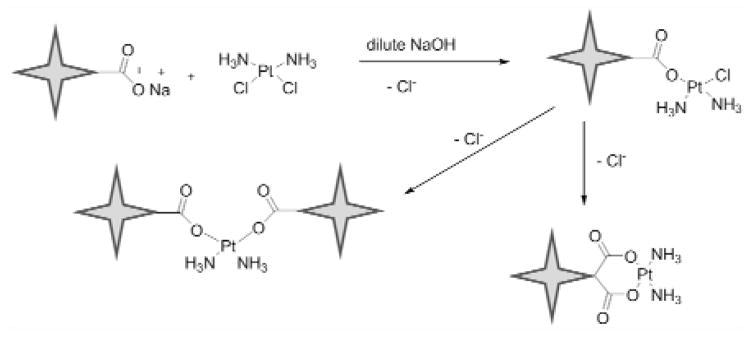
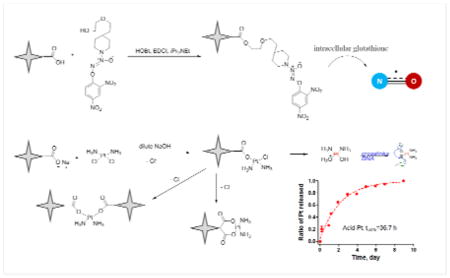
Synthesis of poly(acrylic acid) star polymer-cisplatin conjugates
The poly(acrylic acid) star polymer-cisplatin conjugates (acid-Pt) were synthesized according to the procedure of Cai et al.6. Poly(acrylic acid) star polymer (50 mg) was dissolved in dd H2O (25 mL) in a 50-mL round bottom flask. The pH of the solution was adjusted to 10 using 1-M NaOH solution and cisplatin (25 mg) was added. The solution was stirred under argon protection at ambient temperature for 36 hours. The solution then was dialyzed (10-kDa MWCO tubing, Pierce, Rockford, IL) against distilled water at 4 °C for 2 days with water changes every 6 hours to remove the unbound cisplatin. After dialysis the resulting polymer was concentrated by rotary evaporation to obtain the desired multi-arm poly(acrylic acid) star polymer-cisplatin conjugates (Fig. 5).
Figure 5.
Synthesis of poly(acrylic acid) star polymer-cisplatin conjugates
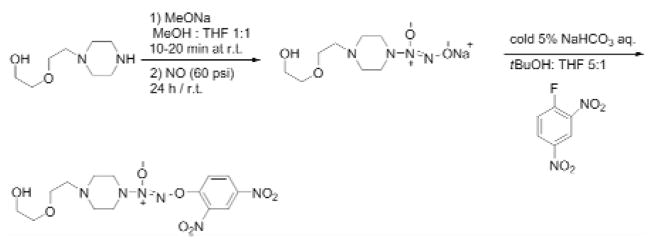
Synthesis of a nitric oxide-donating prodrug, O2-(2,4-Dinitrophenyl) 1-{[4-(2-(2-hydroxyethoxy))ethyl] piperazin-1-yl}diazen-1-ium-1,2-diolate
The nitric oxide prodrug was synthesized according to the procedure of Chakrapani et al 21 (Fig. 6). The 1-[2-(2-hydroxyethoxy)ethyl]piperazine (5.0 g, 27.3 mmol) was dissolved in methanol (2 mL) and treated with 25% w/v methanolic sodium methoxide (6.2 mL) and ether (20 mL). The resulting solution was charged with NO at 60 psi and stirred at ambient temperature for 24 hours, leading to the formation of a white precipitate. The white solid was filtered, washed with ether, and dried under reduced pressure to afford the sodium 1-[2-(2-hydroxyethoxy)ethyl] homopiperazin-1-yl] diazen-1-ium-1, 2-diolate. Subsequently, the white solid was dissolved in 5% w/v ice cold sodium bicarbonate solution (60 mL). The solution was treated with 1-fluoro-2,4-dinitrobenzene (4.67 g, 25.1 mmol) pre-dissolved in a mixture of t-BuOH (30 mL ) and THF (5 mL). A yellow precipitate formed immediately and was purified by silica flash chromatography (CHCl3: EtOAc=9:1) to afford the desired compound. The molecular structure was verified by 1H-NMR and mass spectrometry.
Figure 6.
Synthesis of O2-(2,4-Dinitrophenyl) 1-{[4-(2-(2-hydroxyethoxy))ethyl] piperazin-1-yl}diazen-1-ium-1,2-diolate
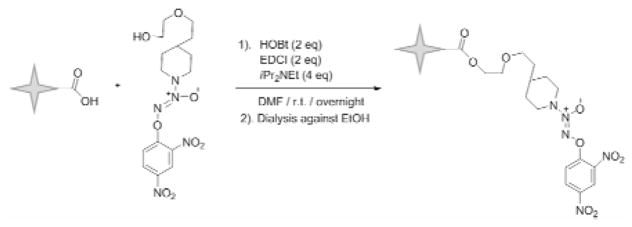
Synthesis of poly(acrylic acid) star polymer-nitric oxide prodrug conjugates
The poly(acrylic acid) star polymer (50 mg) was dissolved in 25 mL of ice cold DMF: dd H2O (25:1 v/v) mixture in a 50-mL round bottom flask (Fig. 7), and EDCI·HCl (1.5 eq) and HOBt·H2O (1.5 eq) were added to the solution. After 5 minutes, O2-(2,4-Dinitrophenyl) 1-{[4-(2-(2-hydroxyethoxy))ethyl] piperazin-1-yl}diazen-1-ium-1,2-diolate (1.0 eq) was added to the solution. The reaction proceeded under argon at 0 °C for 30 minutes, followed by ambient temperature overnight in the dark. The resulting acid-NO conjugate was dialyzed using 10,000 MWCO tubing against a mixture of dd H2O: ethanol (1:1 v/v) at ambient temperature for 12 hours, followed by ethanol for another 12 hours. The desired acid-NO conjugates were obtained after the evaporation of the solvent under reduced pressure. The molecular structure was verified by 1H-NMR.
Figure 7.
Synthesis of poly(acrylic acid) star polymer-nitric oxide prodrug conjugates
Characterization
Size exclusion chromatography
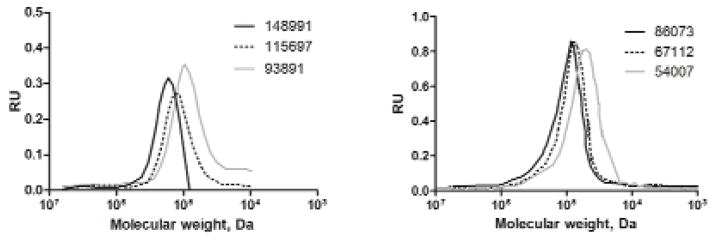
The molecular weights and polydispersity index (PDI) of the multi-arm tert-butyl acrylate star polymers were determined using EZStart 7.4 software and a Shimadzu 2010CHT system equipped with a RID-10A refractive index detector and a TSK gel multipore Hx-M 7.8×30 cm column using 10-mM LiCl in DMF (0.8 mL/min) as the mobile phase. The calibration curve was generated using polystyrene standards ranging from 1,180 to 339,500 g/mol. After deprotection, the resulting multi-arm poly(acrylic acid) star polymer was highly soluble in methanol, water, DMF, and DMSO. The molecular weights of the multi-arm poly(acrylic acid) star polymers were determined by SEC using polyethylene glycol (PEG) standards ranging from 3,070 to 66,100 g/mol (Scientific Polymer Products, Inc) (Table 1).
Table 1.
| Poly(tert-butyl acrylate) star polymers* | Poly(acrylic Acid) star polymers ** | ||||
|---|---|---|---|---|---|
| Mw, SEC | Mn, SEC | PDI | Mw, SEC | Mn, SEC | PDI |
| 148,991 | 130,122 | 1.14 | 86,073 | 72,321 | 1.19 |
| 115,697 | 97,107 | 1.19 | 67,112 | 55,927 | 1.20 |
| 83,788 | 71,209 | 1.16 | 54,007 | 39,992 | 1.31 |
Polystyrene was used as a MW standard.
PEG was used as a MW standard
Determination of drug conjugation
The substitution degree of the NO prodrug on the acid-NO conjugate was determined by 1H-NMR based on the ratio of the aromatic protons of the NO prodrug to the methylene protons of the polymer core. The degree of cisplatin substitution was determined by atomic absorption spectroscopy (AAS) (Varian SpectrAA GTA-110 with graphite furnace) using platinum standards ranging from 100 to 450 ppb (Fisher Scientific) 6. The furnace program was as follows: ramp from 25 to 80°C, hold 2 s, ramp to 120°C, hold 10 s, ramp to 1000°C, hold 5 s, ramp to 2700°C, hold 2 s, cool to 25°C over 20 s. The graphite partition tube was cleaned every 40 samples by baking at 2800°C for 7 s. Argon was used as the injection and carrier gas.
Viscosity
The viscosity parameters were measured using a Stabinger viscometer (SVM 3000, Anton Paar, USA). A series of multi-arm poly(acrylic acid) star polymer samples (MW: 64,000; 75,000; 803, 00; 110,000 g/mol) were prepared by dissolving the polymers in dd H2O at three concentrations including: 1.0 mg/mL, 3.0 mg/mL and 10.0 mg/mL.
In vitro release of drug from acid star polymers
The in vitro release kinetics of cisplatin was determined according to our previously published procedure 6. The acid-Pt conjugate (10 mg) was dissolved in PBS (10 mL), transferred to dialysis tubing (10,000 Da MWCO), and placed in a PBS bath (pH 7.4, 37 °C, 4 L) with or without 10% bovine serum albumin and 0.2% sodium azide. Two hundred microliter aliquots were collected from the tubing at pre-determined intervals and stored at −80 °C until analysis by AAS. The AAS produced a linear concentration curve from 10 to 450 ng/mL (R2=0.9998), with a limit of detection of 5 ng/mL and a limit of quantification of 10 ng/mL (5% standard deviation).
The release half-life of nitric oxide from NO-prodrug and acid-NO conjugates was determined in cell culture. MCF-7 cells were seeded in 96-well plates 24 hours prior to drug treatment (100 μL/well). On the following day, cells were treated with 20 μM of either the nitric oxide prodrug or the multi-arm polymer based nitric oxide prodrug conjugate, acid-NO. Fifty microliters of cell culture media were collected from each plate at 10 minutes, 2, 7, 22, 48 and 96 hours, post treatment to determine the nitrite content (N=5). The Griess reaction was performed according to the manufacturer’s protocol. The absorbance was measured at 535 nm using a UV microplate reader. The nitrite concentration and the corresponding nitrite oxide levels were determined using the nitrite standard curve previously generated.
Cytotoxicity
Cell growth inhibition was determined in 96-well plates. Plates were seeded with 3,000 cells/well in 100 μL of media (12 replicates/sample). Drug or conjugate solutions were applied after 24 hours. Resazurin blue in 10 μL of PBS was applied to each well (final concentration 5 μM) after another 72 hours. After 4 hours, the well fluorescence was measured (ex/em 560/590) (SpectraMax Gemini, Molecular Devices), and the IC50 concentration was determined as the midpoint between drug-free medium (positive) and cell-free (negative) controls.
Results and Discussion
Synthesis of acid-NO and acid-Pt conjugates
The poly (tert-butyl acrylate) star polymers were synthesized via MADIX/RAFT polymerization mediated by xanthates in α, α, α-trifluorotoluene, using AIBN as an initiator. The MADIX/RAFT polymerization approach is of special interest to polymer and drug delivery scientists compared to other synthetic strategies involving free radical reactions, including NMP, ATRP, and RAFT, as a wide range of potential monomers could be readily utilized to generate well-controlled polymeric architectures. The polymerization resulted in 90 % conversion of the monomers after a reaction duration of approximately 14 hours. Reaction byproducts started to form if the reaction was allowed to proceed for longer than 20 hours. Subsequent treatment of the poly (tert-butyl acrylate) star polymers of different molecular weights with TFA acid in dichloromethane yielded the corresponding acid star polymers. The acid star polymer and its corresponding polymer-chemotherapeutic conjugates, including acid star polymer-nitric oxide prodrug conjugate (20% wt drug loading) and acid star polymer-cisplatin conjugates (8% wt drug loading) were successfully synthesized. The acid star polymers were soluble in water, methanol, DMF and DMSO. Besides the multi-arm poly(acrylic acid) star polymers we developed, various other polymeric systems have also been explored for locoregional delivery of anti-cancer drugs, including PEG-graft-α,β-poly [(N-amino acidyl)-aspartamide] polymers 22, PLGA-4-arm-PEG branched polymeric nanoparticles 23, and a core-shell star polymer carrier with a poly(styrene) core 24.
Characterization – size exclusion chromatography
The molecular weights of the poly (tert-butyl acrylate) star polymers and their corresponding acid star polymers were determined by SEC using polystyrene and polyethylene glycol (PEG) as standards, respectively (Fig. 8). The PDIs of poly (tert-butyl acrylate) star polymer and acid star polymer with increasing molecular weights were calculated and reported in Table 1. The PDIs were similar to values reported by other groups using MADIX/RAFT to form structured polymers, including 4-arm star polymers reported by Stenzel and coworkers (PDIs: 1.20 to 1.44) 19. The PDI was in part due to using linear PEGs and polystyrenes as molecular weight standards, since standards with similar architecture were not available. Also, the highly charged nature of the polymers may have led to some interactions with the column stationary phase.
Figure 8.
SEC traces of poly(tert-butyl acrylate) star polymers (Left panel, MWs: 148,991, 115,697, and 93,891 Da) and poly(acrylic Acid) star polymers (Right panel, MWs: 86,073, 67,112, and 54,007 Da)
Characterization – NMR
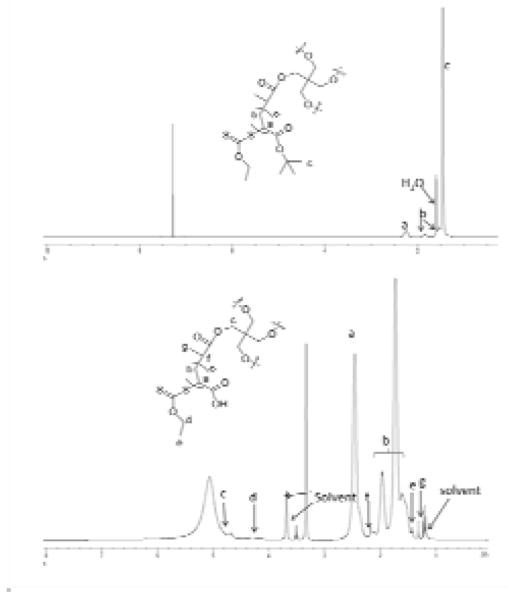
The molecular structures of the star polymers and nitric oxide prodrug were verified using 1H-NMR, 13C-NMR and HR-MS (Fig. 9). All data were consistent with the published results (purity>99%).
Figure 9.
1H-NMR spectra of poly (tert-butyl acrylate) star polymer (top panel, solvent: CDCl3), and poly(acrylic acid) star polymer (bottom panel, solvent: MeOD)
O2-(2,4-Dinitrophenyl) 1-{[4-(2-(2-hydroxyethoxy))ethyl] piperazin-1-yl}diazen-1-ium-1,2-diolate
1H-NMR (CDCl3, 400 MHz): δ = 1.69 (brs, 1H), 2.70 (t, J = 5.3 Hz, 2H), 2.80 (t, J = 5.1 Hz, 4H), 3.63–3.66 (m, 2H), 3.69–3.75 (m, 8H), 7.68 (d, J = 9.3 Hz, 1H). 8.47 (dd, J = 9.2, 2.7 Hz, 1H), 8.90 (d, J = 2.7 Hz, 1H); 13C-NMR (CDCl3, 100 MHz): δ = 50.3, 51.5, 57.0, 61.9, 68.0, 72.3, 117.6, 122.2, 129.1, 137.2, 142.3, 153.9. HRMS (ESI) Calculated for C14H21N6O8 (M + H) +: 401.1421; Found: 401.1413.
Poly (tert-butyl acrylate) star polymer
1H-NMR (CDCl3, 400 MHz): δ = 4.65 (q, 8H), 4.25 (q, 4H), 4.03 (q, 8H), 2.25 (brs), 1.86 (brs), 1.65–1.27 (brs, overlap).
Poly(acrylic acid) star polymer
1H-NMR (CDCl3, 400 MHz): δ = 4.66 (q, 8H), 4.30 (q, 4H), 4.14 (q, 8H), 2.25 (brs), 1.86 (brs), 1.65–1.27 (brs, overlap), 1.30 (t, 12H).
Poly(acrylic acid) star polymer-nitric oxide prodrug conjugate
1H-NMR (CDCl3, 400 MHz): δ = 8.90 (d, J = 8.4 Hz, 1H), 8.57 (dd, J = 8.4 Hz, 1H), 7.68 (d, J = 13.2 Hz, 1H), 4.66 (q, 8H), 4.30 (q, 4H), 4.14 (q, 8H), 3.73 (m, 8H), 3.65 (m, 2H), 2.79 (m 4H), 2.70 (m, 2H), 2.73 (brs), 1.81–1.48 (brs, overlap).
Poly(acrylic acid) star polymer-cisplatin conjugate
1H-NMR (CDCl3, 400MHz): δ= 4.68 (q, 8H), 4.38 (q, 4H), 2.55–2.37 (bs), 2.06–1.89 (bs), 1.84–1.66 (bs), 1.44 (t, 3H), 1.20 (d, 3H).
Characterization – viscosity
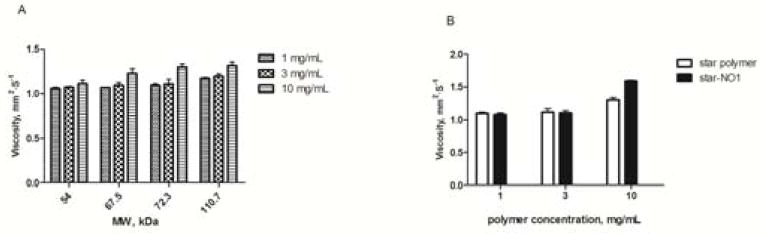
The viscosity of acid star polymers slightly increased with increases in either the molecular weight (54.0 to 110.7 kDa) or the concentration (1 to 10 mg/mL) of the polymer (Fig. 10). The viscosity of the acid-NO conjugates was also evaluated and compared to the acid star polymer itself at three different concentrations. The acid star polymer selected for the drug conjugation had a molecular weight of 72.3 kD. Based on our ongoing studies, star polymers with a molecular weight close to 75 kD exhibit advantageous patterns of lymphatic drainage and retention, compared to star polymers of higher or lower molecular weights, thus, the 72.3-kD star polymer drug carrier may be a potential candidate for localized drug delivery applications. It is worth noting that none of the acid star polymers tested exhibited high viscosity compared to other polymeric injectables for localized drug delivery, including hyaluronic acid, which demonstrated a 3-fold increase in viscosity relative to acid star polymers with a similar molecular weight at 10 mg/mL (data not shown). The FDA recommends that injectables have a viscosity of less than 50 cP, which may be readily injected using a 25- or 27-ga needle. The viscosities of our materials were less than 2 cP, and they can be easily injected using a 31-ga needle. Thus, these polymers are highly suited for a locally administered chemotherapeutics due to their low viscosity at high concentrations, which allows the use of small-bore needles and low injection volumes.
Figure 10.
Viscosity measurements of A) a poly(acrylic acid) star polymer at concentrations of 1, 3 and 10 mg/mL, and B) a star polymer nitric oxide prodrug conjugate (acid-NO, 72.3 kDa)
In vitro release of platinum from acid-Pt
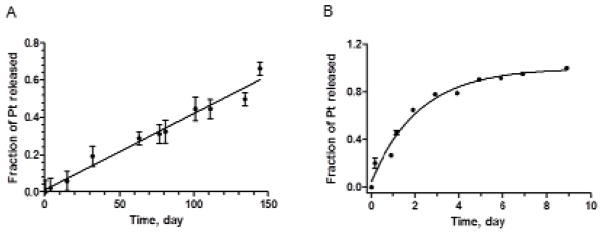
The release kinetics of cisplatin from the acid star polymer backbone was determined in PBS (pH 7.4) at 37°C with or without 10% serum. The half-life was determined by fitting the release data to either a zero order linear regression model (release of Pt in PBS without serum) or a first order decay model (release of Pt in serum-containing PBS) using GraphPad 5 (R2 >0.97 for all fits). The acid-Pt conjugates demonstrated an extended shelf-life in PBS with a platinum release half-life of approximately 120 days (Fig. 11A), which is significantly more stable than other sustained delivery platforms of cisplatin, including cisplatin-incorporating polymeric micelles (release half-life: ca. 4 days) 25, and dextran-based cisplatin conjugates (release half-life: ca. 2 days) 26. The presence of serum expedited the drug release from the acid polymers, which is likely due to the competitive binding between the platinum and the proteins present in the serum. The conjugates were able to sustain the release of cisplatin over 9 days (95% complete) with a release half-life of approximately 36.7 hours in serum-containing PBS (Fig. 11B), suggesting satisfactory stability in plasma in vivo. If this delivery platform could be translated into the clinic, it may be utilized as an adjuvant or maintenance chemotherapy post-surgery, releasing a steady concentration of platinum for an extended period of time, which may eradicate the residual disease and potential nano- or micro-metastases in the surrounding tissues and draining lymph nodes.
Figure 11.
Release of platinum from acid-Pt conjugates in A) PBS, and B) PBS with 10% serum at 37 °C (N=3). The release half-lives were determined to be 120 days and 36.7 hours, using a linear regression or a first order decay model in GrapPad 5, respectively.
In vitro release of NO from acid-NO
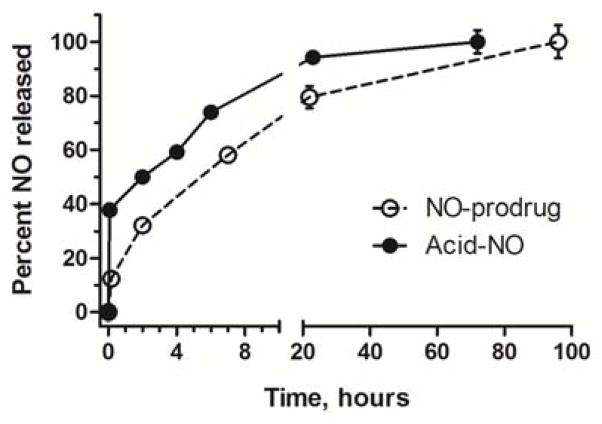
The release kinetics of nitric oxide from both the prodrug (NO-prodrug) and the carrier-nitric oxide prodrug conjugate (acid-NO) were determined in cell culture media. The half-lives were determined be 7.4 and 5.3 hours for NO-prodrug and acid-NO, respectively, by fitting the release data to a first order decay model using GraphPad 5 (R2 >0.97 for all fits, Table 2). The acid-NO exhibited a shorter release half-life compared to the NO-prodrug, which is largely due to the initial burst release of NO (Fig. 12). The poly(acrylic acid) star polymer backbone created a more acidic microenvironment surrounding the conjugates once they were solubilized in cell culture media, which triggered the liberation of NO immediately. Without the burst release, the acid-NO demonstrated a similar t1/2 as the NO-prodrug. Another NO-donating prodrug in preclinical studies, JS-K 11,27,28, has a NO release half-life of approximately 3.2 hours (data not shown), which is shorter than the release t1/2 of either the NO-prodrug or the acid-NO. All of the aforementioned NO-prodrugs tested exhibited significantly longer half-lives of NO release compared to the gaseous nitric oxide, which has a t1/2 of 0.05 to 0.18 milliseconds in blood 29. According to Fetz et al, pretreatment of head and neck cancer cells using NO-donors, including S-nitroso-N-acetyl-penicillamine (SNAP), reverted the cells’ cisplatin resistance via the modulation of survivin30. Thus, a drug delivery platform that generates fast-releasing nitric oxide, along with slow-releasing platinum, may be a potential candidate for the delivery of synergistic cisplatin and nitric oxide combination chemotherapy.
Table 2.
| Drugs/conjugates | IC50 in MCF-7 cells, μM | Release half-life, hours |
|---|---|---|
| cisplatin | 20±4 | - |
| Acid-Pt | >100 | 36.7** |
| NO-prodrug | 48±28 | 7.4* |
| Acid-NO | 64±10 | 5.3* |
Release half-life was determined in PBS with 10% serum at 37 °C.
Release half-lives were determined in cell culture media with MCF-7 cells.
Figure 12.
Release of nitric oxide from NO-prodrug and acid-Pt conjugates in cell culture media at 37 °C (N=3). The half-lives were determined be 7.4 and 5.3 hours for NO-prodrug and acid-NO, respectively, by fitting the release data to a first order decay model using GraphPad 5 (R2 >0.97 for all fits).
Cytotoxicity of acid-NO and acid-Pt conjugates
The in vitro anti-proliferative activities of cisplatin, NO-donating prodrug, and their star polymer-based conjugates, including acid-Pt, acid-NO, and combinational acid-Pt/acid-NO were evaluated in cell culture using a human breast cancer cell line, MCF-7 (Table 2). Acid-NO conjugates demonstrated a similar IC50 to the free NO-prodrug. Release of nitric oxide from the polymeric matrix inhibited the growth of cancer cells. The acid-Pt appeared to be less cytotoxic than cisplatin in the cells. This is likely due to the slow release of the active drug, which was not unexpected for a sustained-delivery platform. One of the obstacles that has not yet been overcome in infusion chemotherapy is the poor tumor penetration and accumulation of small-molecule anti-cancer agents. Usually the majority of the chemotherapeutics have been cleared from systemic circulation, via the kidneys, before a significant concentration of the anti-cancer agent is achieved in the tumorigenic tissues. However, the acid-Pt and acid-NO nanoconjugates may greatly enhance the intratumoral drug concentration compared to the normal tissues due to the Enhanced Permeability and Retention effect (EPR), in which nanoformulations tend to penetrate tumors more effectively via leaky, fenestrated tumor blood vessels. In addition, the acid-Pt and acid-NO formulations could be given subcutaneously as a local injection adjacent to the tumor to eradicate potential metastasis in the tumor-draining lymph nodes; whereas, it is clinically infeasible to inject free cisplatin or nitric oxide subcutaneously due to the severe tissue damage that the anti-cancer agents would create. After local injection of the acid-Pt, or the acid-NO, the nanocarriers diffuse into the surrounding cutaneous tissues and enter the lymphatic vessels filled with lymph fluid; subsequently, they follow the lymph and reach the sentinel lymph node, the first tumor draining lymph node, and deliver the chemotherapeutics to the metastases. Furthermore, both of the star polymer-based conjugates could be given simultaneously as a localized combination therapy. Nitric oxide has been shown to overcome chemoresistance in a cisplatin-insensitive human head and neck squamous cell carcinoma cell line30. The concurrent administration of both the cisplatin- and NO-releasing conjugates may minimize the incidence of acquired chemoresistance during therapy and maximize the efficacy of the combination treatment. Similar to cisplatin, nitric oxide has also been found to sensitize cancer cells to ionizing radiation 31, which may be used as an adjuvant combination regimen post radiation therapy. In addition, nitric oxide was recently found to increase the anti-tumor activity of other chemotherapeutics, e.g. doxorubicin 32. This discovery can be further explored using a combinational polymer-based, sustained-release doxorubicin (hyaluronan-doxorubicin) 33 and nitric oxide (acid-NO) delivery platform that our laboratory developed for treating cancers that are responsive to doxorubicin therapy, including certain types of breast, lung and ovarian cancers.
Conclusion
In summary, we have successfully synthesized a multi-arm poly(acrylic acid) star polymer architecture suited for the multimodal delivery of both hydrophilic and hydrophobic chemotherapeutics, as either a single-drug chemotherapy or a combinational regimen. This strategy has laid the foundation for future investigations of the delivery of chemo-cocktails using multiple anti-cancer agents that possess a synergism in vivo.
Acknowledgments
This work was supported by awards from the National Institutes of Health K-INBRE (P20 RR015563), the American Cancer Society (RSG-08-133-01-CDD), and the Department of Defense Prostate CDMRP. SC acknowledges an Eli Lilly predoctoral fellowship. SD and SC contributed equally to this work.
Contributor Information
Shaofeng Duan, Email: sduan@ku.edu, University of Kansas, 2095 Constant Ave, Lawrence, KS 66047.
Shuang Cai, Email: scai@ku.edu, University of Kansas, 2095 Constant Ave, Lawrence, KS 66047.
Yumei Xie, Email: Yumei.Xie@pnnl.gov, University of Kansas, 2095 Constant Ave, Lawrence, KS 66047.
Taryn Bagby, Email: tarynbagby@gmail.com, University of Kansas, 2095 Constant Ave, Lawrence, KS 66047.
Shenqiang Ren, Email: shenqiang@ku.edu, University of Kansas, 1251 Wescoe Hall Drive, 3012 Malott Hall, Lawrence, KS 66045.
M. Laird Forrest, Email: mforrest@ku.edu, University of Kansas, 2095 Constant Ave, Lawrence, KS 66047.
References
- 1.Etrych T, Strohalm J, Chytil P, Rihova B, Ulbrich K. J Drug Target. 2011;19:874–889. doi: 10.3109/1061186X.2011.622402. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, Liu S. Biomaterials. 2011;32:6595–6605. doi: 10.1016/j.biomaterials.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Wu W, Guo Z, Xin J, Li J. Biomaterials. 2011;32:1759–1766. doi: 10.1016/j.biomaterials.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhou YM, Ishikawa A, Okahashi R, Uchida K, Nemoto Y, Nakayama M, Nakayama Y. J Control Release. 2007;123:239–246. doi: 10.1016/j.jconrel.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Cho HY, Srinivasan A, Hong J, Hsu E, Liu S, Shrivats A, Kwak D, Bohaty AK, Paik HJ, Hollinger JO, Matyjaszewski K. Biomacromolecules. 2011;12:3478–3486. doi: 10.1021/bm2006455. [DOI] [PubMed] [Google Scholar]
- 6.Cai S, Xie Y, Bagby TR, Cohen MS, Forrest ML. J Surg Res. 2008;147:247–252. doi: 10.1016/j.jss.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S, Xie Y, Davies NM, Cohen MS, Forrest ML. J Pharm Sci. 2010;99:2664–2671. doi: 10.1002/jps.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Cai S, Xie Y, Forrest ML. Am J Surg. 2009;198:781–786. doi: 10.1016/j.amjsurg.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai S, Xie Y, Davies NM, Cohen MS, Forrest ML. Ther Deliv. 2010;1:237–245. doi: 10.4155/tde.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Aillon KL, Cai S, Christian JM, Davies NM, Berkland CJ, Forrest ML. Int J Pharm. 2010;392:156–163. doi: 10.1016/j.ijpharm.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurtry V, Saavedra JE, Nieves-Alicea R, Simeone AM, Keefer LK, Tari AM. Int J Oncol. 2011;38:963–971. doi: 10.3892/ijo.2011.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riganti C, Miraglia E, Viarisio D, Costamagna C, Pescarmona G, Ghigo D, Bosia A. Cancer Res. 2005;65:516–525. [PubMed] [Google Scholar]
- 13.Maciag AE, Chakrapani H, Saavedra JE, Morris NL, Holland RJ, Kosak KM, Shami PJ, Anderson LM, Keefer LK. J Pharmacol Exp Ther. 2011;336:313–320. doi: 10.1124/jpet.110.174904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijatovic S, Maksimovic-Ivanic D, Mojic M, Timotijevic G, Miljkovic D, Mangano K, Donia M, Di Cataldo A, Al-Abed Y, Cheng KF, Stosic-Grujicic S, Nicoletti F. J Cell Physiol. 2011;226:1803–1812. doi: 10.1002/jcp.22513. [DOI] [PubMed] [Google Scholar]
- 15.Hakim TS, Sugimori K, Camporesi EM, GA Physiol Meas. 1996;17:267–277. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- 16.Momin EN, Schwab KE, Chaichana KL, Miller-Lotan R, Levy AP, RJT Neurosurgery. 2009;65:937–945. doi: 10.1227/01.NEU.0000356974.14230.B8. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, PIL Mol Pharm. 2010;7:254–266. doi: 10.1021/mp900237f. [DOI] [PubMed] [Google Scholar]
- 18.Jo YS, van der Vlies AJ, Gantz J, Thacher TN, Antonijevic S, Cavadini S, Demurtas D, Stergiopulos N, JAH J Am Chem Soc. 2009;131:14413–14418. doi: 10.1021/ja905123t. [DOI] [PubMed] [Google Scholar]
- 19.Stenzel MH, Davis TP, Barner-Kowollik C. Chem Commun (Camb) 2004:1546–1547. doi: 10.1039/b404763j. [DOI] [PubMed] [Google Scholar]
- 20.Ting SR, Gregory AM, MHS Biomacromolecules. 2009;10:342–352. doi: 10.1021/bm801123b. [DOI] [PubMed] [Google Scholar]
- 21.Chakrapani H, Kalathur RC, Maciag AE, Citro ML, Ji X, Keefer LK, Saavedra JE. Bioorg Med Chem. 2008;16:9764–9771. doi: 10.1016/j.bmc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Tang X, Huang J, Zhang X, Yu J, Zhang Y, Gui S. Colloids Surf B Biointerfaces. 2011;85:280–288. doi: 10.1016/j.colsurfb.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Ding H, Yong KT, Roy I, Hu R, Wu F, Zhao L, Law WC, Zhao W, Ji W, Liu L, Bergey EJ, Prasad PN. Nanotechnology. 2011;22:165101. doi: 10.1088/0957-4484/22/16/165101. [DOI] [PubMed] [Google Scholar]
- 24.Kowalczuk A, Stoyanova E, Mitova V, Shestakova P, Momekov G, Momekova D, Koseva N. Int J Pharm. 2011;404:220–230. doi: 10.1016/j.ijpharm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, Nishiyama N, Kataoka K, Naito S, Kakizoe T. Br J Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichinose K, Tomiyama N, Nakashima M, Ohya Y, Ichikawa M, Ouchi T, Kanematsu T. Anticancer Drugs. 2000;11:33–38. doi: 10.1097/00001813-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S, Carr BI, Kar S, Wang M, Jia L, Ji X, Keefer LK. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 28.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li CQ, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, Strauer BE, Feelisch M, Kelm M. J Clin Invest. 2002;109:1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fetz V, Bier C, Habtemichael N, Schuon R, Schweitzer A, Kunkel M, Engels K, Kovacs AF, Schneider S, Mann W, Stauber RH, Knauer SK. Int J Cancer. 2009;124:2033–2041. doi: 10.1002/ijc.24182. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Zalcenstein A, Oren M. Cell Death Differ. 2003;10:468–476. doi: 10.1038/sj.cdd.4401181. [DOI] [PubMed] [Google Scholar]
- 32.de Luca A, Moroni N, Serafino A, Primavera A, Pastore A, Pedersen JZ, Petruzzelli R, Farrace MG, Pierimarchi P, Moroni G, Federici G, Sinibaldi Vallebona P, Lo Bello M. Biochem J. 2011;440:175–183. doi: 10.1042/BJ20111333. [DOI] [PubMed] [Google Scholar]
- 33.Cai S, Thati S, Bagby TR, Diab HM, Davies NM, Cohen MS, Forrest ML. J Control Release. 2010;146:212–218. doi: 10.1016/j.jconrel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]