Abstract
The lemurs of Madagascar are among the most threatened mammalian taxa in the world, with habitat loss due to shifting cultivation and timber harvest heavily contributing to their precarious state. Deforestation often leads to fragmentation, resulting in mixed-habitat matrices throughout a landscape where disturbed areas are prone to invasion by exotic plants. Our study site, the Mandena littoral forest (southeast Madagascar), is a matrix of littoral forest, littoral swamp, and Melaleuca swamp habitats. Here, Melaleuca quinquenervia has invaded the wetland ecosystem, creating a mono-dominant habitat that currently provides the only potential habitat corridor between forest fragments. We sought to understand the role of this invasive Melaleuca swamp on the behavioral ecology of a threatened, small-bodied folivore, the southern bamboo lemur (Hapalemur meridionalis). We collected botanical and behavioral data on four groups of H. meridionalis between January and December 2013. Our results confirm Melaleuca swamp as an important part of their home range: while lemurs seasonally limited activities to certain habitats, all groups were capable of utilizing this invasive habitat for feeding and resting. Furthermore, the fact that Hapalemur use an invasive plant species as a dispersal corridor increases our knowledge of their ecological flexibility, and may be useful in the conservation management of remaining threatened populations.
Introduction
Deforestation within the tropics is one of the primary threats to global biodiversity [1, 2]. In addition to forest reduction, fragmentation results in extended edges that are often considered entirely distinct ecosystems from forest interiors [3]. Though fragments may persist after deforestation, most are unsuitable habitat for forest species [4, 5]. Within Madagascar, more than 80% of forest areas exist less than 1 km from an edge [6], thus fragmentation is of great concern for the survival of forest fauna and flora species [7, 8]. Decreasing deforestation rates and reforesting fragmented landscapes would help prevent extinctions [9].
Generally, corridors are defined as thin strips of habitat that connect two or more isolated forest fragments, and many studies validate their utilization by organisms (reviewed in [10; 11]). Although fragmentation of populations may result in genetic erosion and increase extinction risk [12], it has been shown that a mosaic of small, suitable habitat fragments may mitigate these negative effects by acting as a single large habitat if the fragments are linked via corridors [13–17]. A network of forest fragments linked by corridors allowing species to disperse may act as a means to maintain biodiversity and ecological processes in anthropogenic landscapes [18, 19].
The primates of Madagascar are the most threatened mammalian taxon in the world [20]. Their survival is continually jeopardized by hunting for bushmeat, as well as habitat loss due to shifting cultivation and timber harvest [20, 21]. As habitat destruction continues to isolate the remaining lemurs in forest fragments, the need for regenerating forests and connecting those remaining fragments is crucial. As such, it is imperative to understand the responses of native plants and animals to disturbance if we are to create effective buffer zones and corridors that combine secondary and natural habitats [7, 22–24].
It is often thought that invasions by exotic species present a critical hindrance to the preservation of endemic biodiversity, as well as ecosystem restoration efforts [25, 26]. In southeast Madagascar, the Mandena littoral forest matrix exists within a seasonally-inundated flood plain that consists of natural littoral swamp and mahampy (Lepironia mucronata) wetlands, a portion of which remains inundated throughout the year. It is here, and in similarly inundated areas [27], that the broad-leaved paperbark tree Melaleuca quinquenervia (Family Myrtaceae), native to Australia, has been an aggressive disperser [28]. These littoral forests and their animal communities are among the most threatened ecosystems in Madagascar [29, 30]. While the viability of non-native tree plantations has been examined to potentially assist in dispersal and fulfilment of partial habitat requirements for the conservation of lemurs [31–33], the role of introduced and possibly invasive tree species has only been minimally examined [34–36]. This is of considerable interest as littoral forest fragments represent critical refuges for the survival and maintenance of biodiversity in the extremes of climatic variability [37]. Riparian habitats often serve as corridors for multiple taxa [38]; therefore, the conservation of these areas and nearby isolated forest blocks is critical to maintaining resilience.
In view of this conflicting situation, we sought to understand the role of an invasive species habitat (Melaleuca swamp) on the behavioral ecology of a small-bodied folivore, the southern bamboo lemur (Hapalemur meridionalis) in littoral forest fragments of extreme southeastern Madagascar. Growing knowledge of the ecological flexibility of bamboo lemurs [39–41] makes this species an excellent model with which to examine its ability to utilize distinct habitats, and potentially corridors, within the anthropogenic landscape. Here, we investigated whether Melaleuca swamp facilitates movement of H. meridionalis between littoral forest fragments and/or natural littoral swamp, and whether this invasive habitat provides additional services, e.g., suitable feeding and resting locations.
Materials and Methods
Ethics Statement
This study was conducted under the Accord de Collaboration between the University of Antananarivo and the University of Hamburg. Research protocols were approved and permits authorized by Commission Tripartite of the Direction des Eaux et Forêts de Madagascar (Autorisation de recherché n.240/12/MEF/SG/DGF/DCB.SAP/SCB du 17/09/2012), adhering to the legal requirements of Madagascar. We captured adults via Telinject® blow darts (administered by an experienced Malagasy technician) containing a hypnotic anesthesia (4 mg/kg of ketamine hydrochloride or tiletamine hydrochloride), so that the animals neither suffered nor recalled the capturing process. All animals recovered from anesthesia within 1.5 hours at the capture site, and there were no injuries as a consequence of capture and animals were followed until regaining full mobility. This process was repeated at the end of the study in December 2013 to remove the radio-collars from the bamboo lemurs.
Study Site and Subjects
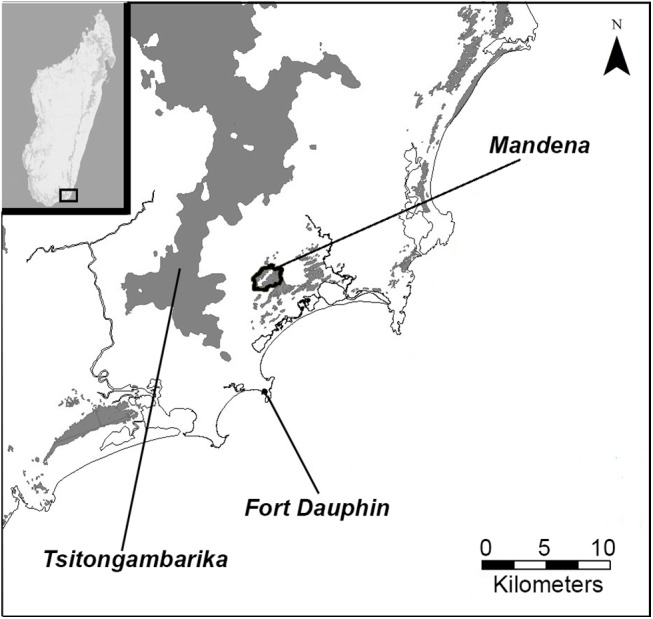
Our study was conducted in the Mandena Conservation Zone (24°95’S, 46°99’E) in southeast Madagascar (Fig 1), a protected area approximately 10 km north of Fort-Dauphin (Tolagnaro). This area consists of 148 ha of fragmented and degraded littoral forest, which is characterized as occurring within 3 km of the coast and growing on sandy substrates with a typically low canopy [42], and approximately 82 ha of interspersed natural littoral swamp and invasive Melaleuca swamp that separates the two littoral forest fragments [41].
Fig 1. Location of Mandena Conservation Zone in southeast Madagascar, relative to the larger, continuous forest of Tsitongambarika.
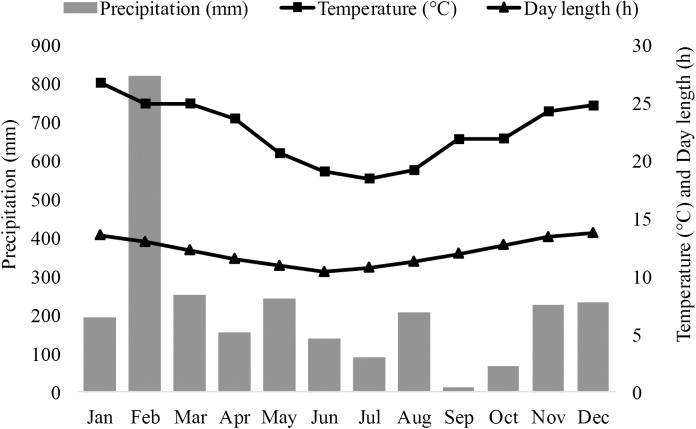
During our study period, temperature (°C) was recorded in 30-mins intervals using Lascar EL-USB-1 data loggers, operated by custom software (EasyLog USB Version 5.45, Lascar Electronics). Precipitation (mm) was measured daily at 6:00h using a rain gauge placed within the study site (Fig 2). Day length (a proxy for season) was calculated as the time between sunrise and sunset, as obtained from the US Naval Observatory Astronomical Calendar (http://aa.usno.navy.mil/data), using geographic coordinates for Mandena.
Fig 2. Monthly total precipitation (mm), mean temperature (°C), and mean day length (h) at Mandena in 2013.
Southern bamboo lemurs (Hapalemur meridionalis) are relatively small-bodied folivorous primates with a mean body mass of 1.072 ± 0.107 kg (X ± SD; N = 15) [41, 43] that maintain a cathemeral activity pattern [44]. This species lives in small social groups with one or two breeding females and typically one breeding male. Within Mandena, H. meridionalis groups average 5.6 ± 1.5 individuals (X ± SD; N = 5). In addition to southern bamboo lemur, the cathemeral collared brown lemur (Eulemur collaris) and nocturnal gray mouse lemur (Microcebus murinus), eastern fat-tailed dwarf lemur (Cheirogaleus medius), greater dwarf lemur (C. major), and southern woolly lemur (Avahi meridionalis) are present within Mandena.
Ten adult H. meridionalis across four neighboring social groups were captured and habituated between October and December 2012. Data were recorded from January to December 2013. As bamboo lemurs are highly cryptic, individuals were fitted with external radio-transmitters with an archival tag (ARC400, Advanced Telemetry Systems, Isanti, USA) that allowed us to more easily follow groups.
Habitat Characterization
To characterize each distinct habitat, we sampled 25 x 100 m2 botanical plots, i.e., 10 in both the littoral forest and littoral swamp, and five in the Melaleuca swamp, the latter requiring fewer plots due to its floristic homogeneity. Within each plot we included all trees with a diameter at breast height (DBH) ≥ 5 cm, recording the scientific binomial name and family name of each so as to measure tree diversity, in addition to their height (m) and crown volume (m3). The latter was estimated as an ellipsoid via the crown height and two crown diameters, i.e., maximum and perpendicular widths. We further conducted vertical-line transects within each plot, so as to detail the structure and canopy cover for each these three habitats [45]. Lastly, we calculated the Shannon index (H′) to determine the species diversity of each habitat. The Mandena littoral forest and littoral swamp that our focal H. meridionalis groups inhabit are legally protected forests; however, much of the Melaleuca swamp falls outside of this demarcation. As such, local people access these unprotected areas daily to harvest wood. To measure the degree to which this occurs, we included felled trees (via tree stumps) in our botanical plots. Lastly, it should be noted that in order for lemurs to access the Melaleuca swamp around Mandena, they must descend and traverse a barren, sandy area that would make them visually conspicuous to any potential predators as they leave the canopy cover of the littoral forest. To examine these crossing sites, we measured the distance (m) traversed where lemurs accessed the Melaleuca swamp.
Behavioral sampling
From January to December 2013, we conducted full-day focal follows (sunrise to sunset) with the aim of acquiring 50hrs/month per group for three social groups. We identified individuals using radio-tracking tags with unique-colored pendants. We collected behavioral data via instantaneous focal sampling [46] at 5-min intervals on broad-level activities (resting, feeding, moving, social, and other) and noted the habitat (littoral forest, littoral swamp, and Melaleuca swamp). In addition, we collected continuous feeding data each time a focal individual fed, recording the specific food item of the species, and duration of consumption measured to the second. All adult individuals in each group were sampled at least once each month. We further noted each occurrence in which the focal animal utilized the Melaleuca swamp corridors connecting the littoral forest fragments.
GIS analysis
We recorded a focal animal’s GPS location in 15-min intervals using a Garmin GPSMAP 62S unit, and noted the specific habitat type. All ranging data were entered into ArcGIS 10.2 (ESRI) using the Geospatial Modelling Environment (GME) spatial ecology interface [47] with R statistical software version 3.1.2 [48]. We determined each group’s territory using a 95% kernel density estimate [49] and further estimated the area (ha) of each habitat type.
Statistical analyses
To determine whether the characterization metrics of habitats differed, we used Kruskal-Wallis analyses for tree DBH, height, and crown volumes. We performed non-parametric tests as the data were not normally distributed, even after transformations. To determine the influence of habitat on bamboo lemur activities, a two-way repeated measures ANOVA was performed for each habitat, assessing the monthly proportion of broad-level activities (limited to rest, feed, and travel). Each habitat (littoral forest, littoral swamp, and Melaleuca swamp) was treated as the within-subjects factor, with groups acting as the between-subjects factor. Additionally, abiotic factors of total precipitation (mm), mean temperature (°C), and mean day length (h) per month were included in the model as covariates. The model errors for the repeated-measures ANOVA (via unstandardized residuals) were found to be normally distributed using the Kolmogorov-Smirnov test, allowing for the continuation of parametric analyses. Adjusted p-values are reported according to the Huynh–Feldt correction when assumptions of sphericity were violated; uncorrected biases from lack of sphericity can otherwise inflate F-statistics [50]. All analyses were performed using PASW v. 21.0 and significance was set at p < 0.05.
Results
Habitats
Compared to the botanically diverse littoral forest and littoral swamp, the Melaleuca swamp comprised six tree species, each from a distinct family (Table 1). While 90.02% was M. quinquenervia, the remainder comprised native Typhondorum lindleyanum (7.52%), Pandanus platyphylus (2.09%), Barringtonia racemosa (0.27%), Ravenala madagascariensis (0.05%), and exotic Acacia mangium (0.05%). Tree analyses found that the three variables were significantly different between habitats (DBH (cm): Kruskal-Wallis H = 363.70, df = 2, p < 0.001; height (m): Kruskal-Wallis H = 195.43, df = 2, p < 0.001; crown volume (m3): Kruskal-Wallis H = 350.33, df = 2, p < 0.001).
Table 1. Comparison of trees (mean ± SD) measured in different habitats within Mandena.
| Habitat | N | Species (N) | Families (N) | DBH (cm) | Height (m) | Crown volume (m3) | Shannon (H′) |
|---|---|---|---|---|---|---|---|
| Littoral Forest | |||||||
| ≥ 5 cm (DBH) | 1454 | 84 | 40 | 9.53 ± 5.09 | 7.22 ± 1.48 | 10.41 ± 18.31 | 3.54 ± 0.05 |
| Littoral Swamp | |||||||
| ≥ 5 cm (DBH) | 2211 | 49 | 32 | 11.66 ± 5.95 | 6.47 ± 1.13 | 3.91 ± 6.68 | 2.92 ± 0.08 |
| Melaleuca Swamp | |||||||
| ≥ 5 cm (DBH) | 2194 | 6 | 6 | 12.11 ± 5.89 | 6.76 ± 2.33 | 4.61 ± 7.64 | 0.39 ± 0.07 |
As a demonstration of human impact on the Melaleuca habitat, we recorded 65 M. quinquenervia with a mean DBH (X ± SD) of 12.85 ± 8.89 cm felled within our five Melaleuca swamp botanical plots between January and December 2013. In addition to timber harvesting and significantly different tree metrics, habitats were further distinguished by their vertical structure (Fig 3). The mean distance of the eight confirmed crossing sites that Hapalemur groups utilized in order to access the Melaleuca habitat from adjacent littoral forest is 9.75 ± 2.71 m (X ± SD).
Fig 3. Vertical structure comparison between each of the three Mandena habitats based on Gautier-transects [45]: littoral forest, littoral swamp, and Melaleuca swamp.
Spatial analysis
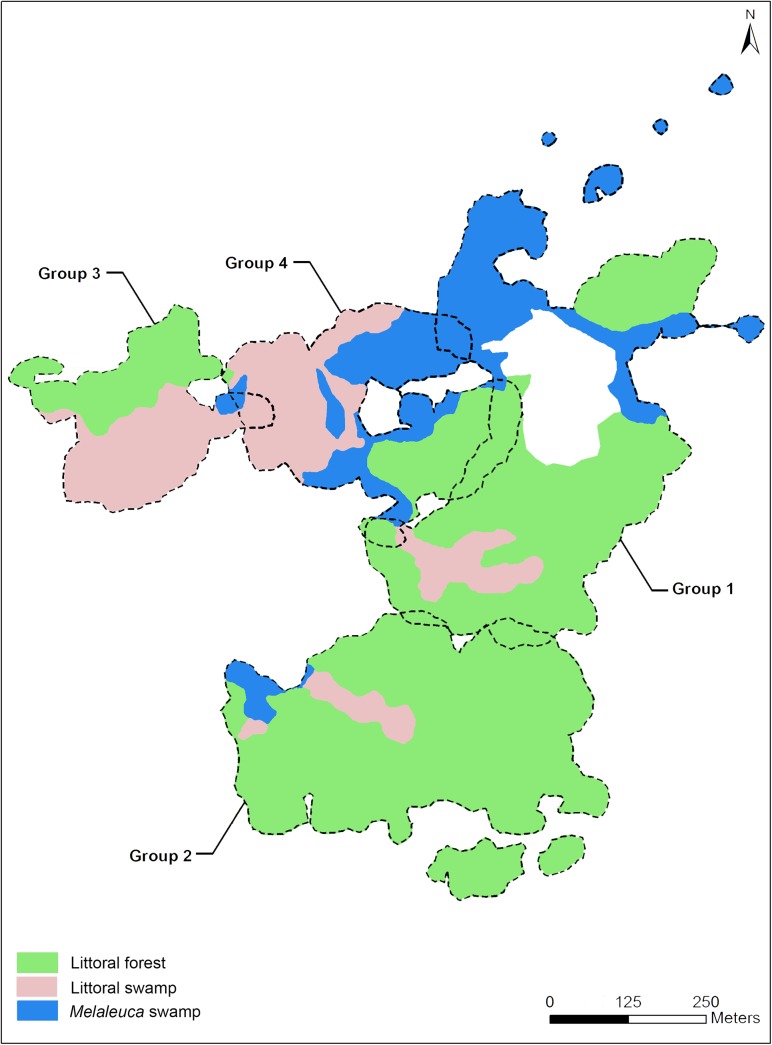
The total area (ha) of both home ranges utilized by groups 1 and 2 were even in size, while the home range of group 4 was substantially smaller (Table 2). The Melaleuca swamp habitat constituted large portions of the home ranges of groups 1 and 4, while it appeared to be minimal for group 2 (Fig 4).
Table 2. Total area (ha) and area per habitat type as obtained via 95% kernel density estimate.
| Group | Forest (ha) | % | Swamp (ha) | % | Melaleuca (ha) | % | Total (ha) |
|---|---|---|---|---|---|---|---|
| 1 | 11.67 | 53.45 | 1.27 | 5.81 | 8.89 | 40.74 | 21.82 |
| 2 | 19.95 | 94.16 | 0.85 | 4.03 | 0.38 | 1.80 | 21.19 |
| 4 | 3.68 | 27.13 | 4.69 | 34.58 | 5.19 | 38.29 | 13.55 |
Group 3 habitat data were not included as they constitute a smaller dataset.
Fig 4. Home ranges (95% kernel) of Hapalemur meridionalis focal groups at Mandena between January and December 2013.
Areas for each habitat (i.e., littoral forest, littoral swamp, Melaleuca swamp) are shown within each.
Activity and Habitat
We observed H. meridionalis for 1,762 hours between January and December 2013 across 194 focal days. Groups differed in the proportion of time (i.e., percentage) spent resting in each habitat (Forest = 27.99 ± 2.21; Swamp = 7.23 ± 1.35; Melaleuca = 4.76 ± 1.02 (X ± SE; N = 36 months)).
There were no differences in overall rates of resting between habitat types (Table 3). Significant interactions were found between temperature and habitat, day length and habitat, as well as group and habitat. Considering the covariates, resting is significantly affected by temperature, but not affected by seasons (i.e., day length). Post-hoc analyses of groups revealed a significant difference between groups 1 and 4 (p = 0.004), while groups 1 & 2, and 2 & 4 were similar in the proportion of time and location they chose to rest.
Table 3. Repeated measures analysis of variance for effects of habitat type on activity (using monthly percentages) of H. meridionalis at Mandena, January-December 2013.
Significant differences indicated in bold.
| Activity | Source of variation | df | F-ratio | p |
|---|---|---|---|---|
| Rest | Within-subjects | |||
| Habitat | 1.786 | 1.303 | 0.278 | |
| Temperature x Habitat | 1.786 | 3.521 | 0.041 | |
| Precipitation x Habitat | 1.786 | 2.170 | 0.129 | |
| Day length x Habitat | 1.786 | 3.756 | 0.034 | |
| Group x Habitat | 3.573 | 23.713 | <0.001 | |
| Covariates | ||||
| Temperature | 1 | 7.144 | 0.012 | |
| Precipitation | 1 | 1.330 | 0.258 | |
| Day length | 1 | 3.271 | 0.081 | |
| Between-subjects | ||||
| Group | 2 | 4.848 | 0.015 | |
| Feed | Within-subjects | |||
| Habitat | 1.995 | 3.523 | 0.036 | |
| Temperature x Habitat | 1.995 | 3.868 | 0.026 | |
| Precipitation x Habitat | 1.995 | 0.991 | 0.377 | |
| Day length x Habitat | 1.995 | 6.249 | 0.003 | |
| Group x Habitat | 3.990 | 25.583 | <0.001 | |
| Covariates | ||||
| Temperature | 1 | 24.282 | <0.001 | |
| Precipitation | 1 | 1.431 | 0.241 | |
| Day length | 1 | 10.154 | 0.003 | |
| Between-subjects | ||||
| Group | 2 | 0.473 | 0.628 | |
| Travel | Within-subjects | |||
| Habitat | 1.995 | 1.396 | 0.256 | |
| Temperature x Habitat | 1.995 | 1.309 | 0.278 | |
| Precipitation x Habitat | 1.995 | 3.454 | 0.038 | |
| Day length x Habitat | 1.995 | 2.108 | 0.131 | |
| Group x Habitat | 3.991 | 27.393 | <0.001 | |
| Covariates | ||||
| Temperature | 1 | 15.279 | <0.001 | |
| Precipitation | 1 | 1.619 | 0.213 | |
| Day length | 1 | 5.504 | 0.026 | |
| Between-subjects | ||||
| Group | 2 | 0.107 | 0.899 |
When considering feeding activity, there was no appreciable difference in the mean proportion of time each group fed; however, there were significant differences in the average proportion of feeding between the habitats (Forest = 25.52 ± 2.75; Swamp = 5.72 ± 1.09; Melaleuca = 11.35 ± 2.31 (X ± SE; N = 36)). Significant interactions were revealed between temperature and habitat, day length and habitat, and group and habitat (Table 3). Furthermore, feeding activity is affected by both temperature and day length, varying seasonally. Post-hoc analyses showed no discernible effect of feeding between groups.
Traveling showed no differences in means between the groups (Table 3), while the main effect of habitat was revealed to have no influence (Forest = 6.69 ± 0.51; Swamp = 2.02 ± 0.37; Melaleuca = 1.41 ± 0.21 (X ± SE; N = 36)). There were significant interaction effects between precipitation and habitat, and group and habitat. Considering the covariates, traveling is affected by both temperature and day length, varying seasonally. Post-hoc analyses showed no discernible effect of feeding between groups.
Melaleuca habitat use
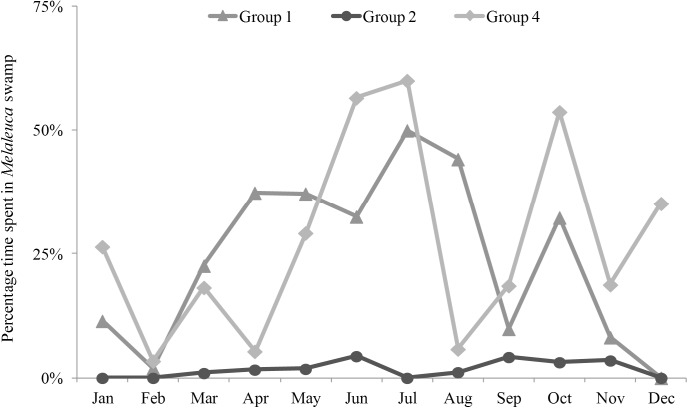
Considering individual focal days, H. meridionalis were observed to access Melaleuca habitat on 54.12% of days, although this only constituted 18.55% of our total observation record (Table 4). Despite this, both groups 1 and 4 accessed this invasive habitat often, while the minimal proportion of Melaleuca within the territory of group 2 was still utilized on greater than 20% of observation days. In terms of monthly percentage of time, however, group 2 utilized Melaleuca less compared to the other lemur groups (Fig 5).
Table 4. Role of Melaleuca swamp habitat on daily activity budget of H. meridionalis.
| Observ. days | Accessed Melaleuca | |||
|---|---|---|---|---|
| Number of days | % of days | % of observation record | ||
| Group 1 | 65 | 37 | 56.92 | 24.22 |
| Group 2 | 63 | 13 | 20.63 | 1.70 |
| Group 4 | 66 | 55 | 83.33 | 30.97 |
| Overall | 194 | 105 | 54.12 | 18.55 |
Fig 5. Monthly percentage of time spent by H. meridionalis groups in the Melaleuca habitat from January-December, 2013.
Two of the three bamboo lemur focal groups fed regularly on the flowers of this invasive species when available. Group 1 was observed to feed on the flowers of M. quinquenervia for 110.65 mins, constituting 0.79% of the annual diet. While group 2 never fed on M. quinquenervia flowers, group 4 spent 2.43% of their annual total feeding record (316.32 mins) selecting for them.
Discussion
Our results show that H. meridionalis use the introduced stands of M. quinquenervia substantially. Melaleuca and swamp habitats were often inundated by water during the warm/wet austral summer, which may restrict lemur use of these habitats to the cooler/drier months. However, examination of the monthly use of Melaleuca habitat by each group shows that while they spend less time here in the warmer months, they are capable of accessing this habitat when inundated. In fact, it is during this inundated period (Oct-Apr) when M. quinquenervia flowers in short, frequent bursts, but availability of this food item does not appear to influence the proportion of time bamboo lemurs spend in this habitat. This is especially true of groups 1 and 4, which spent considerable time feeding on these flowers when available, something that collared brown lemurs (E. collaris), eastern fat-tailed dwarf lemurs (C. medius), and gray mouse lemurs (M. murinus) have also been observed to exploit [51].
There were larger proportional areas of Melaleuca habitat in the territories of groups 1 and 4, thus they spent more time resting, feeding, and travelling in this habitat compared to group 2. Furthermore, these social groups were occasionally found before sunrise sleeping in a Melaleuca tree, typically huddled together at an approximate height of 7m. The overall difference in time-budget between the groups (when controlling for the effect of habitat and the covariates) was similar for feeding or travelling activity categories, but displayed appreciable differences for resting. Precipitation was not influential, except in the case of travelling. Additionally, we observed E. collaris, M. murinus, and southern woolly lemurs (A. meridionalis) travelling and sleeping in the Melaleuca habitat (see also [35]).
Indigenous and/or exotic tree species can provide benefits to both local people and primates [36, 52, 53]; the presence of Melaleuca in Mandena has value as habitat and as timber. Local people have begun to harvest these trees daily with the recent legal protection status of the Mandena littoral forest. Gaps in the Melaleuca canopy would allow for continued growth of terrestrial swamp vegetation, specifically graminoid species, which constitute a large portion of the H. meridionalis diet [41]. Melaleuca may have value as a temporary, fast-acting solution to connecting fragments while more long-term conservation solutions are being put in place, e.g., the Mandena nursery/reforestation efforts [35]. In the case of Mandena, the exotic Melaleuca acts similarly to a plantation forest for native fauna; while not as ideal as natural littoral forest, it provides valuable habitat and may possibly contribute to the conservation of endemic fauna [54]. Many studies from various countries, including Madagascar, have documented that exotic plantation forests can provide habitat for numerous native forest fauna [31, 55–61]. As an example, threatened bird species such as Apteryx mantelli, Casuarius casuarius, and Upupa epops have been known to occur in substantial populations in some exotic plantation habitats [60, 62, 63] (but see [64, 65]). Furthermore, primates such as black howler monkeys (Alouatta pigra) have been reported to thrive in Eucalyptus spp. plantations [66], mantled howler monkeys (A. palliata) are able to use shade-grown coffee (Coffea arabica) as the core of their habitat range [67], while siamang (Hylobates syndactylus) are known to occur in rubber (Hevea brasiliensis) and dammar gum (Shorea javanica) tree plantations [68]. As for Malagasy primates, many lemur genera (including Eulemur, Hapalemur, Indri, Cheirogaleus, Microcebus, and Lepilemur) are known to use old growth eucalypt (Eucalyptus sp.) plantations [31], while some occasionally utilize and feed from mono-stands of invasive guava (Psidium spp.) [39, 69]. Bamboo lemurs (both Hapalemur spp. and Prolemur simus) similarly use an exotic species habitat and appear to be relatively adaptable within anthropogenic landscapes: they have been seen crop-raiding agricultural fields at some sites and even living in a coffee plantation [39, 40, 70] (but see [71]).
That the lemurs utilized invasive Melaleuca for behavioral activities demonstrates its potential role as a riparian corridor to facilitate dispersal. From October 2012 to December 2013 we confirmed three separate Hapalemur dispersals that utilized Melaleuca corridors to emigrate from their natal group, while a fourth dispersal remains unconfirmed. While our data indicate that H. meridionalis are tolerant of habitat degradation and fragmentation, habitat matrix composition and connectivity have been shown to influence dispersal in various birds and mammals [72, 73], e.g., hazel grouse Bonasa bonasia [74], barred antshrikes Thamnophilus doliatus [75], Angola black-and-white colobus Colobus angolensis palliatus [76], and various marsupials [77]. Furthermore, exotic tree plantations/forests have been demonstrated to facilitate dispersal for a wide range of taxa [78, 79], for example, dispersal of the chucao tapaculo (Scelorchilus rubecula) is facilitated by the vertical structure rather than plant species composition of the corridor, in this case shrub fields dominated by 1–2 m tall invasive Baccharis magellanica [80].
While instances of successful dispersal provide a glimmer of hope, the further fragmentation of remaining forests is of great concern if forest species of Madagascar are to persist [8]. Lemurs fulfill important ecological roles, e.g., they are the primary seed dispersers and pollinators, and are essential for maintaining the island’s unique forests; their loss would likely trigger extinction cascades [55, 81]. Although the fate of all lemur species should be considered precarious due to increasing habitat destruction, the knowledge that some lemurs are able to cope with this degradation (to a certain degree) should be seen as positive. Recent studies have begun to alter our view of Hapalemur spp. as dietary specialists: they demonstrate dietary flexibility and some populations are able to subsist on items other than bamboo [39, 41, 82]. Some primate species adapted to narrow ecological specializations may be sensitive to natural or anthropogenic habitat perturbations [83, 84], whereas others have been shown to adjust to these changing environments [75, 85].
The ecological flexibility of the southern bamboo lemurs might provide a model for conservation action to help some of their congeners to survive. Among the most threatened within the genus is the Lac Alaotran gentle lemur (H. alaotrensis), assessed by the IUCN [86] as Critically Endangered (CR B1ab(iii,v)), due to its greatly restricted range that is becoming increasingly populated while the remaining viable habitat continues to shrink [87]. They subsist on a diet limited to sedges and non-bamboo grasses [82], similar to H. meridionalis when it is in the Melaleuca habitat. In captivity, however, H. alaotrensis regularly display a preference for bamboo [88], suggesting little divergence from congeners, with a flexibility that may allow them to persist in habitats outside of Lac Alaotra. As it would appear that all former subspecies of H. griseus maintain some dietary plasticity, in that they are not restricted to bamboo forests [39, 41, 82], perhaps conservationists need to rethink their strategy when considering how to save species of the Hapalemur genus.
Supporting Information
(XLSX)
Acknowledgments
This work was carried out under the Accord de Collaboration between the University of Antananarivo and the University of Hamburg, as well as a collaboration agreement between TME and QIT Madagascar Minerals (QMM). We thank the Madagascar Ministry of the Environment, Water and Forests for approving protocols and granting permission to conduct research. Special thanks to Jacques Rakotondranary and Tolona Andrianasolo for their logistical support and to Katie Hall and Natalie Breden for their assistance in the field. We acknowledge Johny Rabenantoandro and the QMM biodiversity staff for their assistance and provision of on-site logistical support. We thank Clara Scarry and Shauna Burgess Mora for assistance with the spatial analyses. Finally, we thank Steven Goodman for helpful comments and suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work received funding and in-kind support from American Society of Primatologists, Conservation International’s Primate Action Fund, IDEAWILD, Mohamed bin Zayed Species Conservation Fund (Project Number: 11253008), Primate Conservation Inc., and the Primate Society of Great Britain/Knowsley Safari Park. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. QIT Madagascar Minerals provided support in the form of salaries for authors [JBR, FR, LNA, DR, RR], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Asner GP, Rudel TK, Aide TM, Defries R, Emerson R. A contemporary assessment of change in humid tropical forests. Conserv Biol. 2009; 23: 1386–1395. 10.1111/j.1523-1739.2009.01333.x [DOI] [PubMed] [Google Scholar]
- 2. Gibson L, Lee TM, Koh LP, Brook BW, Gardner TA, Barlow J, et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature. 2011; 478: 378–381. 10.1038/nature10425 [DOI] [PubMed] [Google Scholar]
- 3. Laurance WF, Delamônica P, Laurance SG, Vasconcelos HL, Lovejoy TE. Conservation: rainforest fragmentation kills big trees. Nature. 2000; 404: 836–836. [DOI] [PubMed] [Google Scholar]
- 4. Broadbent EN, Asner GP, Keller M, Knapp DE, Oliveira PJ, Silva JN. Forest fragmentation and edge effects from deforestation and selective logging in the Brazilian Amazon. Biol Conserv. 2008; 141: 1745–1757. [Google Scholar]
- 5. Laurance WF, Goosem M, Laurance SG. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol. 2009; 24: 659–669. 10.1016/j.tree.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 6. Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F. Fifty years of deforestation and forest fragmentation in Madagascar. Environ Conserv. 2007; 34: 1–9. [Google Scholar]
- 7. Hannah L, Dave R, Lowry PP II, Andelman S, Andrianarisata M, Andriamaro L, et al. Climate change adaptation for conservation in Madagascar. Biol Lett. 2008; 4: 590–594. 10.1098/rsbl.2008.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganzhorn JU, Wilmé L, Mercier J-L. Explaining Madagascar’s biodiversity In: Scales IR, editor. Conservation and environmental management in Madagascar. New York: Routledge; 2014. pp. 17–43. [Google Scholar]
- 9. Wearn OR, Reuman DC, Ewers RM. Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science. 2012; 337: 228–232. 10.1126/science.1219013 [DOI] [PubMed] [Google Scholar]
- 10. Beier P, Noss RF. Do habitat corridors provide connectivity? Conserv Biol. 1998; 12: 1241–1252. [Google Scholar]
- 11. Hilty JA, Lidicker WZ Jr, Merenlender A. Corridor ecology: the science and practice of linking landscapes for biodiversity conservation Washington, D.C.: Island Press; 2012. [Google Scholar]
- 12. Caro TM, Laurenson MK. Ecological and genetic factors in conservation: a cautionary tale. Science. 1994; 263: 485–486. [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science. 1998; 281: 2045–2047. [DOI] [PubMed] [Google Scholar]
- 14. Bevers M, Flather CH. Numerically exploring habitat fragmentation effects on populations using cell-based coupled map lattices. Theor Popul Biol. 1999; 55: 61–76. [DOI] [PubMed] [Google Scholar]
- 15. Haddad NM. Corridor and distance effects on interpatch movements: a landscape experiment with butterflies. Ecol. Appl. 1999; 9: 612–622. [Google Scholar]
- 16. Haddad NM. Corridor length and patch colonization by a butterfly, Junonia coenia . Conserv Biol. 2000; 14: 738–745. [Google Scholar]
- 17. Mech SG, Hallett JG. Evaluating the effectiveness of corridors: a genetic approach. Conserv Biol. 2001; 15: 467–474. [Google Scholar]
- 18. Hale ML, Lurz PW, Shirley MD, Rushton S, Fuller RM, Wolff K. Impact of landscape management on the genetic structure of red squirrel populations. Science. 2001; 293: 2246–2248. [DOI] [PubMed] [Google Scholar]
- 19. Lens L, Dongen SV, Norris K, Githiru M, Matthysen E. Avian persistence in fragmented rainforest. Science. 2002; 298: 1236–1238. [DOI] [PubMed] [Google Scholar]
- 20. Schwitzer C, Mittermeier RA, Johnson SE, Donati G, Irwin M, Peacock H, et al. Averting lemur extinctions amid Madagascar's political crisis. Science. 2014; 343: 842–843. 10.1126/science.1245783 [DOI] [PubMed] [Google Scholar]
- 21. Golden CD. Bushmeat hunting and use in the Makira Forest, north-eastern Madagascar: a conservation and livelihoods issue. Oryx. 2009; 43: 386–392. [Google Scholar]
- 22. Kremen C, Cameron A, Moilanen A, Phillips SJ, Thomas CD, Beentje H, et al. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science. 2008; 320: 222–226. 10.1126/science.1155193 [DOI] [PubMed] [Google Scholar]
- 23. Irwin MT, Wright PC, Birkinshaw C, Fisher BL, Gardner CJ, Glos J, et al. Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol Conserv. 2010; 143: 2351–2362. [Google Scholar]
- 24. Campera M, Serra V, Balestri M, Barresi M, Ravaolahy M, Randriatafika F, et al. Effects of habitat quality and seasonality on ranging patterns of collared brown lemur (Eulemur collaris) in littoral forest fragments. Int J Primatol. 2014; 35: 957–975. [Google Scholar]
- 25. Braithwaite RW, Lonsdale WM, Esterbergs JA. Alien vegetation and native biota in tropical Australia: the impact of Mimosa pigra . Biol Conserv. 1989; 48: 189–210. [Google Scholar]
- 26. D'Antonio CM, Vitousek PM. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu Rev Ecol Syst. 1992; 23: 63–87. [Google Scholar]
- 27. Dray FA Jr, Bennett BC, Center TD. Invasion history of Melaleuca quinquenervia (Cav.) ST Blake in Florida. Castanea. 2006; 71: 210–225. [Google Scholar]
- 28. Miandrimanana C, Solovavy N, Marinjakasandrata R, Birkinshaw CB. Approche expérimentale de l’utilisation de glyphosate dans le contrôle de Melaleuca quinquenervia (Myrtaceae), une espèce envahissante dans la réserve communautaire de la forêt d’Analalava-Foulpointe (Madagascar). Madagascar Conservation and Development. 2014; 9: 49–53. [Google Scholar]
- 29. Ganzhorn JU, Lowry PP II, Schatz GE, Sommer S The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. Oryx. 2001; 35: 346–348. [Google Scholar]
- 30. Bollen A, Donati G. Conservation status of the littoral forest of south-eastern Madagascar: a review. Oryx. 2006; 40: 57–66. [Google Scholar]
- 31. Ganzhorn JU. A possible role of plantations for primate conservation in Madagascar. Am J Primatol. 1987; 12: 205–215. [DOI] [PubMed] [Google Scholar]
- 32. Ganzhorn JU, Abraham J-P. Possible role for plantations for lemur conservation in Madagascar: food for folivorous species. Folia Primatol. 1991; 56: 171–176. [Google Scholar]
- 33. Ganzhorn JU, Malcomber S, Andrianantoanina O, Goodman SM. Habitat characteristics and lemur species richness in Madagascar. Biotropica. 1997; 29: 331–343. [Google Scholar]
- 34. Ramanamanjato J-B, Ganzhorn JU. Effects of forest fragmentation, introduced Rattus rattus and the role of exotic tree plantations and secondary vegetation for the conservation of an endemic rodent and a small lemur in littoral forests of southeastern Madagascar. Anim Conserv. 2001; 4: 175–183. [Google Scholar]
- 35. Andriamandimbiarisoa L, Blanthorn T, Ernest R, Ramanamanjato J-B, Randriatafika F, et al. Habitat corridor utilisation by the gray mouse lemur, Microcebus murinus, in the littoral forest fragments of southeastern Madagascar. Madagascar Conservation and Development. In press. [Google Scholar]
- 36. Gérard A, Ganzhorn JU, Kull CA, Carrière SM. Possible roles of alien plants in native fauna conservation: Madagascar as a case study. Restor Ecol. 2015; 10.1111/rec.12246 [DOI] [Google Scholar]
- 37. Virah-Sawmy M, Willis KJ, Gillson L. Threshold response of Madagascar’s littoral forest to sea-level rise. Global Ecol Biogeogr. 2009; 18: 98–110. [Google Scholar]
- 38. Hilty JA, Merenlender AM. Use of riparian corridors and vineyards by mammalian predators in northern California. Conserv Biol. 2004; 18: 126–135. [Google Scholar]
- 39. Grassi C. Variability in habitat, diet, and social structure of Hapalemur griseus in Ranomafana National Park, Madagascar. Am J Phys Anthropol. 2006; 131: 50–63. [DOI] [PubMed] [Google Scholar]
- 40. Wright PC, Johnson SE, Irwin MT, Jacobs R, Schlichting P, Lehman S, et al. The crisis of the critically endangered greater bamboo lemur (Prolemur simus). Primate Conservation. 2008; 23: 5–17. [Google Scholar]
- 41. Eppley TM, Verjans E, Donati G. Coping with low-quality diets: a first account of the feeding ecology of the southern gentle lemur, Hapalemur meridionalis, in the Mandena littoral forest, southeast Madagascar. Primates. 2011; 52: 7–13. 10.1007/s10329-010-0225-3 [DOI] [PubMed] [Google Scholar]
- 42. Dumetz N. High plant diversity of lowland rainforest vestiges in eastern Madagascar. Biodivers Conserv. 1999; 8: 273–315. [Google Scholar]
- 43. Eppley TM, Hall K, Donati G, Ganzhorn JU. An unusual case of affiliative association of a female Lemur catta in a Hapalemur meridionalis social group. Behaviour. 2015a; 152: 1041–1061. [Google Scholar]
- 44. Eppley TM, Ganzhorn JU, Donati G. Cathemerality in a small, folivorous primate: proximate control of diel activity in Hapalemur meridionalis . Behav Ecol Sociobiol. 2015b; 69: 991–1002. [Google Scholar]
- 45.Gautier L, Chatelain C, Spichiger R. Presentation of a relevé method for vegetation studies based on high resolution satellite imagery. In: Seyani JH, Chikuni AC, editors. Proceedings of the XIIIth Plenary Meeting AETFAT. Malawi; 1994. pp. 1339–1350. [Google Scholar]
- 46. Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974; 49: 226–267. [DOI] [PubMed] [Google Scholar]
- 47.Beyer HL. Geospatial Modelling Environment. 0.7.2.0 ed. 2012. Available: http://www.spatialecology.com/gme.
- 48.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. Available: http://www.R-project.org/.
- 49. Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989; 70: 164–168. [Google Scholar]
- 50. Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 51. Bollen A. Fruit-frugivore interactions in a Malagasy littoral forest: a community-wide approach of seed dispersal Ph.D. Dissertation, Antwerp University, Belgium: 2003. [Google Scholar]
- 52. Bicca-Marques JC, Calegaro-Marques C. Exotic plant species can serve as staple food sources for wild howler populations. Folia Primatol. 1994; 63: 209–211. [DOI] [PubMed] [Google Scholar]
- 53. Grimes K, Paterson JD. Colobus guereza and exotic plant species in the Entebbe Botanical Gardens. Am J Primatol. 2000; 51: 59–60. [Google Scholar]
- 54. Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J. Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv. 2008; 17: 925–951. [Google Scholar]
- 55. Ganzhorn JU, Fietz J, Rakotovao E, Schwab D, Zinner D. Lemurs and the regeneration of dry deciduous forest in Madagascar. Conserv Biol. 1999; 13: 794–804. [Google Scholar]
- 56. Gascon C, Lovejoy TE, Bierregaard RO Jr, Malcolm JR, Stouffer PC, Vasconcelos HL, et al. Matrix habitat and species richness in tropical forest remnants. Biol Conserv. 1999; 9: 223–229. [Google Scholar]
- 57. Goodman SM. Galidia elegans, ring-tailed mongoose In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago: University of Chicago Press; 2003. pp. 1351–1354. [Google Scholar]
- 58. Andreone F, Guarino FM, Randrianirina JE. Life history traits, age profile, and conservation of the panther chameleon, Furcifer pardalis (Cuvier 1829), at Nosy Be, NW Madagascar. Trop Zool. 2005; 18: 209–225. [Google Scholar]
- 59. Barbaro L, Pontcharraud L, Vetillard F, Guyon D, Jactel H. Comparative responses of bird, carabid, and spider assemblages to stand and landscape diversity in maritime pine plantation forests. Ecoscience. 2005; 12: 110–121. [Google Scholar]
- 60. Barbaro L, Couzi L, Bretagnolle V, Nezan J, Vetillard F. Multi-scale habitat selection and foraging ecology of the Eurasian hoopoe (Upupa epops) in pine plantations. Biodivers Conserv. 2008; 17: 1073–1087. [Google Scholar]
- 61. Simmen B, Bayart F, Marez A, Hladik A. Diet, nutritional ecology, and birth season of Eulemur macaco in an anthropogenic forest in Madagascar. Int J Primatol. 2007; 28: 1253–1266. [Google Scholar]
- 62. Kleinpaste R. Kiwis in a pine forest habitat In: Fuller E, Andrews JRH, Harris-Ching R, editors. Kiwis, a monograph of the family Apterygidae. Auckland: SeTo Publishing; 1990. pp. 97–138. [Google Scholar]
- 63. Keenan R, Lamb D, Woldring O, Irvine T, Jensen R. Restoration of plant biodiversity beneath tropical tree plantations in Northern Australia. Forest Ecol Manag. 1997; 99: 117–131. [Google Scholar]
- 64. Wilmé L. Composition and characteristics of bird communities in Madagascar In: Lourenço WR, editor. Biogéographie de Madagascar. Paris: l’ORSTOM; 1996. pp. 349–362. [Google Scholar]
- 65. Watson JE, Whittaker RJ, Dawson TP. The importance of littoral forest remnants for indigenous bird conservation in southeastern Madagascar. Biodivers Conserv. 2005; 14: 523–545. [Google Scholar]
- 66. Bonilla-Sánchez YM, Serio-Silva JC, Pozo-Montuy G, Chapman CA. Howlers are able to survive in Eucalyptus plantations where remnant and regenerating vegetation is available. Int J Primatol. 2012; 33: 233–245. [Google Scholar]
- 67. Williams‐Guillén K, McCann C, Martínez Sánchez JC, Koontz F. Resource availability and habitat use by mantled howling monkeys in a Nicaraguan coffee plantation: can agroforests serve as core habitat for a forest mammal? Anim Conserv. 2006; 9: 331–338. [Google Scholar]
- 68. Michon G, de Foresta H. The Indonesian agro-forest model In: Halliday P, Gilmour DA, editors. Conserving biodiversity outside protected areas: the role of traditional agro-ecosystems. Gland, Switzerland: IUCN; 1995. pp. 90–106. [Google Scholar]
- 69. Birkinshaw CR, Colquhoun IC. Lemur food plants In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago: University of Chicago Press; 2003. pp. 1207–1220. [Google Scholar]
- 70. Martinez B. Occurrence of bamboo lemurs, Hapalemur griseus occidentalis, in an agricultural landscape on the Masoala peninsula. Lemur News. 2008; 13: 11–14. [Google Scholar]
- 71. Olson ER, Marsh RA, Bovard BN, Randrianarimanana HLL, Ravaloharimanitra M, Ratsimbazafy JH, et al. Habitat preferences of the Critically Endangered greater bamboo lemur (Prolemur simus) and densities of one of its primary food sources, Madagascar giant bamboo (Cathariostachys madagascariensis), in sites with different degrees of anthropogenic and natural disturbance. Int J Primatol. 2013; 34: 486–499. [Google Scholar]
- 72. Wethered R, Lawes MJ. Matrix effects on bird assemblages in fragmented Afromontane forests in South Africa. Biol Conserv. 2003; 114: 327–340. [Google Scholar]
- 73. Pardini R. Effects of forest fragmentation on small mammals in an Atlantic Forest landscape. Biodivers Conserv. 2004; 13: 2567–2586. [Google Scholar]
- 74. Aberg J, Jansson G, Swenson JE, Angelstam P. The effect of matrix on the occurrence of hazel grouse (Bonasa bonasia) in isolated habitat fragments. Oecologia. 1995; 103: 265–269. [DOI] [PubMed] [Google Scholar]
- 75. Gillies CS, St. Clair CC. Riparian corridors enhance movement of a forest specialist bird in fragmented tropical forest. Proc Natl Acad Sci USA. 2008; 105: 19774–19779. 10.1073/pnas.0803530105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Anderson J, Rowcliffe JM, Cowlishaw G. Does the matrix matter? A forest primate in a complex agricultural landscape. Biol Conserv. 2007; 135: 212–222. [Google Scholar]
- 77. Laurance SG, Laurance WF. Tropical wildlife corridors: use of linear rainforest remnants by arboreal mammals. Biol Conserv. 1999; 91: 231–239. [Google Scholar]
- 78. Ferreras P. Landscape structure and asymmetrical inter-patch connectivity in a metapopulation of the endangered Iberian lynx. Biol Conserv. 2001; 100: 125–136. [Google Scholar]
- 79. Wethered R, Lawes MJ. Nestedness of bird assemblages in fragmented Afromontane forest: the effect of plantation forestry in the matrix. Biol Conserv. 2005; 123: 125–137. [Google Scholar]
- 80. Castellón TD, Sieving KE. An experimental test of matrix permeability and corridor use by an endemic understory bird. Conserv Biol. 2006; 20: 135–145. [DOI] [PubMed] [Google Scholar]
- 81. Razafindratsima OH, Dunham AE. Assessing the impacts of nonrandom seed dispersal by multiple frugivore partners on plant recruitment. Ecology. 2015; 96: 24–30. [DOI] [PubMed] [Google Scholar]
- 82. Mutschler T. Folivory in a small-bodied lemur. The nutrition of the Aloatra gentle lemur (Hapalemur griseus alaotrensis) In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM, editors. New directions in lemur studies. New York: Kluwer Academic/Plenum Press; 1999. pp. 221–239. [Google Scholar]
- 83. Harcourt AH, Coppeto SA, Parks SA. The distribution–abundance (density) relationship: its form and causes in a tropical mammal order, primates. J Biogeogr. 2005; 32: 565–579. [Google Scholar]
- 84. Kamilar JM, Paciulli LM. Examining the extinction risk of specialized folivores: a comparative study of colobine monkeys. Am J Primatol. 2008; 70: 816–827. 10.1002/ajp.20553 [DOI] [PubMed] [Google Scholar]
- 85. Nowak K, Lee PC. “Specialist” primates can be flexible in response to habitat alteration In: Marsh LK, Chapman CA, editors. Primates in fragments: complexity and resilience. New York: Springer Science & Business Media; 2013. pp. 199–211. [Google Scholar]
- 86. IUCN. 2012 IUCN Red list of Threatened Species. International Union for Conservation of Nature (IUCN), Species Survival Commission (SSC), Gland, Switzerland and Cambridge, UK: 2012. Available: http://www.redlist.org. [Google Scholar]
- 87. Bakoariniaina LN, Kusky T, Raharimahefa T. Disappearing Lake Alaotra: Monitoring catastrophic erosion, waterway silting, and land degradation hazards in Madagascar using Landsat imagery. J Afr Earth Sci. 2006; 44: 241–252. [Google Scholar]
- 88. Beattie JC, Feistner ATC. Husbandry and breeding of the Alaotran gentle lemur: Hapalemur griseus alaotrensis at Jersey Wildlife Preservation Trust. International Zoo Yearbook. 1998; 36: 11–19. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.